Note: Descriptions are shown in the official language in which they were submitted.
i2~f'~
This invention relates to the preparation of novel rho-
danine derivatives useful for preventing or treating complica-
-tions due to aldose reductase and in particular to intermediate
compounds useful in the preparation of such deravitives.
This application is a divisional application of copend- -
ing application No. 499002 filed January 6, 19~86.
A variety of compounds have been developed for treating
diabetes caused by the increase of blood sugar resulting from the
decrease of insulin secreted from the pancreas. No compound,
however, is known which is sufficiently effective in preventing
or treating complications developing due to aldose reductase in
the course of chronic diabetes, such as diabetic cataract, neu-
ropathy and nephropathy.
Medicaments having a rhodanine skeletone for treatingsuch complications are known, e.g. those disclosed in Japanese
Unexamined Pa-tent Publica-tions No. 28074/1982 and No. 40478/1982
but are unsatisfactory in their efficacy.
The invention of the copending applica-tion is to pro-
vide a class oE compounds which are sufficiently effective for
preventing or treatlng the complicatlon due to aldose reductase
as well as a process for preparing such compounds.
In the copending application No. 499002 compounds of
the formula
~ /
n, Nr~l ~s~S ( 1 )
0
.. ~ .
~.
~ Z ~ 73
wherein Rl is lower alkyl, A is a group of the formula
l3
-CH=C-CH= (in which R3 is hydrogen or lower alkyl) or methylene,
and R2 is hydrogen or a group of the formula -(CH2)nCOO~I (in
which n is an integer of 1 -to 6) or pharmaceutically acceptable
salts of the derivatives are disclosed and claimed.
We have conducted extensive research to develop com-
pounds having a high aldose reductase inhibitory activity and
found that the rhodanine derivatives of the formula (1) and phar-
maceutically acceptable salts thereof exhibi-t outstanding action
of inhibiting aldose reductase, and further show excellen-t
actions of lowering blood sugar level and also decreasing the
level of lipids.
The compounds of formula (1) are novel compounds undis-
closed in literature. The compounds of formula (1) have the
foregoing e~celle.nt actions of inhibiting aldose reductase, and
of lowering blood sugar level and lipid level, and therefore
use~ul as a medicame.nt for treating complications due -to aldose
reductase and a:Lso for treatlng diabetes and related diseases.
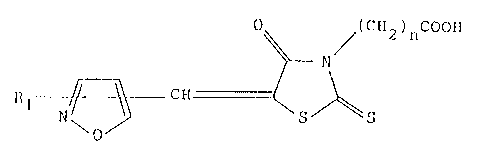
~ preEerred class of the compounds are represen-ted by
the formula
o / ( c 2 ) rl
)~ N
1~1 Ir j~ Cll2 ~ S ~ S (1-2)
wherein Rl is as defined above, and n is as defined above prefer-
ably a group -CH2COOH.
. ; . , ~ , .
., ~ ~ ., . . . -
2~
Examples of lower alkyl groups represented by Rl in the
forrnu:La (1-2) are straigh-t chain or branched chain Cl-C6 alkyl
groups such as me-thyl, ethyl, propyl, isopropyl, bu-tyl, sec-
butyl, ter-t-butyl, iso-butyl, pentyl, sec-pentyl hexyl, etc. The
pharmaceutically acceptable salts of the compounds of formula (l-
2) i.nclude all the salts formed by the reaction of the carboxylic
acid represen-ted by the group -(CEI2)nCOOH withca base, preferably
a base which forms a pharmaceutically acceptable salt. Such
salts include salts with alkali metals such as sodium, potassium
and lithium; alkaline earth metals such as calcium and magnesium;
ammonium; tetra(Cl-C4 alkyl)ammonium such as tetramethylammoniwn,
tetraethylammonium, tetrapropylammonium and tetrabutylammoniwn;
and salts with organic amines including mono- and di-(C1-C4
alkyl)amines such as methylamine, ethylamine, isopropylamine,
tert-butylamine, dimethylamine and diethylamine; C5-C7 cycloaky-
lamines such as cyclopentylamine and cyclohexylamine; phenyl-Cl-
C3-alkylamines such as benzylamine, phenethylamine and phenyl-
propyl.amine; 5- or 6-membered he-terocylcic compounds containing
in its ring structure one or two nitrogen atoms as the het-
eroatom, such as piperidine, piperazine, imidazoline and pyrrole;
mono-- or di-(Cl-C~ alkanol)amines such as monoethanolamine, mono-
propanolarllirle and diethanolamine; basic amino acids such as
lysine, arginine and hystidine; etc.
These salts can be prepared by a conven-tional process,
for example by reacting the compound of the formula (1-2) with a
theoretical amount of a suitable base in an appropriate solvent.
Examples of useful bases are hydroxides or carbonates of alkali
metals and alkaline earth metals, ammonium hydroxide, ammonia,
tetra(C1-C4 alkyl) ammonium halides, or the organic amines exem-
plified above etc. Useful solvents include Cl-C6 monohydric
alkanol and water, etc. The salt can be separated by lyophiliz-
ing the solution when soluble in the solvent used, or by filter-
ing the reaction mixture when insoluble or sparingly soluble in
the solvent used, if required after distilling off the solvent.
~ _ 3 _
73
The rhodanine derivatives of the formula (1-2) can be
prepared, for example, by -the process as shown below in Reaction
Scheme I.
<Reac-tior. Scheme I>
~ ~ / ( C1l2 ) n
lO1 N ~ -CHO ~ <
(2) (3)
O ~ / (CEi2) COOH
~ CH ~ S
~ / (~H2)~COOH
\ O ~ S ~ S (1-2)
In -the foregoing Eormulas, R1 and n are as defined
above,
The present invention provides compounds represented by
the formula
~ / ( CE12 ) JlC~I
1~ Ctt ~S,~s (1')
_ 4 _
'
, ,
.,
`
~ '7~
wherein Rl is lower alkyl and n is an integer of 1 to 6 which are
of course intermediates in the preparation of the preferred corn-
pounds of the formula (1-2).
According to Reaction Scheme I, the condensation is
conduc-ted between the aldehyde compound of the formula (2) and
the compound of the formula (3) under the conv~entional conditions
for aldol condensation or Knoevenagel condensation to give -the
compound of the formula (1'). The compounds of the formula (1'),
lo may be subsequently reduced to obtain the compound of the formula
(1-2).
The aldol condensation and Knoevenagel condensation are
well known, and are described, for example, in Merck Index, 10th
edition, "Organic name reactions". Examples of solvents useful
in the condensation are Cl-C4 aliphatic alcohols such as ethanol,
me-thanol and isopropanol, Cl-C3 alkanoic acids such as acetic
acid, an.hydri.des oE the alkanoic acids such as acetic anhydride,
propi.onic anhydride, di(Cl-C4 alkyl)ethers such as diethyl ether
and d:ipropyl ether, cyclic ethers such as tetrahydrofuran, diox-
ane and the like. Examples of catalysts useful in the condensa-
tion are basic compounds which are conventionally used for aldol
condensation or knoevenagel condensation. Examples of such basic
compounds serving as the catalyst are inorganic bases including
hydroxides, carbonates, hydrogencarbonates, Cl-C6 alkoxides and
Cl-C6 alkanoic acid salts of alkali metals and alkaline earth
metals, such as potassium hydroxide, sodium hydroxide, calcium
hydroxide, sodium carbonate, sodium hydrogencarbonate, sodium
methoxide, sodium ethoxide, magnesium ethoxide and sodium ac-
etate, and ammonia; and organic bases including mono-, di- and
tri-(Cl-C4 alkyl)amines such as methylamine, ethylamine diethy
lamine, triethylamine, aniline, nicotine, pyridine, piperidine
and the like. ~lthough the amounts of the aldehyde derivative of
the formula (2) and the compound of the formula (3) are suitably
determined, it is preferred to use the compound of the ~ormula
(3) in an amount of about 1.0 to about 1.2 moles per mole of the
,
'
~ '73
aldehyde derivative of the formula (2). The catalys-t is used in
a conventional amount of about 0.1 to about 1.0 mole per mole of
the cornpound of the formula (2). The reaction is usually per-
formed with heating and advantageously proceeds as a rule at a
temperature of about 50 to about 150C, preferably at the reflux
temperature of the solvent, and completed in about 1 to about 10
hours. ~ -
The resulting compound of the formula (1') is further
o subjected to a reduction. The reduction is conducted usually inliquid phase under a hydrogen pressure of between atmospheric
pressure and about 10 atm. The solvents to be used in the reduc-
tion are not specifically limited unless they adversely affect
-the reaction, and include wa-ter, methanol, ethanol, acetone and
the like. Water is usually preferred. When the compound of the
formula (1') is insoluble or sparingly soluble in water, it is
preferred to transform the compound (1') into a water-soluble
salt of alkali metal such as sodium, potassium or the like prior
to reduction. While the reduction is more advantageously
effected with the increase in hydrogen pressure, the reaction
sufficiently proceeds under a hydrogen pressure of between about
atmospheric pressure to about 10 atm. The temperature is suit-
ably determined, but ranges generally from about 10 to about
50C, preferably around room temperature. ~seful catalysts
include those effective for hydrogenating the C-C double bond,
such as platinum, palladium, nickel and the like. The foregoing
reaction gives the compound of the invention in which A is methy-
lene. The reduction is usually completed in about 1 to about 10
hours.
The compound of the formula ~1-2) prepared by the aforesaid
process can be easily separated from the reaction product by
conventional separation methods such as column chromatography,
recrystallization method, etc.
The compounds of the formula (3) and the compounds of
'' ' - :-, ,
: ~. :' :. '' .
~ Z 7~
-the formula (2) wherein are known or can be prepared by known
methods.
For use in preventing or treating the diseases caused
by aldose reductase, e.g. diabetic cataract, neuropathy and
nephropathy or in preventing or treating diabetes, the rhodanine
derivatives of formula (1-2) are administered ~to mammals includ-
ing humans in pharmaceutical dosage form such as oral prepara-
tion, injection, rectal suppository or eye drop, in accordance
with the purpose of therapy contemplated. Such preparations can
be formulated in the manner already known in the art, using con-
ventional pharmaceutically acceptable, non-toxic carriers or
excipients. For the formulation of solid preparations for oral
administration, such as tablets, coated tablets, granules, pow-
ders and
- . .
: .
. .
,: .. ... , . , : ..
.. . .
... .
ec~ ule9, excip1ents and, when required, bincler~,
diJLntegr,ltors, lubrieants or glazes, colorirlg aeents,
corri~erlts, etc. can be added to the eompound of this
invel)tiorl. Sucll additives are already l<nown in the art
an~l useful examples are excipients such a~ lactose, white
sll~ar, sodium chloride, glucose solution, ~tarcll, eale1um
earbonate, I<aolin, erystalline eellulose an(l silleie aeld;
bin(~ers such as water, ethanol, proparlol, glueose,
earboxymetilyl cellulose, shellae, methyl cellulo3e,
potassium phosphate and polyvinyl pyrrolidone;
disinteerators sueh as dried starch, sodium alginclte, agar
pow(ler, sodium hydrogenearbonate, ealeium earbonate,
sodiuln lauryl su]fate, glyeeryl monostearate, ~tareh and
laeto~e; lubrieants or glazes sueh as purified tale,
stearie acid salt, borle aeid powder, solid polyethylene
elycol; eorrigents sueh as ~ucro~e, compound bitter orange
peel, citric acid, tartarle acid, etc. For the
f`ormulatiorl of liqulcl preparations for oral
adm~ tratiOIl, 9uch as 301utions for oral adm1ni9tratlon,
~yrups, etc., eonventional eorrlgents, buffers,
~tabilizers, etc. can be added to the compound of the
invention. Sueh preparations can be formulatecl in the
u~llal manner. ~xamples of userul eorrigents are those
exemplifiecl above. Typical buffers inclucle sodlum citrate.
Stabillzer~ include tragacanth, gum arabic, gelatin,
73
etc. rhe pharlnacolo~ical composition3 thus prepared are
ol.ul.l.ly aclllllnlatere(l. I'arenteral solutlon3 can bc
forlnulated in the u3ual manner using distlll~d ~ater for
itljection as the carrier and adding to the compoulld Or t~le
il~vel~tion converltional additives such as pll-adjuatlng
agellts, buffers, stabilizers, isotonlc agent.~, local
anestlletlcs, etc. Examples of the pll-ad~usting agents and
bu~`fera are sodium salts of eitrie acld, acetie aeld and
pl~ospl)orie aeid. l`he stabilizers inelude so(liurn
pyrosulfite (anti-oxidant), EDT~, thioglycolie acid,
tlliolactlc aeid, ete. Example~ of userul local
anesthetics are proeaine hydroellloride, xylocaine
hyclrochloride, lidoeaine hydroeh1oride, etc. Sueh
solutions can be given subeutaneously, intramuscularly or
lntraverlously. For tlle preparation Or reetal
~lu~)l)ositorles, eonventlonal exeipients auch as fat;ty aeid
trLglyeeride ànd like base and lf required, Tween and lil<e
suf`cletants, ete. can be added to the compound Or the
lrlvention, followed by formulatlon in the usual manner.
20 Sucll aupposltories are admini~tered to the reetulll. For
the preparatlon of eye dropa, eonventional dlluents such
aa ~terlllzed distilled water, phisiological saline, ete.
can be u~ed. Eye drops should preferably be made isotonie
to tears by using a eonventional pll-acljusting agent,
25 burrers, etc.
" . . . .
.
.
. !.
: ' ' ' , . ':, .
~ '7~
The amount of the compound of formula (1-2) to be
incorporated in-to the preparations varies wi-th the symptonls of
the patient or with the type of the preparation. Preferably the
amoun-t pe:r administration unit is about 10 to about 300 mg for
oral administration, abou-t 10 to about 50 mg for parenteral
administra-tion, about 10 to about 200 rng for intrarectal
administration, and about 5 to about 50 mg for~admlnistration -to
the eyes. The compound of the present invention is adminis-tered
to the patient in an effective amount for inhibi-ting aldose
reductase or for treating diabetes. The effective amount is
suitably decided in accordance with the knowledge of the medical
art. The dosage per day for an adult, which is variable with the
symptoms, age, sex, and the like, is preferably about 5 to 900
mg/kg of body weight for usual purposes.
The presen-t invention will be described below in more
detail wi-th reference to the following Examples.
-- 10 --
'~ '`' .. ' : ~
.. .
.
Example l
(a) ~ 3.2 g quantity of 3-methyl-5-i30xazolyl-
aL(Iellyde~ 5.6 e of rhodanine-3-acetic aci~ ancl 2.~ g of
sodium acetate were added to 30 ml of acetic anllyclrlcle and
th~ ixture was stirred with heating at ~30 to l50C for
30 mirlutes and poured into ice water. The precipitate wa~
~eparatecl by filtration and recrysta11izecl from
climetllylformamide, giving II.o g of 5-(3-methyl-5-
i.~30xazo1ylrnetlly1ene)rhodanine-3~acetic acicl having a
melting poirlt of 280 to 2830C (decomposition) in a yield
O r ~
Elemel~tary analysis (for Cl0ll8N20l~S2^ll20)
C 1I N
~alc~l.('~) 39.72 3.33 9.27
L;`ourld (%) 39.0~ .3.2~
(b) In lO0 m1 of water were clisso1ve(l 2.0 g Or
5-(3-methy:l-5-lsoxazolyll1lettlylene)rhodanine-3-acetic acid
and l.0 g of` sodlum hydrogencarbonate. To the 301ution
was acldecl l.0 g of lOZ pa11adium charcoal ancl tlle mlxture
was ~ub~ec~ed to a recluction at room temperature and under
a hyclrogen pressure of 3 atm. ~fter completioll of the
reactloll, the catalyst was removed by filtration. To tlle
flltrate wa~ added dilute hydrochloric acid to adJu~t the
pll to l. The precipitate wa3 ~eparated by filtration and
recry3tal1ized from water, giving 0. 6 g of 5-(3-methyl-5-
,
:` - '-
:: , , ' , '` :,
. , ' ' :
~266~3
isoxazolylmethyl)rhodanine-3-acetic acid (Compound la) having a
melting point of 235 to 240C (decomposition) in a yield of 30%.
Elementary analysis (for CloHloN2~S2)
C H N
Calcd.(%) 41.95 3.52 9.78
Found (~) 41.33 3.25 9.57
Example 2
Compounds of lb to lc as shown below in Table 1 were
prepared in the same manner as in Example 1.
- 12 -
. .
. ~
,
'3
oo~ ~0 cor~
~ n ~ ~ t--
Z;
V~ o~o~ o~o~ o~o~
~ ~ Ln ~o ~U
u~ n ~ o ~o ~n ~
:>.~ X (~I ~ ~ r: -
v~ ) -r ~v) ~ r~
, _r =r _r
O O O
n ~ ~ o~
_~ ~ o 7 Z a~
O ~ N C~ O~)
1'~
nJ ~C ~ =r ~: 3 -t ~1~ -t -r
o vv v ~v o v a
~ IJ r~ rl ~
,~ O ~ O O n~ O O ~ O
[~ ~ C) t ~ t ~
_~ o t~. ~ P. o t~.
o _r ~3 ~ F O~ E~
_, ~ O ~ o ~ O
~n a) o~ n Q)
a) E3 ~V ~\I V o~ a
r-
E-~ tL~ U~ V O ' O n
~_D
< \` ~ o) :r~
.I................. 0 5' 0
~ C~
~ ~ t~.
1~ / .
~
~ ~ i~ ~
tL: O
u) ~ n u~
o ~
t~E3
.-
t~ C~
~ ~ ~n
'~
~I Q
~ 13 --
': . ,
'"` ' ' ,