Note: Descriptions are shown in the official language in which they were submitted.
~L26662~
-- 2 --
aackground Discussion
The present invention relates to a method and
apparatus for the concentration of gases and vapours
present in trace quantities in the atmosphere or in some
other gaseous medium. In this specification the gases and
vapours which are of interest will be referred to as
"vapours". Such vapours normally are gaseous forms of
materials which may be gaseous, liquid or solid at room
temperature, or tnose which are emitted from liquids and
solids at room temperature. They will also embrace in
this connection certain gases which are not considered
vapours but which are capable of being adsorbed onto
adsorption powders for removal from an air stream or the
like.
Specifically the invention relates to a device
and method for enhancing the concentration of trace gases
and vapours to such a level that they may be more readily
analyzed by appropriate in~trumentation.
A great deal of work has been done in recent
years to facilitate the work of detecting bombs in various
situations, such as in maintaining security at airports
and in boarding aircraft. X-ray machines and manual
searches are, of course, invaluable but cannot be expected
to detect all types of weapons such as explosives.
The devices already known involve the
preconcentration of vapours in discrete volumes of air. A
measured volume of sample air is passed through a solid or
liquid filter which collects the vapours of interest. The
collected vapours are subsequently retrieved in a much
more concentrated form than originally prevailing in the
atmosphere. If the volume of air sampled is large enough,
sufficient vapour may be recovered to be amenable to
analysis. This basically constitutes a batching-sample
method and is therefore time consuming and generally
unsuitlble for real time requirements.
,~ . . .: , .
:, - .
~Z~i66~1
In U.S. Patent No. 2,493,911, issued on January
10, 1950, to P.L. Brandt, an adsorption powder is
continuously recirculated through cool adsorption and hot
desorption regions to effect separation of the desired
components. The process described by Brandt requires
relatively large quantities of adsorbent powder for its
industrial applications, and it is reLatively expensive to
use. The large amount of powder involved also places a
constraint on the speed of operation of the
adsorption-desorption process. The Brandt patent relies
on the evolution of relatively large quantities of gas
during the desorption phase to transport the adsorption
powder from a low to a high elevation, which would not be
effective when attempting to separate and concentrate
minute traces of gases from the mixture aF. in the present
invention.
Also known in the prior art is the use of a
cyclone separator to remove naturally prevailing dust
particles in the atmosphere for the purpose of subjecting
them to elemental analysis. This is used in geographical
surveys and has been developed, for example, by Barringer
Research Limited of Toronto, Canada, (see U.S. Patent
No. 3,998,734 issued December 21, 1976, of A.R. Barringer).
The present inventor has been involved in an
ongoing research programme to develop the present
invention, as summarized in the proceedings of "New
Concepts Symposium and Workshop", October 30 to November
1, 1978, Reston, Virginia, U.S.A., published by The U.S.
Departments of Treasury, Energy, Justice and
Transportation, pages 265 to 267, the contents of w~ich
are incorporated herein by reference.
Objects of the Invention
The object of the present invention is to provide
a more efficient method and means for preconcentrating
gases and vapours in air or other gaseous media than has
~,
- . .
,- : :~ :: - - , ., ;
. , ~: .: .,- , , ~: .
~ 2~i~6Z~
'neretofore been known, a method that will require a
minimum of expense, and means which allows
preconcentration of gases or vapours on a continuous basis
with little or no time lag.
A further object of the invention is to effect
the transfer of the powder from a test medium to the
carrier gas.
Another ob~ect of one as~ect of the invention is
the continuous recycling of the sample powder from the
desorption region to the adsorption region of the device.
A specific object of the invention is to provide
a device which is readily portable and is suitable for use
in concentrating extremely small quantities of gases and
vapours. Other objects will become apparent as the
detailed description of the invention proceeds.
The device of the present invention collects the
vapours of interest from the sampled air by means of
adsorbent powder. The collection of the vapours on the
powder and their subsequent removal through desorptive
heating and transfer to an auxiliary carrier gas stream
comprises an efficient concentrating effect. One
important feature of this device is that instead of
scrubbing pertinent vapours from discrete volumes of air
by a stationary bed of adsorbent material as shown in the
prior art, resulting in a discontinuous and time-consuming
concentration process, it now becomes possible to achieve
a similar concentrating effect on a continuous and nearly
instantaneous basis. It is, moreover, possible to attain
a high degree of specificity with regard to the vapour
being collected, through selection of the adsorbent
powder, the adsorption temperature, and the desorption
temperature.
Summary of the Invention
The present invention allows for the
preconcentration of gases and vapours present in trace
, . .~ , - , -~
2'1
-- 5 --
quantities in various gaseous media including the
atmosphere and a portable device to enhance the
concentration of t'nese trace gases on a continuous basis
with little or no time lag, such that the resultant sample
is concentrated to such a level that it may be more
readily analyzed by appropriate instrumentation.
An important use of the present invention is for
the detection of hidden explosives, based on analysis of
ambient air for traces of characteristic vapours. The
inventor has found that this device and method is capable
of converting certain trace vapours present in
parts-per-trillion (ppt) (1 ppt = 10 12 mole fraction)
into a much more readily measurable concentration cf
parts-per-billion (ppb) (1 ppb = 10 9 mole fraction) in
a carrier gas.
Thus the present invention provides a method for
the rapid and continuous concentration of trace vapours in
a g~seous medium comprising the steps of entraining an
adsorption powder for the trace vapours in a stream of the
?0 gaseous medium in an adsorption region, passing the
gaseous stream containing the powder and adsorbed vapour
through a particle separator to produce a substantially
powder-free sample gas and to collect the powder
containing adsorbed vapours, then passing the powder
through a desorption region in contact with a carrier gas
stream, the powder being heated sufficiently in the
desorption region to release the adsorbed trace vapours
into the carrier stream, and then recovering the carrier
gas with the desorbed vapours from the powder.
In a preferred embodiment of such a method the
adsorption powder containing the adsorbed vapours i5
passed into a first desorption region heated to a suitable
temperature for removal of the vapours desired to be
tested, and then the powders are passed through a heated
zone at a higher temperature to complete the removal of
~12665Ei~
other vapours therefrom, the carrier gas from the second
heating zone being vented.
In connection with certain preferred embodiments
the temperature of adsorption is approximately room
temperature and the temperature of desorption is 120 to
150C. The carrier stream will normally be nitrogen gas.
The adsorbent powder will normally be in the range of 60
to 120 mesh size.
The heating zones are suitably adapted to heat
the powder, for example by contact with the walls of the
desorption region, or by inductive means (for example
radio frequency or microwave) or radiant heating means.
The walls may suitably be heated by an electrical
resistance heating coil.
In a preferred embodiment of such a method, the
desorption region has two heated zones, the first heated
zone having a first temperature Tl suitable for
desorption of the vapour of principal interest and the
second heated zone has a second temperature T2 which is
higher than T1, and is suitable for removing
substantially all vapours from the aflsorption powder.
The present invention also provides a continuous
trace vapour preconcentrator comprised of a gaseous medium
sample inlet, a vapour adsorption region; a particle
separator, a vapour desorption region: and vapour takeoff
outlet; wherein said inlet allows for the introduction of
a stream of sample gaseous medium and a quantity of
suitable adsorbent powder; said adsorption region provides
mixing of the sample flow resulting in contact of the
powder with the sample stream; said separator adapted to
separate the powder with adsorbed vapours from the gaseous
sample stream, said desorption region having a heating
zone and means for the introduction of a low flow carrier
stream; and a vapour takeoff outlet for the removal of the
carrier stream with enriched vapour concentrate, said
- : '', ' ~ , :
~ .~ : . ::.: - ,
-- 7 --
carrier stream having a low flow rate relative to the flow
rate of the gaseous sample stream.
The preconcentrator is normally further provided
with a means for collection of the desorbed powder and
means for recycling the powder to the inlet for reuse.
Brief Description of the Drawings
In the accompanying drawings which illustrate, by
way of example, embodiments of the present invention:
Figures 1 and l(a) depict schematic
representations of embodiments of a continuous trace
vapour preconcentrator, also referred to herein as a
continuous-action preconcentrator, or CAP.
Figure 2 shows chromatograms resulting from
analysis of ethylene glycol dinitrate (EGDN), which is a
typical vapour associated with explosives of the type of
concern in airports.
Figure 3 illustrates response time when a flame
ionization detector (FID) i9 coupled to the continuous
action preconcentrator of Fig. l.
Detailed Disclosure
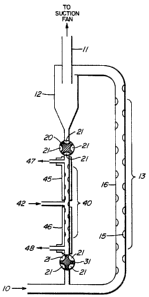
In the embodiment shown in Figure l, sample air
is drawn through the inlet (10) from the area to be
tested. This can be achieved by a suction pump connected
to the outlet (ll) of the cyclone particle separator (12)
or by a positive action blower fan in the circuit at the
sample air inlet. A quantity of a suitable adsorbent
powder is introduced into the sample stream at the s~mple
air inlet and is swept along by the flow. The powder will
normally be in the 60 to 120 mesh size range. Suitable
powders include usual chromatographic packings and solid
adsorbents. Examples of such powders are TENAX-GC (trade
mark), CHROMOSORB (trade mark) coated with a silicone type
stationary phase such as OV-l or 0V-17 (trade marks);
charcoal, silica gel, molecular sieve, nickel powder; or
any of the other packings conventionally used in gas
.
... .
i6Z lL
-- 8 --
chromatography. The choice of the particular adsorbent
powder will depend on the parameters of the system,
including the type of gas or vapour to be adsorbed and the
conditions of temperature and the like to be used in the
system.
In the embodiment shown in Figure 1 some increase
in turbulence is induced and promoted in the adsorption
region (13) by indentations (15) in the walls of the flow
tube (16) for intimate contact of the powder with the
sample stream. The adsorption region will normally be
operated at room temperature. For certain specific
applications it may be warmed or alternatively cooled for
s~lectivity in adsorption of vapours. The choice of such
conditions will be obvious to a person skilled in the art
or will be determined by ordinary experimentation.
The adsorption powder loaded with the trace
vapours from the sample air is then passed to a cyclone
particle separator (12) where the powder is removed from
the air straam and alls to the bottom of the cyclone
separator. The cyclone separator is a simple device of
known construction and operation. The air stream is
passed out of the system through outlet (11), and will not
normally be of any further interest.
In the embodiment shown in Figure 1, the
adsorbent powder which has been removed from the air
stream and has collected at the bottom of the cyclone
particle separator (12) is then passed into a desorption
region. In the embodiment shown in Figure 1 there is a
valve (20) driven by a motor, suitably at about one
revolution per second. With the four valve pockets (21)
shown in Figure 1 this provides a flow of powder from the
sample air to the desorption region (40) without any
significant flow of sample air into the desorption
region. In fact some slight flow of nitrogen carrier gas
from the desorption region (40) to the particle separator
.
: : . : ., . -: - : -
:. ~ . . : - , : ,. ::
~Z66621
(12) may occur as a result of the positive pressure of
nitrogen carrier gas.
The desorption region (40) consists of two heated
zones (45) and (46), in the embodiment shown in Figure 1.
~eated zone (45) is heated to temperature Tl, which is
sufficient to cause the desorption of the particular trace
vapours desired, which are taken up by the carrier gas,
such as nitrogen, introduced in carrier inlet (42), and
are taken off through vapour takeoff (47). The vapour
takeoff will of course go to an analyzer. This may be any
real ~ime analyzer, such as a mass spectrometer, ion
mobility spectrometer, or ionization detector.
~ eated zone (46) in this particular embodiment is
at a somewhat higher temperature T2 for "cleanup" of the
vapours and materials which may be adsorbed in the
powder. Vent (48) can be adjusted for bleeding off a
sufficient amount of the nitrogen carrier from heated zone
T2 to provide a suitable amount of vapour takeoff at
outlet (47), containing the vapours of principal
interest. The powder which has by then fallen to the
bottom of the desorption region (40) is then fed back into
the sample air inlet for recycling. A motor driven valve
(31), ~imilar to valve (20), may suitably be used for this
purpose, to provide positive displacement of the powder
without any appreciable air flow. This cycle of powder
entering the air inlet flow, passing through the
adsorption region, the cyclone particle separator, and the
desorption region, can occupy as little as a few seconds.
Thus this provides a continuou~ly available reading of the
vapour concentrations in the incoming air.
An estimate of the vapour enrichment achievable
with the device is readily calculated. If the sample air
containing a concentration Ci(air) of a particular trace
component i is sampled at a rate of F(air), the maximum
enrichment ratio
~,
"
~. ...
: , ~ j: :: ,: .
~L2~62~
-- 10 --
Ci(N) F(air)
Ci(air) F(N)
where Ci(N) refers to the vapour concentration at the
takeoff point and F(N) is the flow rate of the nitrogen
stream at the same exit. If both the adsorption and
desorption processes are complete, that is to say all of
the i-component is removed from the sampled air flow and
retrieved in the Tl zone, then for typical operating
flow rates of F(air) = 100 L/min (litres per minute) and
F(N) = 0.1 L/min
Ci(N)
= 103
Ci(air)
Thus, in this example, there has been a
thousandfold increase in the concentration of the desired
trace component.
The choice of the arrangement and temperatures
involved in heated zone Tl and T2 can be varied.
Normally, heated zone 46 is at a sufficiently high
temperature T2 to remove all vapours and gases 50 that
the powder leaving the desorption region is substantially
free of adsorbed material. However, the temperature T2
of heated zone (46) may be chosen for removal of a second
desired vapour (or gas as mentioned earlier) and, in fact,
one or more additional heated zones may be provided with
various temperatures and levels of removal of vapours from
the powder.
In some cases the use of different temperatures
Tl and T2 may be advantageous, for desorption of
thermally labile vapours such as EGDN at a lower
temperature, and for desorption of higher boiling-point
vapours such as DNT at a higher temperature, in the
embodiments shown in the Figures.
The embodiment shown in Figure 1 employs gravity
to pass the powder through the desorption region and
: .
-
i2~
collect it at the end of that region. By suitablevariationS of the structure and method, an embodiment can
be provided which is not gravity dependent.
The carrier gas introduced at carrier inlet (42)
will normally be an inert gas, such as nitrogen. This can
have certain advantages over the use of air as the carrier
gas, since some adsorption powders and some vapours will
be degraded by oxidation after repeated passes through a
'neated zone in the presence of air.
Although almost all of the adsorption powder is
recovered in the cyclone particle separator (12) there
will be a certain amount of powder lost through the sample
outlet (11). It is possible to extend the operational
life of the system before requiring an introduction of
additional powder by providing a powder reservoir at the
bottom of the desorption zone (40) immediately above valve
(31).
In another embodiment shown in Fig. l(a) the
nitrogen carrier is introduced through input ports (32)
and (33). The vapour t~ke-off port is shown as (36). The
heated zones (45) and (46) will normally be at the same
temperature, suitable for desorbing the vapours of
interest, in thls embodiment. The chromatograms and
tracings of Figs. 2 and 3 resulted from operation of the
CAP shown in Fig. l(a).
Although a motor driven valve with four pockets
is shown in Figures 1 and l(a), other means may be used
for introducing the powder into the desorption region
without permitting significant flow of sample air therein,
and likewise for re-introducing the powder into the sample
air stream.
EXPERIMENTAL VERIFICATION
The concept of vapour enhancement embodied in
this continuous action preconcentrator invention has been
validated experimentally using a rudimentary, nonoptimized
.. .- . .,~ :,, .
- 12 -
configuration of the device shown schematically in Figure
l(a), constructed in large part with readily available
standard components. Some of the test procedures employed
and data obtaine~ are outlined below.
A. Determination of Enrichment Ratio. A trace vapour
generator, similar to that described by Krzymien and Elias `-
in J. Phys. E: Sci. Inst., 9, 584 (1976), (the contents of
which are incorporated herein by reference) was used to
provide known, controllable concentrations of ethylene
glycol dinitrate (EGDN) vapour in an air stream. The
latter, flowing at a rate in excess of 500 L/min,
contained part-per-trillion (ppt) levels of EGDN (1 ppt =
2 mole fraction), which was determined by gas
chromatography, using the sampling and analysis protocol
described in NRC Laboratory Technical ~eport LTR-UA-27,
(National Research Council - Canada) January 1975 - L.
Elias and M. Krzymien, (the contents of which are
incorporated herein by reference).
The continuous action preconcentrator was
operated with its inlet (10) in the spiked air stream,
while samples were taken and analyzed of the nitrogen
carrier stream at the outlet end (36) (the Vapour Takeoff
port) of the device. The ratio of EGDN concentration at
the outlet C(N) to that at the inlet C(air) comprised the
observed enrichment ratio.
Two series of tests are summari~ed in Table I, in
which enrichment factors of 2-3 orders of magnitude were
achieved. Although well below the theoretical maximum
(based on F(air)/F(N)), the results clearly illustrate the
capability of the invention to concentrate trace vapour
levels in ambient air to very much higher values.
Figure 2 presents typical chromatograms obtained
from analysis of the inlet and outlet streams.
Figures 2a and 2b show respectively the EGDN
concentrations in the inlet air and the outlet carrier gas
.,
. .
, ., .. ,:. ~
. :.: ~ . :: . .:
-: .: ~ . ,
: - . . .: ,. . .
~tE;6;2~
- 13 -
(nitrogen) and it can be seen that there is a thousand
fold increase in concentration. In Fig. 2(a) 3.2
picograms (pg; 1 pg = 10 12 grams in a sample volume of
400 cm3 were analyzed. In Fig. 2(b) chromatographic
analysis yielded 87 pg of EGD~ in a sample volume of 10
c~3.
Figure 2c shows a calibration run involving 86 pg
in 8 microliters standard solution. The area under the
peak at 1.74 minutes (min) shows quantitatiYely the
concentration of the vapour.
B. Speed of Response. To test the response time of the
continuous action preconcentrator (CAP) to a sudden change
of the incoming vapour concentration, a flame ionization
detector (FID) was connected to the outlet port to
continuously monitor C(N). Target vapours utilized in
these trials were acetone and propane. CAP operating
conditions were: adsorbent powder - Tenax GC; Tl/T2 -
200/200C; F(N) - 50 mL/min; F(air) - 53 L/min.
In one experiment, 0.1 mL of propane (Figure
3(a)) was rapidly fed into the inlet while the FID signal
was recorded. In another experiment a 20mL headspace
sample from acetone (Figure 3b), obtained wit-n a
ga~-sampling syringe, was rapidly injected into the inlet
of the CAP while operating in normal room air. Tracings
from these trials are shown in Figure 3.
An overall time lag of 3-5 seconds is evident
from Figure 4, most of which may be due to the time
required to transport the vapours through t'ne connecting
line of the FID.
As can be seen from Figures 3a and 3b,
introduction of propane and acetone into the system
resulted in a very prominent response at about 3 seconds,
peaking at about 6 or 7 seconds, and tapering off over the
next 18 to 20 seconds.
~'''; ' ' . ~ ' ' , ',
662~
- 14 -
TABLE I
Determination of Enrichment Ratio C(N)/C(air) for EGDN Vapour
, _ . .
RunEGDN Conc. C~N1 No. Adsorbent Tl/T2 F~N) F(air) C(air) C(N) C(air)
mL/min L/min ppt ppt
_
R117 Tenax*-GC 120/120C 20 72 4.1 970 240
118 Tenax*-GC 120/120 20 72 4.11200 300
119 Tenax*-GC 120/120 20 72 4.11100 270
301 Chromosorb* 150/150C 12 53 1.3 1700 1300
310 Chromosorb* 150/150 1253 1.3 L400 1100
312 Chromosorb* 150/150 1253 1.3 1900 1500
320 Chromosorb* 150/150 1253 1.3 2100 1600
* trade marks.
As will be understood by any person skilled in
the art, the present invention is amenable to variations
on the structural details ~nd parameters of operation of
the present invention.
~ . .. : ~ . : ~: . .
~2~
- SD15 -
SUPPLEMENTARY DISCLOSURE
In the embodiment shown in Fig. 4, a
constriction (53~ is shown in place of the prPvious rotating
valve ~20) at the inlet of the desorption zone, while a
powder reservoir (52~ and metering valve (51~ are shown in
place of the prevîous rotating valve (31) at the bottom of
the desorption zone. When a blower fan (50) or some other
positive pressure device is employed at the inlet (10) to
provide a sample air stream, positive pressure is maintained
in the cyclone particle separator (12), and by suitable
choice of carrier gas pressure introduced at carrier inlet
(42), the bulk of the sample air will be forced out of the
sample air outlet (11) and only a very minor portion of it
will descend into the desorption region (40) with the powder.
Similarly, in the embodiment shown in Fig. 4.
the powder reservoir (52) acts as a boundary to keep separate
the incoming sample air stream from the carrier stream at the
bottom of the desorption zone (40). The powder metering
valve (51) functions as a means of introducing adsorbent
powder into the system at a desired rate.
The ratio of gaseous medium flow to carrier gas
flow is in the range of 10:1 to 106:1 and more specifically
could be in the range of 100:1 to 10,000:1 or 1,000:1.
In one embodiment the vapour in a gaseous medium
is 2,4 dinitrotoluene (DNT) and the desorption is carried
out at a temperature in the range of 130 to 180C.
The desorption region has an effective diameter
of 2 to 8 mm, and a length of 30 to 100 cm.
In other cases those sizes are for diameter of
1 to 3 cm, and for a length of 70 to 200 cm.
,, ' ~ , -;. ."" '' , ~ ,; :