Note: Descriptions are shown in the official language in which they were submitted.
~2~42~3
1 B 33273
Gas separation
This invention relates to gas separation and in
particular to producing synthesis-ready ammonia synthesis gas
from a raw synthesis gas.
Such a raw synthesis gas is generally prepared,
using a hydrocarbon feedstock, for example natural gas or
naphtha, by the sequence of primary and secondary steam
reforming or partial oxidation (with air, or oxygen-enriched
air being employed in the secondary reforming or partial
oxidation step) to give a gas stream containing hydrogen;
nitrogen and argon (from the air); carbon oxides; steam (as
an e~cess of that required for reforming or as produced in
the partial oxidation); and, generally, a small amount of
methane. This gas mixture is then subjected to the shift
reaction wherein carbon monoxide reacts with steam to produce
hydrogen, thereby increasing the hydrogen content of the gas,
and carbon dioxide. The shifted gas is then cooled, eg to a
temperature below 50C to condense the steam present as
liquid water which is then separated. The resultant gas is
the raw synthesis gas.
For use in ammonia synthesis the synthesis-ready
gas should contain hydrogen and nitrogen, should be
essentially free of gases such as steam, carbon dioxide, and
carbon monoxide, which deactivate the ammonia synthesis
catalyst, and desirably is essentially free of inerts such as
methane and argon in order to minimise any purge from the
ammonia synthesis loop.
The ammonia synthesis reaction involves the
reaction of 1 mole of nitrogen with 3 moles of hydrogen and
so, in order to minimise any purge from the synthesis loop,
the synthesis-ready ammonia synthesis gas desirably has a
; H2/N2 molar ratio near to 3, for example in the range 2.5 to
3.1. However it is often advantageous to conduct the
aforementioned secondary reforming or partial oxidation steps
:
''`~
-:
. . . , , ~ . . ~ . :
,. : ~
: .,, : :: ~ -
. .
~2~
2 B 33273
with such an amount of air, or oxygen-enriched air that in
the raw synthesis gas there is an e~cess of nitrogen over
that required in the synthesis-ready ammonia synthesis gas.
The raw synthesis gas thus usually contains
hydrogen, carbon monoxide, carbon dioxide, methane, argon,
and nitrogen, the latter often being in an excess of that
required for ammonia synthesis.
Carbon monoxide, nitrogen, and the inerts such as
methane and argon, have boiling points, at atmospheric
pressure, in the range -100 to -200C: such gases are
hereinafter termed medium boiling gases and are hereinafter
referred to as ~B.
It is therefore necessary, in order to convert the
raw synthesis gas into synthesis-ready ammonia synthesis gas,
to separate from that raw synthesis gas carbon oxides and, ln
order to minimise the purge, if any~ from the ammonia
synthesis loop, it is desirable to separate from the raw
synthesis gas any excess of nitrogen and other MB gases.
It has been proposed in GB-A-2126573 to carry out
such a separation by a pressure swing adsorption (PSA)
process but the percentage recovery of hydrogen is low
(72.4%) and the PSA product gas has to be methanated and
dried before it can be contacted with an iron ammonia
synthesis catalyst. Other proposals, su h as in GB-A-2103199
or European Chemical News 1978, 20 October, 39 - 47, have
involved feeding a N2-free raw gas to the PSA system and
adding N2 in the course of the PSA cycle or thereafter; such
proposals are unsatisfactory in requiring an external supply
of substantially pure nitrogen. It appears that recovery of
the C02 from such proposed processes is inefficient owing to
the low pressure and/or purity of the CO2-containing waste
stream from the PSA system, and consequently they do not
provide an attractive route to an integrated ammonia/urea
process or to manufacture of solid or liquid C02.
.,
::
:
''~ : i : . ' ;- . : . :
:
. ~ -
42~3 A
3 B 33273
In the present invention these disadvantages are
overcome by the use of two PSA stages.
According tc the invention there is provided a
process for the production of synthesis-ready ammonia
synchesis gas from a raw gas containing hydrogen, carbon
dioxide and medium boiling gas including nitrogen comprising
removing carbon dioxide and unwanted medium boiling gas by
pressure swing adsorption characterised by
(a) subjecting the raw gas to a first PSA system
effective to remove carbon dioxide while leaving hydrogen and
medium boiling gas largely unadsorbed; and
(b) subjecting the unadsorbed gas to a second PSA
system effective to separate therefrom a waste gas stream
containing said unwanted medium boiling gas, including the
excess, if any, of nitrogen; and wherein, if the unadsorbed
gas leaving the first PSA system contains carbon monoxide,
subjecting that unadsorbed gas to chemical removal of carbon
monoxide, preferably by catalytic methanation, prior to
subjecting said unadsorbed gas to said second PSA system.
~ach PSA system can be broadly of the type wherein
there is a plurality of adsorbent beds and each bed takes
part, successively, in steps including adsorption, pressure
equalisation, depressurisation to exterior~ and
repressurisation, with an optional purge step between
depressurisation and repressurisation.
In the ensuing description of the preferred PSA
systems, the terms "inlet" and "outlet" refer to the
direction of flow of gas during the adsorption step, and the
terms "co-current" and "couater-current"~mean towards such
outlet and inlet respectively.
In the first PSA system the pressure of the raw gas
entering a bed undergoing adsorption duty is preferably in
the range 25 to 50, especiaily 30 to 40, bar abs. The
temperature in the fiFst~PSA~syste= is preferablY higher than ~ ;
'.. ; ,
... . ..
~6~2~
4 B 33273
in conventional PSA systems in order to increase the purity
and/or pressure of the C02-rich stream evolved in the final
depressurisation to exterior or in the purge step. Suitable
temperatures are up to 200C, especially in the range 60 -
150C. Such a PSA process is among those described in our
European application and US application claiming priority
from UK application 8426393. However, conventional
temperatures of up to about 50C can also be used
satisfactorily, especially if, as described hereinafter,
waste gas from the second PSA system is fed to the first PSA
system.
The adsorption step in the first PSA system is
preferably terminated sufficiently before the C02-front has
reached the bed outlet in order to ensure that the C02
content of the unadsorbed gas, integrated over the adsorption
step, is at the required level, typically under 0.5 and
preferably under 0.2% V/v. The C02 content of the gas tends
to rise towards the end of the adsorption step as its
adsorption tail approaches the bed outlet. The ~2/MB molar
ratio of the unadsorbed gas has a maximum value at an
intermediate part of the adsorption step, because at the
beginning MB gas adsorbed in preceding equalisation steps and
any purge step, if those steps are counter-current, becomes
desorbed; and because at the end the MB adsorption front has
reached or passed the bed outlet.
~ fter the adsorption has proceeded to the desired
extent in a bed, the pressure in that bed is reduced by one
or more pressure equalisation steps in which gas from the
outlet of the bed that has finished its adsorption duty is
released, co-curren~ly out therefrom and is fed, preferably
counter-currently, into a recipient bed that has been
depressurised and may have been purged and may have been
partly repressurised.
There may be one or more presure equalisation
.. , ~ .
~ ,
~2~
B 33~73
steps. Thus, as in ~he relevant part of the 4~bed system
depicted in flgure 2 of USP 3430418, on which the system
described hereinafter is based, ~here may be a single
equalisation step. Alternatively, as in the relevant parts
of the modified 4-bed system depicted in figure 2 of USP
3564816 or of the 5-bed system depicted in figure 3 of USP
3430418, there may be two equalisation steps. Alternatively,
as in the relevant parts of the syRtems using 6 or more beds,
for example the 8-bed and 10-bed systems depicted in USP
3986849, there may be three equalisation steps. Although the
pressure equalisations are illustrated by those references,
as described below, the PSA cycles that may be used in the
process of the invention differ from the disclosed cycles in
other respects.
After the pressure equalisation step or steps, the
bed is subjected to depressurisation. The depressurisation
can be conducted in a variety of ways. For example, the
depressurisation may be effected in one step, to de orb C02,
and to release the relatively small amount of MB and H2
remaining in the bed, to the exterior. Alternatively the
depressurisation may be effected in a plurality of steps:
thus the depressurisation may be conducted, preferably co-
currently, in one or more steps, (hereinafter termed the
penultimate depressurisation) to an intermediate pressure
level releasing to the exterior an MB-rich/C02-lean gas and
then in a final step, preferably counter-currently, to
release to the exterior an ~B-lean/C02-rich gas.
Alternatively, in a multiple step depressurisation, in the
penultimate depressurisation, the released gas may be used,
as described below, to purge another bed: in this mode o~
operation the gas released to the exterior as the purge from
said another bed is enriched in C02 by the C02 purged from
said another bed: as described above, in the final
depressurisationj the bed is depressorised, preferably
~,
.:
..
~ 34~8
6 B 33273
counter-currently.
The ter~inal pressure of co-current penultimate
depressurisation with product release is preferably at least
2 bar abs and typically up to 10 bar abs. The gas released
in this step is leaner in CO2 the higher the pressure. The
terminal pressure of final, countercurrent, depressurisation
is conveniently in the range 1 to 3 bar abs. However to
ensure more complete C02 desorption the final
depressurisation may be to a ter~linal sub-atmospheric
pressure. The C02-rich
stream released in this final depressurisation step is richer
in C02 the lower the terminal pressure of the penultimate
depressurisation. However the lower the latter pressure, the
less will be the proportion of the C02 in the raw gas that is
recovered in the final depressurisation C02 product stream.
After final depressurisation the bed is optionally
purged. ~or example, as described above, the bed can be
purged, preferably counter-currently, with a through-current
of gas fed counter-currently out of a bed undergoing
penultimate depressurisation. Alternatively the bed can be
purged, preferably counter-currently, with a through-current
of ~B-rich/C02-free gas from a depressurisation in the second
PSA system. As a further alternative the bed can be purged,
preferably counter-currently, with a through-current of gas
of low or zero C02 content from an exterior source. If two
or more such purges are used, they are preferably effected
in the above order.
Ater final depressurisation, and after purge, if
used, the bed is repressurised by gas released from a bed
undergoing pressure equalisation as described above and by a
feed of unadsorbed gas from the outlet line of a bed, or
beds, on adsorption duty. The latter repressurisation may be
operated during, as well as after, the repressurisation
resulting from a bed undergoing pressure equalisation, in
order to avoid excessive fluctuations in the flow rate of
,
: ~
. . .
: . . ,
.: ' ''~: ''` ~ , :
: : ~' .,~ ',' ' `
,, ~ . ,.
28
7 B 33273
unadsorbed gas leaving the bed, or beds> undsrgoing
adsorptio~ duty. Alternatively or addieionally, the final
repressurisation may be by gas recycled from the second PSA
system: this gas may be waste g8S or product gas fro~ the
second PSA syste~.
If the unadsorbed, essentially C02 free> gas from
the first PSA system contains C0 as an ~ gas> this
unadsorbed gas is then sub~ected to chemical removal o~ C0.
This chemical removal is preferably a catalytic methanation
process to convert all the C0 present to methane. This
methanation process may also convert most> or all, of any C02
present ln the unadsorbed gas from the first PSA systPm. The
methanation catalyst can be of the well-tried supported
nickel and/or cobalt type> containing for example 5 _ 70% W/w
of such metal (calculated as monoxide) on a refractory
comprislng alumina> spinel> cement or aluminosilicate. If
desired> however> a supported ruthenium catalyst can bs used.
The temperature need not be controlled to prevent methanation
of C2 and thus is conveniently in the range 250 - 400C at
the catalyst outlet. Whereas methanation produces by-product
water vapour> most of this water vapour can conveniently be
removed by coolin~ to condense the water vapour as llquid
water wbich is readily separated. Any residual water vapour
can be removed by the second PSA system. In some cases the
second PSA system can remove all of the water vapour produced
by the methanation step. Generally no separate adsorptive
water vapour removal step is necessary.
The second PSA system may be of the type described
in European application 85301022, which w;ll Dublish as
Eæ'A-157480 and which corresponds to USSN 703531, operated und~r
conditions to adjust the H2/MB molar ratio to that required in
the s~nthesis-ready amm~nia synthesis gas.
As in the first PSA sy.stem, each bed successively
,
342~3
8 B 33273
undergoes adsorption, pressure equalisation, depressurisation
(preferably in two stages, co current followed by counter-
current), and repressurisation with an optional purge step,
preferably using co-current depressurisation gas from an
other bed, between the depressurisation and repressurisation
steps.
In order to limit the magnitude of fluctuations in
the composition and flow rate of unadsorbed gas in the
adsorption step of each PSA system, each &ystem preferably
includes enough beds to permit at least two beds to be used
on adsorption duty simultaneously, but out of step with one
another. Likewise there are preferably sufficient beds to
permit beds to be on simultaneous, but out of step, duties in
those other steps, ie depressurisation and purge (if any),
where gas is released from the PSA system.
The waste-gas from the second PSA will be
essentially C02 free, particularly where there is a
methanation step in which any C02, as well as C0, is
methanated, between the first and second PSA systems. This
waste-gas will also be MB-rich/~2-lean. As mentioned above,
this waste gas from the second PSA system can be used in the
first PSA system. It is therefore possible that the second
PSA waste gas can be taken at a higher pressure than is
disclosed in the aforesaid European application 85301022. A
waste gas pressure of at least 2 bar abs. and up to one
quarter of the second PSA system gas inlet pressure is
preferred. This waste gas may required compression and/or
heating to the first PSA system operating conditions but,
even so, such use of the second PSA system waste gas may be
advantageous.
In one mode of operation waste gas from the second
PSA system is fed into a bed of the first PSA system after
depressurisation to exterior as part of the repressurisation
of that bed. This has the advantage that it returns to the
: '
: : .. '-.
.: : - . -, : . ,
,::: :: : ,, :
.
"
:,
8~2i~3
9 B 33273
process any a2 in the waste gas of the second PSA system.
Part of the waste gas from the second PSA system may be
vented in order to prevent accumulation of MB gas in the
unadsorbed gas from the first PSA system.
In another mode of operation, a bed of the first
PSA system, that has finished its~ adsorption duty but has not
been depressurised to exterior, is swept by a through
current, preferably co-currently, of waste gas from the
second PSA system, directly, or indirectly, into a recipient
bed of the flrst PSA system after that recipient bed has been
depressurised (and purged, if used) and before that recipient
bed has finished its adsorption duty. The first PSA system
bed may be s~ept before or after its pressure equalisation
step. The effect of this sweep is to displace the unadsorbed
gas, which contains H2 and MB and has a H2/MB ratio greater
than that of the waste gas from the second PSA system, that
is still present in that bed of the first PSA system into the
recipient bed: in this way the H2 of the unadsorbed gas in
the bed being swept is not lost during subsequent
depressurisation to exterior or pur~e (if used).
~ The waste gas from the second PSA system can be a
gas from co-current depressurisation, counter-current
depressurisation or purge or more than one of these. Since
by the invention a2 is retained in the process, that gas need
not be taken at minimal pressure in order to minimise H2
loss. However, at the preferred inlet and equalisation
pressures of the first PSA system, it is usually necessary to
compress the waste gas from the second PSA system before
feeding it to the first PSA system. ~ ;
If desired the gas used to sweep the bed of the
first PSA system can include one or more other streams of
suitable composition, namely C02-lean or C02-free, possibly
containing MB gases tbat will be subsequently purged, and
advantageously containing H2. Such other streams include
;
. - , . : . .
28
B 33273
intermediate pressure waste gas from the first PSA, or
ammonia synthesis purge gas.
There are three preferred ways of effecting a first
PSA system sweep with waste gas from the second PSA system:
two of these preferred ways involve sweeping after the
pressure equalisation step of the first PSA system and are
termed ~intermediate pressure sweep while the third way,
termed high pressure sweep , involves sweaping before
pressure equalisation.
In the first intermediate pressure sweep mode of
operation, the waste gas from the second PSA system i9 fed
into the inlet of a bed of the first PSA system that has just
undergone pressure equalisation and is still connected to the
recipient bed undergoing repressurisation. By this means gas
still in the bed after equalisation is swept into the
recipient bed and replaced by second PSA waste gas. The
extent to which gas is replaced depends on how much waste gas
is available from the second PSA system and on the pressure
to which (if at all) it is necessary or convenient to
compress it. After this step both the post-equalisation bed
and the recipient bed are at a pressure between feed and
equalisation; the recipient bed contains unadsorbed product
gas and the post-equalisation bed contains second PSA waste
gas and may contain some unadsorbed product gas. The post-
equalisation bed can be equalised with a fully regenerated,
ie depressurised and optionally purged possibly partly
repressurised, bed if one is available~ or can be
depressurised to waste, possibly stagewise at 2 or more
pressure levels.
One further advantage of the use, as described
above, of the waste gas from the second PSA system, or
unadsorbed product gas swept out thereby, for
repressurisation is that less, possibly none, of the gas from
the unadsorbed product line of the first PSA system need to
:;
.
-:: :: -: . ,: .. ,. ::: ;
:: :;.:."., :
~- : :.:: .: :.
~;2 6842~3
11 B 33273
be diverted into repressurisation, and hence the flow of
unadsorbed product gas from the first PSA system is subject
to less fluctuation.
In the second intermediate pressure sweep mode of
operation, the waste gas from the second PSA system is fed
into a bed that has undergone pressure equalisation, has been
disconnected from the recipient bed, and has been con~ected
to the inlet of a compresser, the outlet of which feeds into
the raw gas inlet line of the first PSA system. By this
means gas still in the bed after equalisation is swept into
the PSA raw gas inlet and replaced by second PSA waste gas.
The extent to which gas is replaced depends on how much gas
is available, but less gas is needed than in the first
intermediate pressure sweep mode because it is used only at
equalisation pressure, not at pressures up to feed pressure.
Since the swept out gas passes into the raw gas inlet, a
destination for it is continuously available. After this
step, the post-equalisatlon bed contains second PSA waste gas
and may contain unadsorbed product gas if the quantity of
second PSA waste gas was insufficient to sweep out all the
unadsorbed product gas. If more tha~ this sufficient second
PSA waste gas is available, it can be passed into the
compressor and raw gas feed inlet, and thereby ~ in the
second PSA waste gas will be retained in the process. After
the sweep, the post-equalisation bed can be equalised with a
fully regenerated, possibly partly repressurised, bed if one
is available, or can be depressurised to~waste, possibly
stagewise at 2 or more pressure levels.
In the high pressure sweep mode of operation, the
waste gas from the second PSA system is fed into a bed that
has completed its adsorption step, but has not been
equalised, and has been connected to the raw gas inlet line
of the first PSA system, possibly by way of a booster
compressor recovering the pressure-drop through the bed.
- ,~ ..,: ::: . : ::
"
, . ., : ,: ; ~ . ;, : '- , ' , :
.. : :: - -
~8~
12 B 33273
Thereby unadsorbed product gas stlll in the bed is swept into
the raw gas inlet line and recovered.
Since the bed is at feed pressure, the quantity of
waste gas from the second PSA system required is almost
double ehat for the above described second mode of
intermediate pressure sweep in which almost half the
: unadsorbed product gas in the bed is recovered by
equalisation. After high pressure sweep the bed can be
equalised with a fully regenerated, possibly partly
repressurised, bed; such a bed is available if a system of 4
or more beds is used. As an altecnative, the swept bed can
be depressurised to waste, possib:Ly stagewise at 2 or more
pressure levels; in such a system there is an energy penalty
in tha. gas is let down from the highest pressure in the
system after having been compressed, but for some users the
penalty may be mieigated if there is a use for waste gas at
relatlvely high pressure or if there is reason for having
only 2 or 3 beds instead of the 4 or more required when
equalisation is practised.
In the aforementioned `'sweeping" modes of
operation, the waste gas from the second PSA system is
preferably passed through the bed being swept co-currently in
order to minimise deso~ption of C02. This is more critical
for the first mode of intermediate pressure sweep since any
desorbed C02 would be adsorbed at the outlet of ehe recipient
bed (assuming flow into lts outlet as in equalisation) and
thus would contaminate the unadsorbed gas stream fed to the
second PSA system in the next adsorp~ion step using that bed.
Alternatively in this mode of intermediate pressure sweep,
the swept out gas is received co-currently, and this is
preferred.
In the second intermediate, and in the high,
pressure sweep modes of operation, any desorption of C02 is
less important since this desorbed C02 will be returned to
- : :. : :.; :.: .: ~ : -
- :. . .: . :. .-~
:' . . ` '
~;8~28
13 B 33273
the raw synthesis gas inlet line.
If sweeping by second PSA waste gas is continued
until such waste gas has passed into a bed undergoing
repressurisation or into the raw gas feed, there will be an
accumulation of MB gases in the combination of the two PSA
sys~ems. The same will occur if second PSA waste gas is used
directly in repressurisation.
If the adsorbent is correctly choqen and operated,
the increased MB partial pressure may result in increased
adsorption, so that such accumulation is limited. Otherwise
it may be desirable to maintain a purge of ~B-rich gas at a
suitable point in the system.
Especially in the second intermediate pressure, and
in the high pressure, sweep modes, wherein the waste gas from
the second PSA system is accumulated in the first PSA system
under raised pressure, a subsequent depressurisation to purge
another bed can be carried out at two or more pressure
levels, so as to produce first a waste gas rich in fuel
values but lean in C02 and finally a waste gas rich in C02.
It will be appreciated that the waste gas from the
second PSA may be used both for sweeping, prior to
depressurisation, and also for repressurisation as described
above.
In an another alternative, a bed in the first PSA
system that has completed is adsorption duty is swept with a
C02-rich gas and the swept-out gas is returned to the raw
synthesis gas inlet line. After depressurising the bed to
recover C02, the depressurised bed is purged with waste gas
from the second PSA system.
The C02-rich gas used for this C02-sweeping is
conveniently derived from the C02-rich gas recovered from the
depressurisation of another bed. If desired, the required
C02-rich gas can be supplied by an autonomous circulation
system. More conveniently, the requirement of the recipient ~ ~
:
:
. - ::: .:: .,,:,..,.: . . : .: ::
:: . : . : : .- .
~61~428
14 B 33273
bed is provided from the storage capacity of pipework and any
reservoirs in the C02 collecting system or, in a suitably
designed system, from a bed undergoing desorption of C02-rich
gas in the same time period. The C02-rich gas may need to be
compressed, depending on the pressure of the gas to be swept
out. Often the C02-rich gas will be compressed in the course
of use further downstream in a process sequence making for
example solid or liquid C02 or urea; thus the gas used in
sweeping can conveniently be takea from such a downstream
source.
The bed subjected to C02-sweeping has preferably
been at least once downwardly pressure-equalised with another
bed. In such equalisations, carried out with co-current flow
from the bed, the C02 front stays in the bed and thus the gas
passed into the recipient bed or beds is H2 + MB gas low in
C2 and, since it enters the recipient bed(s) counter-
currently, drives back the C02 front in such bed(s). By
sweeping after equalisation the pressure of the C02-rich gas
need not be so high as would be necessary for sweeping before
equalisation.
C02-sweeping is continued preferably co-currently
and until the C02 front has moved towards the bed outlet but
ramains within the bed. A small C02 content in the swept-out
gas is not harmful if that gas is fed to the raw synthesis
gas inlet tlme of the first PSA system and then a balance may
be struck between the advantage of more complete sweeping and
the disadvantage of adding more C02 to the inlet gas.
The effect of the C02-sweeping step is to expel H2
and M3 gases from the void space in the bed and also to
desorb H2 and ~B gases. Consequently the gas desorbed in the
subsequent depressurisation consists almost entirely of C02
and is very suitable for further processing.
For a C02-sweepino step the C02-rich gas needs to
be compressed, for example to 6 to 12 bar abs, but such
- ': . ~ ,. . .: -, :
:
~26~2~3
15 B 33273
pressures would com~only be needed for the further processing
already mentioned. For C02 recovery the depressurisation,
which is preferably counter-current, is for example from 6 -
12 bar abs and can go down, if desired, to less than 1 bar
; 5 abs., f or example to 0.1 bar abs., depending on the extent to
which C02 is required.
.~fter the C02-sweep and the depressurisation with
C02-recovery, the bed is purged, preferably counter-
currently, with waste gas from the second PSA system. The
effect of this purge is to desorb still more C02 since the
waste gas from the second PSA system is substantially C02-
free and thus subjects the bed to a still lower C02 pressure
than would be attained in normal depressurisation to below
atmospheric pressure. Since the purge gas is not obtained
from another bed in the first PSA system, its supply does not
depend on a step occurring in another bed of the first PSA
system; therefore purging need not be rigidly synchronised
with steps occurring in other beds.
The purge outlet gas consists mainly of MB gases, a
s~all percentage of H2 and a content of C02 depending on the
extent to which C02 was recovered in the depressurisation to
C2 recovery step. If it is desired to keep down the C02
content Gf the purge outlet gas but very high C02 recovery is
not required, the void space gas present after the
depressurisation can itself be swept out wholly or partly to
waste, by means of the second PSA waste gas to be used as
purge gas.
The purging pressure, whatever the C02 content
intended in the outlet purge gas, can be superatmospheric,
and then the outlet purge gas can be used as a source of
power, by heating it and expanding it (preferably with
combustion) in a gas turbine; the gas turbine exhaust can
supply at least part of the heat required before expansion.
To obtain the required purging pressure the second
: - ~ :, ;
''' ., . : :
.
B~ 8
16 B 33273
PSA waste gas can if necessary be compressed. However the
waste gas may be taken from the second PSA system at
superatmospheric pressure, for example in the range 2 - 10
bar abs. and the inlet and outlet ~2/N2 ratios of the second
PSA system chosen accordingly.
By the use of a C02-sweep, and purge with waste gas
from the second PSA system, a large extent of C02 desorption
is possible. It is therefore possible to use adsorber beds
in the first PSA system of unconventionally small volume in
proportion to the flow rate of gas to be purified.
Alternatively or additionally, aclsorption step times can be
unconventionally long, for example 4 - 8 minutes, and thus
time is available durlng each adsortion step for several
short steps, of which equalisations, sweeps and
depressurisations are examples.
Since the C02-sweep step returns H2 to the process,
the first PSA system does not rely on multiple pressure
equalisations to decrease ~2 loss and therefore need not
include a large number of beds. It appears that the most
convenient number will be 4 through 6 or possibly 8 if it is
desired to have overlapping operation of successive
adsorbers.
Whereas it was indicated above that the first PSA
system could advantageously be operated "hot" with inlet
temperatures above 40C in order to maximise the C02 content
of the stream of recovered C02, where a C02-sweep and second
PSA waste gas purge are employed, such higher temperature
operation can be avoided.
The adsorbent charged to the PSA beds can be any of
those considered suitable for PSA, including silica gels,
active carbons and zeolites. The adsorbent for at least the
beds of the first PSA system preferably includes a zeolite,
since zeolites are capable of adsorbing C02 much more
preferentially to the MB gases than the other adsorbents.
- ,
:
17 B 33273
Such specificity is less at the high temperatures that may be
used in the first PSA system than at conventional PSA
temperatures, but is fully adequate. When using a high
temperature first PSA system, with a zeolite adsorbent, the
effect is to produce a C02 adsorption isotherm that resembles
the ambient temperature isotherms for active carbon and
silica gel. A suitable zeolite is of the A type, for example
calcium A (Zeolite A i9 defined in D W Breck's "Zeolite
Molecular Sieves" and is available from several manufacturers
under different trade names). The following table sets out
values of the adsorption constants K, where
K _ kg. mols of adsorbed gas per kg of adsorbent
pressure in bar abs.
for C02 and N2 and of the ratio KC02/KN2 for a zeolite and an
active carbon. The values at 30, 70 and 100C are quoted
from page 565 of "Gas Purification" by A L Kohl and S C
Riesenfeld, 3rd ed. 1979 (Gulf Publications, Houston, Texas,
USA) and those at 150C are obtained by Clausius Clapeyron-
~ype calculation.
Table
_ . I
; K, kg. mol kg 1 bar 1 at TC
T = 30T = 70 T = 100 T = 150
Zeolite N2 26 x 10 5 11.9 x 10 5 7.4 x 10-5 3.9 x 10 5
C2 0.1610.041 0.0179 0.0058
ratio 619344 241 148
. . _
Carbon N2 41.5 x 10 5 23.8 x 10 5 16.9 x 10 5 10.7 x 10 5
C2 4.46 x 10-3 1.45 x 10-3 0 73 x 10-3 0.29 x 10-3
ratio 10.7 6.1 4.33 2.71
. . _
Whereas, in the absence of a purge by second PSA
.
. .
:' ~ . . : :
2~3
18 B 33273
system is preferably mainly carbon, when such a purge is used
that adsorbent is preferably zeolite.
The present invention is of particular use in
combinations wherein
(a) the C02-containing gas released in the first PSA
system is fed to a C02 purification system such as an
adsorptive or liquid-absorption or distillation C02 recovery
system; for this purpose the product C02-containing gas can,
if desired, be compressed; and/or
tb) with or without the above-mentioned C2
concentration, the C02-containing product gas is fed to urea
synthesis; and/or
(c) unadsorbed gas from the second PSA system is fed to
ammonia synthesis, the latter advantageously being the source
of ammonia for urea synthesis where, as mentioned above, the
C02-containing product gas is used for urea synthesis.
Usually the product gasesj ie C02-product gas
released in the first PSA system, and the synthesis ready
ammonia synthesis gas produced as the unadsorbed gas stream
in the second PSA system have to be compressed before further
use.
Since the efficiency of compressors tends to vary
: as the molecular weight of the gas being compressed varles it
is advantageous, particularly in the case of the second PSA
system to have sufficient beds that at least two beds are on
adsorption duty at any one time, but out of phase with one
another, in order to minimise fluctuations in the composition
of the gas fed to the compressor.
The chemical step preceding the first PSA system is
preferably a catalytic shift step preferably decreasing the
C0 content to at most 1, preferably und~r 0.5, % V/v on a dry
basis. Cooling and liquid water sep~aration are effected
before the first PSA system, but the residual water vapour
can be removed in the PSA system, without a separate drying
A ~
,
:; '.; ~: '~:: ,, : ', ;
:: .
~842~3
,
19 B 33273
operation.
Upstream of shift and water removal the preferred
stage is hydrocarbon air-steam reforming ln which catalytic
primary steam reforming is effected in indirect heat exchange
with catalytic air reforming and the product raw gas has an
(H2 + CO)/(~B - CO) molar ratio iu the range 1.25 to 2.5,
especially 1.5 to 2.1, a C02 content in the range 10 to 25
V~v, and at least 90% V/v of the ~ is N~.
Preferred embodiments of the invention are shown in
the accompanying drawings in which
Figure 1 is a block diagram of the overall
flow sheet,
Figure 2 is the flow sheet of the second PSA
system, and
Figures 3 - 10 are flow sheets of the first PSA
system showing various alternative embodiments
making use of the waste gas from the sacond PSA
unit as follows:
for final repressurisation (Figure 3);
as a counter-curren~ purge (Figure 4);
as a co-current sweep then counter-current purge
(Figure 5);
as a co-current sweep then intermediate co-current
repressurisation (Figure 6);
as a co-current intermediate pressure sweep with
recycle (Figure 7);
as a co-current high pressure sweep with recycle
(Figure 8);
as a counter~current purge in a cycle using a sweep
with C02-rich gas to recycle (Figure 9); and
as in Figure 9 but for a 6 bed PSA unit (Figure
10) .
In the flow sheets the dotted horizontal lines represent
divisions between successive time intervals of the PSA cycle.
: ,..
:
... .
.: :, : - ~ :
: ~ . - :- ~,.
-: ., ~: ,.
4~8
20 B 33273
With the 4-bed units of Figures 2 to 9 there are four such
intervals labelled Tl to T4 while in the 6-bed unit of Figure
10 there are 6 time intervals Tl to T~.
The connections to the beds are positioned, in the
flow sheets, so that gas entering the left hand side (LHS),
and/or leaving the right hand side (R~S), of a bed is flowing
co-currently. Conversely gas entering the R~S, and/or
leaving the L~S, of a bed is flow:Lng counter-currently~
In the flow sheets and ensuing description the
following abbreviations are employed:
AD - adsorption duty (co-current)
El = intermediate equalisation (co-current - may
be omitted).
E2 = final equalisation (co-current - may be the
only equalisation as shown in Figures 2 -
8)
Dl = intermediate depressurisation (co-current -
omitted in Figures 5, 9 and 10)
D2 - final depressurisation (counter-current)
PU = purge (counter-current)
SW - sweep (co-current)
Rl = initial repressurisation (counter-current)
R2 = intermediate repressurisation (counter-
current - omitted in Figures 2 - 5, 7 and 8)
: 25 R3 = final repressurisation (co-current )
IG = inlet raw synthesis gas
W = waste gas from the second PSA system
SR = synthesis ready ammonia synthesis ~as
U = unadsorbed gas from the first PSA system
~ = unadsorbed gas from the first PSA system
after methanation and water removal.
CR = C02-rich gas separated in first PSA system
~IR = C02-lean/MB-rich gas separated in first PSA :
system
:~ . .. ,. ., . .. :,.
~2~
21 B 33273
P ~ product gas (CR plus MR where these are not
taken off separately).
In the process of these embodiments a stream of raw ammonia
synthesis gas, IG, from the process sequence of natural
gas/steam primary reforming, air secondary reforming, heat
exchange between the primary and secondary reforming,
catalytic shift, cooling, and water separation, is fed, via
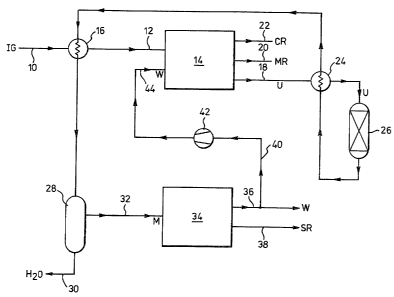
line 10 to the inlet gas manifold 12 of a firs~ PSA unit 14,
optionally via a heat exchanger 16 where a hot inlet gas, IG,
is required. PSA unit 14 has a plurality of beds, eg 4, 6, 8
or 10, of adsorbent and programmeld valve actuators providing
an uninterrupted succession of regenerated adsorbent beds and
steps of pressure equalisation, depressurisation, purge, and
repressurisation with an optional sweep step after adsorption
but before depressurisation.
The raw synthesis gas, IG, contains H2, N2, C0, Ar,
C~4 and C02 and the PSA unit 14 is effective to give a
stream, U, of unadsorbed gas, which is essentially free from
C02, leaving PSA unit 14 via manifold 18. PSA unit 14 also
gives one or two gas streams containing the C02 removed from
the synthesis gas IG. As shown in Figure 1, a C02-lean/~B-
rich stream MR (which will contain a little H2) and a C02-
rich/~-lean stream CR (which may contain a small amount of
H2), leave the PSA unit 14 via manifolds 20 and 22
respectively. Alternatively, as will be described there may
be a single product gas stream P containing the C02 and some
~B and a little ~2 leaving PSA unit 14: for convenience of
description its manifold is also designated 22.
Where separate MR and CR streams are produced, the
MR stream is taken from manifold 20 and may be used as a fuel
for a gas turbine (not shown) driving, for example, one or
more compressors. The CR stream or P stream, is taken from
manifold 22 of PSA unit 14 to C02 recovery steps (not
shown).
-, ,,- ~ ,
- .' :: '~ ~ '
' ' ' ' ~ , - , ' . ~ .: : ~ .,
~;8~2~3
22 B 33273
If the temperature at which PSA unit 14 is operated
is above the critical temperature of C02 and high enough to
limit adsorption of MB gases, the C02 content of the
unadsorbed gas stream, ie stream U, is low, for example 0.5%
V/v or less, but not much of the MB and H2 of the raw
synthesis gas are separated in the PSA unit 14.
The unadsorbed gas stream, ie stream U, is fed from
manifold 18 to a heat e~changer 24 wherein it is heated and
passed to a methanator 26 in which it encounters a supported
nickel catalyst. The temperature of the methanation inlet
gas is controlled at such a level that both C0 and C02 are
metha~ated. The resulting methanated gas is cooled in heat
exchanger 24 as the source of heat for heating the gas fed to
methanator 26, cooled further in heat exchanger l6 as the
source of heat for the raw gas, IG, entering PSA unit 14
where the latter is of the hot type, and finally cooled in
cooler/separator 28 wherein liquid water is separated and
removed via line 30. From cooler/separator 28, the
methanated gas from which water has been separated, ie gas
stream M, is fed to the inlet manifold 32 of a second PSA
unit 34. In PSA unit 34, which is operated atJ for e~ample,
30C, the methanated gas ~ is separated to give a waste gas W
containing CH4, N2, and Ar, as its main components, and the :
synthesis ready ammonia synthesis gas SR which has an H2:N2
ratlo within the range 2.5 to 3.1. The waste gas W will
contain a little H2. The W and SR gases leave PSA unit 34
via manifolds 36 and 38 respectively.
PSA unit 34 is of the same general type as that of
unit 14: a flo~ chart for a 4-bed PSA unit 34 is shown in
Figure 2.
During the first time interval Tl, bed A is used
for adsorption duty. In this duty the inlet of bed A i3
connected to the inlet manifold 32 for the methanated gas M
and its outlet is connected to the outlet manifold 38 ;
: ., ,,'.: : , .-
-:
" : :: ' :
34Z13
23 B 33273
supplying the synthesis ready ammonia synthesis gas SR.
In the second time interval, wherein bed D is used
for the adsorption duty, bed A is first subjected to a
pressure equalisation step E2 wherein the inlet to bed A is
closed and its outlet is connected to the outlet of bed B
which has just been purged in step PU. When the pressures in
beds A and B are equal, the outlet of bed A is disconnected
from that of bed B and is connected to the outlet of bed C
whose inlet is connected to the ~aste gas manifold 36. Bed A
is thus subjected to a co-current depressurisation Dl wherein
the gas in bed A passes co-currently out of bed A to bed C
through which it passes, counter-currently, as a purge PU.
In the third time interval T3, wherein bed B is
: used for adsorption duty, the outlet of bed A is closed and
its inlet ls connected to the waste gas manifold 36: the bad
is thus subjected to a counter-current depressurisation D2.
The outlet of bed A is then connected to the outlet of bed D,
which has just undergone the equalisation step E2, so that
the gas released during the co-current depressurisation step
Dl of bed D flows counter-currently through bed A as a purge,
ie step PU.
In the fourth time interval T4, wherein bed C is
used for adsorption duty, the inlet of bed A is closed and
its outlet is connected to the outle of bed B (which has just
finished its adsorption duty AD) so that the gas released
from bed B during its equalisation step E2 effects counter-
current repressurisation ~1 of bed A. Finally the outlet of
bed A is closed and its inlet connected to the SR gas outlet
manifold 38 (in Figure 2, since this manifold 38 is being
supplied, in this time interval, with gas from bed C, the
connection to bed A is shown from the SR outlet time fro~ bed
C), to effect co-current repressurisation, step R3.
Bed A is thus ready to recommence adsorption duty.
As shown in Figure 2 the other beds go through the same cycle
.,, '
:: :, : : : . -
,: : :.:
:
~. ~'' ,', ,
~%6~3~28
24 B 33273
but out of phase with one another.
Some or all of ehe waste gas W from PSA unit 34 ~ay
be used as a fuel in a furnace or gas turbine, possibly in
admixture with the MR (or P) gas stream from PSA unit 14.
~owever, it i5 preferrred that at least part of the waste gas
W from PSA unit 34 is fed back to PSA unit 14 via line 40 in
Figure 1. Unless PSA unit 34 is operated under conditions
giving a waste gas W at a suffic:Lently hlgh pressure for its
intended use in PSA unit 14, it will generally be necessary
to compress waste gas W in compressor 42 before it is
supplied to the waste gas inlet manifold 44 of PSA unit 14.
Various uses of the waste gas W from PSA unit 34 in
the PSA unit 14 are shown in the flow sheets of Figures 3 to
10.
The cycle of the PSA unit 14 is similar to that
described above for PSA unit 34. However, as shown in
Figures 3 - 5, 9, and 10, the gas released from the bed in
the final, counter-current, depressurisation step D2, and the
purge step PU may bs collected separately to give,
respectively a C02-rich gas stream CR and a MB-rich gas
stream ~R. Such separate collection of CR and MR can also be
adopted, if desired, in the embodiments of Figures 6 - 8.
Alternatively, in the arrangements of Figures 3 - 5, the
final depressurisation and purge gases can be collected as a
single stream of product gas, P, as show~ in the embodiments
of Figures 6 - 8.
In the embodiment of Figure 3, the waste gas W from
PSA unit 34 is used for the co-current repressurisation step
R3 instead of using gas from the manifold 18 supplied with
gas from a bed undergoing adsorption duty AD. This has the
advantage that H2 otherwise lost in the waste gas W from PSA
unit 34 is returned to the system.
Alternatively, in an embodiment not illustrated,
this final rapressurisation, or at least the initial part
,:
' .~ . " : ' "' . "'' ' .
2B
B 33273
thereof, may be affected with part of the SR gas leaving PSA
unit 34 instead of with the waste gas: this has the
advantages that the amount of MB returned to YSA unit 14 in
this repressurisation step R3 is minimised and also that
less, if any, compression of this returned gas is required.
In the embodiment of Ei~ure 4 the waste gas W from
PSA unit 34 is used as the purge gas: thus the gas leaving
the bed undergoing the co-current depressurisation stap Dl in
PSA unit 14 is taken directly to the ~R gas outlet manifold
20 while the waste gas W from PSA unit 34 is fed to the
outlet of the bed of PSA unit 14 undergoing the purge step
PU. This has the advantage that the MR gas has a lower C02
content than in the embodiment of Figure 3. Consequently in
this embodiment generally the CR and ~R gases will be
collected separately, as shown, rather than combined to give
a single product gas P.
In the embodiment of Figure 5, the co-current
depressurisation step Dl is replaced by a sweep step SW
wherein the waste gas W from PSA unit 34 is fed to the inlet
of a bed that has undergone the equalisation step E2. The
outlet of the bed undergoing the sweep step SW is connected, .
as in the embodiment of Figure 3, to the outlet of a bed
undergoing the counter-current purge PU. This has the
advantage that the CR gas produced in the counter-current
depressurisation step D2 following the sweep step SW will
contain virtually no hydrogen.
In the embodiments of Figures 6 and 7 a co-current
sweep step SW using the waste gas W from PSA unit 34 is
interposed between the equalisation step E2 and the co-
current depressurisation step Dl. In the Figure 6 embodimentthe gas swept from the bed is used for an intermediate, co-
current, repressurisation step R2. This has the advantage
that H2 in the waste gas W is retu~ned to the system.
However it will be seen that the pressure at which the waste
,. ~ ,
- ,
~:
, :
4~
26 B 33273
gas has to be supplied increases, as in the embodiment of
Figure 3 as repressurisation proceeds. Thus during the -
course of the sweep the pressure has to increase from that
remaining after equalisation towards the adsorption pressure.
In the embodiment of ~igure 7 the gas swept from the bed is
returned, via a compressor 46, to the inlet gas manifold 12.
This has the advantage of returnLng the ~2 as in the Figure 6
embodiment with the further advantage that, unlike the Figure
3 and Figure 6 embodiments, the compressor 42 in the waste
gas W inlet line 40 is not subject to a fluctuating load.
In the embodiment of F:Lgure 8 the cycle is similar
to that of Figure 7 except that thé sweep step SW is before,
instead of after, the equalisation step E2. This is more
advantageous than the system of Figure 7 where the waste gas
W from PSA unit 34 is at a higher pressure.
In the embodiment of Figure 9 the waste gas W from
PSA unit 34 is used as purge gas, as in the Figure 4
embodiment, but also a co-current sweep step SW is employed
in place of the co-current depressurisation with the swept
gas being returned to the inlet gas manifold 12 via
compressor 46 as in the Figure 7 embodiment. In this case
the gas used for the sweep step SW is C02-rich gas CR taken,
via compressor 48, from the manifold 50 supplied with gas
from the counter-current depressurisation step D2. Although
in this embodiment there is no bed undergoing step D2 at the
time of the sweep step SW, a sufficient reservoir of C~ gas
wil~ probably be availabIe in the pipework and/or a small
reservoir vessel can be provided. Alternatively the CR gas
used for the sweep can be gas from external processing of the
CR gas exported from PSA unit 14. By means of this C02-rich
gas sweep, the CR gas produced in the subsequent counter-
curren~ depressurisation will be particularly pure.
In this embodiment two equalisation steps E2 and E1
are shown: before the final equallsation E2 in which the
: ~ :
-:.. ,: ;: :
.
~2~342~
27 B 33273
released gas effects initial counter-current repressurisation
Rl of a bed that has been purged, an intermediate
equalisation step E1 is employed for intermediate counter-
current repressurisation R2 of another bed that has already
been subjected to the initial repressurisation Rl by gas
released from a final equalisation step E2. Such multiple
equalisations may also be adopted where appropriate in the
embodiments of Figures 3 to 8.
In the embodiment of Figure 9 each time interval is
typically about 6 minutes. Referring to bed A, at the end of
interval Tl, the C02 adsorption front is well short of the
bed outlet. In intervcal T2 the two equalisation steps are
first effected with the result that bed B has been
repressurised to over half the adsorption pressure and bed C
to over one quarter of ths adsorption pressure. Beds B and C
have had such C02 adsorption fronts as remained moved back
towards the bed inlets. Up to three quarters of the ~2 left
in bed A at the end of the adsorption step, ie in the void
space and as adsorbed gas, has been returned to the process.
In the sweep step SW in interval T2 the gas is
allowed to flow until most of the H2 and ~B gas has been
swept out of the bed, but is stopped before significant C02
breaks through. The gas in bed A is now almost exclusively
C02. In the counter-current depressurisation step D2 also in
interval T2, the major proportion of the gas is exported as
CR gas and only a minor proportion is used for the sweep. It
will be appreciated that the CR export can be taken from the
mani~old 50 before compressor 48 if desired: however since
the exported CR gas will normally have to be compressed in
its subsequent processing, it is often more convenient to
effect the compression of all the gas released in the
counter-current depressurisation step D2 and to export the CR
gas, as shown, from manifold 22 after compressor 48. After
the counter-current depressurisation step D2, the purge step
,
~, .
'''`'
.
-. ...
~26~42~3
28 B 33273
is effected to purge the remaining C02 from the bed: since
the time remaining in interval T2 is unlikely to be
sufficient to effect completion of the purge, ~he purge can
continue, as shown, into interval T3.
In the embodiment of Figure 10, a 6 bed arrangement
for effecting the cycle of Figure 9 is shown. There it is
seen that a bed is undergoing counter-current
depressurisation D2 while the sweep SW is taking place and so
no reservoir for CR gas is required. Since in the Figure 10
embodiment each time interval only includes 1 or 2 steps,
whereas in the Figure 9 embodiment: up to 5 steps were
required in each time interval, the time intervals ln the
Figure 10 embodiment can, if desired, be shorter than those
of the Figure 9 embodiment.
In a further embodiment, not shown in the Figures,
the arrangement of Figure 9 is modified by the omission of
the equalisation steps El and E2 and the corresponding
initial and intermediate repressurisation steps Rl and R2.
In this embodiment the final repressurisation R3 is
preferably using some of the synthesis ready ammonia
synthesis gas from the second PSA unit. This embodiment has
the advantage of reducing the number of beds required in the
first PSA system, in some cases to as few as two, at the
expense of increased power requirement Pg in compressors 46
and 48.
PA/CG/MP/L201
- ,::. :, ,. .,.: . . - ::: : : ' ~, :