Note: Descriptions are shown in the official language in which they were submitted.
~6~
--1--
~ his invention relates to a process of
producing a mixture of methanol and hiher alcohols
having 2 to 7 carbon atoms per molecule from a synthesis
gas containing hydrogen and carbon oxides by a reaction
carried out at a temperature in the range from 200 to 320 C
and under a pressure in the range from 50 to 100 bars on a
copper, zinc and aluminum con~aining catalyst.
~ aid-open German Application 33 10 540
discloses such a process in which the catalyst employed
contains essentially copper, cobalt, aluminum and at
least one alkali metal or alkaline earth metal. Zinc may
also be contained in the known catalyst.
~ mith and Anderson have reported in the
Canadian Journal of Chemical Engineering, Vol. 61
(February 1983), on pages 40 to 45 on investigations
~69~0~
concerning the synthesis of higher alcohols on a CuO-ZnO-
Al2O3 catalyst which contains alkali metal. When the
synthesis gas is caused to flow in contact with the
catalyst, methanol and higher alcohols are formed and the
content of higher alcohols in -the liquid product is
distinctly dependent on the content of the K2CO3 activator
in -the catalyst. The highest yield of higher alcohols has
been obtained with a catalyst containing 0.5 wt.% K2CO3.
With catalysts having a lower content of K2CO3, the yield of
higher alcohols dropped ~uickly.
It is an object of the invention to carry out the
process described first hereinbefore in such a manner that
the yields of methanol and higher alcohols are increased and
a long life of the catalyst is achieved. Such methanol-
alcohol mixtures are used in technology to improve the knock
rating of fuels and as solubilizers for water in a mixture
with natural fuels and as solubilizers for water in a
mixture with natural fuels for Otto cycle engines.
Furthermore, it is desirable to minimize the water content
of the product of the synthesis in order to avoid the need
for a purifying distillation, which would be difficult
because of the formation of an azeotropic mixture.
In meeting these and other objects, the present
invention provides a process of producing a mixture of
me-thanol and higher alcohols having 2 to 7 carbon atoms per
molecule. The process comprises contac-ting a synthesis gas
having a H2:CO mole ratio of 0.3 to 1.9 and a CO2 content
not in excess of 2 vol. %, at a temperature in the range
from 50 to 100 bars, with a copper-, zinc- and alumimum-
containing catalyst. This catalyst is derived from a partly
reduced catalyst precursor which contains 35 to 65 wt.% CuO,
15 to 45 wt.% ZnO and 5 to 20 wt.% Al2O3, and which has a
total content of alkal~i metal and/or alkaline earth metal
~Z~4C~1
- 2a -
not in excess of 0.25 wt.%. The oxides of the catalyst
precursor are reduced, at least in part, before the process
begins. The catalyst has a Cu:Zn weight ratio in the range
from 1:1 to 2.4:1.
B
L26~
-- 3 --
In accordance with the present invention the
alcohols produced in contact with the catalyst may consist
of about 50 to 99.8 % methanol and about 0.2 to 50 % C2 to
C7 alcohols.
In accordance with the present invention, CO2
may be removed from the synthesis gas by means of methanol
contained in the reaction product or by means of a methanol-
alcoho~ mixture before the synthesis gas is used for the
synthesis.
In accordance with the present invention a mix-
ture of methanol and higher alcohols may be formed in a
first reaction stage, the content of higher alcohols in the
liquid product obtained from said mixture may be in excess
of 10 wt.%, hydrogen is admixed to the residual gas to form
a synthesis gas, and the latter is used in a succeeding
second reaction stage to produce methanol, from which a
liquid product may be derived which contains less than
0.5 wt.% higher alcohols.
It has surprisingly been found that a product
having a very low water content will be obtained whenever
the synthesis gas contacted with the catalyst has a low CO2
content and a H2:CO mole ratio which is distinctly lower
than 2. The gas stream leaving the catalyst contains con-
densible liquid products which usually consists of the
following components:
Methanol 55 to 88 wt.%
Higher alcohols (C2 to C7) 10 to 45 wt.~
Water 0.1 to 1.5 wt.%
The process in accordance with the invention is
preferably carried out at temperatures in the range from
~'
12~9~
- 3a -
250 to 300C; in said process the catalyst obviously has a
high importance. In the catalyst precursor and in the
partly reduced catalyst used for the synthesis the weight
ratio of Cu:Zn is suitably in the range from 0.7 to 4.8 and
preferably in the range from 1.0 to 2.4.
In view of -the previously published investigations,
such as that by Smith and Anderson, it was not to be pre-
dicted that a Cu-Z~-A1 catalyst whic
~z~
stantially free of alkali would produce excellent results
also in the simul-taneous synthesis of methanol and higher
alcohols. It had previously been believed that additions of
activating alkali metals or alkaline earth metals were
required to accomplish that object.
Higher yields of higher alcohols will be
particularly obtained if catalysts are used in which the
total content of alkali metal and alkaline earth metal is
not in excess of 0.1 wt. ~. The content of alkaline earth
metal may be virtually zero. The alkali metals enter the
catalyst in most cases as impurities during the production
process and include lithium, sodium, potassium, rubidium
and/or caesium. The corresponding alkaline earth metals are
magnesium, calcium and/or barium.
The production of higher alcohols can be further
increased in that the catalyst is contacted with a synthesis
gas which contains methanol vapor, e.g., in a proportion of
3 to 15 vol. ~.
The preferred catalyst can be produced, e.g., in
that the catalytically active copper oxide and zinc oxide
components are precipitated from an aqueous solution of the
corresponding salts, such as the nitrates or acetates, by an
addition of alkaline substances in the presence of colloidal
alumina or colloidal aluminum hydroxide (in the form of a
gel or sol). The resulting mixture or precipitation product
may be processed in known manner by drying, calcining,
pressing to shapes and, if desired, reduction. The alkaline
precipitants preferably consist of an alkali or ammonium
carbonate or bicarbonate, preferably the carbonate or
blcarbonate of potassium. It has been found that in the
synthesis also of higher alcohols a low potassium content up
to a certain limit will not be disturbing and will be much
more favorable than a corresponding sodium content.
To improve the porous structure of the catalys-t
~r
~z~g~
-- 5 --
the precipi-tation of the copper oxide and zinc oxide
components is in the presence of the colloidal alumina or
aluminum hydroxide is preferably carried out in the presence
of a dilute solution of the alkaline substances, such as a
solution of 10 to 20 wt. 96 alkali carbonate, and at low
temperatures and at pH values in the neutral or slightly
acid range. The precipitation is suitably affected at a
temperature of 25 to 65C, preferably of 30 to 40C. The
addition of the alkalies is usually terminated at a pH value
in the range from 6.5 to 7.5.
A higher yield of higher alcohols can be achieved
by the use of a catalyst which in its oxidic form has a void
volume of 0.25 to 0.5 cm3/g. The distribution of the pores
of that catalyst precursor is of great importance. 50 to
85% of the avoid volume may be constituted by pores which
are 0.014 to 0.08 ~Im in diameter, 15 to 50% by pores which
are less than 0.014 lum in diameter, and not in excess of 4g6
of the void volume by pores which are in excess of 0.08 Jum
in diameter. That void volume is determined by mercury
poroslmetry (Literature: R. Anderson, Experimental Methods
in Catalytic Research, Academic Press, New York, 1968).
To a person skilled in the art it will be apparent
that the catalysts of the type explained hereinbefore can be
used also for the synthesis of methanol accompanied by very
small ~[uantities of higher alcohols. This has been proved
by experiments (see Example I). In that case a high-
hydrogen synthesis gas is used.
Examples of the Preparation of the Catalyst
Catalyst A
Two solutions are prepared for the precipitation
of the catalyst precursor.
Solution
418 g copper nitrate and 50 g zinc oxide are
6~
- 6 -
dissolved in 1.6 liters water and 148 g 52% nitric acid.
Thereafter, a colloidal aluminum metahydrate gel is added.
Solution 2
535 g potassium carbonate are dissolved in 3317 g
water.
The solutions are mixed at 40C with strong
stirring in such a manner that the pH value during the
precipitation is 6.9. The precipitation is terminated when
the pH value is not in excess of 7.1. Thereafter the
precipitate is filtered off and washed with water until
alkali metal (potassium) can be no longer detected in the
filtered effluent. The filter cake is dried at 120C and is
subsequently calcined at 280C for 8 hours. The calcined
product is reduced in size and is compacted after 2 wt. %
graphite have been added.
The calcined catalyst precursor is free of
graphite and contains 65 wt. % CuO, 23 wt. % ZnO and 12
wt. % A12O3. The catalyst precursor A thus produced
contains 0.06 wt. % potassium. The resulting tablets have a
size of 5 mm x 5 mm and the following physical properties:
Bulk density of tablets: 1015 g/liter
Void volume: 0.36 cm3/g
Pore size distribution determined by Hg porosimetry
Pores 0.08 to 0.014 lum
25in diameter 78% of void volume
Pores less than 0.014 ~um
in diameter 21% of void volume
Pores in excess of 0.08 lum
in diameter 1~ of void volume
Catalyst B
Catalyst precursor B is made in a process which is
similar to the preparation of catalyst precursor A with the
single difference that the filter cake which has been
filtered off is washed with water only twice so that the
o~
filtered effluent is no-t free of alkali metal.
The calcined catalyst precursor is free of
graphite and con-tains 65 wt. % CuO, 23 wt. % ZnO and 12
wt. % Al2O3 and has a residual alkali metal content of 0.31
wt. % potassium. As regards physical properties, catalyst B
does not exhibit detectable differences from catalyst A.
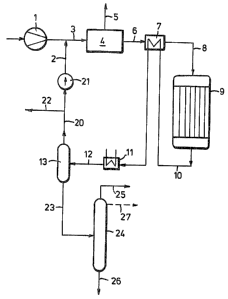
An embodiment of the process will now be explained
with reference to the drawing~
A synthesis gas which contains hydrogen and carbon
oxides is supplied by the compressor 1 and is mixed with
residual gas from line 2. The resulting mixture is supplied
in line 3 to a process stage 4 for removing CO2. In that
stage, substantially all CO2 is removed from the mixed gases
in known manner. The separated CO2 gas conducted in line 5
may desirably be used in the production of the synthesis gas
if the latter is derived, e.g., from natural gas. CO2 may
be removed by a scrubbing with methanol or with a mixture of
methanol and higher alcohols and a product of the present
process may be used for that purpose.
The gas which is substantially free of CO2 is
supplied in line 6 to a heat exchanger 7, in which the gas
is heated and from which the gas is conducted in line 8 to
the synthesis reactor 9. The reactor 9 consists of a
tubular heater, in which the tubes contain the catalyst
consisting mainly of copper, zinc and aluminum. A liquid
coolant, such as water, is disposed between the tubes.
The synthesis gas supplied to the reactor 9
consists mainly of H2 and CO in a mole ratio in the range
from 0.3 to 1.9. the CO2 content is not in excess of 2 vol.
% in the catalyst in the catalyst-filled tubes of the
reactor the temperatures are in the range from 200 to 320C,
preferably at about 250 to 300C, and the pressure is 50 to
150 bars. In the reactor, the synthesis gas is reacted to
form methanol and higher alcohols.
~26~
The product of the synthesis is withdrawn from the
reactor 9 in line 10 and is first indirectly cooled in the
heat exchanger 7 with the cold gas from line 6 and is cooled
further by water in a cooler 11. The partly condensed
product is conducted in line 12 to the separator 13, in
which residual gases are separated and then withdrawn in
line 20. Part of said residual gases is recycled through
the circulating compressor 21 and the line 2. A branch
stream of residual gases is withdrawn in line 22.
The liquid product obtained in the separator 13 is
conducted in line 23 to a distilling stage 24, in which the
low-boiling components, particularly dimethylether, methyl
formate and acetone, are separated and withdrawn overhead in
line 25. The sump product of the distillation column 24
consists of methanol and higher alcohols and contains water
not in excess of about 2 wt. % and is withdrawn in line 26
and is suitable as a motor fuel, particularly as an additive
to regular-grade or premium gasoline for Otto cycle engines.
The concentration of higher alcohols in the
product withdrawn in line 26 may be increased, if desired,
in that methanol is also distilled in the distilling stage
24 and is withdrawn either overhead in line 25 together with
the low-boiling components or is separately withdrawn from
the column 24 through a lateral outlet 27.
The yield of higher alcohols can also be increased
in that the synthesis gas supplied to the catalyst of the
reactor contains methanol vapor, e.g., in a proportion of 3
to 15 vol. %. That methanol may be produced in that the
methanol contained in the product conducted in lines 10 and
12 is not completely condensed so that the residual gas in
line 2 contains methanol vapor. Besides, methanol may be
injected into the synthesis gas conducted in line 8.
The partial stream of residual gas in line 20 may
be used entirely or in part for a second methanol synthesis,
,....
~Zi~314~
which is not shown. That second methanol synthesis may be
carried out in known manner so that its product consists
mainly of methanol and has only a low content of higher
alcohols. The catalyst used for such methanol synthesis may
consist of copper, zinc and aluminum, such as the catalyst
used in the reactor 9. Because the residual gas in line 20
has substantial contents of CO and also of CO2, that
residual gas must be enriched with hydrogen before entering
the second methanol synthesis so that the H2:CO mole ratio
is at least 2Ø
In the process illustrated in the drawing it is
also possible to form in the synthesis reactor 9 a product
consisting mainly of methanol without appreciable quantities
of higher alcohols if the H2:CO mole ratio in the fresh
synthesis gas is higher than 2. That fresh synthesis gas is
sucked by the compressor 1 and its use has the result that a
synthesis gas having a high H2 content is contacted with the
catalyst.
ZO EXAMPLE 1
100 cm3 of catalyst precursor A are placed into a
tubular heater, which is cooled by a water jacket and in
which the catalyst is reduced under normal pressure by a
treatment with a mixture of 1 vol. % H2 and 99 vol. % N2.
The temperature in the reactor is raised in steps -to 240 C.
When a temperature of 240C has been reached, the reactor is
supplied with a synthesis gas consisting of 4 vol. % CO2, 10
vol. % CO, 75 vol. % H2 and 11 vol. % inert gas. Under the
selected experimental conditions, namely, a pressure of 50
30 bars, an hourly space velocity of 10,400 standard liters per
liter of catalyst, a liquid product of 1,105 kg/h per liter
of catalyst is obtained after an operation of about 100 to
150 hours. That product has the following composition:
~r
94~)~
- 10 -
Methanol 94~25 wt. %
Ethanol 0.08 wt. %
Propanols 0.04 wt. %
Butanols 0.03 wt. %
l~2O 5.6 wt. %
After an operation for 150 hours, the temperature is
increased to 270C and the pressure to 100 bars~ If the
catalyst is contacted at an hourly space velocity of 4,500
standard liters per liter of catalyst with a synthesis gas
composed of 0.5 vol. % inert gas, the liquid product
obtained after the 150th hour of operation at a rate of 1.02
kg/h per liter of catalyst has the following composition:
Methanol 84.7 wt. %
Ethanol 6.0 wt. %
Propanols 3.2 wt. %
Butanols 3.5 wt. %
Pentanols and other
higher alcohols2.3 wt. %
Water 0.3 wt. %
It is apparent from this example that a catalyst
which is almost free of alkali metal (0.06 wt. % potassium)
and alkaline earth metal may be used to produce either a
mixture of methanol and higher alcohols (e.g., 15 wt. %
higher alcohols) or methanol having a low content of higher
alcohols (0.15 wt. %).
E~AMPLE 2
100 cm3 of catalyst precursor B having an alkali
metal content of 0.31 wt. % potassium are placed into the
reactor used in Example 1 and are reduced therein as
described in Example 1.
Operating at 270C and 100 bars, the reactor is
contacted at an hourly space velocity of 4500 standard
liters per liter of catalyst with a synthesis gas consisting
~L26940~
-- 11 -
of 0.5 vol. % CO2, 48 vol. % CO, 50 vol. % H2 and 1.5 vol. %
inert gas. Between the 150th and 200th hours of operation,
the liquid product obtained in the reactor at a rate of 0.98
kg/h per liter of ca-talyst has the following composition:
Methanol 90.9 wt. %
Ethanol 3.5 wt. %
Propanols 1.8 wt. %
Butanols 1.9 wt. %
Hexanols and other
higher alcohols1.7 wt. %
Water 0.2 wt. %
It is apparent from this experiment that the use
of catalyst precursor B which contains 0.31 wt. % potassium
under the same conditions results almost in the same yield
of liquid product as the use of the catalyst precursor A,
from which alkali metal has been removed to the practical
limit (residual potassium content 0.06 wt. %).
The decisive difference resides in that use of the
product obtained by the use of catalyst A having a low
content of alkali metal and/or alkaline earth metal has much
higher contents of C2 to C7 alcohols (15.0 wt. %) the
product obtained by the use of catalyst B, which has a high
alkali metal content and results in a liquid product which
contains 8.9 wt. % C2 to C7 alcohols.
EXAMPLE 3
Catalyst precursor A is placed in-to the reactor
used also in Example 1. When the catalyst has been reduced,
it is supplied first with a synthesis gas having a low CO2
content (gas I) and subsequently with a synthesis gas having
a high CO2 content (gas II). The experimental conditions
and the results obtained are listed hereinafter:
~6~
Gas IGas II
.
Synthesis gas
CO2, vol. % 1.4 11.4
CO, vol. % 61.0 60.1
H2, vol. % 30.0 26.1
Inert gas, vol. % 7,6 2.4
Pressure, bar 50 50
Temperature, C 75 75
Hourly space velocity,
standard liters per liter2650 2700
Rate of liquld product
kg/1-h 0.21 0.16
Composition of liquid product
Methanol, wt. % 80.0 86.4
Higher alcohols, wt. % 19.8 12.1
Water, wt. % 0.2 1.5
It is apparent from the above table that the
contacting of the catalyst with a synthesis gas having a
higher CO2 content results in a distinct decrease of the
yield of higher alcohols and in a higher water content of
the liquid product so that the highest permissible water
content in the mixed fuel may be exceeded.
~`~
1.