Note: Descriptions are shown in the official language in which they were submitted.
The presen-t invention relates to a method of destroying
household and industrial waste to form a leach-proof slag and a
gas in which the combustible constituents consist essentially
only of H2 and CO.
Nowadays such waste, besides by dumping, is disposed of
~ almost exclusively by complete combustion. A relakively low
temperature is obtained in the combustion step due to low thermal
value, high water content and uneven composition. This means
that combustion is incomplete and that heavier hydrocarbons are
formed. Incombustible constituents are discharged as ash in
which the constituents are dissolved or not bound at all, which
gives rise to dumping problems such as dust and harmful
substances being easily leached out of the ash.
1'; The present invention provides a process which
eliminates tha above mentioned drawbacks and offers a pro-
environmental process, the residual product being substantially
free from any unbound pollutants and a combustible gas being
produced in which the combustible constituents are essentially
only H2 and CO.
The method according to the present invention comprises
the steps of: a~ supplying the waste material at the top of a
shaft furnace while simultaneously supplying energy in the form
?~ of hot oxidizing gas at the bottom of the shaft furnace, b)
discharging liquid slag from the bottom of the furnace shaft and
withdrawing the gas generated at the top of the furnace shaft,
and
3U -
3~
-- 1 --
~' ~
~7~
c) supplying the gas generated, to a subsequent reac-
tion chamber while simultaneously supplying energy
in the form of a hot gas.
The gas generated in the furnace shaft contains pollutants
such as heavy hydrocarbons. The supply of energy to the
subsequent reaction chamber and the presence of water which
has vaporized from the waste material causes the hydrocar-
bons to be thermally cracked to form CO and H2.
According to one embodiment of the invention oxidizing gas,
preferably air, heated in a plasma generator, is used to
supply energy to the bottom of the shaft furnace~ The tem-
perature can thus be accurately and rapidly controlled to
the desired level following variations in the composition
of -the waste.
According to a further embodiment of the invention the hot
gas supplied to the subsequent reaction chamber is heated
in a plasma generator. The use of a plasma generator to
heat the gas gives it extremely high energy density and
the volume of gas required for the desired amount of ener-
gy is therefore relatively small.
According to a further embodiment of the invention, finelypulveri~ed coke and/or water vapour is injected into the
subsequent reaction chamber to compensate a too low con-
tent of C and/or H2O.
According to a further embodiment of the invention, the
gas is also subjected to a catalytic cleaning step to re-
move any remnants of heavy hydrocarbons. The gas is con-
ducted through a chamber containing a catalyst. The cata-
lyst is preferably lime or dolomite, but other catalysts
are also feasible such as nickel.
.
The process is preferably controlled so that the tempera-
ture of the gas leaving the shaft furnace is at most 800C
and that of the gas mixture leaving the subsequent reac-
tion chamber is more than 1000C, preferably approximately
1200C. The high temperature in the subse~uent reaction
chamber produces substantially complete thermal disinte-
gration of heavy hydrocarbons present in the gas.
A temperature exceeding the melting poin~ of the slag is
maintained in the lower part of the shaft~ When the slag
solidifies incombustible constituents become glass-encased,
thus enabling the slag to be safely dumped.
According to yet another embodiment of the invention
chlorine compounds are removed from the gas by conducting
it after cooling through a chamber containing quicklime.
The quicklime is preferably taken from a previous cataly-
tic purifying step in which limestone/dolomite has been
calcinated due to the high initial temperature of the gas.
Other advantages and features of the invention will be re-
vealed in the following detailed description with refe-
rence to the accompanying drawings in which
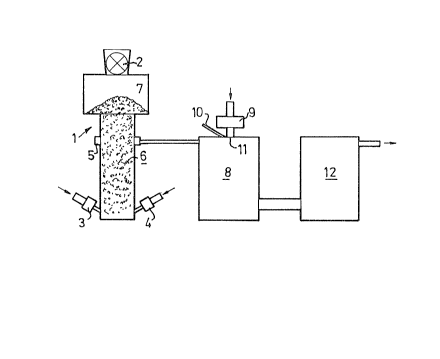
Figure 1 shows schematically a view of a plant for per-
forming the process according to the invention,
and
Figure 2 shows a means for catalytic disintegration of
heavy hydrocarbons and removal of chlorine com-
pounds from the gas generated at pyrolysis of
the waste.
The waste material is supplied through suitable sluice
means 2, to a shaft furnace 1. Energy and oxidant are
30 supplied at the bottom of the shaft furnace, by means of
, '
, ' .
one or more arrangements for the supply of hot air in the
embodiment shown. These arrangements may be plasma genera-
tors, for instance. The gas generated at pyrolysis is with-
drawn through a bustle-pipe S, arranged in the embodiment
shown so that the gas is withdrawn below the surface 7 of
the waste material 6 in the shaft furnace.
The gas thus generated is then conducted to a subsequent
reaction chamber 8. Energy is supplied by means of a hot
gas which in the preferred embodiment shown is heated in
a plasma generator 9. All or a part of the gas may be
passed through the plasma generator. Finely pulverized
coke and/or water vapour may also be supplied if necessary,
through lances 10 close to the inlet 11 for the gas heated
by the plasma generator. Impurities, primarily in the form
of heavy hydrocarbons, are thermally disintegrated in this
reaction chamber.
After thermal disintegration the gas may be subjected to
further cleaning in a means 12, indicated only schemati-
cally in the drawing, in the form of an empty chamber.
Finely pulverized lime, for instance, may be injected in
this chamber for catalytic disintegration of any heavy
hydrocarbons remaining in the gas. Alternatively the gas
may be conducted through a filler of lime in lump form,
or some other catalyst for the disintegration process.
The gas may then be subjected to a chlorine purification
step, described in more detail with reference to Figure 2,
and mercury be removed therefrom by condensing in a final
step.
The gas-purifying equipment illustrated in Figure 2 com-
prises a first shaft 13 containing a limestone and dolo-
mite filler 14, supplied to the shaft through a sluice
arrangement 15. The gas from the subsequent reaction cham-
` :
': :
' "'
~7~
ber is supplied, possibly after heat-exchanglng, through
a gas inlet 16 at the bottom of the shaft and is with-
drawn through outlet 17 at the top of the shaft after
having passed through -the filler. An out-feed table or
the like is arranged at the bottom of the shaft for par-
tially or wholly calcinated limestone being discharged
through a gas-tight sluice arrangement 18.
The partially or wholly calcinated limestone is then
carried on a conveyor belt or the like, indicated at 19
in the drawing, through a sluice arrangement 21 to form
a filler 22 in a second shaft 20.
The gas emitted from the shaft 13 passes through a pipe
23 to a heat exchanger 24 and is heat-exchanged, prefer-
ably with air, enabling the physical heat from the gas
to be utilized in earlier process steps or for other pur-
poses. The gas is then conducted through the pipe 25 to
a lower gas inlet 26 in the second shaft and passed
through the filler 22 before being withdrawn through
a gas outlet 27 located at the top of the shaft 20.
An out feed table or the like is arranged at the bottom
of the shaft for discharging the product formed during
the chlorine-purification process, via a gas-tight
sluice arrangement 28.
The temperature of the gas introduc~d at the bottom of
the first shaft shall exceed about 800C. At these tem-
peratures the limestone is calcinated and forms CaO -~ CO2.
The content of heavy hydrocarbons of the gas such as tars
or the like, is cracked with the aid of H2O and/or CO2
with CaO as catalyst. The grade of limestone should be
selected according to the prevailing gas temperature
since different types of limestone are calcinated at
different temperatures. In this first step, thus, the
.: , .
.:,
'
. . .
lime acts only as catalyst in the cracking and is not
affected by the chemical composition of the gas. The
calcinated limestone discharged will still be in lump
form, but considerably more porous.
When the tars or the heavy hydrocarbons have been removed
the gas can be heat-exchanged without difficulty. This
is preferably performed using cold air and the hea-ted
air can then be utilized in one or more of the preceding
process steps.
The calcinated limestone is transported further to the
second shaft to give a filler which is used to clean the
gas from chlorine compounds and/or chlorine. CaO and 2HCl
react here to form CaC12, for instance. This reaction
should take place at a temperature below the melting
point of CaC12 in the form in which it occurs.
The gas leaving is thus free from hydrocarbon compounds
and chlorine compounds and, after possibly condensing
out mercury, the only combustible constituents will be
CO and H2. After combustion the gas, now containing only
CO2, H2O and N2, can be released to the atmosphere.
The method according to the present invention thus enables
all the harmful, anti-environmental substances which nor-
mally cause great problems in conventional processes cur-
rently in use, to be taken care of and converted to harm-
less products, possibly even useful products such as
CaC12 .
~'`' ~ `'
. . .
.