Note: Descriptions are shown in the official language in which they were submitted.
lZ7064~
Case 5250-Plus
FUEL COMPOSITIONS
Compression ignition fuel compositions and
additive mixtures of organic nitrate ignition
accelerator and hydrocarbyl-substituted succinimide, in
5 amounts sufficient to resist the coking tendencies of
compression ignition fuel compositions when used in the
oDeration of indirect injection diesel engines.
Throttling diesel nozzles have recently come into
widespread use in indirect injection automotive and
10 light-duty diesel truck engines, i.e., compression
ignition engines in which the fuel is injected into and
ignited in a prechamber or swirl chamber. In this way,
the flame front proceeds from the prechamber into the
larger compression chamber where the combustion is com-
15 pleted. Engines designed in this manner allow for
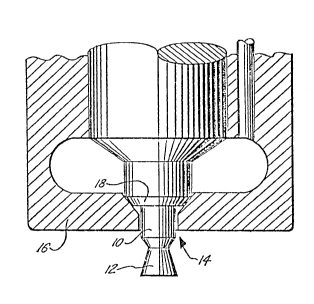
quieter and smoother operation. The Figure of the
Drawing illustrates the geometry of the typical
throttling diesel nozzle (often referred to as the
n pi ntle nozzle").
Unfortunately, the advent of such engines has
given rise to a new problem, that of excessive coking on
127~i642
-- 2
the critical surfaces of the injectors that inject fuelinto the prechamber or swirl chamber of the engine. In
particular and with reference to the Figure, the carbon
tends to fill in all of the available corners and
5 surfaces of the obturator 10 and the form 12 until a
smooth profile is achieved. The carbon also tends to
block the drilled orifice 14 in the injector body 16 and
fill up to the seat 18. In severe cases, carhon builds
up on the form 12 and the obturator 10 to such an extent
10 that it interferes with the spray pattern of the fuel
issuing from around the perimeter of orifice 14. Such
carbon build up or coking often results in such
undesirable consequences as delayed fuel injection,
increased rate of fuel injection, increased rate of
15 combustion chamber pressure rise, and increased engine
noise, and can also result in an excessive increase in
emission from the engine of unburned hydrocarbons.
~ hile low fuel cetane nu~ber is believed to be a
major contributing factor to the coking problem, it is
20 not the only relevant factor. Thermal and oxidative
stability (lacquering tendencies), fuel aromaticity, and
such fuel characteristics as viscosity, surface tension
and relative density have also been indicated to play a
role in the coking problem.
An important contribution to the art would be a
fuel composition which has enhanced resistance to coking
64Z
tendencies when employed in the operation of indirect
injection diesel engines.
In accordance with one of its embodiments, this
invention provides distillate fuel for indirect injection
compression ignition engines containing at least the
combination of (a) organic nitrate ignition accelerator, and
(b) hydrocarbyl-substituted succinimide or succinamide, or
the combination of (a) organic nitrate ignition accelerator,
(c) hydrocarbyl amine having from 3 to 60 carbons and from 1
to 10 nitrogens and (d) N,N'-disalicylidene-1,2-
diaminopropane, or the combination of (b) hydrocarbyl-
substituted succinimide or succinamide, (c) hydrocarbyl amine
having from 3 to 60 carbons and from 1 to 10 nitrogens and
(d) N,N'-disalicylidene-1,2-diaminopropane, said combinations
being separately present in an amount sufficient to minimize
coking, especially throttling nozzle coking, in the
prechambers or swirl chambers of indirect injection
compression ignition engines operated on such fuel.
Another embodiment of the present invention is a
distillate fuel additive fluid composition comprising (a)
organic nitrate ignition accelerator, and (b) hydrocarbyl-
substituted succinimide or succinamide, or (a) organic
nitrate ignition accelerator, (c) hydrocarbyl amine having
from 3 to 60 carbons and from 1 to 10 nitrogens and (d) N,N'-
~:~ rn/
3 2~Q64~2
disalicylidene~l,2-diaminopropane or (b) hydrocarbyl-
substituted succinimide or succinamide, (c) hydrocarbyl amine
having from 3 to 60 carbons and from 1 to 10 nitrogens and
(d~ N,N'-disalicylidene-1,2-diaminopropane in an amount -
sufficient to minimize the coking characteristics of such
fuel, especially throttling nozzle coking, in the prechambers
or swirl chambers of indirect compression ignition engines
operated on such fuel.
Since the invention also embodies the operation of
an indirect injection compression ignition engine in a manner
which results in reduced coking, a still further embodiment
of the present invention is a method of inhibiting co~ing,
especially throttling nozzle coking, in the prechambers or
swirl chambers of an indirect injection compression ignition
engine, which comprises supplying said engine with a
distillate fuel containing at least the combination of (a)
organic nitrate ignition accelerator, and (b) hydrocarbyl-
substituted succinimide or succinamide, or the combination of
(a) organic nitrate ignition accelerator, (c) hydrocarbyl
amine having from 3 to 60 carbons and from 1 to 10 nitrogens
and (d) N,N'-disalicylidene-1,2-diaminopropane or the
combination of (b) hydrocarbyl-substituted succinimide or
succinamide, (c) hydrocarbyl amine having from 3 to 60
carbons and from 1 to 10 nitrogens and (d)
rn/
127(~64~2
-- 5
N,N'-disalicyclidene-1,2-diaminopropane, said combina-
tions being separately present in an amount sufficient
to mini~ize such coking in an engine operated on such
fuel.
A feature of this invention is that the
combination of additives utilized in its practice is
capable of suppressing coking tendellcies of fuels used
to operate indirect injection compression ignition
engines. Such behavior was exhibited in a series of
standard engine dynamometer tests conducted as des-
cribed in Examples I, II and III hereinafter.
A wide variety of organic nitrate ignition
accelerators, component (a), may be employed in the
fuels of this invention. Preferred nitrate esters are
15 the aliphatic or cycloaliphatic nitrates in which the
aliphatic or cycloaliphatic group is saturated, con-
tains up to about 12 carbons and, optionally, may be
substituted with one or more oxygen atoms.
Typical organic nitrates that may be used are
20 methyl nitrate, ethyl nitrate, propyl nitrate, isopropyl
nitrate, allyl nitrate, butyl nitrate, isobutyl nitrate,
sec-butyl nitrate, tert-butyl nitrate, amyl nitrate,
isoamyl nitrate, 2-amyl nitrate, 3-amyl nitrate, hexyl
nitrate, heptyl nitrate, 2-heptyl nitrate, octyl
25 nitrate, isooctyl nitrate, 2-ethylhexyl nitrate, nonyl
nitrate, decyl nitrate, undecyl nitrate, dodecyl
~Z7C~6~Z
-- 6
nitrate, cyclopentyl nitrate, cyciohexyl nitrate, methyl-
cyclohexyl nitrate, cycloclodecyl nitrate, 2-ethoxyethyl
nitrate, 2--(2-ethoxy-ethoxy)ethyl nitrate, tetra-
hydrofuranyl nitrate, and the like. Mixtures of such
materials may also be used. The preferred ignition
accelerator for use in the fuels oE this invention is a
mixture of octyl nitrates available as an article of
commerce from ~thyl Corporation under the trade mark
DII-3 ignition improver.
The hydrocarbyl-substituted succinimides,
component (~) of the fuels of this invention, are well
known. They are readily made by first reacting an
olefinically unsaturated hydrocarbon of the desired
molecular weight with maleic anhydride to for~ a
hydrocarbyl-substituted succinic anhydride. Reaction
temperatures of 100-250C are used. ~ith higher boiling
olefinically-unsaturated hydrocarbons, good results are
obtained at 200-250C. This reaction can be promoted by
the addition of chlorine. Typical olefins include
cracked wax olefins, linear alpha olefins, branched
chain alpha olefins, polymers and copolymers of lower
olefins. These include polymers of ethylene, pro-
p-ylene, isobutylene, l-hexene, l-decene and the like.
~seful copolymers are ethylene-propylene copolymers,
ethylene-isobutylene copolymers, propylene-isobutylene
copolymers, ethylene-l-decene copolymers and the like.
lZ7~:16~2
Hydrocarbyl substituents have also been made from
olefin terpolymers. Very useful products have been made
from ethylene-C3_l2 alpha olefin - C5_l2 non-
conjugated diene terpolymers; such as ethylene-
propylene-1,4-hexadiene terpolyrner; ethylene-propylene-
l,5-cyclooctadiene terpolymer; ethylene-propylene-
norbornene terpolymers and the like.
Of the foregoing, by far the most useful hydro-
carbyl substituents are derived from butene polymers,
especially polymers of isobutylene.
The molecular weight of the hydrocarbyl sub-
stituent can vary over a wide range. It is desirable
that the hydrocarbyl group have a molecular weight of at
least 500. Although there is no critical upper limit, a
preferred range is 500-500,000 number average molecular
weight. The more preferred average molecular weight is
700-5,000 and most preferably 900-3,000.
Hydrocarbyl-substituted succinimides and
succinamides are made by reaction of the desired
hydrocarbyl-substituted succinic anhydride with an amine
having at least one reactive hydrogen atom bonded to an
amine nitrogen atom. ~xamples of these are methyl
a-nine, dirnethyl amine, n-butyl amine, di-(n-dodecyl)
amine, N-(arninoethyl) piperidine, piperazine, ~-(3-amino-
pro~yl) piperazine, and the like.
~27Q~i~2
Preferably, the amine has at least one reactiveprimary anine group capable of reacting to form the
preferred succinimides. Examples of such primary amines
are n-octyl amine, N,N-dimethyl-1,3-propane diamine,
N-(3-aminopropyl) piperazine, 1,6-hexane diamine, and
the like.
Hydroxyalkyl amines can also be used to make the
succinimide-succinamide components of the invention
which contain some ester groups. These amines include
ethanol amine, diethanol amine, 2-hydroxypropyl amine,
N-hydroxyethyl ethylenediamine and the like. Such
hydroxyalkyl amines can be made by reacting a lower
alkylene oxide, such as ethylene oxide, propylene oxide
or butylene oxide with ammonia or a primary or secondary
amine such as ethylene diamine, dethylene triamine,
triethylene tetramine, tetraethylenepentamine and the
like.
A more preferred class of primary amines used to
make the succinimide, succinamide or mixtures thereof
are the polyalkylene amines. These are polyamines and
mixtures of polyamines which have the general formula
H2N-~ R - NH-~ H
~,7Q6~
wherein R is a divalent aliphatic hydrocarbon group
having 2-4 carbon atoms and n is an integer from 1-10
including mixtures of such polyalkylene amines.
In a highly 2referred embodiment, the poly-
alkylene amine is a polyethylenearnine containing 2-6
ethyleneamine units. These are represented by the above
~ormula in which R is the group -CH2CH2- and n has a
value of 2-6.
The amine used to make the succinimide,
succinamide or mixture thereof need not be all amine. A
mono or poly-hydroxyalcohol may be included in the
reaction. Such alcohols can be reacted concurrently
with the amine or the two alcohol and amine may be
reacted sequentially. Useful alcohols are methanol,
ethanol, n-dodecanol, 2-ethyl hexanol, ethylene glycol,
propylene glycol, diethylene glycol, 2-ethoxy ethanol,
trimethylol propane, pentaerythritol, dipentaerythritol
and the like.
Useful amine-alcohol products are described in
U.S. 3,184,474; U.S. 3,576,743; U.S. 3,632,511; U.S.
3,804,763; U.S. 3,836,471; U.S. 3,936,480; rJ.s.
3,948,800; U.S. 3,950,341; U.S. 3,957,854; U.S.
3,957,855; U.S. 3,991,098; U.S. 4,071,548 and U.S.
4,173,540.
The reaction between the hydrocarbyl-substituted
succinlc anhydride and the amine can be carried out by
lZ7~642
- -- 10 --
~ixing the com~onents and heating the mixture to a
temperature high enough to cause a reaction to occur but
not so high as to cause decomposition of the reactants
or products or the anhydride may be heated to reaction
temperature and the amine added over an extended
period. A useful temperature is 100-250C. Best
results are obtained by conducting the reaction at a
temperature high enough to distill out water formed in
the reaction.
A preferred succinimide-succinamide component is
available as an article of commerce from the Ed~in
Cooper Company under the trade mark HITEC ~-644. This
product comprises a mixture of active ingredients and
solvent. Thus, when HITEC~ E-644 is used as component
(b) in formulating the fuels of this invention, the
product as received should be used at a concentration of
at least about 40 PTB (pounds per thousand barrels)
0.11436 grams per liter - to insure that the Einished
blend contains an adequate quantity of the foregoing
20 succinimide-succinamide ingredient although smaller
amounts may be successEully employed.
Tne nitrate ignition accelerator--component
(a)--should be present in an amount of at least 100 to
1000 PTB (pounds per thousand barrels) - 0.2859 to 2.859
grams per liter - of the base fuel. Preferably, the
~Z7~)6~
concentration of the ignition accelerator is 400 to 600
PTs (1.1436 to 1.7154 grams per liter).
It is not believed that there is anything
critical as regards the maximum amount of components (a)
and ~b) used in the fuel. Thus, the maximum amount of
these components will probably be governed in any given
situation by matters of choice and economics.
The coking-inhibiting components (a) and (b) oE
the invention can be added to the Euels by any means
known in the art for incorporating small quantities of
additives into distillate fuels. Components (a) and (b)
can be added separately or they can be combined and
added together. It is convenient to utilize additive
fluid mixtures which consist of organic nitrate ignition
accelerator and hydrocarbyl-substituted succinimide-
succinamide agents. These additive fluid mixtures are
added to distil:Late fuels. In other words, part oE the
present invention are coking inhibiting fluids which
comprise organic nitrate ignition accelerator and
hydrocarbyl-substituted succinimide-succinamide.
Use oE such fluids in addition to resulting in
great convenience in storage, handling, transportation,
blending with fuels, and so forth, also are potent
concentrates ~hich serve the function oE inhibiting or
mininizing the coking characteristics oE compression
~Z7~6g~
- 12 -
ignition distillate fuels used to operate indirect
compression ignition engines.
In these fluid compositions, the amount of com-
ponents (a) and (b) can vary widely. In general, the
fluid compositions contain S to 95~ by weight of the
organic nitrate ignition accelerator component and 5 to
95% by weight of the hydrocarbyl-substituted
succinimide-succinamide component. Typically, from .01
by weight llp to 1.0% by weight of the combination will
be sufficient to provide good coking-inhibiting proper-
ties to the distillate fuel. A preferred distillate
fuel composition contains from 0.1 to 0.5% by weight of
the combination containing from 25% to 95% by weight of
the organic nitrate ignition accelerator and from 75~ to
5% by weight of the hydrocarbyl-substituted succinimide-
succinamide component.
The additive fluids, as well as the distillate
fuel compositions of the present invention may also
contain other additives such as, corrosion inhibitors,
antioxidants, metal deactivators, detergents, cold flow
improvers, inert solvents or diluents, and the like.
Accordingly, a more preferred distillate fuel
composition includes a hydrocarbyl amine in combination
with the present additives.
~7~642
r~hile a variety of hydrocarbyl amines may be used
in the fuel connositions of this invention, a primary
aliphatic amine, the aliphatic group of which is
tertiary, e.g., an amine of the fonnula:
R-NH2
wherein R is one or a mixture of tertiary aliphatic
groups containing 8 to 18 or more (preferably 12-16)
carhon atoms is preferred. Most preferably, these
tertiary aliphatic groups are tertiary alkyl groups. It
10 is also preferred that hydrocarbyl amine component (c)
include in addition to the above-depicted amine one or
more hydrocarbyl amines differing therefrom.
U.S. Pat. ~o. 3,909,215 gives a description of
the various hydrocarbyl amines having from 3 to 60
carbons and ~rom 1 to 10 nitrogens which may be employed
in the fuels of this invention. A few additional
examples of desirable amines include 2,6-di-tert-
butyl-~-dimethylamino-p-cresol, N-cyclohexyl-N,.I-
dimethylamine, ~nd N-alkyl,N,N-dimethylamines in which
20 the alkyl group is one or a combination of al~yl groups
pre~erably having 8 to 18 or more carbon atoms.
A particularly preferred hydrocarbyl amine is
available commercially ~rom the Rohm and Haas Company
under the trade mark Primene 81R. The Primene 81R*is
* trade mark
~75;~6~2
- 14 -
believed to be a mixture of primary aliphatic amines in
which the aliphatic groups are predominantly Cl2 and
Cl4 tertiary alkyl groups.
The fuels of this invention should contain at
least 1.5 to 40 PTB (0.00429 to 0.1143 grams/liter of
component (c), the hydrocarbyl amine.
Accordingly, another embodiment of the present
invention is distillate fuel for indirect injection
compression ignition engines containing at least the
combination of (a) organic nitrate ignition accelerator,
(b) hydrocarbyl-substituted succinimide, and (c)
hydrocarbyl amine, said combination being present in an
amount sufficient to minimize coking, especially
throttling nozzle coking in the prechambers or swirl
chambers in indirect injection compression ignition
engines operated on such fuel.
Also included as a further embodiment of the
invention is a distillate fuel additive composition
com2rising (a) organic nitrate ignition accelerator, (b)
hydrocarbyl-substituted succinimide and (c) hydrocarbyl
amine in an amount sufficient to minimize the coking
characteristics of such fuel, especially throttling
nozzle coking in the prechambers or swirl chambers in
indirect injection compression ignition engines operated
on such fuel.
~Z7~64;~
- 15 -
In general, these additive fuel compositions ~ill
contain as much as 50% by weight of the combination of
organic nitrate ignition accelerator and hydrocarbyl-
substituted succinimide and up to 50% of the hydrocarbyl
amine or other additives when they are present.
In a still further embodiment of the invention
there is provided a method of inhibiting coking,
especially throttling nozzle coking in the prechambers
or s-~irl chambers of an indirect injection compression
ignition engine which comprises supplying said engine
~ith a distillate fuel containing at least the com-
bination of (a) organic nitrate ignition accelerator,
(b) hydrocarbyl~substituted succinimide and (c)
hydrocarbyl amine, said combination being present in an
amount sufficient to minlmize such coking in an engine
operated on such fuel.
Another additive which can be used to advantage
in the present invention i5 a metal deactivator.
Examples oE these are salicylidene-o-aminophenol,
20 disalicylidene ethylenediamine and disalicylidene
propylenediamine. ~ particularly preferred metal
deactivator is N,N'-disalicylidene-1,2-diaminopropane
(80 weight percent active in 20 weight percent toluene
solvent) which is available as an article of commerce
from Ethyl Corporation under the trade mark . ~ '~thyl" MDA.
~27~642
- 16 -
The fuels of this invention should contain at
least 0.2 to 5 PTB (0.00572 to 0.012 grams per liter) of
component (d), the me~al deactivator, preferably N,N'-
disalicylidene-1,2-diaminopropane.
Accordingly, another embodiment of the present
invention is distillate fuel for indirect injection
compression ignition engines containing at least the
combination of (a) organic nitrate ignition accelerator,
(b) hydrocarbyl-substituted succinimide, (c) hydrocarbyl
10 amine, and (d) N,N'-disalicylidene-1,2-diaminopropane,
said combination being present in an amount sufficient
to minimize coking, expecially throttling nozzle coking
in the prechambers or swirl chambers in indirect
injection compression ignition engines operated on such
15 fuel.
Also included as a further embodiment of the
invention is a distillate fuel additive composition
comprising (a) organic nitrate ignition accelerator, (b)
hydrocarbyl-substituted succinimide, (c) hydrocarbyl
20 amine, and (d) N,N'-disalicylidene-1,2-diaminopropane in
an amount sufficient to minimize the coking charac-
teristics of such fuel, especially throttling nozzle
coking in the ?rechambers or swirl chambers of indirect
injection compression ignition engines operated on such
25 fuels.
In general, these additive fuel compositions will
contain as much as 50% by weight of the combination of
lZ7Q6~
organic nitrate ignition accelerator and hydrocarbyl-
substituted succinimide-succinamide and U2 to 50~ of the
combination of hydrocarbyl amine and N,N'-disalicylidene-
1,2-diaminopropane or other additives when they are
present.
In a still further embodiment of the invention
there is provided a method of inhibiting coking,
especially throttling nozzle coking in the prechambers
or swirl chambers in an indirect in]ection compression
ignition engine which comprises supplying said engine
with a distillate fuel containing at least the com-
bination of (a) organic nitrate ignition accelerator,
(b) hydrocarbyl-substituted succinimide, (c) hydrocarbyl
amine and (d) ~,~'-disalicylidene-1,2-diaminopropane,
15 said combination being present in an amount to minimize
such coking in an engine operated on such fuel.
In another embodiment of this invention, the
coking-inhibiting components (a), (c) and (d) of the
invention can be added to the fuels by any means known
in the art for incorporating small quantities oE
additives into distillate fuels. Components (a), (c)
and (d) can be added separately or they can be combined
and added together. It is convenient to utilize
additive fluid mixtures which consist oE organic nitrate
ignition accelerator, hydrocarbyl amine and metal
deactivator agents. These additive fluid mixtures are
127 L?~4Z
- 18 -
added to distillate fuels. In other words, part of the
~resent invention are coking inhibiting fluids which
comprise organic nitrate ignition accelerator,
hydrocarbyl amine having Erom 3 to 60 carbons and from 1
to 10 nitrogens and metal deactivator, preEerably
disalicylidene-1,2-diaminopropane.
In these fluid compositions, the amount of
components (a), (c) and (d) can vary widely. In
general, the fluid compositions contain 10 to 97.9% by
10 weight of the organic nitrate ignition accelerator
component, 2.0 to 75% by weight of the hydrocarbyl amine
and 0.1 to 15% by weight metal deactivator. Typically,
from O.Ql~ by weight up to 1.0% by weight of the
combination of the components (a), (c) and a(d) will be
15 sufficient to pro~ide good coking-inhibiting properties
to the distillate fuel. A preferred distillate Euel
composition contains from 0.1 to 0.5~ by weight oE the
combination containing rom 50 to 97.9% by weight of the
organic nitrate ignition accelerator, from 2.0 to 45~ by
20 weight of the hydrocarbyl amine and Erom 0.1 to 5.0% by
weight of the metal deactivator component.
In another embodiment of this invention, the
coking-inhibiting components (b), (c) and (d) of the
invention can be added to the fuels by any means known
25 in the art for incorporating small quantities of
additives into distillate fuels. Components (b), (c)
127~6~2
- 19 -
and (d) can be added separately or they can be combined
and added together. It is convenient to utilize
additive fluid mixtures which consist of hydrocarbyl-
substituted succinimide-succinamide agents, hydrocarbyl
5 amine and N,N ' -disalicylidene-1,2-diaminopropane These
additive ~luid mixtures are added to distillate fuels.
In other words, part of the present invention are coking
inhibiting fluids which comprise hydrocarbyl-substituted
succinimide-succinamide, hydrocarbyl amine having from 3
10 to 60 carbons and 1 to 10 nitrogens, and metal deacti-
vator, preferably N,N'-disalicylidene-1,2-diaminopropane
In these fluid compositions, the amount of
components (b), (c) and (d) can vary widely. In
general, the fluid compositions contain 10 to 97.9% by
15 weight of the hydrocarbyl-substituted succinimide-
succinamide component, 20 to 75% by weight of the
hydrocarbyl amine and 0.1 to 15~ by weight metal
deactivator. Typically, from 0.01% by weight up to 1.0
by weight of the combination will be sufficient to
20 provide good coking-inhibiting properties to the dis-
tillate fuel. A preferred distillate fuel composition
contains from 0.1 to 0.5% by weight of the combination
containing from 50% to 97.9% by weight of the hydro-
carbyl succinimide-succinamide component and from 2. OQ
25 to 45~ by weight of the hydrocarbyl amine and from n.
to 5.0% by weight of the metal deactivator, preferably
N,~'-disalicylidene-1,2-diaminopropane.
1;27~6~2
- 20 -
The practice and advantages of this invention
will become still further apparent from the following
illustrative example.
EXA~PL~ 1
In order to determine the effect of the fuel
compositions of the present invention on the coking
tendency of diesel injectors in indirect injection
compression ignition engines, use was made of a com-
mercial diesel engine operated on a coking test cycle
developed by Institute Francais Petrole and as prac-
ticed by Peugeot S. A. The amount of coking together
with a ~uantitative indication of the adverse consc-
~uences of such coking was determined by means of ~i)
injector air flow performance, (ii) emission of unburned
hydrocarbons, (iii) englne noise, and (iv) injector
deposit ratings. The engine employed in the tests was a
1982 Peugeot 2.3 liter, 4-cylinder, turbo-charged XD2S
diesel engine connected to a Midwest dynamometer through
an engine clutch. This engine is equipped with Bosch
injectors positioned within prechambers, and is deemed
representative of the indirect injection compression
ignition engines widely used in automobiles and light-
duty trucks.
The base fuel employed in these engine tests was
a commercially-available diesel fuel having a nominal
cetane rating of 42. PIA analysis indicated the fuel
~Z7~64Z
was composed by volume of 31.5~ aromatics, 3.0% olefins
and 65.5~ saturates. Its distillation range (ASTM
~-158) was as follows:
~arometer 29.46 inches of Hg (0.9987 ~ars)
Initial 406F - 207.78C
_ vaporated at F - at C
439 226.11
450 232.22
456 235.56
463 239,44
480 248.89
499 259.44
521 271.67
545 285.0
70 572 300.0
603 317.22
85 621 327.22
90 643 339.44
95 678 358.89
Final 678F 358.89
Recovery 97.5%
Residue 2.5
Loss None
127(~64~
- 22 -
Other inspection data on the base ~uel were asfollo~s:
~iner,latic Viscosity, (ASTM D-445) . . . 3.50 Centi-
stokes, 40C
Pour Point (ASTM D-97). . . . . . . . .-26C
Cloud Point (ASTM D-97) . . . . . . . . 33C
- Flash Point (ASTM D-93) . . . . . . . 91C
Steam Jet Gum . . . . . . . . . . . . . 2.4 mg~l00 ml
Aniline Point (ASTM D-611). . . . . . . 143.4F (61.89C)
10 Total Sulfur. . ~ . . . . . . . . . . . 0.41 wt. %
Ramsbottom Carbon, ~ (ASTM D-524) . . . 0.1460 on 10
Residuum
Gravity (ASTM D-287). . . . . . . . . . 31.8 API
Specific Gravity @ 25C . . . . . . . . 0.86
Cetane rating . . . . . . . . . . . . . 41
A test blend was prepared from this base fuel
(Fuel A). Fuel A contained a combination of (i) 506 PTB
(1.447 grams/liter) of mixed octyl nitrates (a com-
mercial product available ~rom Ethyl Corporation under
20 the trade mark DII-3 Ignition Improver), (ii) 41 PT3
(0.117 gram/liter) of HITEC E-644, a product o~ Edwin
Cooper, Inc., believed to be a hydrocarbyl
succinimide-succinamide made by reacting two moles of a
polyisobutenyl succinic anhydride (PIBSA) ~ith one mole
of a polyethylene amine mixture having an average
composition corresponding to tetraethylene pentamine,
~Z70642
- 23 -
(iii) 14 PT3 (0.04 grams/liter) of a hydrocarbyl amine
available commercially from Rohm and Haas Company under
the trade mark Primene 81~ and (iv) 1.7 plrB (o.on4s6
grams/liter) of Ethyl* Metal Deactivator, a product of
Ethyl Corporation, the active ingredient of which is
N,~'-disalicylidene-1,2-diaminopropane. The manu-
facturer gives the following typical properties foe its
HITEC~ E-5 44 product:
Appearance Dark brown viscou.s
liquid
Nitrogen, wt. % 2.0
Specific Gravity
at 60/60F o. 928
Viscosity at 210F, cs 340
(9s.89oc)
The Primene 81~*is believed to be a mixture of
primary aliphatic amines in which the aliphatic grouos
are predominantly C12 and C14 tertiary alkyl groups.
The manufacturer gives the following typical
properties for its Ethyl* metal Deactivator:
Form Liquid
Color Amber
Density, at 68F
g/ml 1.0672
lb/gal 8.91
Active ingredient, wt % 80
* trade mark
127~64t2
- 24 -
Solvent vehicle (toluene), wt % 20
Flash point, open cup, F 84 (28.89C)
Fire point, F 100 (37.78C)
Solubility
In gasoline (Typical) Saturated solution
contains 94~ M~.
In water, wt. % 0.04
Shell Rotella T*, an SAE 30, SF/CD oil was used
as the crankcase lubricant.
Before starting each test, new Bosch DNOSD -
1510*nozzles were installed using new copper gaskets and
flame rings. The fuel line was flushed with the new
test fuel composition to be tested and the fuel filter
bowl and fuel return reservoir were emptied to avoid
additive carry-over from test-to-test.
At the start of each test, the engine was
operated at 1000 rpm, light load for 15 minutes. Aeter
this warm-up, the engine was subjected to the following
automatic cycle:
Event RPM Beam Load Minutes EGR
1 750 0 4 off
2 2750 12.0 6 on
3 1500 6.2 6 on
4 4000 16.2 4 o~f
* trade mark
lZ7Q~
- 25 ~
The above 20-minute cycle was repeated 60 times and the
test was completed by running the engine at idle for
another.30 minutes. The total elapsed time was thus
20.5 hours per test.
~hen passing from one event to the next event in
the above cycle, some time, of course, was re~uired to
enable the engine to accelerate or decelerate from one
speed to the next. Thus, more specifically, the above
cycle was programmed as follows:
10SegmentSeconds ~m Beam Load
1 2 750 0
2 200 750 0
3 3* 2500 12
4 7* 2750 12
15 5 350 2750 12
6 3* 2275 6.2
7 7* 1500 6.2
8 330 lS00 6.2
9 3* 3500 16.2
2010 7* 4000 16.2
11 230 4000 16.2
12 3* 2000 0
13 7* 750 0
14 30 750 0
25 * Represents two mode periods for acceleration or deceleration
to the next condition.
lZ~Q64Z
- 26 -
Hydrocarbon exhaust emissions were measured at
the start of each test (after the first 20-minute
cycle), at the 6-hour test interval and at the end of
the test. These measurements were made at 750, 1000,
and 1400 rpm idle. Noise level readings were made at a
location three feet Erom the engine exhaust side. The
measurements were made at the start and at the end of
the test while operating at three idle speeds, viz.,
750, 1000 and 1400 rpm.
10 After the test operation, the injectors were
carefully removed from the engine so as not to disturb
the deposits formed thereon. Measurements were made of
air flow through each nozzle at different pintle lifts,
and pintle deposits were rated using the C~C deposit
rating system.
The most significant test results are given in
Table I, in which air flow is expressed as cc/min an~
hydrocarbon emissions as ppm.
TABLE I
Air Flow Pintle Obturator Hydrocarbon
@ 0.1 mm Deposits Noise, ~B Emissions
Fuel LiEt (10 = clean) EOT* INC~. EOT* Incr.
Base 36 8.0 83.8 3.0 577 406
A 38 8.6 81.4 1.9 275 1~3
* Value at end of test; the increase (Incr.) shown is in
comparison to the value at start of test.
1Z7(~64Z
- 27 -
The results presented in Table I show that there
were less coking deposits (higher air flow rate and
fewer deposits), less engine noise and less hydrocarbon
emissions with Fuel A, the fuel of the invention, as
compared to the Base Fuel.
E~AMPLE II
A test blend was prepared from the base fuel of
Example I (Fuel B). Fuel 3 contained a combination of
(i) 506 PTB (1.447 grams per liter) of mixed octyl
nitrates (a commercial product available from Ethyl
Corporation under the trade mark DII-3 Ignition
Improver), (ii) 13.2 PTB (0.0377 grams per liter) of a
hydrocarbyl amine available commercially from Rohm and
Haas Company under the trade mark Primene 81R and (iii)
1.7 PTB (0.00486 grams per liter) of Ethyl* Metal
Deactivator, a product of Ethyl Corporation, the active
ingredient of which is ~,~'-disalicylidene-1,2-diamino-
propane
The test engine was operated under the same con-
ditions as those of Example I.
The most significant test results are given inTahle II, in which air flow is expressed as cc/min and
hydrocarbon emissions as ppm.
.
* trade mark
:127Q64Z
- 28 -
T~BIE II
J
Air Flow Pintle Obturator Hydrocarbon
@ 0.1 mm Deposits Noise, ~B Emissions
Fuel Lift (10 = clean) EOT* I~CR. EOT* Incr.
Base 36 8.0 83.8 3.0 577 406
3 49 8.4 81.3 2.2 282 51
* Value at end of test: the increase (Incr.) shown is in
comparison to the value at start of test.
The results presented in Table II show that there
were less coking deposits thigher air flow rate and
fewer deposits), less engine noise and less hydrocarbon
emissions with Fuel ~, the fuel of the invention, as
compared to the Base Fuel.
EXAMPLE III
A test blend was prepared from the hase fuel of
Example I (Fuel C). Fuel C contained a combination of
(i) 41 PTB (0.117 grams per liter) of HITEC E-644, a
product of Edwin Cooperr Inc., helieved to be a hydro-
carbyl succinimide-succinamide made by reacting two
moles of a polyisobutenyl succinic anhydride (PI3SA)
with one mole of a polyethylene amine mixture having an
average composition corresponding to tetraethylene
pentamine, (ii) 14 PTB (0.04 grams per liter) of a
hydrocarbyl amine available commercially from Rohm and
Haas Company under the trade mark Primene 81~, and
1~7Q642
- 29 -
(iii) 1.7 PTB (0.00486 grams per liter) of Ethyl# Metal
Deactivator, a product of Ethyl Corporation, the active
ingredient of which is N,~'-disalicylidene-1,2-diamino-
propane.
The test engine was operated unde the same con-
ditions as those of Example I. The most significant
test results are given in Table III, in which air flow
is expressed as cc/min and hydrocarbon emissions as ppm.
TABLE III
Air Flow Pintle Obturator ~ydrocarbon
@ 0.1 mm Deposits Noise, DB Emissions
Fuel Lift (10 = clean) EOT* INCR. EOT* Incr.
Base 36 8.0 83.8 3.0 577 406
C 40 8.5 83.2 3.0 513 278
* Value at end of test; the increase (Incr.) shown is in
comparison to the value at start of test.
The results presented in Table III show that
there were less coking deposits (higher air flow rate
and fewer deposits), less engine noise and less
hydrocarbon emissions with Fuel C, the fuel of the
invention, as compared to the Base Fuel.
# trade mark