Note: Descriptions are shown in the official language in which they were submitted.
1~'7~
The present invent~on relates to a me~ho~ of manufactur~ng a copper
radiator which is especially useful in motor cars. In partLcular, the
corrosion reslstance of fins has bPen improved and the lightening in weight and
hi8h performance of a radiator have been made possible by the invent~on.
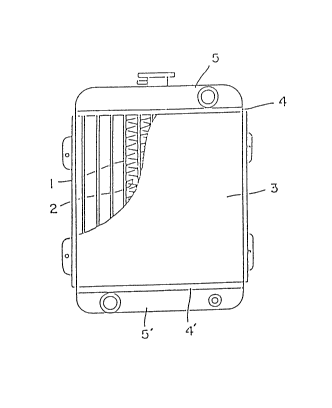
In the drawing, Fig. 1 is a front ~levatlonal view of a typlcal radiator.
The radiator for a motor car is one to cool the heat-exchanging medlum by a
stream of air. It is constructed generally as ghown ln Fi~. 1, wherein fins
(2) are provided between many flat tubes (1), at leazt onP ~urface of the tubes
or fins being covered with brazing material, with the tubes and fins belng
assembled temporarily. Then this temporary assemblags is dipped lnto a flu~
solution, or the ~lux solution is coated onto the surface theraof, and
thereafter the assembla~e ls heated in the atmosphere. The brazing material is
allowed to melt by this treatment and the molten brazing material is spread all
over the contact places of tubes with fins. It then solidifies and the bonding
o~ tubes with fins is made flrm to form the core S3)-
The flux on the surface of the temporary assemblage has the function of
removing the oxidized film produced on the surface thereof and lmproves the
wettability of the brazing material w~en heating the temporary assemblage in
the atmosphere.
Purthermore, the seat plates ~4~ and (4') are fitted to the ends of core
(3) by soldQring and the tanks (5) and (5') are connected to these seat plates.
In general, for the tubes, copper alloys such as brass, etc. are used; for the
fins, thin plates made from high heat-conductive copper or copper alloys such
as Cu-Sn, Cu-Cd, Cu-Zr, Cu-Ag, etc. and corrugated or louvered, are used; and
for the seat plates, br~ss plates are used. Also, for the tanks, those made
from brass have been used and connected by sol~ering, but reslnous tanks as
recently used to achie~e a lightening in weight are connected by mechanical
crimpin~.
Recently, from the requlrement for liKhtening in we1ght and incr,~aslng
performance of the car, the lightening and the efficiency of the radistor for
motor cars has also been examined. As a result, the thlnning and the high
densification of fins are regarded as effective means and, for the flns, a
number of thin plates (thic~ness: 0.02-0.05 mm) made from hi~h heat-conductive
copper alloys aforementioned are used. Although copper and copper alloys are
excellent in corrosion resistance, with the recent advent of the use of
PAT 6369~
4 l- r
chloride as a snow-melting ggent, the corrosion wastage ~ue to snow damage has
b~come a serious pro~lem with the radiator. ~amely, the snow-meltlng ag~nt
scattered in large quantities sticks to ~e radiator and corrode~ the fins a'c
an extraordinarily high rate to decrease the effective radlation araa, so
result~ng ln a drastic lowering in the performance of a radlator during a short
psri~d.
Moreover, by the method as described above, since the temporsry assemblage
is beated in the atmosphere, rslatively large amounts of oxidized film are
prod~ced on the surface of the temporary assemblage for a ~hort time. Hareby,
there ar~ses a problem that if the oxidized film ls produced ln large amounts,
more flux becomes necessary and th~ mora the flux, the more the amount of flux
is thermally decomposed, induc~n~ bad odors.
Horeover, it is also considared that when the molten brazing material is
solidified and the bonding of tubes with fins has bean completed, this
assemblage should be washed to wash out the flux remaining bshind on the
surface thereof; but, as described above, if the amount of flux ~sed becomes
large, the heavy metals in the flux flow out lnto the wash effluent at an
incrQased rate, so producing effluent contamination.
In order to prevent thess problems, various methods have been investigated,
but all of them have been insufficient. For example, coated fllm with a
thickness of more than 0.01 mm becomes necessary to prevent corrosion, however,
this is infer3Or bacause of an increasa in tha weight and a riss in the cost.
Moreover, if fins are formed with Cu-10% Ni alloy, known as a corrosion-
resistant copper alloy, to make fins more corrosion-resistant, the radiation
property decreases remarkably in 8 plate of the same thicknass. Namely, when
comparin~ by the electroconductivity proportlonal to the thermal conductivity,
the relation being known as the Wiedemann-Franz's law, Cu-10% Ni alloy shows
less than 10% IACS to 90 to 80~ IACS with usual fin materials.
As a result of extensive investigations in view of the situatio~ methods
of manufacturing an economical copper radiator for motor cars have been
developed by the invention, wherein high performance is kept withstanding the
wastage due to the snow damage, and it is poss~ble to comply wlth the request
for llghtening and there are no bad odors and effluent contamination.
The heat-exchanger of this invention is characterlzed in that in a radiator
wherein fins are fitted to the outsids of a plurality of tuba~ by bonding with
PAT 6369-1
-- 2 --
solder to form a copper core, and a s~at plate is fitted to at least one end of
said core by bonding wlth solder to connect a tan~ thereto, the thickness of
oxidized fllm on ths surface of the fins i9 not more than 1200 A.
Uoreover, one of the manuacturing methods of the invent~on is
characterlzed in that in the process aforementioned, the soldering for the
formation of the core is carried out in a nonoxidizirlg atmosphere and/or the
core is submltted to a rPduction treatment by heating in a reducing atmo~phere
during assembling the radlator after soldering for the formation of core The
thlckness of oxidized film on the surface of fins is not more than 1200 A after
assembling the radiator.
~urthermore, another one of the manufacturing msthods of the invention is
characterized ln that in the assembling of the radlator, the core is submitted
to a dipping treatment in a copper oxide-dissolvable or reducing solution in
the process for the assembling of the radiator after the formation of core, to
mske the thickness of oxidized film on the surface of fins not more than 1200 A
after assem~llng the radiator.
Further, the third characterist~c of the inventlon lies in that following
ths process aforementioned, rust inhibitor i8 adsorbed in, or adhered to the
surface of fins.
In this invention, the reason why the thicknQ~s of oxidlzed film on the
surface of fins was made not more than 1200 A after as~embling the radiator is
due to the fact that as a result of overall diligent experimental analyses of
the actual situation of corroqion due to salt damage aforementioned and various
factors concerned therein, it has been known that the oxidized fllm produced on
the surface of fins is a si~nificant factor in the promotion of corrosion. In
consequence of further experlmentsl snalysis, it has become e~ident thst when
the thickness of oxidized film exceeds 1200 A, the corrosion due to salt damage
is accelerated and the extent thereof increaqes with an increase in the
thickness of film.
Moreover, the fact that in the process of manufacturin~ the radiator
aforementioned, the bonding wlth solder for the formation of the core is
carried out in a nonoxidlzing atmosphere and~or the core is reduced by heatlng
in a reducing atmosphere durinK assembling the radiator after soldering for the
formation of core, or that the core is dipped into a copper oxide-dissolvable
or reducing _olution is to maXe the thlckness of oxidized film on the surface
PAT 6369-1
-- 3 --
~q~
of fins not more than 1200 A after assem~lin~ the ra~lator.
Namely, in the manufacturin~ process, the temperature of tne hi~h-
temperature furnace, where the soldering ~s done, is 300 to 400c and oxidized
film wlth a thickness of 2000 to 10,000 R is produced. Although the in~ide of
the urnace is dlluted somewhat with the vapor of flu~, etc., lt is a virtually
atmospheric environment. Therefore, the ~ins are oxidized easily. The
oxidation of fins is prevented by carryin~ out tho sol~ering ln a nonoxidizin~
atmosphere, and/or ~he oxidized film produced on the sur~ace of ~ins is r~duced
by submittin~ the core to a reduction treatment by beating in a reducing
atmosphere during assembling the radiator a~ter solderin~ for the formation of
core. For the nonoxidlzin~ atmospheres, N2, H2, C0, C02, H20 or
mixtures of these ~ases are used. For the reducing atmospheres, H2, C0 or
~ases having these as effectivo inKredien~s are used, ana the reduction is
conducted by heating to hi8her than 150C.
The pravention of the oxide film can also be attalned by submitting the
core to a dippin~ treatment for the dissolution of reduction into a solution of
dissolvable or reducible copper oxlde durin~ assembling the radiator.
The copper oxide-dissolvable solutions can be such 28 dilute aqueous
solutions of sulfuric acid, hydrochloric acid atc., or complex-formable aqueous
solutions of ammonia, cyanides, ethylenedlaminetetrascetate (EDTA), methyl-
aminenitrilotriacetate (NTA), etc. Also, the copper oxide-reducible solutions
can be such as aqueous solutions of hydrazine, methylhydrazine, methyl alcohol,
etc. The treatment is possible at normal ambient temperaturos, however, the
treatment time can be shortened by warmine. In particular, at the time of the
reduction treatment, it is preferable to warm the solution. ~oreover, the
treatment time can also be shortened by raisln~ the tamperature of the coro or
radlator by soldering. Through such treatment, the thickness of oxidized film
on the surface of fins can be decr2ased to less than 100 A.
Moreover, since the build up of oxidized film on the surface of fins can be
inhibited by submitting the surface of the fins to adsorption or aahèrence
treatment by a rust inhlbltor, the corrosion of the fins due to salt dama8e can
be more effectively reduced. Su,ch inhibitors can be benzotriazole (BTA),
tolyltriazole (TTA) and ethylbenzotriazcle and reactlon products theroof wlth
amines, carboxylic acids, etc., hi~her alkylamines ~uch a~ dodecylamine,
stearylamine9 etc., mercaptobenzothiazole, and the like. Many various
PAT 6369-1
-- 4 --
~2~
compounded chemicals can also be obtained commercially. Moreover, these
chemicals may be used in the form of an aqueous solution or in solution with an
organic solvent.
With the radiator assembled by the manufactur~ng methods of the invention
as described above, the corrosion due to the salt dama~e can be suppressed by
about 20 to 50% compared with a conventlonal radiator. It is known that copper
oxide has a protective proper~y agains~ atmospheric oxidation or sulfidizing
corrosion, it promo~es corrosion due to a salt environment. Althou~h the
raason for this is not clearly understood, it is con~idered that copper oxide
has cracXs and pores, and these act as cathodes electrochemically a~ainst the
copper ground. Accordin~ to the manufacturing methods of the invention, by
makin~ the thickness of oxidized film on the surface of fins of the radiator
not more than 1200 A, the corrosion due to ~alt has been prevented effectively.
Furthermore, lf using the manufact~ring method wherein the fo~nation of
core is csrried out in a nonoxidlzin~ atmosphere, the thickness of oxidized
fllm produced on the surfaces of the tubes and fins can be made thin and the
quantity of flux required can be lowered. As a result, problems such as bad
odors and effluent contamination, which are attributed to flux, can be avoided.
Moreover, since the oxidized fllm produced on the surface of tubes and fins
is produced gradually in the air after soldering ln a nonoxidizing atmosphere,
the oxidized film is a very dense thin film and it is posslble to make the
surfaces of tubes and fins very ~mooth which contributes to the improvement in
the corrosion reslstance.
The invention will now be illustrated in detail based upon the following
examples.
Rxam~
Flst brass tubes (thickness of wall: 0.12 mm, width: lOmm, thicXness:
3mm) covered with solder, and fins, corrugated from thin plates tthicXness:
0.04 mm, width: 8.5mm) of Cu-0.15% Sn-0.01~ P alloy were superpose~ and bonded
with solder by holding it with an iron frame snd keeping it for 10 minute~ at
-310C in a nonoxidizin~ atmosphare consistin~ of ~2-1% H2. After bein~
Xept for a further 15 minutes ~t 120C in the same atmosphere, they were taken
out to form the core.
Kx~:~
In Example 1, a nonoxidizin~ atmosphere of 100% N2 was used in place of
PAT 6369-1
-- 5 --
N2- 1% H2 .
~Rmpl~ 3
The oore formed according to example 1 was aipped for 1 mlnute into a 0.25
aqueous solution of sTA and then drled.
l~xa~pl0
The core formed accordin~ to Example 1 was dipped for 1 minutes into a 0.57
alcoholic solution of mercaptobenzothiazole and then dried.
xampl~ 5
Flat brass tubes and corrugated flns were superposed ~imilarly to ~xample 1
10 and bonded with solder in the atmosphere by holdin~ i~ with an iron frame to
form the core. Then the core was kept for 5 minutes at 180C in a reducin~
atmosphere consistin~ of H2-50~ C0 and, after bein~ Xept for a further 10
minutes at 120C in the same atmosphere, was taken out into the atmosphere for
reducin~ treatment of the core.
~ample 6
The core formed according to Example 5 W~8 dipped for 1 minute into a 0.25
aqueous solution of BTA and then drled.
~xal~ 7
~rom a comm~rcial radlstor manufactured by comblnin~ corrugated thin plates
20 of Cu-0.15~ Sn-0.01~ P alloy with a thickness of 0.04 mm, w~th brass tubes
covered with solder, a piece of core havin~ a width of 10 cm and a length of 10
cm was cut off and dipped for 10 seconds at 40C lnto a 1% aqueous solution of
H2S04. Then it was washed with water and dried.
xample 8
Following the treatment in Example 7, the core was dipped for 5 seconds at
room temperature into a 0.25h slcoholic solution of BTA and then dried.
example 9
The core was dipped for 25 seconds at 40C into a 10~ aqueous solution (pH:
11.5) of EDTA, then washed with water and dried.
~xa~le 10
The core was dipped for 10 seconds at 40C lnto a 4% aqueous solution of
NaCN, then washed with water snd dried.
exasnpl~ 11
Follow~n~ the treatment in Example 10, the core was dipped for 10 ~econds
at 60C into a 0.1~ aqueous solutlon of dodecylamlne ana then dried.
PAT 6369-1
-- 6 --
Bxample 12
The core was dipped for 10 ssconds at ~0C into a 5% solutlon of
NH2.NH2 and then dried.
ConvQntional ~ethod
In Example 1, the bonding with solder was made in the air in place of non-
oxidizing atmosphere consistin~ of ~2-1% H2
Of the respective cores thus manufactured, the thicknes~ of oxidized film
on the surface of fins was measured. Then a spray test with a 5~ saline
solution on the basis of the procedure JIS Z-2371 was conducted for 0.5 hours
and a moistening test at 8 temperature of 60C and a humidity of 95% was
conducted for 23.5 hours, 40 times. A portion of the fins was cut off and the
amount of corrosion of the fin portion was determined. Moreover, cooling fluid
was circulsted through the cores manufactured by the respective method~, while
the spray test with saline solution was being carried out to evaluate the
corrosion resistance of the tube by measuring the time until ths tube begins to
lea~ fluid.
Results are shown in Table 1. The thickness of oxidized film on the
surface of fin was measured by the cathodic reduction method, and the amount of
corrosion was calculated from the difference of weights befors and after
dipping when dipped for 1 minute into a S~ aqueous solution of H2S04 and
applying ultrasonic waves to the llquid.
PAT 6369-1
-- 7 --
Table 1
Time until the
Thickness of Amount of corrosion leakage of
~nufacturing method ¦ oxidized film Of fin fluid fron tube
(R) (%) (hr)
.. . _ _
Example 1 Nonoxidative 210 7.3 730
Soldering
2 390 7.2 500
" 3 Dipping, BTA 180 6.6 680
" 4 Dipping, Mercapto- 180 6 9 590
benzo~hiazole
" 5 Postreduction 160 6.9 720
" 6 5 + Dipping, BTA 140 6.4 750
" 7 Acid pickling 80 7.2 640
" 8 7 + Dipping, BTA 50 6.6 710
" 9 Dipping, EDTA 60 7.0 560
" 10 Dipping, NaCN 80 7.4 630
" 11 10 + Dipping, 60 6.85 670
Dodecylamine
" 12 Dipping, Hydrazine 60 7.0 590
Conventional method ' 4200 12.6 . . _ _ _ _
PAT 6369-1
~2~d2~87
Next, the cores in Example 1 aforementiong~ Were submltted to the oxldatlon
treatment for 1 to 30 minutes zt 350C ln an alr bath and, th~reafter, the
th~c~ness of oxldlzQd fllm and the amount o~ corroslon were measured s~mllarly
to investl~ate the relatlonship between the thickness of oxidlz~d film an~ th~
amount of Corrosion. Results are shown ~n Table 2.
Table 2
j
I Time kept ¦ 1 min 12 min 10 minl 30 m1n
Thickness of oxidized film (R? ¦ 800 1 1400 3200 1 9800
Amount of corrosion (%) ! 8.1 9.9 ' 11.9 15.1
Furthermore, the cores manufaGtured accordln~ to Examples 1, 3, 4, 7, 8 and
11 and the conventional method were k~pt for 300 hour~ ln a molstened state at
a temperature of ~0C and a humidity o~ 95~, and the thickness of oxidized fllm
was measurQd. Then the spray test with sallne solutlon aforement~oned and the
moistenln~ test were repeated 40 times to det0rmlno the amount o~ corroslon.
Results are shown in Table 3.
Table 3
~ Thickness of , Amount of
Manufacturing method oxidized film . corrosion
(R) ¦ (%)
¦ Example 1 820 1 7,9
.. 3 350 6 9 "
! ~ 7 1100 1 8.8
, " 8 300 7,4
~ 420 7.4
PAT 6369-1 . i
Conventional method 4200 12.5
. . _ ~
~7
As e~ident from Table 1, it can be seen that in the case of the
conventional method, ~he amount of corrosion of the fin 19 12.5~, whereas in
the casss of ~xamples 1 throu~h 12 o~ the inventlon, it is as low as about 7
in all cases. Moreover, it is also recognlzed that the time until the
fotmation of holes in a tube is as short as 180 hours according to the
conventional method, whereas it amounts to more than 500 hours according to
examples of the invention. ~Irthermore, in Examples 1 and 2, the amounts of
flux could be decreased to less than abou~ half compared with that used in the
conventional method.
Moreover, from Table 2, it can be seen that the amount of corrosion of the
fin increases wlth increasing thickness of oxidized film on the fin and, in
particular, it increases remarkably in the range wherein the thickness of
oxidized film is more than 1400 R. Furthermore, as evident from Table 3, by
following the manufacturing methods of the invention, the amount of corrosion
becomes less, so that deterioration of the surface can be restrained under the
environment from the shipment of the radiator to the end use. Besides, the
effects shown by the foregoing examples are not conflned to Cu-Sn alloy, and
the same thing can be said of Cu-Cd, Cu-Zn, Cu-Ag and others.
As described above, according to the invention, the corrosion due to the
salt damage can be prevented effectively by suppressing the formation of
oxidized film in the manufacturing process. Therefore, it has become possible
to manufacture a high-performance radiator economically and lighten the car, so
having a significant industrial effect.
PAT 6369-1
-- 10 --