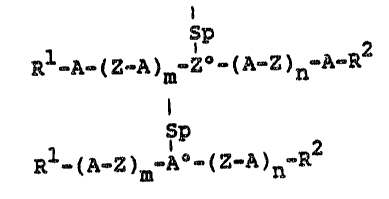Note: Descriptions are shown in the official language in which they were submitted.
-- 1 --
Merck Patent Gesellscha~t
mit beschrankter Haftung
6100 ~ a r m s t a d t
Polymer mater;aLs having liquid-crystalline phases
S The invention relates to polymer materials which
have liquid-crystalline phases and the noesogenic groups
of which ~re bonde~ laterally to the polymer backbone.
A nu~ber of liqu;d-crystalLine side-chain polymers
are already known. Thus~ for example, German Of~enlegungs-
schr;ft 2,944,591 and European Patent Specification 0,060,335
describe organopolysiloxanes, and German Offenlegungs-
schrift 2~831,909 and Springer and Weigelt, Makromol.
Chem. 184 (1983) 14~9, describe polymethacryLates posses-
sing mesogenic side groups.
The common feature of these kno~n side-chain poly-
mers is that their mesogenic groups are bonded to the
polymer backbone, if appropriate via a spacer, at the
4-position in the direction of the longitudinal molecular
axis~ and hence analogously to the customary wing groups~
Such polymer materiaLs frequently have nematic phases at
temperatures aboYe 100C. In many cases, such materials
also exhibit crystalline behaviour, associated with ~he
lack of mesomorphic properties.
The object of the present invention was to find
polymer materials ~hich have licluid-crystalline phases
and which possess the disadvantages described only to a
slight extent, if at aLlO
To date~ only comparatively small lateral sub-
stituents have besn considered to be compatible with the
occurrence of liquid-crystalline properties (cf. Gray in:
The Molecular Physics of Liquid Crystals (editors G.R.
Luckhurst and G.W. Gray), London-New York-San Francisco
1979, page 1). The fact that it has now been found that
pclymer materials in which the mesogenic groups are bonded
laterally to the polymer backbone possess surprisingly
wide mesophase ranges, a birefringence wh;ch can be varied
~ithin ~ide limi~s and a positive as well 3S negative dia-
magnetic anisotropy is therefore all the more surprising.
~7~
-- 2
They may furthermore be readily processed to articles of
any shape with anisotropic properties, and have high
chemical stability.
The invent;on relates ~o polymer mater;als which
have l;qu;d-crystalline phases and the mesogenic groups
of ~hich are bonded to the polymer backbone laterally,
that is to say not terminally via a ring group or bridging
group of t~e mesogenic un;t. Such mesogenic groups corre-
spond to the formula Ia and/or Ib
Sp Ia
Rl ~A- ( Z-A ) ~Z -1 A- Z ~ nWA-R~
Sp
Rl~(A-Z) -A-(Z~A)n-R2 Ib
~herein
R1 and R2 are alkyl hav;ng 1-15 C atoms, ;n wh;ch
furthermore one or t~o non-adjacent CH2 groups
can be r~placed with -0-, -C0-, -0-C0-, -C0-9-
and/or -CH=CH-, or one of the radicals R1 and R2
may furthermore be H, F, Cl, ar, NCS, N02~ CN or
R -A-Z~
R3 is alkyl having 1-15 C atoms, in which further-
more one or two non-adjacent CH~ groups can be
replaced with -0 , -C0-, -0-C0-, -C0-0- and/or
-CH=CH-, or is H, F, Cl, ~r, NCS or CN,
Sp ;s a covalent bond or a radical of the formula
_Q~_W1_~2_~2_
Q1 and Q2 independently of one another are each a
chem;cal bond and/or an alkylene group having 2 to
25 C atoms, in wh;ch furthermore one or more non-
adjacent CH2 groups can be replaced with -0-, -S-
or -N(C1-C6-alkyl)-,
~1 and w2 independently of one another are each a
chemical bond and/or a functional grouping from
the group Gonsist;ng of -0 , -S-, -S0-, -S02 ,
-N(C1-C6-alkyl)-, -C0-, -C0-0~, -0-C0-, -0-C0-0-,
-C0-S-, -S-C0-, -C0-NH~C1-C6-alkyl)-~ ~NH(C1-C6-
-- 3
alkyl)-C0~ -3 C0-NH(C1-C6-alkyl)- or -~H~C1~C6-
alkyl)-C0-0 and Cl-C~-alkyl,
A in each case is a 1,4-cyclohexylene group, in
which furthermore one or t~o non-adjacen~ CU2
groups can be replaced with -0- and/or -S-, and/or
which can be sub~tituted in the 1-position by
C1-C4-alkyl, F, CL, 8r, rF3 or CN, or is a
pi~eridine-1,4 diyl or 1~4-bicyclo~2.?.2~octylene
group or a 1,4-phenylene group wh;ch is unsubsti-
tuted or substituted by one or t~o f and/or Cl
atoms and/or CH3 groups and/or CN groups and
in which furthermore one or more CH groups can
be replaced ~ith N~
A-Sp- is a rad;cal of the formula (1) or (2)
Sp ~; .
_ ~ (1) ~ (2)
wherein furthermore one or t~o non-adjacent CH2
groups can be replaced ~ith -0- and/or -S-, and/or
a -CHz-CH group can be replaced with -N=C- or
-CH=C- and/or which can be substituted in the
Z0 1-position by C1-C4-alkyl, F, Cl~ ~r, CF3 or CN,
or is a radical of the formula ~3)
J
~p
(3)
wherein q is 0 to 2 and wherein furthermore one
or more CH groups can be replaced with N, and/or
Z5 which can furthermore be substituted by one or
two F and/or Cl atoms and/or CH3 groups and/or C~
groups, or is a radical (4), (5) or (6)
~p Sp
S~ >--~ ~
~ (4) ~ ~ (5) ~N N- (6)
. , '~.
3~
-- 4
n and m are each 0 to 3,
Z in each case is -C0-0-, -0-C0 ~ CH2CH2-,
-CHCN-CH2 , ~CH2-CHCN-, -CH=CH-, -OCM2-, -CH20-,
-CH~N-, -N=CH-, ~N0=N-, -N=N0- or a single bond,
and
~-Sp is -CH2CHSp-, -CSpCN-CH2-, -CHCN-CHSp-,
-CSp=CH-~ -CHSp-0- or -CSp-N-,
~it~ the proviso that m ~ n ;s 0 to 3.
AboYe and belo~, R1, R2, R3, A, A~ , n,
Sp, Q1, QZ, ~ 2, z and Z have the stclted meanings,
unless expressly stated otherw;se.
The compounds of the formula Ia/b accordingly
comprise co~pounds having two rings, of the partial for-
mulae Iaa to Iba, where;n (Sp-) is a spac~r ~hich Possesses
the stated meanings and is bonded to the above r;ng group
or bridging group:
R1-Atsp-)-z-A-R2 Iaa
R -A (Sp-)-A-R Iab
R1-A-Z(Sp-)-A~R2 Iba,
csmpounds possess;ng three r;ngs, of the partial
formulae lac to Ibg:
R1-A(Sp~)-A-z-A-R2 Iac
R1 A A(S ) Z A R2 Iad
R1-A-A 7-~~Sp-)-R2 Iae
25 R1-A(Sp-)-Z-A-Z-A-R2 laf
R1_A_~_A(Sp-)-Z-A-R2 Iag
R3-A-z-A($p_)-z-A-~2 Iah
R3-A-Z-A-Z-A~tSp-)-R~ Iai
R3-Ao(5p_)-A-~-A-R2 Iaj
30 R3-A-Qo(sp~-z-A-R2 Iak
R3_A-A-Z-A(SP-)-R2 Ial
~1_A~(sp_~-A-A-R2 Iam
R1-A-A(Sp-)-A-R~ I an
R1-A-A-Z(Sp-)-A-R2 Ibc
35 R1-A-Z~Sp-)-A-z-R2 Ibd
~3_A_Z(sp-)-A-z-A-~2 Ibe
R3_A_z_A~z(Sp-)-A-R2 Ibf
R3_A-A-Z(Sp-)-A-R2 Ibg,
compounds possessing four rings, of the partial
^3~
-- 5
formuLae lao to Ib
R3_Ao(sp-)-z-A-z-A-z-A-R3 lao
R3-A-Z-Ao(Sp-)-Z~A-Z-A R3 Iap
R3-h(Sp-)-A-Z-A-A-R3 laq
S R3-A A(Sp-)-z-A_A_R3 Iar
R3-Ao(sp-)-z-A-A-A-R3 Ia~
R3-A-Z-Ao(Sp-)-A-A-R3 Iat
R3-A-Z-A-A~(sp-)-A-R3 lau
R3-A-z-A-A Ao~Sp-~-R3 Iav
10 R1-A(Sp-)-A-z-A-5_A_R3 Iaw
R1-A-Ao(Sp-)-Z-A-Z-A-R3 Iax
R1_A-A-Z-Ao(Sp-)-Z-A-K3 Iay
~1_A_A_z_A_z_A(5p~)~R3 Iaz
~3_Aotsp-)-z-h-A-z-A-R2 Iaaa
15 R3-A-Z-Ao(sp-)-A-z-A-R2 Iaab
R3-A-Z-A-Ao(Sp-)-Z-A-R2 Iaac
R3_A-Z-A-A-Z-A(Sp-)-R2 Iaad
R1_Ao(sp-)-A-A-A-R3 Iaae
R -A-A (Sp-)-A-A-R Iaaf
20 R1-A-A-Ao(sp-)-A-~3 Iaag
R1-A-A-A-Ao(Sp-)-R3 Iaah
R1 Ao(sp-)-A-A-z-A-R3 Iaai
R1-A-A(sp-)-A-z-A-R3 Iaaj
R1-A-A-Ao~sp-)-~-A-k3 Iaak
Z5 R1-A-A-A-Z-Ao(Sp-)-R3 Iaal
R1_Ao~sp-)-A-2-A-A-R3 I aam
R1-A-Ao(sp-)-z-A-A-R3 Iaan
R 1 _ A-A - ~ - A ( S p- ) - A-R3 Iaao
R1_A_A_z_A_A~(sp-)-R3 Iaap
30 R3-Ao(Sp-)-A-A-Z-A-R2 Iaaq
R3-A-Ao(sp-)-~-z-A-R2 Iaar
R3-A-A-Ao~sp-)-z-A-R2 Iaas
R3_A-A-A-Z-A(Sp-)-R2 Iaat
R3-A-zo(sp-)-A-z-A-z-A-R3 Ibh
3 5 R 3 - A - X - A ~Z(Sp-) A-Z- A - R 3 I b i
R3_A-A-z(Sp-) A-A-R3 Ibj
R3-A-z0(sp-~-A-A-A-R3 Ibk
R -A-A-Z (Sp-)-A-Z-A-R Ibl
R1_A-A-Z-A-zo(sp-)-A~R3 Ibm
7 ~
-- 6
R3-A-Z~(Sp-)-A-A-Z-A-R2 Ibn
R3 A-z-A-A-ZSSp-3-~-R2 Ibo
R1_A_A_A_zO~Sp_~_A_R3 Ibp
R1-A-A-~(~p-)-A-A-~3 Ib~
5 R3-A~A-A-Zo~sp~) A R3 Ibr,
and compounds possessing five rings, of the part;al for-
mulae Iaau to Ib~
R3-AotSp-)-Z-A-A-Z-A-Z-A-R3 Iaau
R3-A-Z-Ao(sp-)-A-Z-A-Z-A R3 Iaav
10 R -A-Z-A-A (sp~ -A-Z-A-R3 Iaa~
R3-A-Z-A-A-Z Ao(Sp-)-Z-A-R3 Iaax
R3-A-Z-A-A-Z-A-Z-A~tSp-)~R3 Iaay
R3-A(Sp-)-A-A-h-A-R3 Ia~z
R3-A~Ao(sp-)-A-A-~-R3 Iaba
15 R3-A-A-Ao(Sp-)-A-A-R3 Iabb
R3-~o(sp-)-A-A-z-A-A-R3 Iabc
R3 A Ao(sp-)-A-z-A-A-R3 Iabd
R -A-A-A (Sp-)-Z-A-A-R Iabe
R3_~_A_A-Z-A(Sp-) A-R3 Iabf
2~ R3-A-A-A-Z-A-Ao(SP-)-R3 Iabg
R3-A-Zo(Sp-)-A-A-Z-A~Z-A-R3 Ibs
R3-A-Z-A~A-Zo(Sp-)-A-Z-A-R3 Ibt
R3-A-Z-A-A-Z-A-~otSp-)-A-R3 Ibu
R3-A-A-A-~o(Sp-)-A-A-R3 Ibv.
In the compounds of the formulae above and belo~,
R1, R~ and R3 are preferably alkyl, but ~ay also be
alkoxy.
Other preferred compounds of the for~ulae above
and below ar~ those ;n which one of ~he radicals R1, R2
and R3 is CN, F or SCN.
A and A independently of one another are each
preferably 1~4-cyclohexyl~ne, 1,4-phenylene, 1,3-di~xan-
2,5-diyl or pyr;din~-2,5-diyl.
Sp is preferably a radical -Q~ a2-~l2- Q1
and Q independently of one another aré each preferably
alkylene having 2 to 15 C ato~s. W1 and ~Z independently
of one another are ~ach pref~rably -0-, -S-, -0-C0~
-CO-0- or -CO-NH-~ n and 0 independently of one another
are each 0 or 1, and in par~icular n ~ ~ is 0 to 2.
~L~7~-3~ 3
The radicals Z independently of one another are
each preferably single bonds and are also preferably -CO-O-,
O-CO- or -CH2CH2- groups. Z-Sp is preferably CH~-CHSp-,
-CSp=CH- or CHSp-O-.
R , R2, R3, A, A, Sp Q1 Q2 w1 w2 ~ o
and n ~ay be identical; however, they are preferably in-
dependent of one another and d;fferent.
~re R1, R2 and/or R3 are alkyl radicaLs and/or
alko~y radicals, they may be straight-chain or branched.
Preferably, they are straight-chain~ have 2, 3, 4, 5, 6
or 7 C atoms and are accordingly preferably ethyl, propyl,
butyl, pentyl, hexyl, heptyl, ethoxy, propo~y, butoxy,
pentyloxy, hexylo~y or heptyloxy, or methyL, octyl, nonyl,
decyl, undecyl, dodecyl, tridecyl, tetradecyl, pentadecyl,
methoxy, octyloxy, nonyloxy, decyloxy, undecyloxy, do-
decyloxy, ~ridecyloxy or tetradecyloxy.
Oxaalkyl is preferably straight-chain 2-oxapropyl
(= methoxymethyl), 2- t= ethoxymethyl) or 3-oxabutyl (= 2-
methoxyethyl), 2-, 3- or 4-oxapentyl, 2-, 3-, 4- or S-oxa-
hexyl, 2-, 3-, 4-, 5- or 6-oxaheptyl, 2-, 3-, 4-, S-, 6-
or 7-oxaoctyl, 2-, 3-, 4-, S-, 6-~ 7- or 8-oxanonyl or
2-, 3 , 4-, 5-, 6-, 7-, ~- or 9 oxadecyl.
Where R1, R2 and/or R3 are alkyl radicals in
which a CH2 group is replaced with -CH=CH-, ~hese radi-
cals can be straight-chain or branched. They are pref-
erably straight-chain and have 2 to 10 C atoms. Accord-
ingly, they are, in particular, vinyl, prop-1-enyl, prop-
2-enyl, but-1-enyl, but-2-enyl, but-3-enyl, pent-1-enyl,
pen~-2-enyl, pent-3-enyl, pent-4-enyl, hex-1-enyl, hex-2-
enyl, hex-3-enyl, hex-4-enyl, hex-5-enyl, hept-1-enyl,
hept-Z-enyl, hept-3-enyl, hept-4-enyl, hept-5-enyl, hept-
6-enyl, oct 1-enyl, oct-2-enyl, oct 3-enyl, oct-4-enyl,
oct-S-enyl, oct-6-enyl, oct-7-enyl, non-1-enyl, non-2-
enyl~ non-3-enyl, non-4-enyl, non-5-enyl, non-6-enyl,
non-7-enyl, non-8-enyl, dec-1-enyl, dec-2-enyl, dec-3-
enyl, dec-4-enyl, dec-S-enyl, dec-6-enyl, dec-7-enyl,
dec-8-enyl or dec-9-enyl~
~ ecause they possess better solubility in the
customary liquid-crystalline base ~aterials, compounds
-- 8
of the formulae I having branched wing groups R1, R2
and/or R3 may sometimes be important; however, they are
particularly important as chiral dopants when they are
optically active~ Smectic compounds of this type are
suitable components for ferroelectr;c materials, for chi-
ral t;lted smectic phases and as components ~f nematic
liquid-crystalline phasas, in particular for avoiding
reverse t~ist~
Branched groùps of this type contain, as a rule~
no ~nore than one cha;n branch. Preferred branched radicals
R1, R2 and/or R3 are isopropyl, 7-butyl (= 1-methyl-
propyl), ;sobutyl (= 2-methylpropyl), 2-methylbutyl, iso-
penty( 5= 3-methylbutyl~, 2-~ethylpentyl, 3-methylpentyl,
2-ethylhe~yl, Z-propylpentyl, isopropo)ty, 2-methylpr
2-methylbutoxy, 3-methylbutoxy, 3-methylpentyloxy, 3-
methylpentyloxy, 2-ethylhexyloxy, 1~methylhexyloxy and
1-methylheptyloxy.
The for1ulae Ia and Ib embrace both the racemates
of these ~ompounds and the optical antipodes and their
ZO mixtures.
Preferred co~pounds among those of the for~ula
Ia/b and Iaa to Ibv are those ;n ~hich at least one of
the radicals present has th~ stated pre~erred mean;ngs.
Compounds corresponding to the mesogenis radicals
Z5 of the formula Ia or Ib and in which the valence through
which the spacer Sp is bonded is saturated with hydrogen
or a radical -Q1-H or ~ H are known liquid crystals and
are described in, for exa~ple~ German Patent Applications
P 33 15 295, P 33 46 175, P 34 01 320, P 34 01 321,
3G P 24 04 116 and P 34 11 571; in German Offenlegungs-
schriften 2,167,252, 2,2S7,588, 2,429,093, 2,547,737,
2~b41,724, Z,944,905, 2,951,099, 3,140,868 and 3,228,350,
and in ~uropean Published Specification~ 0,014,885~
OyO84,194, 0,104,011, 0~111,695, 0,122,3a9 and 0,126~883.
M~sogenic groups which are suitable for la~eral
bonding to poly~ers preferably possess the basic struc~
tures a to an, in which Phe is 1,4-phenylene, Cyc is trans-
1,4-cyclohexylene, Dio is 2,5-dioxan-1,3-diyl and F'ym
;s pyri0;d;ne-2,5-diyl, and R1 and R2 have the meanings
given in formula Ia/b:
a R1 Phe~he-R~
b R1-Phe Cyc R
c R1 -Cyc -Cyc: ~R
d Rl~Phe-Phe-Ph~-R~
e Rl~cyciPhe Phe~R2
f Rl-C~c-Phe Cy~
g RL-Cyc~Phe-Phe-Cyc~R2
h Rl PheiC~2C~2-Phe R2
i R -Cyc-CH2C~2-Cyc-R2
10 j R~-Phe-Cyc~OE12C~ Phe-R2
k Rl-Cyc~C~2C~2~Phe Phe-~2
1 Rl Phe CH2Ca2 Ph~ P 2
m R -Cyc C~(CN)-C~-Cyc-R
n Rl-Ph~-COO-Phe-R
lS o Rl-Cyc-COO-Phe-R~
p Rl-Cyc-COO-Phe-Phe~R2
~ Rl-Cyc-Phe-COO-Phe R~
r Rl~Cyc-Phe OCO-Cyc~R2
. s Rl Phe-Cyc~COO Phe R~
2 O t Rl ~-Phe-Cyc~OCO-Phe-R2
u Rl~Phe-Cyc-COO-Cyc-~R2
v Rl~Phe-Phe-COO-Phe-Phe R2
w Rl-Cyc~COO-Phe Phe~R2
~c Rl-Phe~Phe-COO-Phe R2
y Rl-Cyc-COO-Phe-COO Phe~R2
z Rl-Phe-~20 Phe-R2
aa Rl-Phe-Phe C~20-PheR2
ab Rl~Cyc~Phe-C~O-Phe-R2
ac Rl~Phe~Cyc-C~20~Phe~R2
ad Rl-Di~-Phe~-R~
ae ~l-Dio-Cyc_R2
af Rl Cyc-Dio-Phe-R2
ag R ~Dio~Cyc-Phe R
ah Rl~D.io-Phe-COO-Phe-R2
ai ~1 Dio~Cyc-COO-Phe-R2
~:7~3~
-- 10 --
a j Rl-Pym ~ Phe E~.~
ak Rl-Cyc-Pym Pne-R2
al Rl-Pym~Phe-COO~Phe~R2
am Rl-P~n~-Ph~C~20-Phe-R;~
an Rl-~Pym-Phe~OCO~Phe~R2
The spacer Sp can be bonded either to one of the
ring grou~s Phe, Cyc, Dio or Pym or to a bridging group
-CH20 , -CH2CH~- or -CH(CN)-CH2-.
The invention furthermore relates to a process
for the preparation of polymer materials according to
Clai~s 1 and 2.
Thus, compounds of the formula II
Y-M II
~herein M is a mesogenic group of the formula ~a/b and Y
is a functional group capable of poly~erization or of being
grafted, can be polymerized, provided that Y is an alkyl-
ene group ~hich has 2 eo s carbon atoms and is present in
the ~- or (~ position. Y can be bonded to the spacer
Sp directlY t-~1-W1-Q2-W2-Y) or via a functional group
_Q1_w1_Q1_w2_y).
Particularly prefPrred groups are -O-, -CO-O-,
-CO-NH- and -S-, in part;cular -O- and -CO-O-.
The poly~er materials according to the invention
2û can be prepared from compounds of the formula II, wherein
r iS an alkylene group which has 2 to 5 C atoms and is pre-
sentin the ~- or (~-1)-position also by copolymerization
with other olefinically unsaturated monomers. Examples
of suitable comonomers are C1-C20-alkyl esters of acrylic
and/or methacrylic acid~ styrene, ~-methylstyrene, 4-
~ethylstyrene, acrylonitrile, methacrylonitrile and
methylene~alonates.
The polymerization is carried out in a manner
kno~n per se, by reaction of radiant energy, heat energy
electrical energy and by the reac~ion of ~ree radical or
ionic catalystsp as described ;n, for example, Odian~ -
Princ;ples of Polymerization, McGraw-Hill~ New York~ 1970.
.
3~
Suitable sources of radiant energy are UV radiation, laser
beams, X-ray beams and radioactive radiationu Electrical
energy can b~ generated, for example, by el~ctrolysis pro
cesses. E~amples of ~he free radic3l catalysts are potas-
S sium persulphate, diben~oyl peroxide, azobisisobutyro-
nitriLe, di-tert-butyl peroxide and cyclohexanone peroxide.
Ion;c catalysts are organic alkali metal compounds, such
as phenyll~ithiu0 anJ naphthalenesodium, and Lewis acids,
such as ~F3, AlCl3, SnCl4 and TiCl4, or metal complexes in
the form of aluminium or titanium compounds. The monomers
can be polymerized in solution, suspen~ion or emulsion or
as suchc
~ here Y is a hydroxyl, amino, mercapto, epoxide
or carboxyl group or one of their reactive derivatives,
the compounds of the formula II can be grafted onto a
polymeric backbone~ Here~ Y is particularly pre~erably
OH, NH2, coaH or a reactive derivative, in particular OH
or a reactive derivative of the carbaxyl group. This
grafting reaction can be carried out by methods ~hich are
known per se~ such as, for example, esterification~ amid-
ationf transesterification~ transamidation, acstalization
or etherification, ~h;ch are described in the literature
Cfor example, in standard works such as Houben-~eyl,
Methoden der Organischen Chemie (Methods of Organic
Chemistry), ~eorg~Thieme-YerLag, Stuttgart, or C.M. Paleos
et al~, J. Polym. Sci~ Polym. Chem. 19 (1981~ 1427].
A preferred grafting reaction is the reaction of
monomers carrying mesogenic groups o~ the formula I a/b
with organopolysiloxanes. For this purpose, linear or
cyclic organohydropolysiloxanes are react~d, as described
in~ for example, European Patent Specification 0,ObO,335,
with ethylenically unsaturated, mesogenic monomers of the
~ormula II (Y is an alkylene group which has 2 to 5 C atoms
and is present in ~he ~- or (~ position), in about equi-
molar amounts, based on the amount of siloxane-hydrogen,
in the presence of a catalyst which promotes the addition
of silane hydrogen at alipha~ic multiple bonds.
Sui~able polymeric backbones are in principle
all polymers the chains of which possess a certain degree
1~ 7~
of flexibility. These may be linear, branched or cyclic
polymer chainsO The degree of polymerization is usually
at least 10, preferably 20-100. ~owever, oligomers, in
particular cyclic oligomers, having 3 ~o 15~ in particular
4 to 7, monomer units are also suitable.
Polymers having C-C main chains, in particular
polyacrylates, polymethacrylates, poly-~-haloacrylates,
poly-~-cya~noacrylates, polyacrylamides, polyacrylonitriles
and polymethylenemalonates, are preferred. Polymers having
heteroatoms in their ~ain chain, for ~xample polyethers,
polyesters, polyamides, polyimides or polyurethanes, or in
particul3r polysiloxanes, are also preferredu
Other particularly suitable polymeric backbones
are liquid-crystalline main-chain polymers, as described
by, for exampl~, R.W. Len2 in L.L. Chapoy (editor), Recent
Advances in Liquid Crystalline Polymers, London ~ New
York, 1985, page 3.
Compounds of the formula II which possess appro-
priate terminal functional groups can be prepared by
methods known per se, as described in the literature,
tfor example in the standard works such as Houben-Veyl,
Methoden der Organischen Chem;e (Me~hods of Organic
Chemistry), ~eorg-Thieme-Yerlag, Stuttgart), the prepara-
tion being carr;ed out under react;on cond;t;ons which
are fam;l;ar, and suitable for the stated reactions.
Variants ~hich are known per se but not mentioned here
can also be used.
Preferred starting materials are compounds of
the formu(a III a/b,
xl~
RlA- I Z-A )m~Z ~ ( A-Z )n-R2 I I I a
x1,2
Rl (A-z)m-Ao~(z-A)n-R~ IIIb
h i A A A1 R1 R2 z, z, m and n have the
meanings given formula Ia/b and
x1 is a carboxyl groups or one of its reactive
derivatives, or an epoxide~ halogen, haloalkyl,
~i~7~
- 13 -
sulphonate or isocyanate group~ and
x2 ;5 an amino, alcohol or ~hiol group or a halo-
gen atomO
To prepare compounds of the formula Ia/b, compounds
of the formula IlIa/b containing a functional group X1
can be subjected to esterification, etherifica~ion, a~ida-
tion or a transition metal-catalysed coupling reaction
with a co~pound which contains a functional grouP x2 and
is suitable as a spacer~ The corresponding reaction of
compounds of the formula IIla/b which contain functional
groups x2 w;th compounds which contain functional groups
x1 and are suitable as spacers is also suitable.
Other compounds which are preferred for the pre-
paration of compounds of the formula Ia/b are compounds
of ~he formulae IV and/or V
Sp-H
Ri~ Z) -A~ 2 IV X2~l_A_(Z-A)n-R2 V
wherein A, A, R1, R2, Sp, Z, Z, m and n have the meanings
given for formula lalb, and X1 and x2 have the meanings
given for for~ula IiIatb.
Compounds of the formula IV containing a functional
group X1 can be subjected to esterification, etherifica-
~ion, a~.dation or a transition metal-catalysed csupling
reaction with compounds of the formula V containing a
functional group of the formula X2. The corresponding
react;on of compounds of the formula IV which contain the
functional groups x2 ~ith compounds of the formula V
which contain funct;onal groups X1 is also suitable.
Other preferred compounds for the preparation
of compounds of the formula Ia~b are compounds of the
formulae VI and/or VII,
Sp-~ 0~
~l - ( A ~ ) m A-C~; O}~ VI Rl - ( A- z ) m~A~ ~2 - ( A- z ) n; R V I I
wherein A, R~ R~, Sp, Z, m and n have the mean;ngs given
for formula Iatb.
Alcohols of the formula Yl can be reacted ~;th
4 --
compounds of the formula V which co~tain a functional
group X1 having the meaning given for formula IIIa/b to
give polymerizab~e compounds of the forlnula II ~hich are
suitable for the preparation of the polymer materials
according to the invention.
Alcohols of the ~ormula VII can be reacted with
compounds which contain functional groups X1 and are suit-
able as s~acers, to give prepolymers of the formula II.
Some of the compounds of the formulae IIIa/b,
IV, V, VI and VII which are suitable for the preparation
of in~ermediates which can be polymerized to give materials
according to the invention, of the formula Ia/b, are known,
but th~ majority are new. They are prepared by ~ethods
known per se, as described in the ~iterature (for example
in standard ~orks such as Houben-Weyl~ Methoden der
Organischen Chemie ~Methods of Orsanic Chemistry), Georg-
Thieme-Verlag, Stuttgart), the preparation being carried
out under reaction conditions which are familiar, and
suitable, for the stated reactions. Variants which are
known per se but not men~ioned here may also be used.
Thsy are used for the preparation of the liquid-crystalline
polymer materials according to the inv@ntion.
The processes stated for the preparation of IIIa/b
to YII are known per se (for example from standard works
such as Houben-Weyl, Methoden der Org. Chemie (Methods
cf Organic Chemistry3, Georg-Thieme-Verlag, Stuttgart).
Usually, ~he reaction conditions kno~n for the stated
reactions are maintained. However, variants ~hich are
known per se but not mentiorled here may also be used.
In particular, the processes stated for the preparation
of the parent substance which is not provided with a poly-
mer;zable 5ide chain and is described by the formulae a
to an can be used.
Some of the low moLecular weight compounds of the
formula II have broad mesophase ranges. However, com-
pounds of the formula II ~hich do not exhibit any meso-
ph~ses are also suitable for the preparation of the poly-
mer mater;als according to the invention.
The preparation of the homopolymers or copolymers
from the polymerizabLe compounds of the forrnula II or
their polymerizable derivatives is preferably carried out
by free radical polymeri~ation~ The reaction is started,
for example, by means of UV irradiatiQn or free radical
formers. The monomers can be polymerized in solution or
as such.
CopoLymer materials exhibiting liquid-crystalline
phases a~cording to the inventirJn are obtained by copoly-
merization of the polymerizable co~pounds of the formula
10 II or their polymerizable derivatives with mono~ers ~hich
carry no mesogenic radicals, which carry other mesogenic
radicals (for e~ample disc-like: German Pat~nt Specifica-
tion 3,430,482~, ~hich carry chiral radicals (far example
German Offenlegungsschrift 2,a31~909~ or which carry dye
radicals (German Qffenlegungsschrift 3,211~400).
Starting from a monomer mixture having the concen-
tration X1, copolymerization with such monomers leads to
a copolymer in which the monomer is incorporated ;n a
concentrat;on X1 only when the copolymerization parameters
of the monsmer components are of comparable orders of mag-
nitude. This is particularly important when it is desired
to prepare a copolymer of a part;cular composition ~ithout
difficulties, for exa~ple without taking into account the
reaction kinetics. Hence, preferably chosen monomer com-
ponen~s are those wh;ch haYe comparable copolymerizationparameters, for e~a~ple alkyl acrylates or alkyl meth-
arrylates, which differ pr;marily by virtue of the SUD-
stituents in the alkyl chain.
Copoly~erization with monomers which do not carry
any mesogenic radical generally leads to a reduction in
the glass transition temperature and in the clear point.
8y a suitable choice of the spacer, it is often possible to
bring the mesophase range into ~he temperature range s~it
able for the particular intended use.
Suitable monom~rs possessing a chiral radical are
;n principle all compounds of this type which possess asym
~etric C ato~s. Mowever, compounds of the formula II or
their polymerizable derivatives~ in which M is a mesogenic
group of the formula la or Ib, wherein one of the radicals
- 16 -
R1, R2 and/or R3 is an alkyl group in which one CH2 group
is replac~d with -CHCH3-, are preferably empLoyed.
Finally, a large number of further possible vari-
a~ions arise from the fact that the compounds according to
the invention combine liquid crystalline properties with
typical polymer properties, such as the ability to form
layers, f;lms and fibres, easy deformabil;ty, etc. These
properties can be modified in a manner known per se, by
copolymerization or mixing ~ith other components, by vari-
ation of the molecular weights, by adding a very wide var-
iety of inorganic or organic additives and metals, and by
many other treatments fam;liar to the skilled worker in
the f;eld of polymers.
The polymer materials according to the invention
can be used as starting materials for the production of
organic glasses having aniso~ropic properties which can
be modified over wide ranges.
Applications of this type occur, for example, in
the sector comprising light collectors and solar collec-
tors or in connection with organic phototropic glasses.Furthermore, an important area of application has opened
up in the field of optical memory.
Other possible applications are being opened up in
the field of magnetic memories~ The materials according
to the invention are also particularly suitable as a ma-
trix for substances having non-linear optical properties,
for the production of "non-linear" optical components.
The polymer materials according to the invention
ar~ also suitable for amplitude modulation and/or frequency
modulat;on of laser beams~
The examples ~hich follow serve to illustrate ~he
invention, G denoting glass state, C denoting crystalline,
N denoting nematic and I denoting isotropic (phase tran-
sition temperatures in degrees Kelvin in each case)~
Example 1
a) 2~ Hydroxyundecyl)-hydroquinone obtained fro~
Z-~10-carboxydecyl)~hydroquinone (prepared from p-benzo-
quinone and methyl undec-10-enoate by using d;borane and
then carrying out hydrolysis) by reduction ~ith lithium
~;~7~3~ ~
- 17 -
alu~inium hydride is esterified ~Jith methacrylic acid to
give 1 (11-methacryloyloxyundecyl)-hydroquinorle.
34.8 9 of the above co~pound are dissolved in
500 ml of d;chloromethane. 20 9 of triethylamine are
S added, after ~hich 34.1 9 of 4-methoxybenzoyl chloride are
introduced slowly at 0C. When the addition is complete,
stirring is continued for 1 hour at room temperature, after
which the~mixture is washed with ~ater and freed from the
sol~ent under reduced pressure. The bis~(4-~ethoxyphenyl)
ester ot 2-(11-methacryloylo~yundecyl)-hydroquinone is
obtained. In the supercoc,led state, the monomer exhibits
a meta-stable ~onotropic ne~atic phase: C (336) N 2~6 I.
b) The bis(4-methoxyphenyl) ester of 2-(11-methacry-
loyloxyundecyl)-hydrociuinone ob~ained according to Example
1a is polymerized in an approx;mately 1 molar solution in
benzene, in the presence of 1 mol % of azobisisobutyro-
nitr;le at 60C. The resulting polymer exhib;ts a stable
l;qu;d-crystall;ne phase: G 312 N 337 I~
Exa~ple 2
a) 2-(11-Methacryloyloxyundecyl)-hydroquinone is
ester;fied with 4-hexyloxybenzoyl chloride~ analogously to
the procedure descr;bed ;r Example 1a.
In the supercooled state~ the resulting bis(4-
hexyloxyphenyl) ester of 2~ methacryloyloxyundecyL)-
hydroquinone has a monotropic metastable nematic phase:C (322) N 319 I.
b) ~he bis(4-hexyloxyphenyl) ester of 2-(11 methacry-
loyloxyundecyl)-hydroquinone obta;ned ;n Example Za is poly-
mer;zed in solution in benzene, analogously to the pro-
cedure described in Example 2. A polymer which exhibits 3stable liquid-crystalline phase is obtained: ~ 282 N 335 I.
Example 3
a) 2-(9-Carboxynonanoyl)-4-butylphenol obtained from
4-heptylphenol, sebacic anhydride and aluminium chloride
by known processes is reduced to 2-(10-hydroxydecyl)-4-
heptylph~nol ~ith lithium aluminium hydride/aluminium
chloride.
b) 2-(10 Hydroxydecyl)-4-heptylphenol (0.1 mol) pre-
pared according to Example 3a is reacted~ ~ith methacryloyl
chloride (Oo1 moL) in 300 ml of pyridine at 5C to give
2-(10-methacryloyloxydecyl)-4-heptylphenol 9
c) 0.1 moL of the phenol 3b obtained above, in 250 ml
of d;chloromethane, and 0.1 mol of triethylamine are trea-
ted ~;th 0.1 mol of 4-propoxyben~oyl chloride at 0-5C.
The m;xture is stirred for 2 hours at this te~perature,
after which it is washed neutral with ice ~ater, dried
over sodium sulfate and evaporated dowrl. 2-(10-Methacryl-
oyl-oxydecyl)-4 haptylphenyl 4-propoxytenzoate remains in
the form of an oil.
d) A solu~ion of 0.05 mol of 2-(1C-methacryloyloxy-
decyl~-4-heptylphenyl 4-propoxybenzoate in 100 ml of tol-
uene is heated at 90C for 10 hours, after the addition
of 0.5 9 of azobisisobutyronitrile. The polymer separates
out as a gel on cool;ng. After the add;t;on of ethanol,
the f;lterable amorphous product which exhibits a nematic
liquid-crystall;ne phase ;s obta;ned~
Example 4
a) 2-(10-Methacryloyloxydecyl)-4-heptylphenol ~0.2
mol) obta;ned according to 3b ;s d;ssolved in 5~0 ml of
dichloromethane and 0.1 mol of triethylamine. 0.1 mol of
trans-4-propylcyclohexanecarbonyl chloride is added drop-
wise at +5C and the mixture is kep~ at this ~emperature
for a further 30 minutes and then stirred for 1 hour at
Z5 room temperature. It is washed neutral with water, dried,
and freed from the solvent under reduced pressure and under
mild cond;t;ons. Oily 2-(10-methacryloylo~ydecyl)-4-heptyl-
phenyl 4-propylcyclohexanecarboxylate remains.
b~ A mixture of 0.1 mol of 2-(10-methacryloyloxydecyl)-
4-heptyLphenyl 4-propylcyclohexanecarboxylate, O.OOS mol
of azob;sis~butyronitr;le and 400 ml o~ benzene is heated
under reflux for 8 hoursO The st;ll hot polymer is then
prec;pitated by stirring the m;xture ;nto 1 litre of
methanol~ The polymer possesses liqu;d-crystall;ne
properties.
Exa~ple S
a) 27.7 9 of 2-(3-cyano-4-fluorophenyl)-S-pentyl-1,3-
dioxane (obtainable from 3-cyano-4-fluorobenzaldehyde and
2-pen~yl-1~3-propanediol) ;n 380 ml of d;oxane are aclded
7~ 3~
- 19 -
dropwise, in the course of 2 hours, to a boiling suspen-
sion of 4~0 9 of lithium aluminium hydride in 300 ml of
the same solvent. ~hen the addition is complete, heating
is continued for a further hour and decomposition is then
S effected with water. The solu~ion is ~filtered off from
the precipitated hydroxide and evaporated do~n under re-
duced pressure. Z-53-Aminometllyl-4-flllorophenyl)-5
pentyl-1,~-dioxane is obtained.
b) A mixture of 14.0 9 of 2 (3-aminomethyl-4-fluoro-
phenyl)-S-pentyl-1,3-dioxane and 11.7 ~ of ethoxycarbonyl-
octanoyl chloride in 250 ml of pyridine is stirred for 3
hours at room temperature~ It is then poured onto 2 litres
of ice wzter~ and the precipitaeed 2-(3-(8-ethoxycarbonyl-
octanoylamidomethyl)-4-fluorophenyl)-5-pentyl-1,3-dioxane
is filtered off under suction~
c) 0.1 mol of the above amido ester Sb is introduced
in portions into a boiling suspension of 7.5 9 of lithium
aluminium hydride in 400 ml of tetrahydrofuran. Heating
for 1 hour, decomposing with ~ater and working up as de-
scribed in 5a) give 2-S3-(9-hydroxynonylaminomethyl)-4-
f luorophenyl)-5-pentyl-1,3-dioxane~
d) 15 9 of an acrylic acid/acrylate poly~er conta;n-
ing free carboxyl groups are converted to the acid chlor-
ide with 10 9 of thionyl chloride in a custo~ary manner.
The residue obtained af~er remov;ng excess thionyl chlor-
ide is suspended in pyridine, and the suspension is stir-
red overnight ~ith 5 9 of 2-(3-(9-hydroxynonylaminomethyl)-
4-fluorophenyl)-S-pentyl-1,3-dioxane. Filtration and
washing with water give a polymer material which exhibits
liquid-crystalline properties.
~xample 6
a~ 15 9 of trans-4-(4-(4-pentylcyclohexyl)-phenyl)-
ethylbenzene in 50 ml of glacial acetic acid are slowly
added dropwise, at -35C, to a m;xture of 50 9 of fuming
nitric acid and 50 9 of acetic anhydride. Stirring is
cont;nued for a further 2 hours at this temperaeure, af~er
which the mixture is poured onto 1 litre of ;ce water, the
pale yellow precipitate ;s f;ltered off and the resulting
mixtures of the mononitro-4 (4-(4-pentylcyclohexyl)-phenyl-
~L ~ 72 ~3 ~ r ~
- 20 -
ethylbenzenes are dried.
b) The nitro compound obtained above is hydrogenated
in a customary manner in ethanolic solutiorl over palladium/
activated carbon.
0.1 ~ol of amino-4-t4-(4-pentylcyclohexyl)-phenyl-
ethylbenzene prepared in this manner is heated for 6 hours
at 90-95C with 0.1 ~ol of dimethyl brassylate in 300 ml
of toluen~, with removal of the resulting methanol by dis-
tillation~ When the reaction is complete, the product is
freed fro~ the solvent under reduced pressure. (12-Methoxy-
carbonyldodecanoyla~ino)-4-(4-(4-pentylcyclohexyl)-phenyl)-
ethylbenzene is obtained.
c) 0.25 mol of the amidoester 6b is reduced with 0.5
mol of LiAlH~ as described in Example 5c to give (13
hydroxydodecylamino)-4-(4-(4-pentylcyclohexyl)-phenyl)-
ethylben~ene, and then grafted, in the manner described
under 5d, onto a copolymer containing carboxyl groups. A
polymer material exhibiting a liquid-crystalline phase is
obtained.
Exampl~ 7
a) 21.0 g of 5-butoxysalicylic acid, 22.2 9 of 5-
heptylpyri~idine-Z-carbonyl chloride and 20~0 9 of tri-
ethylamine in 500 ~l of dichloromethane are subjected to an
esterification reaction for 2 hours at +5C.
b) 2-Carboxy-4-butoxyphenyl 5-heptylpyrimidine-2-
carboxylate obtained according to 7a (0.1 mol) is conver-
ted to the acid chloride ~ith 15.0 g of thionyl chloride
in a customary manner. The crude product obtained after
excess thionyl chloride has been removed by distillation
is taken up in 450 ml of dichloromethane, 20~0 9 of tri-
ethylamine are added and a solution o~ 0.1 mol of 2 (2-
(2~hydroxyethoxy) ethoxy)-ethyl acrylate is then intro-
duced slo~ly. The ~ixture is stirred at room temperature
for 1 hour and then washed with water, dried over sodium
sulfate and freed from ~he solvent under reduced pressure.
2-(2-(2-(2-Acryloyloxyethoxy)-ethoxy)-ethoxycarbonyl)-4-
butoxyphenyl 5-heptylpyrimidine-2-carboxylate is obtained.
c) 0.1 mol of the e~ter 7b into which an acryloyl
group has been introduced, in 120 ml ot benzene~ ;s heated
~7~3~
under reflux for 6 hours, after the addition of 2.5 9 of
azobisisobutyronitrile. After the mixture has b~en cooled,
an equal volume of methanol is added. A polymer ~hich
exhib;ts a liquid-crystalline phase is ;solated by fil-
tration.Example ~
a) 1-(4'-Pentylbiphenyl~4-yl)-2-~4-propylcyclohexyl)-
etheneO o~tainable from 4-propylcyclohexanecarbo~aldehyde
and 4'-triphenylphosphono-4-pentylbipher1yl broMide by a
Wittig reaction, is hydroborated with diborane in a ~us-
tomary manner and onverted to 1-(4-propylcyclohexyl)-2-
~4'-pentylbiphenyl-4-yl)-ethanol ~ith alkaline hydrogen
pero~ide.
b) 0.1 mol of 1-(4-propylcyclohexyl~2-(4'-pentyLbi-
phenyl-4-yl)-ethanol is stirred w;th 0.1 mol of sodium hy-
dr;de in 350 ml of toluene at room temperature until evolu-
tion of hydrogen is complete. 0.1 mol of allyl 9-bromo-
pelargonate (obtained from allyl bromide and 9-bromo-
pelargonic acid in dimethylformamide using potassium car-
bonate) is then added, and the mixture ;s kept at 60C for3 hours. After ~ool;ng, the mixture is washed with water
and dried over sodiu~ sulfate, and the solvent is removed
under reduced pressure to give allyl 9-(1-(4-propylcyclo-
hexyl)-Z-(4'-pentylbiphenyl-4-yl~-etho~y)-pelargonate.
2S c) 10 9 of the allyl ester Bb in Z00 ml of xylene are
kept at 90~C for 3 hours, 200 mg of azobisisobutyronitrile
being added. The polymer obtained on cooling is isolated
by fil~ration. It exhibits liquid-crystalline properties
above the glass transition te~perature.
Example 9
a) A mix~ure of OD1 mol of 4~heptylbenzene, 0.1 mol
of sebacic anhydride and 0.2 ~ol of aluminium chloride in
400 ml of carbon disulfide is heated under reflu~ for ~
hours~ The reaction mi~ture is hydrolysed by add;ng 200 ml
of 10% hydrochloric acid. The residue obtained after the
organic phase has been separated off and the solvent re-
moved by distillation is 4~sebacoyl-heptylbenzene.
b) 0.1 mol of 4-sebacoyl-heptylbenzene, 0.1 mol of
sodium borohydride and 300 ml of methanol are stirred for
5 hours at 0 to +5C. The mixture is then evaporated to
dryness under reduced pressure, the residue is taken up in
water, and the product, 10-(4-heptylphenyl)-lO-hydroxy-
decanoic acid, is precipitated by adding dilute hydro-
chloric acid.c) The corresponding methyl ester is prepared from
0.2 mol of the acid 9b described abave in a customary man-
ner by heating in ~ethanol with the addition of sulfuric
acid.
63.2 9 of methyl 10-(4-heptylphenyl~-10-hydroxy-
decaroat~ are converted to the sodium alcoholate with 10 9
of a 50% sodium hydride/liquid paraffin dispersion in
500 ml of dimethyl sulfoxide. When the evolution of hydro-
gen is complete, a sr,lution of 69.6 9 of 4-(4-mesyloxy-
cyclohexyl)-butylcyclohexane (prepared from ~he corre-
sponding cyclohexanol with mesyl chloride/triethylamine in
dichloromethane) in 120 ml of dimethyl sulfoxide is added
dropwise as 20C. Stirring is continued for a further 2
hours at 50C, and the mixture is poured onto 2 litres of
ice water and extracted with dichloromethane. The residue
obtained after removal of the solvent is chromatographed
over silica gel, using chloroform as the eluant. Methyl
10-(4-t4-butylcyclohexyl)-cyclohexyloxy)-10-(4-heptyl-
~henyl)-decanoate is isolated.
ZS d) 0.1 mol of the decanoa~e obtained in 9c is hydro-
lysed by boiling for 4 hours in a mixture of 300 ml of
m~thanol, 100 ml of water and 25 9 of potassium hydroxide.
The reaction mixture obtained is acidified by adding semi-
concentrated hydrochloric acid. The precipitate is fil-
tered off and dried at elevated temperature. To convert;t to the acid chloride, the crude acid prepared as des-
cribed above is heated under reflux for 3 hours in the same
amount by we;ght of th;onyl chlor;de. Volatile components
are removed under reduced pressure, and 10 (4~(4-butyl-
cyclohe~yl)-cyclohexyloxy)-10-(4-heptylphenyl)~decanoyl
chloride is ;solated ;n this manner.
e) 15 9 of a hydroxylated po~yacrylate in 5 9 of acid
chloride 9d in 200 ml of pyridine are stirred for 24
hours at room temperature, after the addition of 1 9 of
- 23 -
4-dimethylaminopyridine.
The mixture is filtered, and the residue is washed
with water and dried. The polymer prepared in this manner
exhibits a liquid-crystalline phase.