Note: Descriptions are shown in the official language in which they were submitted.
7~
,
The pre~ent invention i~ concerned ~ ch a proce~s
and a re~g~nt for ~che dete~na~ion of ~he lu~eini ~ing
hormolle, as well a~ with rnonoclonal antibodie~ suitable
therefor.
The detennination of the luteini~ing honD~ne (LH~
in body fluid~, for exan~ple urin~ erlLm ~nd pla~ma,
i 3 mainly em~loyed in order to be able to a~ he
endocrinological ~a~us o:~ th~ hypthalamu~, hypophy~i~
and gonads. The~e inve~tis~ations ~erve, in particular9
for the differential diagno~i~ of hypogonadiam,
infertility and the like., In addition, the L~I dater-
mination i8 used in order to determine the ovulation
time point in the case of an ~ nduction of pregnancy.
- - - In all th~se ca~e~, the ~erum values lie within
15 the physiological range. Furthermore, the concentration
of ~H in ~he ~erum is also measured ~olely ~or the
purpo-4e of obtaining evidence regarding the biological
effectivene~s of ~hi~ hormone. Of dia~nostic import-
ance i~, therefore, e~entially a knowledge of the
serum level of the native hormone.
The physiological concentration of human LH in
the 9erwm lie~ in the following ranges:
men ~ 4 - 24 mIU/ml.
women before menopause, cycle 10 ~ 20 mIU/ml~
women ovulation peak 80 - 100 mlU/ml.
w~men after menopau~e 80 - 150 mIU/ml.
~t73
`:
-- 2 --
For the de~ermina~ion o~ h~, there are aspec-
ially ~uitable in~nunological ~e~3t proce~e~ in whicll
the hormone i~ determined a~ an~igen with one or more
antibodie~ directed again~t it. The obtaining of
antibodle3 wlth these polypeptide hormone~ lnvolve~
difficulties since all polypeptide honmone3 are
poorly immunogenic. Becau2~e of ~he homology between
LH and other glycopro~ein hormone~, for exampl2 the
follicle-~timulating hormone (FSH), ~hyroid-
3timulating honmone (TSH) and human chorionic gonado-
tropin (hCG), it i9 very dif~icult to obtain specific
antibodie3 against one of the3e hormone~. An a~tibody
directed against one o~ these glycoprotein hormon23
usually display3 more or les~ cross-reactivity with
the other glycoprotein hormones.
Clinica Chemica Acta 133, 263-274,/1983 descri}~
monoclonal antibodies again . t LH which cro~s-react
with TSH and hCG up to 10% and with FSH up to 3%.
Briti~h Patent Specification No. 2,111,201 al~o de~-
cribes a monoclonal antibody again~t LH~ However,this react ju~t a3 well with hCG. A monoclonal anti-
body which i8 specifically directed against LH and
display~ no cross-reactivity with the other glyco-
protein hormones i~ hitherto not known. Therefore,
at the moment, it i~ not po~sible to determine LH
immunologically without other glycoprotein hormone~
being mor~ or less included.
"0
~7~
- 3
The present invention~seeks to
provide a new in~unological proces~ and reagent with
the help of which LH can be ~pecifically determined
even in the pre~ence of other glycoprotein hormones~
Thu8, according to the pre~ent invention, there
is provided an immunological proce~ for the determin-
ation of the luteini~ing ho~mone (LH), ~herein at least
one monoclonal antibody i9 u~ed which i5 specificalJy
directed again~t LH and cross-reacts with other glyco-
pxotein hormones at an extent of le~s than 3%.
The present invention al80 provide3 a reagen~
for the determination of the lllteini~ing hormone,
wherein it contain3 at least one monoclonal antibody
which is directed against LH and cro~s-reacts with
other glycoprotein hormones to an extent of les~
than 3%.
Aq immunological determination methods, there
can, in principle, be used all available immuno-a~says,
quch a~ radio-immuno-assay, enzyme-immuno-as~ay,
fluorescence-immuno-assay and the like. Furthermore,
all process variants, such a~ competitive immuno-
assay, sandwich proce3s and the like can be used. For
labelling, there can be used the agents which are
conventional for the particular determination methods.
Thus, in the case of a radio-immuno-as~ay, there are
used radioi~otope~, for example 125I, ~or the labelling.
For an enzyme-immuno-a~ay, there can be used all
.~,:;;. j,
~ ~73;~
~,
enzyme~ u~ually e~ployed fo~ this purpose, for exaT~ple
pexoxida~ or ~-galacto~ida~3e. For a fluorescence-
immuno-a~ay, the u8ual fluorsscing group~ can be
u~ed as labels. Detail~ of the~e variou~ te3t method~
5 and process variants are well known. Te~t variant3
are advantageous in which at lea~t two %~onoclonal
antibodie~ a~cording to the present invention ar~
employed which are directed against differ~nt antigenic
determinant~ of the LH and at lea~t one o~ which cros~-
10 reacts with o~her glycoprotein hormones to an extento~ less ~han 37~.,
For the determination of LH, it ha~ proved to
be especially preferable to use the sandwich process
in which the antigen to be determined i~ brought into
contact with a carrier-bound and a labelled antibody.
For such a determination proces~, a 9pecific mono-
clonal antibod~ according to the present invention
can~ for example, be bound to the solid phase. In a
~ir~t incubation ~tep, this i~ incubated with the
sample which contains the LH to be determined, a~ well
aq, in gener~l, other ~lycoprotein honmone~, LH there-
by being selectively bound by the ~pecific antibody.
After the u~ual washing step~ it i8 incubated with
the labelled antibodyO Thi8 mu~t not necessarily be
~pecifically directed against LH but can al~o cross-
react wi~h other glycoprotein hormones.
-`` 3L~7~
-- 5 --
- Tbe procesa can al 80 b~ carried out wlth a non-
~pecific carrier~bound antibody. ~owever, it i~ ~hen
nece~ary that, aa labelled antibody, there ia employed
an antibody according to ~he pre~ent invention ~hich
directed ~pecifically against LH.
Variant~ of the sandwnch proces3 can al30 be
u~ed for the determlnation of LH. Thus, for example,
a soluble ~andwich complex can first be formed wi~h a
non-labelled, ~oluble antibody and the labelled anti-
body. Thi 8 i g subsequently made insoluble with thehelp of a carrier-bound anti~ody which is directed
again~t the FCY part of the non-labelled soluble anti-
body. In the ca~e of this proce3s variant, at lea~t
the non-labelled, soluble antibody mu~t be an antibody
accordiny to the present inventionO The labelled
antibody is thereby preferably employed in exce~s.
The e3sence of the pre~ent invention is to be
seen in that it i~, ~urprisingly, possible to make
available for these immunological proces~es monoclonal
antibodies which are specifically directed against LH
and which, therefore, maXe po3~ible a specific deter-
mination of LH
Therefore, the present invention al~o provides
monoclonal antibodies against hH, ~he cro~s-reactivity
of which with other glycoprot~in honmones amounts to
le3~ than ~%.
For obtaining the monoclonal anti~odie~ accord-
ing to thP present invention, experimental animal~,
for ex~mple mice~ are immunised with LH. For the
immuni~atlon, the immunogen 1~ administered in the
usual way, for example in combiIlation with an adjuvant.
A~ adjuvant, it i9 preferred to U~e aluminium hydroxide~
together with ~ ~ or Freund'~
adjuvant~ The immunisation preferably takes place
over several months with at lea~t four ~mmuni3ation~
10 ~t 4 to 6 week intervala (intral?eritoneal injection3.
From thus i~muni ~ed animal 9 are obtained B-
lymphocytes which are fu~ioned wqth a permanent myeloma
cell line. ~he fusioning take~ place according to the
known proce~s of ~ohler and Milstein (Nature 256, 49$-
4~7/1975). ~he primary cultures of hybrid cellq
thereby formed are cloned in the usual way, for
example with the u~e of a commercially available cell
~orter or by "limiting dilution". In each ca~e, tho~e
culture~ are further worked up which, in an appropriate
te~t proces~, for example an enz~e-immuno-as3ay
~ELISA proce~s~, react positively against LH and
negatively or only a little wlth the other gly~o-
protein hormone~. ~here are thu~ obtained ~everal
hybridoma cell lines which produce the monoclonal
25 antibodie~ according to the present invention. Accord
ing to known method~, the~s cell line~ can be cultured
and those of them producing monoclonal antibodi2s are
i ~olated.
1',2~33~
-- 7 --
By wsy of example, for- cell lines obtained in
thia way, there are mentioned:
clone 369 (NCACC 84122001) and
clone 799 (NCACC 84122005).
The cell lines have beèn deposited under the
given number at the NCACC depository (National
Collection of Animal Cell Cultures).
The ~o obtained noclonal antibodies have a
very high affinity (affinity constants of the order
of magnitude of 109 to 101 l/mol) against LH and
croaa-react with other glycoprotein hormone~ to an
extent of leoJ than 3%. Preferred monoclonal anti-
bodieJ diJplay a crosJ-reactivity toward~ other glyco-
protein hormoneJ, Juch as hCG, FSH and TSH, of leaJ
than 1% and eapecially of le~ than 0.1%. For the
determination of the affinity and of the croaJ-
reactivity with other hormones, there can be used the
proce~JeJ known for thia purpose.
The noclonal antibodies according to the
preaent invention are outstandingly useful for the
apecific determination of the hormone LH in the
preaence of other glycoprotein honmones in a sample,
for example aerum or plasma. For these determination
proceJJea, there can be used the monoclonal antibodies
aJ Juch or fragment~ thereof which poJaeaa the corres-
ponding immNnological propertieJ, for example Fab
fragmentJ, Therefore, the expre~sion ~monoclonal
.;. ,, , .-. , :
~ ;~7~ 6
antibodies" is to be understood to Mean not only the
complete antibodies but also the fragments thereof.
In accordance with another aspect of the
invention a kit for determining the presence of
luteinising hormone comprises separate components of
reagents at least one of which is a monoclonal
antibody of the invention.
The following Examples are given for the
purpose of illustrating the present invention and in
which reference will also be made to the accompanying
drawings in which:
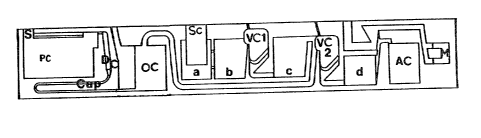
FIGURE 1 illustrates schematically a rotor
insert element which may be employed in the deter-
mination of LH, and
FIGURE 2 is a calibration curve for sera of
known LH content.
... .
~3
Balb/c mice, 8 - 12 week~ old, are i~uni sd
intraperitoneally with 100 ~g. hLH (obtain2hle from
Immunex), ad~orbed on alumlnium hydroxide and
pertu~8i9. After 6 week~, three further immunisations
are carried out at 4 week intervals. 50 ~g~ LH,
adsorbed on aluminium hydroxide and ~ pertussi~,
are thereby, in each case, administered intraperitoneally.
~bout four month~ after the last immuni-Qation,
fusioning is carried out. Four day~ and three day~
bef~re fusioning, in each ca~e, immuniQatiOn i3 carried
out again with 100 ~g. LH/PBS (phosphate buffered
~aline~ intraperitoneally or intravenou~ly.
For the fusioning, with referen~e to the method
deQcribed by Galfre (Method~ in ~nzymology, 73, 3/1981)
108 ~pleen cells of an immunised mouse are mixed once
with 2 x 10 myeloma cells (P3x63Ag8-653, ATCC-CRL 8375)
and subsequently centrifuged for 10 minute-~ ~300 g, 4C.).
20 The cells are again washed once with B5S (balanced 3alt
solution) and centrifug~d at 400 g. The supernatant
is r~moved. The cell 3ediment i~ mixed wqth 1 ml. 5~%
P~G solution (M.W. 4000, Merck)0 Thereafter, at ambient
~;~'7;~
-- 10 --
t~mperature, 5 ml. ~PMI 164Q medium (RPMI ~ ~osew~ll
Parker Memory Institu~e) wi~hout fo~tal calf ~erum
(FCS) and ~ub~equently once again 5 ml. RPMI 1640
medium with 10% FCS, are ~lowly added dropwise thereto,
the mixture iq made up with medium to 50 r~. and
centrifuged for 10 minute~ at 400 g. The aedimented
cells are taken up in RPMI 1640 m~dium with 1~% FCS.
2 x 105 spleen cellq are each ~eeded into 24 ~11 cell
culture plate3 ~obtainable from ~unc)O To each culture
are added 1 x 105 ~pleen cells or 5 x 104 peritoneal
-exudate cells as feed cells. On the following day,
hypoxanthine-azaserine selection medium (100 mM
h~poxanthine, 1 ~gc/ml. azaserine) i~ added thereto.
After akout 7 - lO days, many clones are already
vi~ible. The supernatent of the primary culture~ i~
tested according to the ELISA proces~ de~cribed in
Example 2. Primary culture~ which contain antigen-
specific antibodies are further cloned with the help
of a fluorescence-activated cell sorter on 96-well
culture plates (obtainable from Nunc). As feed cells,
there are used l x lO peritoneal exudate cells or
2 x 104 spleen cells per 96-well of the culture.
In thi~ way, there can be isolated the two
hybridoma cell lines of clone 369 and clone 799, which
have been depo~ited at the NCACC depoqitory (National
Collection of Animal Cell Cultures) under ~he deposit
numker~:
~CACC 84122001 (cl~ne 369) and
NC~CC 84122005 (clone 799~.
For ~he production of ascite3, 5 x 106 hybrid
cell~ are injected ~ntraperitoneally to muce which had
previously been pre-treated 1 to 2 time~ with O.S ml.
pristane. One to three week~ thereafter, ascite~ fluid
can be obtained from the mice and the antibodie~ can be
isolated herefrom in the usual way. Theae monoclonal
antibodies are ~pecifically directed against LH and
~how no or only a ~mall cro~q-reactivity wqth other
glycoprotein hormone~. In the followQng, they are
de~ignated a~ MAB 369 (from clone 369) and MAB 799
~from clone 799).
The tw~ monoclonal antibodies belong to the sub-
clas~ IgGl/~. They po~sess the following affinities:
MAB 369 = 7.9 x 101 l/mol
MAB 799 = 9.5 x 1011 l/mol.
For the determination of the affinitie~, compet-
ition curves are determined wnth the homologou~ antigen
according to the method of Scatchard (Ann~ ~.Y. Acad.
Sci. 51, 660/1949) and evaluated. The mea~urement~
necessary therefor are carried out analogou~ly to
Example 2.
~.
~
In order to recognise the pre3ence and specificity
of antibodies again~t LH in the serum of immuni3ed mice
or in ~he culture ~upernatant of ~he hybrid cell~ or
in a~cite~, th~re i~ u~ed an ELISA pro~e~s a~ test
princ~ple: Microtitre plates are coa~ed oYernight
with 1 ~g. hH/ml. of coa~ing buffer (0.2 M ~odium
carbonate/bicarbonate, pH 9.3 ~ 9 7 5) at 37C. and then
after-treated for 10 minute~ with 0.~% ~odium c~loride
solution and 1% albumin solution and subsequently
wa3hed with 0.~% ~odium chloride ~olution. Sub~e-
quently~ incubation i~ carried out at 37C. for one
hour wnth 100 ~1. of ~2mple and again waRhed with 0.~%
~odium chloride solution. There follow~ a further
incubation for one hour at 37~. with 100 - 150 mU/ml.
of a 3heep anti-mou~e-IgG~peroxidase conjugate. After
a further wa~hing ~tep with 0.9% ~odium chloride
qolution, the peroxidase activity i~ determined in the
usual way (for example wqth AB~S, 30 minutes at ambient
temperature, ~here being read off the extinction
difference, ~ m~, at 405 nm).
The ELISA te~t can al90 be carried out as
followq:
~ he microtitre plates are first coated with a
~heep anti-mou~e IgG (20 - ~0 ~g./ml. coating buffer,
one houx to overnight, 37C.). Thereafter, further
treatment i8 carried out a~ describ2d above, the sample
solution i8 add~d and again wa3hed. Finally, incubation
i3 carried out with 250 mU/ml. of an LH-peroxida~e
con~ugate for 1 hour at 37C. After again washing,
~ ~7;3;~
-13-
th~ peroxida~e activity i~ de~ermined, or example
wi~h ABTS~
~.
~
The procedure de3cribed in Exa~ple 2 i9 u~ed.
The reactivity of LH is fir~t determined. Then, to
the particular monoclonal antibodies, there iS3 in
each ca~eO added the antigens (hCG, TSH, FSH) to be
te~ted for cro~q-reaction, in increa~ing concentration.
~ he cros~-reactions are subsequently calculated
according to the following equation~
x 100 = % cro~-reaction
C (cros~-reac~ing antigen)
C = concentration of the antigen which 1~ necessary for
the achievement of 5~ of the maximum ~ignal
In the following Table 1 are ~et out the mea~ured
value3 for MAB 369 and MAB 799:
~ 3~;
.
-14--
Table
_ ~___ __ ___
glyco- origin max.conc. conc.range MAB 369 MAB 799
hormone ~Firm) in the in the te~t % cross % cro3s
~erum to cro~9- react. react.
__ ~_ . .. . . ....... ......... _
5 hCG UCB 200 0 - 250 ~ 0,l < Ool
~elgium IU/ml. IU/ml.
_~ __ ~ _~ . . .
FSH UCB 400 0 - 9 ~ O.l ~ O.l
. ~elgium mIU/ml. IU/ml.
____ ~_ __ ~ _
-TSH Boehringer lO00 0-9000 ~ O.l ~ O.l
Mannheim ~IU/ml. ~IU~ml.
_ ___ ~_~ _
~-TgH Boehringer lO00 0-9000 ~ O.l < O.l
MaTmheim ~, IU~ml. ,~IU/ml.
.. . . ~ , ~ ~ ~
Example 4.
~ ~ .A microtitre plate i~ coated for 2 hours at 37C.
or ove~night at 4C. with 10 ~g./ml. ~heep antibody
again~t the Fc~ region of a mou~e antibody in 0. 2M
carbonate buffer (pH 9.6). Thereafter, it i~ wa~hed
with PBS~0.1% Tween~20 (pH 7.35). Sub~equently, there
are added thereto 100 ~1. of a monoclonal antibody
(MAB 1~ ~onc~ntration lO ~g./ml.~ in incubation buffer
~PBS, 0.1% bovine serum albumin (BSA), 0.1% Tween 20)
and incubated for 2 hours ~t 37C.
An L~-peroxida~e conjugate (lO0 mU/ml.) i pre-
incubated overni~ht with lO ~g./ml. of a #econd mono-
trade mark
-15-
clonal antibody (MAB 2) in solution at 4C.
After the incubation of the plate with MA~ 1,
exce~ M~B 1 i~ removed by wa~hing with PBS-Tween 20.
The plate iA then after-coa~ed for 30 minutea at
ambient te~perature with 1% mou~e normal serum in
F~S-~SA. 100 ~1. of the pre-incubated ~AB 2/L~
peroxidase complex are applied to th~ plate and
incubat~d for 2 hours at 37C. ~he bound peroxidase
activity i~ made vi~ible with ~BTS ~8 ~ubstrate. The
mea~ured colour inten~ity i~ directly proportional to
~he MAB 2~LH-peroxida~e conjugate bound to MAB 1. For
MAB 799 as MAB 1 and MAB 369 as MAB 2, the bound
activity amounts to 34% of the non-competing LH-POD
activity.
The re~ults found show that the tw~ noclonal
antibodie~ are directed against various epitope3 of
~he antigen LH.
~52a~ -
Determination of LH
~)
13 _~L~ bY~S~:
15 mM sodium phosphate buffer, pH 7.4
15 m~ ~odium chloride
S n~ EDTA
0.~% bovine serum albumin, pH 7.4
5 mM o-nitrophenylgalactoside
-16-
2) Rece tor l solution~
A~ receptor 1, there i~ employed a monoclonai
mouBe anti-hLH antibody according to the pre~ent
invention. The ascite~ fluid containing thi~ antibody
5 i~ mixed ad 1.~ M with anmlonium gulphate. The precipit-
ate i8 taken up in a bu~fer of 15 mM sodium phosphate
(pH 7.0) and 50 mM sodium chloride. ~he solution ~o
obtained is ~ubjected to a pa~3age over D~A3-cellulo~e~
3) RecePtor 3 solution:
As receptor 3, there i3 also employed a monoclonal
mouse anti-hLH antibody according to the pre~ent
invention whi~h, however, recogni~es a dif~erent anti-
genic determinant than receptor l. ~he ascites fluid
containing thi 9 antibody i 9 purified as described in
2) aboveO m e complete antibody is split up in known
manner into the Fab fragment. The Fab frag~nt3
obtained are coupled wnth ~-galactosidase according to
the method of R.R. Porter (Biochem. J., 73, ll9/1959~.
43 Activated recePtor 2 olution:
Sheep anti-mou3e FC~ antiserum is mixed ad 1.8 M
wnth ammonium sulphate. The precipitate is taken up
in a buf~er of 15 mM sodium pho~phate (pH 7.0) and
50 mM sodium chloride. The solution ~o obtained i~
subjected to a pas~age over DEA~-cellulose.
B) Prod
1) ~L~
3~:3
_17-
40 ~1. of a solution which contain~, per ~.
100 ~mol sodlum phosphate (pH 7.3, 37 ~.),
2 ~mol magne~ium chloride,
O.9% sodium chloride,
.5 0.~ bovine ~erum albumin,
5 ~g.~ml. anti-hLH monoclonal antibodie3
from u~e ~receptor 1 ~olution)
100 mU anti-hLH antibody (mou~e) Fab ~ragment-
~-galacto~idase conjugate
( receptor 3 ~olution 3
i~ applied dro~wise to a fleece which ~on i~t~ of
commercial polyester paper. Sub~equen~ly, it i~ dried
at ambient t~mperature. Until used, the~a fleece are
3tored at 4C. and at a relative atms~spheric humidity
of 209~.
~) ~D~ '
Sheep antibodies againAt the Fc'~ part of mou~e
antibodies (activated receptor 2 colution) are fixed
on to cellulose fleece according to the known cyanogen
bromide activation proces~ (see Federal Republic of
Germany Patent Specification No. 1768512), ~ereby,
per g~ of fibre material, there are provided 10 ~g.
of ~ntibody for f ixiny . Uncoupled antibody i 9 removed
by washin~ and the fleece iB gently dried at ambient
temperatuxe~ The 50 obtained 1eece i~ 3tored
analogou~ly to r~gent carrier 1.
L2~73~
- 18 -
The determination with the help of these two re-
agent carriers l and 2 is further described with
reference to Figure l and takes place with the use of
the device for carrying out analytical determinations
described in Federal Republic of Germany Patent Speci-
fication No. 34250080 This describes a rotor insert
element for centrifugal automatic analysers comprising
a formed body which contains a sample application
chamber, which is in connection with a plurality of
reagent fields, each of which contains an absorbent
carrier material impregnated with a particular reagent,
at least one mixing valve chamber and a measurement
chamber which together form a sample liquid transport
path which leads from radially inwardly to radially
further outwardly when the insert element is fixed on
the rotor and also has at least one further chamber for
the reception of a liquid and a transport path which
leads from this chamber to the measurement chamber and
is at least partly identical with the sample liquid
transport path. With reference to Figure l the sample
liquid transport path thereby leads from a sample
application chamber (Sc) via a chamber (a) filled with
absorbent material containing buffer, a chamber (c) and
a first valve chamber (VCl) arranged between the chambers
ta)- and tc), to a second valve chamber tVC2) and from this,
_ a chamber (d) and via a collection chamber (AC),
to a measurement chamber (M). For the reception of a
further liquid, there is provided a substrate chamber
.. . .
73;306
-- 19 --
(PC) constructed as pump chamber which is connected
with the second valve chamber (VC2) via a dosing device
comprising a dosing chamber (DC) and a capillary (Cap),
and an overflow chamber (OC). In operation reagent
carrier 1 is placed on field c of the disposable insert
element and reagent carrier 2 in field d. 40~1. of
sample are thereby pipetted through an opening on the
upper edge directly on to the field a. The sample is
undiluted. 270 ~1. of substrate solution are pipetted
into chamber PC. By means of an appropriate programme
where high speeds of rotation alternate with stopping,
sample and substrate solution are then conveyed in the
- direction of the separation matrix and cuvette. In
the course of the programme, the receptors 1 and 3 are
thereby eluted by the sample liquid from the field c
and the homogeneous mixture is subsequently brought to
reaction. On field d, the complexes formed are bound
to the receptor 2. The tran~fer of the sample from
field c to field d takes place within a very short
period of time.
The substrate solution is divided into portions
by the dosaging chamber DC, the first of which serves
for washing out excess, non-complexed conjugate.
The ~-galactosidase activity bound to d via
complex formation is proportional to the amount of hLH
contained in the sample. This activity is determined
~'~7~3~
-20-
with a further substrate portion, the substrate
thereby being reacted in a 5 minute reaction to give
coloured products. The colour formed is measured in
the cuvette at 410 nm.
A chamber (b) connected to chamber (a) and
first valve chamber (VCl) is optional and extends the
area of use of the insert element.
The insert element further includes an entry S _
to substrate chamber PC.
The calibration curve according to Figure 2 of
the accompanying drawings is obtained from calibra-
tion sera of known LH content which cover the range
from 0 to 25 mU hLH/ml. (standardised according to
the first IRP standard for hLH 68/40) and makes
possible a sufficiently sensitive measurement of hLH
in serum or plasma. On the basis of this calibration
curve, there can be determined the unknown content of
hLH in body fluids, for example serum or sample. The
finding again of standards made up with 100 IU/ml.
hCG (lst IRP = 1st international reference prepara-
tion of WHO) amounts to 96 to 104~.
~q
~;~'73;~
The ordinate in Figure 2 xepresents the extinc-
tion which is measured in a cuvet-te at 410 nm accor-
ding to Example 5; the ex-tinc-tion has no units but is
based on the Beer-~ambert Law:
S E log _o
in which E is the extinction, Io is the incident
intensity of light and I is the intensity of emerging
light. E varies between 0 and 1; if E is 0 there is
no interference between the sample in the cuvette and
a light beam, whereas if E is 1 no light passes
through the sample.
Example 6.
Determination of LH according to the sandwich prin-
cipl~ ~
100 ~ul. of a solution which contains a mono-
clonal antibody against LH according to the present
invention in a coating buffer (0.2 M sodium
carbonate/bicarbonate, pH 9.4) in a concentration of
50jug./ml., are introduced into each recess of a
microtitre plate and incubated for one hour at
ambient temperature. Subsequently, it is after-
coated with incubation buffer (1% bovine serum
albumin, 0.9% sodium chloride) and
-22
ineubated for 30 mlnute~ at ambient temperature. After
washing with wa~h buffer (0.9YO ~odium chloride, 0.1%
Tween 20), there are introduced into each recesR
100 ~1. of ~ample which contains the hH to be deter-
mined and incubated for 30 mlnutes at ~bient temper-
ature. After again wa~hing with wash buffer, it i~
loaded with 100 ~1. of a conjugate o peroxida~e
(activity 100 mU~ml.~ and a further monoclonal antibody
according to the pre~ent invention which, however, i~
directed against a different epitope of ~ and incubated
for 1 hour at ambient temperature.
For the preparation of the conjugate, there i B
used horseradish peroxida~e (EC 1.11.1.7). The con-
jugate i8 prepared by oxidation with periodate and
Qub3equent reduction with boron hydride according to
the procedure of P.K. Nakane (M.B. Wil~on and
P.X. ~akane, in W. Knapp ed. "Immunofluorescence and
Related Staining Techniques", 1978, Elsevier/North
Holland, Biomedical Press, pages 215 - 224).
After wa~hing with wa~h buffer, it i~ loaded with
100 ~1. ABTS sub~trate solution and, after a one hour
colour reaction~ the extinction i~ measured at 405 nm
in an ELISA reader (~ynatec).
For the production of a calibration curve, in
~ 25 the case of the above-described proces~, instead of
t the ~ample~ olutions are used which contain LH in
different, definite concentrations.
* trade mark
-23
The Patent publications referred to herein
are more fully identified below:
Fed. Rep. of Germany Patent 1,768,512,
published (Offenlegungstag) October 14, 1971,
R.E.A.W. Axen et al, Pharmacla AB.
Fed. Rep. of Germany Patent Publication
~Offenlegungsschrift) 3,425,008 filed July 6, 1984,
- published (Offenlegungstag) February 6, 1986, Sigmar
Klose et al, Boehringer Mannheim GmbH.
U.K. Patent Application 2,111,201, filed
December 9, 1982, published June 29, 1983, W.M. Hunter
et al, Celltech Limited.
~ ' ~"`' '' , ,'