Note: Descriptions are shown in the official language in which they were submitted.
~73~3~
Compounds useful as starting compounds in the preparation
o tetrahydrophthalimides
The present application has been divided out of
Canadian Patent Application Serial No. 575,317 filed August
l9, 1988. That application was divided out of Canadian
Patent Application Serial No. 487,286 filed July 23, 1985.
This invention relates to compounds useful as
starting compounds in the preparation of tetrahydro~
phthalimides.
It is known that certain kinds of tetrahydro-
phthalimides are useful as herbicides. For instance, the
herbicidal use of 2-(4-methoxyphenyl)-4,5,6, 7-tetrahydro-
2H-isoindole-1,3-dione is disclosed in U.S. Patent No.
3,878,224. However, the herbicidal effect of these
compounds is not always satisfactory.
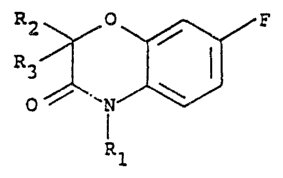
According to the invention there is provided a
lS compound of the formula:
2 ~ 0 ~ F
O `l
R
wherein Rl is a hydrogen atom, a Cl-C5 alkyl group,
a C3-C4 alkenyl group, a C3-C4 alkynyl ~roup, a
Cl-C4 haloalkyl group, a C3-C4 haloalkenyl group,
a C3-C4 haloalkynyl group, a Cl-C2 alkoxy
33;~
(Cl-C2) alkyl group or a C1-C2 alkoxy (Cl-C2)-
alkoxy(Cl-C2)alkyl group and R2 and R3, which may
be the same or different, each represents a hydrogen atom,
a halogen atom, a C1-C3 alkyl group or a phenyl group.
These compounds are useful in the preparation of compounds
of the formula (I):
( R ~ n ~
I (I)
Rl
wherein Rl, R2 and R3 are as defined above, X is a
hydrogen atom, a chlorine atom or a fluorine atom, n is an
integer of 0 or 1 and A is an amino group or a nitro group.
The term "halogen" used herein includes chlorine,
bromine and fluorine.
An amino compound of the formula (I), i.e. a
compound wherein A is an amino group, can be reacted with
a 3,4,5,6-tetrahydrophthalic anhydride in a solvent at a
temperature of 80 to 200C for a period of 1 to 24 hours
to obtain a compound of the formula:
~ ~ n ~ ~ (II)
I
3;~ 3
~herein ~, R3, X and n are as defined above.
In the reaction, the 3,4,5,6-tetrahydrophthalic anhydride is
used in a~ amount of 1 to 3 equivalen's to 1 equi~alent of
the amino compound (I) . Examples of the solvent are
aliphatic hydrocarbons (e.g. hexane, heptane, ligroin),
aromatic hydrocarbons (e.g. benzene, toluene, xylene),
ethers (e.g. diisopropyl ether, dioxane, ethylene glycol
dimethyl ether), ratty acids (e.g. formic acid, acetic acid,
propionic acid), water, and mixtures thereof.
After completion o~ the reaction, the reaction
mi~ture is subjected to ordinary post-treatment. For
instance, the reaction mixture is, if necessaxy, admixed
with water, and the precipitated crystals are collected by
filtration. Alternatively, the reaction mixture is op-
tionally admixed with water, followed by solvent extraction
or concentration. Further, if necessary, the purification
by chromatography or recrystallization mav be applied.
It has been eound that the tetrahydrophthalimideS
o~ formula ~II) show a high herbicidal activity against a wide
variety of weeds including broad-leaved weeds, Graminaceous
weeds, Commelinaceous weeds and Cyperaceous weeds in agricul-
tural plowed field by foliar or soil treatment without produc-
ing any material phytotoxicity on various agricultural crops
such as wheat, barley, corn, soybean and peanut. Examples of
broad-leaved weeds are wild buckwheat ( ~ convolvulus),
ladysthumb (P lyqonum persicaria), pale smartweed (~yg~
lapathiEolium), common purslane (Portulaca oleracea), common
3;~;3'3
chickweed (Stellaria media), common lambsquarters ( enopodium
album), redroot pigweed (Amaranthus retro~lexus), radish
(Raphanus sativus)~ wild mustard (Sinapis arvensis), hemp
sesbania (Sesbania exaltata), sicklepod (Cassia obtusi~olia),
velvetlea~ (Abutilon theophrasti), prickly sida (Sida spinosa~,
field pansy (Viola arvensis), catchweed bedstraw (Galium
aparine), ivyleaf morningglory (Ipomoea hederacea), tall
mocningglory (Ipomoea purpurea), henbit (Lamium am?lexicaure),
jimsonweed (Datura stramonium), black nightshade (Solanum
~ ), persian speedwell (Veronica pe~sica), common cocklebur
(Santhium pensylvanicum), common sunflower (Helianthus annuus),
scentless chamomile (Matricaria erforate), pineappleweéd
(Matricaria matricarioides), oxeye daisy (ChrYsanthemum
leucanthemum), corn marigold (ChrYsanthemum seqetum), sun
spurge (Euphorbia helioscopia), etc. ~xamples o~ Graminaceous
weeds are Japanese millet (Echinochloa frumentacea), barnyard-
grass (Echinochloa crus-qaLli), sicklepod (Cassia obtusifolia~,
large crabgrass (Digitaria sanquinalis), annual bluegcass -
(Poa annua), blackgrass (AloPecurus myosuroides), oats (Avena
sativa), wild oats (Avena fatua), johnsongrass (Sorqhum
halepense), etc. Examples o~ Commelinaceous weeds are asiatic
dayflower tCommelina communis), etc. Examples of Cyperaceous
weeds are yellow nutsedge (CY~ esculentus), etc.
Upon the pre-emergence soil treatment, the tetrahy-
2; drophthalimides (II)exhibit a particularly strong herbicidal
activity against broad-leaved weeds such as catchweed bedstraw,
common chickweed, ield pansy, persian speedwell, scentless
.
"3
-- 5
chamomile, pale smartweed, ladysthumb, wild mustard,
pineappleweed, oxeye daisy, common lambsquarters, black
nightshade, field bindweed and redroot pigweed in the
field of wheat or barley while exerting no or little
chemical injury to wheat or barley; they also exhibit a
marked herbicidal activity against broad-leaved weeds such
as velvetleaf, common cocklebur, tall morningglory,
sicklepod, prickly sida, jimson weed, hemp sesbania,
redroot pigweed, common lambsquarters and black nightshade
in the field of soybean or peanut while exerting no or
little chemical injury to soybean or peanut.
Further, some of the tetrahydrophthalimides (II)
of the invention are effective in exterminating paddy
field weeds including Graminaceous weeds such as
barnyardgrass (Echinochloa oryzicola) and broad-leaved
weed such as commonfalsepimpernel (Lindernia ~
indian toothcup (Rotala indica) and waterwort (Elatine
triandra) without any phytotoxicity to rice plants on
flooding treatment.
Among the compounds of the invention, preferred
are those wherein Rl is a Cl-C4 alkyl group, a
C3-C4 alkenyl group, a C3-C4 alkynyl group, a
C3-C4 haloalkynyl group or a Cl-C2 alkoxymethyl
group and R2 and R3 are each a hydrogen atom, a methyl
group or an ethyl group. More preferred are those wherein
Rlis a Cl-C3 alkyl group, a C3-C4 alkenyl group,
a C3-C4 alkynyl group or a halopropynyl group. Most
preferred are those wherein R2 is a hydrogen atom or a
methyl group and R3 is a hydrogen atom, especially when
Rl is a C3-C4 alkenyl group or a C3-C4 alkynyl
group.
3~3
- 5a -
Specific preferred tetrahydrophthalimides of he
formula (II) are 2-[4-(2-propynyl)-2H-1,4-benzoxazin
-3(4H)-on-6-yl]-4,5,6,7-tetrahydro-2H-isoindole-1,3-dione,
2-[2-methyl-4-(2-propynyl)-2H-1,4-benzoxazin-3(4H)-on-6-yl]
-4,5,6,~7-tetrahydro-2H-isoindole-1,3-dione, 2-[7-fluoro-4-
(2-propynyl)-2H-1,4-benzoxazin-3(4H)-on-6-yl]-4,5,6,7-
tetrahydro-2H-isoindole-1,3-dione, 2-[7-fluoro-2-methyl-4-
~2-propynyl)-2H-1,4-benzoxazin-3(4H)-on-6-yl]-4~5,6,7,-
tetrahydro-2H-isoindole-1,3-dione, etc.
Practical and presently preferred embodiments for
production of the compounds of the invention, compounds of
the formula (I) described in Canadian Patent Application
Serial No. 575,317, as well as the tetrahydrophthalimides
of the formula (II) described in the parent application,
Canadian Patent Application Serial No. 487,286 are
illustratively shown in the following examples.
~.~7333~3
( Example 1
A mixture of 6-amino-4-(2-propynyl)-2H-1,4-benz
oxazin-3(4H)-one (0.8 g), 3,4,5,6-tetrahydrophthalic an-
hydride (0.61 g) and acetic acid (20 ml) was heated under
reflux for 2 hours. After being allowed to cool, water was
added to the mixture, and the precipitated crystals were
collected by filtration and washed with water. Recrystal-
lizat~on from ethanol gave 2-~4-(2-propynyl)-2H-1,4-benz-
oxazin-3(4H)-on-6 yl]-4,5,6,7-tetrahydro-2~-isoindole-1,~-
dione (0.4 g). m.p., 205 - 206C.
l~-NHR (CDC13) ~ ppm: 1.8 (4H,-m), 2.2 (lH, t),
2.4 (4~, m~, 4~6 (2H, s), 4.62 (2~, d), 7.0 - 7.3 (3H, m).
Example 2
A mixture of 6-amino-7-fluoro-4-(2-propynyl)-2H-
1,4-benzoxazin-3(4H)-one (0.31 g), 3,4,5,6-tetrahydro-
phthalic anhydride (0~28 g) and acetic acid (3 mL) was
heated under reflux for 2 hours. After being allowed to
cool, water was added to the mixture, which was then
extracted with ethyl acetate. The organic layer was washed
with wzter, neutralized with sodium bicarbonate solution,
dried and concentrated. The residue was purified by silica
gel thin layer chromatography using a mixtuxe of ethyl
acetate and hexane (1 : 2) as an eluent to give 2-[7-
fluoro-4-(2-propynyl)-2H-1,4-benzoxazin-3(4H)-on-6-yl]-
4~5~6~7-tetrahydro-2H-isoindole-l~3-dione (0.12 g). m.p.,
196.0C.
lH-NHR ~CDCl3) ~ ppm: 1.81 (4H, m), 2.4 (4H, m),
2.53 (lH, t), 4.62 (2H, s), 4.73 (2H, d), 6.88 (lH, d, J =
73
10 Hz), 7.04 (lH, d, J - 6 Hz).
Exam~le 3
~ mixture of 5-amino-3-(2-pr~opynyl~-3H-benz-
oxa~ol-2-one ~0.50 g), 3,4,5,6-tetrahydroph~halic znhydride
(0.53 g) and acetic acid (5 ml) W25 heated a~ 100 to llO~C
under refLux for 3 hours. After being allowed to cool,
water was added to the mixture, which was then extrac.ed
with ethyl acetate. The organic layer was washed witn
water, neuralized with sodium bicarbonate solution, dried
and concentrated to give 2-t3-(2-propynyl)-3X-benzoxazol-
2-on-5-yl]-4,5,6,7-tetrahydro-2H-isoindole-1,3-dione (0.30
g). m.p., 285.5C.
lH-NHR (CDC13) ~ ppm: 1.8 (4H, m), 2.22 (lE, t),
2.4 (4X, m), 4.60 (2H, d), 7.0 - 7.3 (3H, m).
In the same manner as above, the tetrahydrophthal-
imides ~s shown in Table 1 were obtained.
~7;~
-- 8 --
~ .
1-- 0 ~ ~I cn (n ~ W N 1' 3 ~
l :J
:~ ~ ~3
~ ooooo ~ \~\~ ~
~--Z o
~ 1 ,_ ~
~ ,., ., ' 8 8 (' \~
W ~ 3: ~ ~ ~ ~ ,
3 ~ W ~ ~ , , , ~=~
~ X
. o~ ~o
, 3 ~ , ~
~ ~ ~ x
3 3 3 3 3 ~3 3 3 3 ~1 3
~:1 ra ~ ~ ra '~J ~ ~:
. . . . . . . . . . .
w ~ ~n ~ ~ u7 co pJ
W O 0 1-- 1~ ~ Ul ~_
I I . I I ~ I I I .
~ ~ O ~ ~ ~ n
,~ ~ I ~ o ~ ~ 9 0 O
C~
. o . ~n o o o o o o
~ ~o o ~
. _ . _
~ ~7~.3;3~
_ , _ . Z .,
NIV ~ ~ ~ 1~ ~ O O
n ~ W t~ ' 0 ~1:7 CO ~IJl ~ W N ~0
~ C
) . ~
2~ N
3 Ul 11 ~ O ~Jl O
I O ~ O
:~
~1
_:: X
~n w w ~
W~
x
3 3 3 3 3 3 3 3 3 3 3 3 3 3 U:~ 3
1 ~ ~ p, ra s :~
. . ~ Ul Ul ~
~ .
w ~ o ~ w
,~ I w cn ~ ~o O ~ o
I~ o ~ ~
I I o ~I o ~I o ~ ~ ~n I o c,~ ~n O
~ ~ ~ :~
O u~ o o o C~ o o Ul
Vl ~ ~ Uol ~ ~o ~ ~o
, ~ :~
o - - - -
73;~
-- 10 --
'
(
W W W ~ W ~ W W W t~ 0 3
.` .
. ,
,~ ~
~ ~ Q ~ n
' `~ ~' b ~' b '~ ~
~, ~, ~, ~, ~,
$ ~ ~ w~ ~ :~
$ ~, W W ~
X
3 3 ~ 3 3 3 ~ 3 3 kl O
. . . ~ ~ 5 :~
aw ~ L~ r~,
W W C~ W ~1 C
I ~ Ul ~ _,
~ o I O
W ~ O l-
, ~
n ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ O g
o ~ o ~o ~
~ ~c
. ~ ~
w ~ n
llt ~ ~) N
~ n ~ n
~ ~ _ N ~ I_
n ~
n
~ 1
~ X ~ ~
.
~: ~ t ~ t ~ t x
-t 3 3 ~ 3 3 ;3 3t~3 . gN . 5 o
~n ~ ~ ,t
o ~ t_ 3
I~ N 1~ tl C
O D ~.Jl 1-'~I 1~ ~ 1-- ~1 1~1 tD
~I O ~ ~ N O ~ ' W I_ ~
. I . . . I IUl I ~1 1 _
I l_ O I I ~n I co ~ co ~ 47
I-- N ~ N ~ O O N 0' ~ 3
~1 o O U~ O N O tll
n
O O
73;~
- 12 -
The production of the amino compound is
summarized in the following scheme:
3~
- ~ 13 --
( ~2~C~X
O I ~Ta2
Rl f t /7 al ~ n - 1
retuc- \ %i~ (`7)
~2~~ a2~
O N 2 . O N N~2
Ri (~) reduc- ~ (XV)
Bl~r (V) ~
( ~D. ~ ~ =
O N N2 R O 0;~ NOz
~ IV)
~4Z
2 (2`'-e,c~ .Cl)~
,<~3~2' 2~2'
(VI) (VIII) (X)
SO~
~o~ s2~ 0 ~s t (~
~2N R4~ 02N ao~x
(VI~) (IX) ~ (~
~.~7;~
wherein Rl, R2, R3, X and n are each as defined above, X'
is a fluorine atom or a chlorine atom, Y is a halogen atom,
Z is a hydroxyl group, Q is a halogen atam, Rl is a Cl-C5
p, C3 C4 alkenyl group, a C3-C alkynyl grou
a Cl-C4 haloalkyl group, a C3 C4 haloalkenyl group, a
C3-C4 haloalkynyl group, a Cl-C2 alkoxy(C1-C2)alkYl group
or a Cl-C2 alkoxy(Cl-C2)alkoxy(Cl-C2)alkyl group and R4
and R5 are each a lower alkyl group.
The above conversions will be explained further
in detail below.
(1) Production of the compound (IV) from the
compound (III)
The compound (rv) is obtainable by reacting the
compound (III) with the compound ~V) in a solvent in the
presence of a dehydrohalogenating agent at a temperature of
0 to 80C, preferably of 10 to 30C. The amounts of the
compound (V) and the dehydrohalogenating agent are respec-
tively 1.0 to 1.5 and 1.0 to 1.5 equivalents to one equiva-
lent of the compound (III). Examples of the solvent are
aromatic hydrocar~ons (e.g. toluene, benzene), amides (e.g.
N,N-dimethylformamide), sulfur compounds (e.g. dimethylsul-
foxide), nitriles (e.g. acetonitrile), water, and their
mixtures. Examples of the dehydrohalogenating agent are
sodium hydride, sodium hydroxide, potassiu~ hydroxide, etc.
A~ter completion of the reaction, the reaction
mixture is subjected to ordinary post-treatment such as the
addition of water, extraction with an organic solvent and
~ ~73;3.~3
-- 15 --
concentration. If desired, any conventional purification
procedure such as recrystallization or chromatography ma~ be
adopted.
(2) Production of the compound from khe
compound (IV)
The compound is obtained by reducing the
compound (IV~ with 2.0 to lO equivaLents of iron in the
presence of an acid in a solvent (e.g. acetic acid, water,
alcohoi, tetrahydrofuran) at a tempera~ure of 20 to 100C.
~ fter completion of the reaction, the residue is
collected by filtration and extracted with an organic
-solvent. The extract is washed wi~h wa~er ana sodium
bicarbonate solution and concentrated. ~f necessary, the
reaction mixture may be purified by recrystallization or
chromatography as conventionally employed.
(3) Production of the compound (III)
i) Production of the compound (III) (n = 0; X =
H) from the compound (VI):-
The compound (III) (n = 0; X = H) can be producedfrom the compound (VI), i.e. 2-amino-4-nitrophenol, by ~he
method as described in J.Am.Chem.Soc., 71, 1265 (1949) and
J.Pharm.Sci., 53, 538 (1964).
ii) Production of the compound (III) (n = l; X =
~) from the compound (VI):-
The compound (III) (n = l; X - ~) can be produced
from the co~pound (VI), i.e. 2-amino-4-nitrophenoL, by the
method as described in Synthesis, 1982, 986.
iii) Production of the compound ~III) ~n = 0, X =
7~;33~
- 16 -
or Cl) from the compound (VII) through the compound
(VIII):-
The compound (VIII) is obtainable from the
compound (VII) by the method as described in J.Pharm.Sci.,
53, 538 (1964), and the resuLtant compound (VII) i5 ni~ratea
by 1.0 to 1.2 equivalents of 60 % nitric acid in 80 ~
aqueous sulfuric acid solution a~ a temperature of ~5 ~o 5C
to give the compound (III).
iv) Production of the compound (III) (n = 1, X =
F or Cl) from the compound (IX) through the compound (X):-
The cQmpsund (X) is ob~ained by reducing tne
compound (IX), which is produced by the method as described
in J.~m.Chem.Soc., 81, 94 (1959), with 2.0 to 10 equi~alents
of iron in the presence of an acid in a solvent (e.g. acetic
acid, water, alcohol, tetrahydrofuran) at a temperature of
20 to laOC.
After completion of the reaction, the residue is
collected by fiLtration and extracted with an organic
solvent. The extract is washed with water and sodium
bicar~onate solution and concentrated. If necessary, the
reaction mixture may be purified by recrystalllzation or
chromatography as conventionally employed.
The compound (X) thus obtained is subjected to
nitration with a mixture of sulfuric acid and nitric acid at
a temperature o -10 to 10C so as to selectively nitrate
the 6-pasition of the benzoxazine ring to give ~he compound
(III) ~n - l; X - F or CL). Sulfuric acid and nitric acid
are respectively used in amounts of one equi~alent to large
~ ~7..~ 3
- 17 ~
e~cess and of 1 to 1.2 equivalents ~o the compound (X). The
concentrations of sulfuric acid and of ni~-ic acid are
preferred to be 80 X and 60 %, respectiveiy.
After completion of the reaction, the reaction
mixture is poured into ice water~ and the precipitated
crystals are collectea by filtration and washed with water.
If necessary, any purification method suc:~ as recrystal-
lization or chromatography may be adopted~
(4) Production of the compound ~XII) from the
compound (XI)
The compound (XII) is obtainable from the compound
(XI) by the method as described in Rec.Trav.Chim., 76, 128
(1957) and J.Am.Chem.Soc., _ , 1555 (1943).
(5) Production of the compound (XIV) from the
compound (~II) through the compound (XIII)
The compound (XIV) is obtainable from the compound
(XII) through the compound (XIII) by the method as described
in Gazz.Chim.Italiana, 22, I, 242 (1892) and J.Am.Chem.SocO,
81, 94 (1959).
(6) Production of the compound (XV) from the
compound (XIV)
The compound (XIV) is reduced with 5 to 15 equi-
valent amounts of iron powder in a solvent at a ~emperature
of 60 to 150C to give the compound (XV). As the solvent,
there may be employed an aliphatic carboxylic acid (e.g.
acetic acid, propionic acid), if necessary, with an ester
(e.g. ~thyl acetate), an alcohol (e.g. methanol, ethanol,
isopropanol3 or water.
~ ~ ~73;3;3~3
- 18 -
After completion of the reaction, the reaction
mixture is subjected to ordinary post-treatment such as
extraction with an organic solvent and concen~ration. When
desired, purification by recrys~allization or chromatog_aph~
may be also adopted.
(7) ~roduction of the compound (n = 1) from
the compound (XV)
The compound (n = l) is obtainable by react-
ing the compound (XV) with 1.O to 2.0 equivalents of the
compound (V) in the presence of 1.0 to 1.5 equivalents of a
dehydrohalogenating agent in a sol~ent a~ a temperature of 0
.o 80C, preferably at 10 to 30C. Examples of the solvent
are aromatic hydrocarbons (e.g. toluene, benzene), amides
(e.g. N,N-dimethylformamide), sulfur compounds (e.g.
dimethylsulfoxide), nitriles (acetonitrile ), water and their
mixtures. As the dehydrohalogenatins zgent, there may be
employed sodium hydride, sodium hydroxide, potassium
hydroxide, etc.
After completion of the rea~-tion, the reaction
mixture is subjected to ordinary post-treatment such as
addition of water, extraction with an organic solvent and
concentration. When desired, purifica~ion by recrystal-
lization or chromatography may be also adopted.
The compounds (I), (III~, (IV) and (XV) are novel
and can be summarized by the formula:
73;~
- 19 -
R3~¦ ~ ~XVI)
wherein A is an amino group or a nitro group and R1, R2, R3
and X are each as defined above.
~ urtner, the compound (X) wherein X is a fluorine
atom is also novel and may be representable by the formula:
~ 2~ C)n ~ ~XVII)
wherein R2 and R3 are each as deLined above.
Typical examples for production of the starting
compounds are illustratively shown in the following
Examples.
Example 4
Production of the compound from the compound
(IV):-
Electrolytic iron (11.39 g) was suspended in 5 %aqueous acetic acid solution (22 ml) and heated to 80C. To
the suspension, a solution of 5-nitro-3-(2-propynyl)-3H-
benzoxazol-2-one (4.15 g) in acetic acid (20 ml) and ethyl
acetate (20 ml) was dropwise addeà, and the resultan~
mixture was stirred at 70C for 3 hours. After being
allowed to cooL, wa~er was added to the mixture, which was
then extracted with ethyL acetate. The extract was washed
3;~
20 -
with water and sodium bicarbonate solution, dried anà
concentrated to give 5-amino-3-(2-propynyL)-3Y~-~enzoxazol-
2-one ~1.95 g). m.p., 102.1C.
lH-NMR (CDC13) ~ ppm: 2.32 llH, t), 3.2 - 3.8
(2H, broad~, 4.47 (2~, d), 6.7 - 7.2 (3H, m).
Example 5
Production of the compound from the compound
(IV):-
A solution of 2-methyl-6-nitro-4-(2-propynyl)-2H-
1,4-benzoxazin-3(4H)-one (5 g) in acetic acid (50 ml) and
ethyl acetate (S0 ml) was dropwise added to a mixture of
iron powder (6 g~ and 5 % aqueous acetic acid (50 ml), and
the resultant mixture was heated under reflux for 1 hour.
After being allowed to cool, iron powder was removed by
filtration and the filtrate was admixed with water. The
aqueous layer was extracted with ethyl acetate, and the
extract was combined with the organic layer, washed with a
saturated sodium bicarbonate solution, dried and concen-
trated. The residue was crystallized from methanol to give
6-amino-2-methyl-4-(2-propynyl)-2H-1,4-benzoxazin-3(4H)-one
(2.2 g). m.p., 158 - 159C.
lH-N~R (CDC13) ~ ppm: 1.5 (3H, d), 2.Z (lH, t),
3.8 (2~, m, NH2).
~ xample 6
Production of the compound from the compound
(IV):-
Iron powder (l.05 g) was suspended in 5 ~ aqueousacetic acid (2.0 ml) and heated to 80C. To the suspension,
~.~7~t~ 3
a solution of 7-fluoro-6-nitro 4-(2-propynyl)-2H~ -benz-
oxazin-3(4H~-one (0.47 g) in acetic acid (l.9 ml) and ethyl
acetate (1.9 ml) was dropwise aaded, and the resultan~
mixture was hea~ed under reflux at 60 to 80C for 3 hours.
A~ter being allowed to cool, water a~d ethyl ace~ate were
added to the mixture. The residue was removed by filtr-
ation, and the filtrate was extracted wi~h ethyl acetate.
The extract was washed with water and aqueous sodium bi-
carbonate solution, dried and concentrated to give 6-amino-
7-fluoro-4-¦2-propynyl)-2H-1,4-benzoxazin-3(4~)-one (0.31
g). m~p. r 183 - 185C.
H-NMR (CDC13 ~ DMSO D6) o ppm: 2.60 (lH, t), 4.0
- 4.6 (2H, broad), 4.48 (2H, s), 4.58 (2H, d), 6.64 (lH, d,
J = 10 Hz), 6.70 (lH, d, J = 6 Hz).
In the same manner as above, there are produced
the compounds (III), of which typical examples are shown in
Table 2.
~'7;~t~ 3
-- 22 --
( Table 2
R2~ ~3~X
O N NHz
1 1
n R1 R2 R3 Physical constant
, ~
0 CH-CCH2- X m.p. 102.1~C
1 CX3 H H H m.p. 152-153.5C
1 C2H5 X H ~ m.p. 118-119C
1 C2H5 H X F m~p. 108.5-109.0C
1 C2H5OC~2- H H F m.p. 133~
1 CH-CCH2- H H F m.p. 183-185C
1 CH-CCH2- CH3- H H m.p. 158-159C
1 CH2CCH2- C~3- H F m.p. 154.7C
1 CH_CCH2- C2H5- H H m.p. 126.5-128C
1 CH-~CCH2- ~H3- C~3 H m.p. 89-91C
1 CH~CCH2- C2H5 H F m.p. 121-122.5C
1 CH-CCX2- C~3- CH3 F m.p. 106-107C
1 CH_CcH2- 3 7 H H m.p. 124.5-125.5aC
1 C~ 2 H H 8 m.p. 97.5-99.5C
C=C~
. /C=C\ H H H m.p. 137.5-138.5C
¦ H/C C~cl ~ F m.p. 133-134C
,
.~7~ 3
(Continued)
_ _ R2 R; X Physical constant ¦
~ H F m.p. 109.5-110.5C !
Cl/ Cl ~ j
1 /C~2~ m.p. 142-143C
. CH2=C~Cl
CH2=~ 2 H H F m.p. 108-109C
\Cl
l CX_CCH2- ~ ~ H m.p. 168-170C
1 CH3(Cl)C=CHCH2- H ~ ~ m.p. 113-115C
1 CH3tCl)C=CHCH2- ~ H F n24 0 1.5848
1 ClCH=CHCH2- H ~ ~ m.p. 102-104C
1 ClCH=CXCH2- H H F m.p. 138-139C
1 Cl2C=CHCH2 H H H resinous
1 Cl2C=CHC~2- H H F m.p. 81.5-82~C
1 BrC-CCH2- H ~ ~ m.p. 126.5-127.5C
1 BrC-CCH2- H ~ r m.D 139.5-140.5C
Example 7
Production of the compound (IV) from the compound
(III):-
A suspension of sodium hydride (a . 06 g) in N ,N-
dimethyl~ormamide (3 ml) was cooled to 0C. 7-Fluoro-6-
nitro-2H-1,4-benzoxazin-3(4H)-o~e (0.57 g) was added thereto
at O to 5C, and the resultant mixture was stirred for 30
minutes. Propargyl bromide ~0.35 g) was added to the
mixture, which was gradually heated to room temperature, and
~ 73;~3~
- ~4 -
the reaction was continued for 6 hours. After addition o~water, the resultant mixture was extracted with ethyl
acètate, and the extract was wzshed with water, dried and
concentrated. The residue was purified ~y silica gel thin
layer chromatography using a mixture o' ethyl acetate and
n-hexane (1 : 1 ) as an eluent to give 7-fluoro-6-nitro-
4-(2-propynyl)-2H-1,4-benzoxazin-3(4H)-one (0.47 g). m.p.,
109.1C.
l~_NMR (CDC13) ~ ppm: 2.42 (lH, t), 4.75 (2H, d),
4.79 (2H, s), 6.94 (lH, d, J = 10 ~z), 7.95 (lH, d, J = 6
~z) .
In the same manner as above, there are produced
the compound (IV), of which ~ypical examples are snown in
Table 3.
Table 3
( R ~ n ~ NO2 (IV)
Rl
_ _ .
n Rl R2R3 X Physical constant
0 CH3 H m.p. 120.8C
1 CH3 H H H m.p. 189-191C
1 C2H5 H H H m.p. 130-131~C
1 n-C3H7- H H H m.p. 72-73.5C
1 C2H5 H H F m.p. 131.6C
1 n-C3H7- ~ H F m.p. 103.7C
, ~
~.~73;~
- _ '1 5
( (Continued)
Rl Rz R3 X PhysLcal cons~ant ¦
. 1 ~ CH-CCH2- H H F m.p. 109.1C
1 C2H5OCH2 H H F m~p. 101.6C
1 CH-CC~2- c~3 H F resinous
1CH-CCH2- C2H5 ~ H n22.6 1 5626
1C~=CC~2- C~3- C~3 H n22.6 1 5630
1CH_CCH2- C2H5- ~ F n23.8 1 5661
1CH-CCX2- C~3- CH3 F m.p. 105.3C
lCH-CCH2- C3 7 H H m.p. 124.5-125.5C
1 CH=CCH2- ~ ~ Cl m.pO 157.4C
. 1CH-CCH2- ~ ~ ~ 24-2 1 5967
=C\ 2 H H H n24.2 1 5~19
. /C=C~ 2 H H F ~4-2 1.S926
1Cl\ c/cH2 H H H m.p. 97.5-99.5C
H/ \Cl
1H\C /CH2- H H F m.p. 125-126C
Cl/ \Cl
2 ~ H H. H m.p. 110-111C
Cl
CH2=C/ 2 H H F m.p. 106-107C
, \Cl
1ClCH2~H-CHCH2- H H H n26.4 1 6068
1ClCH2CH=CHCH2- H H F n26.4 1.5870
~"~7,,V,~;3;~3
- 26 -
( ~Continued~
~ ~ ~ R2 R3 X Physical cons~a~ ¦
-- _ -- , , . .. .
1 BrCH2CH=CHCH2- K H ~ m.p. 81~5-83C
1 BrC~2CH=CHCH2- H H F m.p. 10~-109C
1 CH3(cl)c=cHcH2- H H H m.p. 117-118C
1 CH3(cl)c=c~c~2- ~ ~ F m.p. 113 115C
1 ClCH=C~C~2- H H ~ m.p. 103.5-105C
1 ClCH=C~C~2- H ~ F m~p. 67-69C
1 C12C=CHC~2- ~ H H m.p. 80-82C
1 C12C=C~CH~- H H F m.p. 83.5-85.5C
l BrC_CCH2- H H H m.p. 115.5-117C
. 1 BrC-CC~2- H H F m.p. 152-153C
xam~le 8
Production of the compound (III) from the compound
(VIII):-
A solution of 6-fluoro-2(3H)-benzoxazolone (1.0 g)
in 80 % aqueous sulfuric acid (6.5 g) was cooled to 0 IO
5C, and 60 ~ nitric acid (0.8 g) was gradually added
th~reto at 0 to 5C. The resultant mixture was stirred at
the same temperature for 30 minutes and poured onto ice
water. The precipitated crystals were collected by filtr-
ation, washed with water and dried to give 6-fluoro-5-
nitro-2(3H)-benzoXazolGne (1.1 g). m.p., 175.4C.
lH-NMR (CDC13 + DMSO-D6) ~ ppm: 4.0 (lH, broad),
6.8 - 7.9 (3H, m)O
Production of the compound (III) from th~ compound
- 21 -
(X):--
A solution of 7-fluoro-2H-1,4-benzoxazin-3(4EI)-one
l2.0 g) in 80 % aqueous sulfuric acid (30 ml) was cooled to
0 to 5C, and 60 ~ nitric acid (1.6 g) was aradually added
thereto at 0 to 5C. The resultant mixture was stirred at
the same temperature for 30 minutes and poured onto ice
water. The precipitated crys~als were collected by filtr-
ation, washed with water and dried to give 7-fluoro-6-
nitro-2H-1,4-benzoxazin-3(4H)-one (2.1 g) as pale brown
c~ystals. m.p., 205.9C.
lH-N~R (CDC13 + DMSO-D6) ~ ppm: 3.2 (lH, broad),
4.62 (2H, s), 6.76 (lH, d, J = 10 Hz), 7.6 (1~, d, J = 6 Hz).
In the same manner as abové, there are produced
the compounds (III), of which typical examples are shown in
Table 4.
Table 4
0 // ~ ~ NO (III)
n ~ ~ R3 X Physical constant
_ .
0 F m.p. 175.4C
1 H H F m.p. 205.9C
1 C~3- H F m.p. 233.6C
1 C2H5- H H m.p. 138-139C
1 C~3- _ H m.p. 162C
~ ~,7~ 3~
- 28 -
( (Continued)
_ . . . _ . ._ . . ..
n R2 R3 X Physical constant
___ _ ~ .,_ ..... ... _ . _ ..... _ _
1 2H5 EI F m.p, 149-151C
1 CH3- ' CH3- F m.p. 134.5-136C
1 CEI3C~I2C~2- H H m.p. 165-157C
1 (CX3)2CH- H ~ m.p. 120~C
1 CH3CH2CH2 H F m.p. 164-165C
1 ~ H m~p. 148-150~C
1 ~ ~ F m.p. l35.5-137'C
.
Exam~le 10
Production of the compound (X) from the compound
(IX):-
Iron powder (36.42 g) was suspended in 5 ~ aqueousacetic acid (69 ml) and heated to 80C. To ~he suspension,
a solution of ethyl 5-fluoro-2-~itrophenoxyacetate (15.86 g)
in acetic acid (65 ml) and ethyl acetate (65 ml) was drop-
wise added, and the resultant mixture was heated at 60 to
80C under reflux for 3 hours. After removai of residue by
filtration, the filtrate was extracted with ethyl acetate.
The extract was washed with water and sodium bicarbonate
solution, dried and concentrated to give 7-fluaro-2H-1,4-
benzoxazin-3(4H)-one (6.82 g). m.p., 186.7C.
H-NMR (CDC13 ~ DMS0-D ) ~ ppm: 4.2 (1~, broad),
4.51 (2H, s), 6.5 - 7.0 (3H, m~.
In the same manner as above, there are produced
~ ~7~ '3
- 29 -
( the compo~lnd (X), of which typical examples are shown in
Table 5~
Table 5
R2 ~ ~ / F
R3 ~ ~ (X~
O ~
H
R2 R3 Physical constant
- ....... _.
~ H m.p. 186.7C
CH3 H m.p. 151.3C
C2H5 ~ M.p~ 121-123C
C~3 C~3 m.p. 133-134C
n C3~7 ~ m.p. 99-101C
E m.p. 153-155C
Example 11
Production of ~he compound (XV) from the compound
(XIV):
A mixture of iron powder (2.4 g) in acetic acid (1
g) and water (20 ml) was refluxed, and a solution of methyl
2,4-dinitrophenoxacetate (2.24 g) in ethanol (20 ml) and
ethyl acetate (10 ml) was dropwise added thereto. The
res~ltant mixture was stirred for 1 hour and concentrated
under reduced pressure to e~aporate ethanol. ~o the
residue, water and ethyl acetate were added, and extraction
was carried ~ut. The organic layer was dried and concen-
trated, and the residue was com~ined with ether. The
~ ~ t7 3~ 53
. ,
- 30 -
( precipitated crystals were collected by filtration to give
S-amino-2H-1,4-benzoxazin-3(4H)-one (1.1 g).
lH-NMR (DMSO-d6) C ppm: 4.1 (2~, m, ~H2), 4.4
(2H, s), 6.25 (lH, d, d), 6.3 (lH, d), 6.7 (lH, d), 10.3
(lH, m, -CNH).
Exam~le 12
Production of the compound from the compound
(XV):--
Sodium hydride (0.08 g) was suspended in dry
N,M-dimethylformamide (3 ml), and the suspension was cooled
to 0C. While stirring, 6-amino-2H~1,4-benzoxa~in-3(4H)-one
(0.5 g) was portionwise added to the suspension at 0C, and
the resultant mixture was stirred at the same tempeature for
30 minutes. To the mixture, propargyl chloride (0.25 g) was
dropwise added, and the mixture was stirred at room temper-
ature for 6 hours. Water was added to the reaction mixture,
which was then e~tracted with ethyl acetate. The extract
was washed with water, dried and concentrated to give
6-amino-4-~2-propynyl)-2H-1,4-benzoxazin-3(4H)-one (0.36 g).
m.p., 260.1~C.
~ ~73;~;~<:~
- 31 -
( On the practical usage of the tetrahydrophthal-
imides (II),they may be applied in any preparation form such
as èmulsifiable concentrates, wettable powders, suspensions,
granules, etc. in combination with conventional solid or
liquid carriers or diluents as well as sur~ace active agents
or auxiliary asents.
~ he content or the tetrahydroph~halimides(II) as
the active ingredient in such formulation form is usually
within a range of 0.05 to 90 ~ by weight, preferably of 0.l
to ~0 ~ by ~eight~
Examples of the solid carrier or diluent are
fine powders or granules of kaolin clay, a~tapulgite clay,
bentonlte, terra alba, pyrophyll-ite, talc, diatomaceous
earth, calcite, walnut powders, urea, ammonium sulfate and
synthetic hydrous silicate, etc. As the liquid carrier or
diluent, there may be e~emplified aromatic hydrocarbons
(e.g. xylene, methylnaphthalene), alcohols (e.g. iso-
propanol, ethylene glycol, cellosolve), ketones (e.g.
acetone, cyclohe~anone, isophorone), soybean oil, cotton
seed oil, dimethylsulfoxide, N,N-dimethylformamide, aceto-
nitrile, water, etc.
The surface active agent used for emulsification,
dispexsion or spreading may be any of the anionic and
non-ionic type of agents. Examples of the surface active
agent include alkylsulfates, alkylarylsulfonates, dialkyl-
sulfosuccinates, phospha~es of polyoxyethylenealkylaryl
ethers, polyoxyethylene alkyl ethers, polyoxyethylene
alkylaryl ethers, polyoxyethylene polyoxypropylene bLock
-- 32 --
copolymer, sorbitan fatty acid esters, polyoxyethylene
sorbitan fatty acid esters, etc. Examples of the auxiliary
agents include ligninsulfonates, sodium alginate, polyvinyl
alcohol, gum arabic, CMC (carboxymethyl cellulose), PAP
(isopropyl acid phosphate), etc.
Practical embodiments of the herbicidal composi-
tion according to the invention are illustratively shown in
the following examples wherein parts are by weight. The
compound number of the active ingredient corresponds to the
one in Table 1~
Formulation Example 1
Fifty par.s of Compound No. 1, 15 or 20, 3 parts
of calcium ligninsulfonate, 2 parts of sodium laurylsulfate
and 45 parts of synthetic hydrous silicate are well mixed
while being powdered to obtain a wettable powder.
Formulation Exam~le 2
Five parts of Compound No. 5, 9, 13 or 21, 14
parts of polyoxyethylenestyrylphenyl ether, 6 parts of
calcium dodecylbenzenes-llfonate, 30 parts of xylene and 45
parts of isophorone are well mixed while being powdered to
o~tain an emulsifiable concentrate.
Formulation Exam~le 3
Two parts of Compound No. 4, 10 or 20, 1 part of
synthetic hvdrous s~licate, 2 parts of calcium lignin-
sulfonate, 30 parts of bentonite and 65 parts o kaolin clav
are well mixed while being powdered. The mixture is then
kneaded with water, granulated and dried to obtain granules.
Formulation Example 4
7~ '3
- 33 -
( Twenty-five parts of Compound ~o. 2, 15 or 22 is
mixed with 3 parts of polyoxyethylene sorbitan ~onooleate, 3
parts of carboxymethyl cellulose and ~9 parts of water and
pulverized until the particle size of the mixture becomes
less than 5 microns to obtain a suspension.
Formulation ExamPle 5
Five parts of Compound No. 2, 5, 9, 13, 15, 16,
20, 21 or 25, 14 parts of polyoxyethylenestyrylphenyl ether,
6 parts of calcium dodecylbenzenesulfonate, 30 parts of
- xylene and 45 parts or N,N-dimethylformamide are well mixed
while being powdered to obtain an emulsifiable concentrate.
Formulation Exam~le 6
Eighty parts of Compound No. 2, 16, 22 or 50, 3
parts of calcium ligninsulfonate, 2 parts of sodium lauryl-
sulfate and lS parts of synthetic hydrous silicate are well
mi~ed while being powdered to obtain a wettable powder.
Formulation Example 7
0.1 Part of Compound No. 10 or 15, 0.9 part of
synthetic hydrous silicate, 2 parts of calcium lignin-
sulfonate, 30 parts of bentonite and 67 parts of kaolin clay
are well mixed while being powdered. The mixture is then
kneaded with water, granulated and dried to obtain granules.
The tetrahydrophthalimides (II) thus formulated in
any suitable formulation orm are useful for the pre-
emersenc2 or post-emergence control of undesired weeds by
soil or foliar treatment as well as flood fallowing
treatment. These treatments include the application to the
soil surface prior to or a~ter the transplanting or the
-- 3~ --
incorporation into the soil. The foliar treatment may be
effected by spraying the herbicidal composition containing
the tetrahydrophthalimides (II) over the top of the
plants. It may also be applied directly to the weeds if
care is taken to keep the chemical off the crop foliage.
The tetrahydrophthalimides (II) may be used
together with other herbicides to improve their activity
as herbicides, and in some cases, a synergistic efect
can be expected. Further, they may be applied in
combination with insecticides, acaricides, nematocides,
fungicides, plant growth regulators, fertilizers, soil
improvers, etc.
Furthermore, the tetrahydrophthalimides (II) can
be used as herbicides applicable to agricultural plowed
field as well as paddy field. They are also useful as
herbicides to be employed for orchard, pasture land,
lawn, forest, non-agricultural field, etc.
The dosage rate of the tetrahydrophthalimides
(II) may vary on prevailing weather conditions,
formulation used, prevailing season, mode of application,
soil involved, crop and weed species, etc. Generally,
however, the dosage rate is from 0.02 to 100 grams,
preferably from 0.50 to 50 grams, of the active ingredient
per are. The herbicidal composition of the invention
formulated in the form of an emulsifiable concentrate, a
wettable powder or a suspension may ordinarily be employed
by diluting it with water at a volume of 1 to 10 liters
per are, if necessary, with addition of an auxiliary
agent such as a spreading agent. Examples of the
- 35 -
spreading agent include, in addition to the surface active
agents as noted above, polyoxyethylene resin acid ~ester),
lig`ninsuLfonate, abietylenic acid salt, dinaphthylmethane-
disulfona.e, pzra.~~in, etc. The composition formulated in
the form of granules may be normally applied as such without
dilution.
The biological data of the tetrahydrophthalimides
tII) as herbicides will be illustratively shown in the
following Examples wherein the phytotoxicity to crop plants
and the herbicidal activity on weeds were observed visually
as to the degree of germination as well as the growth
inhihition and rat~d with an index 0, 1, 2, 3, 4 or 5, in
which the numeral n Ow indicates no material difference is
seen in comparison with the untreated plant and the numeral
"S" indicates the complete inhibition or death of the test
plants.
The compounds shown in Table 6 below were used for
comparison.
Table 6
Compound
No. Chemical structure Remarks
A O U.S. patent
ll 3,878,224
C 3 ~ ~
O
B ~Cl Commercially
Cl ~ ~ N02 hVr lcbde;
1 nitrofen"
~7;:~;3;~1~3
-- 36 --
( Compound
~lo. Chemical structure Remarks
.
C O Commercially
~S ¦l / H available
I /~ N-C-N ~ herbicide
1~ ¦ ~C~3 "metabenz
CH3 thiazuron"
O Commercially
~ ¦¦ / CH3 available
Cl / \~NH-C-N herbicide;
Cl ~ ~OCH3 ~linuron"
Test Example 1
Cylindrical plastlc pots (diameter, 10 cm; height,
10 cm) were filled with upland field soil, and the seeas of
Japanese millet, tall morningglory and velvetleaf were sowed
therein and covered with soil. A designed amount of the
test compound formulated in an emulsifiable concentrate
according to Formulation Example 2 or 5 was diluted with
water, and the dilution was sprayed onto the soil surface by
means of a small hand sprayer at a spray volume of 10 liters
per are. The test plants were further grown in a greenhouse
for 20 days, and the hexbicidal activity was e.Yamined.
The results are shown in Table 7.
3~3
-- 37 --
( ~able 7
Compound Dosage ¦ Herbicidal activi~y
No. (g/are) ~ -
! Japanese ~aLl morning- IVelvet- !
_ I millet glory ileaf
1 20 , 5 i 5 5
. 10 4 1 5 5
i 120 5 I 5 ' 5
3 20 5 5 5
4 5 , 5
4 20 5 5 5
, 5
7 20 55 5 5
9 lo 5 5 1 5
! lo 4 4 1 5
0 5 55 1 5
11 20 5 5 5
12 20 5 5 1 5
13 20 S 5 i 5
14 10 54 5 5
16 10 4 55 55
17 20 5 5 5
18 22o S 55 5
21 10 S 55 55
23 10 55 55 5
24 100 5 s55 5
27 250 55 45 5
28 20 4 4 5
29 200 - 5 ~ 5 S
- 38 -
( (Continued)
Compound Dosage Herbicidal activity
No. (g/are) ~
Japanese Tall morning- Velvet-
_ _ millet glory~ lea'
36 20 3 S 5
39 20 4 5 5
4 5 S
43 20 4 4 5
44 20 4 4 5
4 4 5
46 20 5 5 5
48 loo S 5 5
49 10 5 5 5
. . ~ _ _ _
. D I ~ ~ 1 ~ 2
Test Example 2
Cylindrical plastic pots ~diameter, 10 cm; height,
10 cm) were filled with upland field soil, and the seeds of
JapaAese millet, radish and velvetl~af were sowed therein
and cultivated in a greenhouse for 10 days. A designed
amount of the test compound formulated in an emulsifiable
concentrate accordinq to Formulation Example 2 or 5 was
diluted with water containing a spreading agent, and the
dilution was sprayed over the foliage of the test plants by
means of a small hand sprayer at a spray volume of 10 liters
per are. The test plants were further grown in the
greenhouse 'or 20 days, and the herbicidal activity was
examined.
The results are shown in Table a.
~73;~3~
( Table 8
¦ Compound Dosage Herbicidal activity
No. tg/are) _ ~
Japanese Radish Ve~vetlea~ !
! l millet
1 I S 5 ~ 5 ~ 5
3 ~ 5 4 ~S I 5
87 S 4 1 5 1 5
5 5 S 5 I S
2 5 4 1 55 - S
. 13 5 4 5 5
! 14 . 5 5 5 S
. 2.5 4 S 5
3~
~7~;33~
- 40 -
( (Continued)
, ~
Compound Dosage Herbicidal activity
No. tg/are) Japanese ¦ Radish I Velvetleaf !
millet
34 5 _ 4 1 5
38 5 4 5 5
1 39 5 5 5 5
! 4l 5 5 5 5
43 5 5 5 5
44 5 4 5 5
SO 5 _ 5 5
2.5 0 0 1
Test Exam~le 3
Cylindrical plastic pots (diameter, 8 cm; height,
12 cm) were filled with paddy-field 50il, and the seeds of
barnyardgrass (Echinochloa orYzicola) and broad-leaved weeds
(e.g. common falsepimpernel, indian toothcup, waterwort)
were sowed in 1 to 2 cm depth. Water was poured therein to
make a flooded condition, and rice seedlings of the 2-leaf
stage were transplanted therein and grown in a greenhouse.
Six days (at that time the weeds began to germinate) there-
after, a designed amount of the test compound formulated in
an emulsifiable concentrate according to Formulation Example
2 or S and diluted with water (5 ml) was applied to the pots
by perfusion. The test plants were grown for further 20
3~
days in the greenhouse, and the herbi~idal activity was
examined.
The results are shown in Table 9.
~able 9
Compound Dosage Herbicidal activity
No. (g/are) . _
Rice plant Barnyard- Broad-leaved
_ grass weed
~0 _ 5
1 ~ 5 5
24 10 O 5 5
A 10 1 ~ 2 2
Test Example 4
Vats (33 cm x 23 cm x 11 cm) were filled with
upland field soil, and the seeds o soybean, peanut, corn,
common cocklebur, tall morningglory, velvetleaf, redroot
pigweed, ~lack nightshade, barnyardgrass and green oxtail
were sowed therein to 1 to 2 cm depth. A designed amount of
the test compound formulated in an emulsifiable concentrate
according to Formulation Example 2 or S was diluted with
water, and the dilution was sprayed onto the soil surface by
means of a small hand sprayer at a spray volume of 10 liters
per are. The test plants were further grown in a greenhouse
7~ 3~
- 42 -
( for 20 days, and the herbicidal activity was examined.
The results are shown in Table 10.
~.~7 ~ s~3
,
-- 43 --
~ , ' ' o~ g I
~ ~ ~ ~ ~ ~ ~ . q
~ ~ W ~ ~- o C~ W ~-
_ . C~ ~ ~
... . ... ....... . t~ o~
U~ n ~ ~ ~D ~J I_
. ~ O
_ ,_ o~
OoOOOI-OOOOOOOOOOOOOOOO~ cr
_ _ _ ~
oooooo~oooooooooooooooIIo
, _ .
>~ ~ o ~ ~ o ~ ~ o
. _ _ __ 1--n g
I ~ ~ ~n I u~ I ~n ~ ~.n ~ Ul I ~ I ~A ~ ~n I I ~ I ~ ~n ~ C :~ 3
~ 0~o~ ~D-
~ Ul ~ ~n ~ ~n ~ ~n ~ ul U~ ~n ~ ~ ~ U- ~n ~n ~ ~ I ~ ~ ~n ~ O 1- n
l C~
l-: ~
~ ~ w ~:
- ~ - - 3- ~ ~
- - ~- -
, I ~ n O~
_ _ _ _. _ , ~ ~ Cl
~ - - ~ --
'3;~
-- 44 --
ol :- ~ z3
, _ ~ ~
N ~1 N /~1 N ~ ~tl 3 3
Ul ~,11 _ O ,0~
OOOC~ 0-0 ~=e,
o o o o o _ = n
~ tll 3
O O O O ~ ~A ~ O ~ I_ n
~. . =~c 1~
O ' O N U~ n 1--~ e C
O _ O ~ ~ ~n ~ e:
--O-- ~ n ~ 50 Sl
_ .__. . G~
0~0- ~n I r
000_ ~ ~ =
- 45
Test Exam~le 5
Vats (33 cm x 2~ cm x 11 cm) were filled with
upland field soil, and the seeds o~ wheat, barley, catchweed
bedstxaw, persian speedwell, common chickweed, common
lambsauarters, pale smartweed, wild buck~7heat and annual
bluegrass were sowed therein to 1 to 2 cm depth. A designed
amount of the test compound formulated in an emulsifiable
concen xate according to Formulation Example 2 or 5 was
diluted with water~ and the dilution was sprayed onto the
soil surface by means of a small hand sprayer at a spray
volume of 10 liters per are. The test plants were further
grown in a greenhouse fox 27 days, and the herbicidal
activity was examined.
The results are shown in Table 11.
-- 46 --
. __
~- :, ~ o ,- o a~ o v~ ~ ~
_ - - , . ~
. ~ . ~ O, , ~ ~ ~ O, ~ ~ O, ' O, ~ ,-' ~ ~, ~ ~ ~ ~ ~ ~ ~D
~n cn W ~ ~ ~n ~ u- Vl ~ Ul ~ ~n ~ ~n ~ ~ n ~ ,_
. _ _ _ _
0000 0001 Ol-OOC~ oocloo_o~ ~
. , .
O O O O O O O ~ I I O I ~ O O C~ O O r~ 1-
_ _ ~ n
0 0 0 0 I ~ V~ I VI ~ n
. _.___ . ~
0 0 0 0 U~ n VI ~ n ~ _
. _ _ _~ S ~ ~
O O O O ~ n Ul I Vl ~n ~n ~.n ~n ~ ~ ~ ~n I ~ ~ ~n Ul C~ ~ ~
_ _ . . ~ n n
. g ~
O O O ~ ~ n V~ n ~ ~ 'C
_ _ . _ _ _ .
000~ ~n~n~n~n~U1~7 1 ~n~nu~n~n ~ Dl
, _ _.
O O O O ul ~n vl ~n ;~ Ul ~ ~n ~n ~n ~ ~n ~ ~ ~ ~ ~n ~ ~ ~n ~ ~n ul a~ c
. _ _ _ _. 0
OOOO I~I~IulI~I~I~nIII~I~I~I~
._ .. . _. _
~7~
- ~7 -
( Test Example 6
Vats (33 cm x 23 cm x 11 cm~ were filled wi~h
upland field soil, and the seeds of corn, whea~, common
cocklebur, velvetleaf, black nightsnade, tall morningglory,
common lambsquarters and green foxtail were sowed therein
and cultivated for 18 days in a greenhouse. A designed
amount of the test compound formulated in an emulsifiable
concentrate according to Formulation Example 2 or 5 was
diluted with water containing a spreading agent, and the
dilution was sprayed over the foliage of the test plants by
means of a small hand sprayer at a spray volume of S liters
per are. The test plants were further grown in the green-
house for 20 days, and the herbicidal activity was examined.
At the time of the application, the test plants were
generally at the 1 to 4 leaf stage and in 2 to 12 cm height,
although growing stage of the test plants varied depending
on their species.
The results are shown in Table 12.
.. . ~
~. ~7.'~;~3~
-- 48 --
. ~ ~ t~
3o
~n ~ W ~ ~ O cr~ O Ul ~ i~ , r~a
O O O O O O O O O O O O O O O O O O O O O O O O O O ~ ~ ~D
~--W I--W I--W 1~ w I~ W 1~ W 1--W ~ W I--W I--' W ID tD
-. .~ _
I o I ~ 1 0 ~ 0 ~ I 11
~_ I o I l~I o~ I 3'
I~
Ul Ul U7 Ul Ul Ul Ul Ul I Vl ~ n IJ'I Ul u~ 11 1 ~ I Ul I ~n c ~ ~
_ ~1 1 0 ~
:
n U~ n ~ U~ Vl Ul ~n ~ m ol = ~D
n
~2 3 ~ t~
d=~ 3 3
l~ r~
-- 49 --
_. , O ~
tI1 :~ G
oo--~
W W ~ ~
. o
oooo
oooo ~ .
: o o
oooo
o~-oo ~ O
~ I Q
o~oo ~ ~
_ ~ 3 ~; ~:'
oooo LC3i- ~:
_l _ O
~00 ~D10
X ~
oooo ,' _
7 ~
- 50 -
( Test Example 7
Vats (33 cm x 23 cm x 11 cm) were filled with
up1and field soil, and the seeds of soybean, peanut, sic~le-
pod, hemp sesbania, velvetleaf, prickly sida, jimsonweed and
large crabgrass were sowed therein to 1 to 2 cm depth. A
designed amount of the test compound formulated in an
emulsifiable concentrate according to Formulation Example 2
or 5 was diluted with water, and the dilution was sprayed
onto the soil surface by means of a small hand sprayer at a
spray volume of 10 liters per are. The test plants were
further grown in a greenhouse for 21 days, and ~he herbi-
cidal activity was examined.
The results are shown in Table 13.
~7;~ 3
_ ~ O Ul 0 3
. __ o~ I'-c3
~n _ O ~0 ~
OC~ OC~C10~0 ~C
00 OOC~OOO ~
00 ~ ` :~ 3
00 =~'0 0'
00 (J~ /IU 3 1- 1''
O_ V~ Jl ~ `C
O ~- ~ ~3
_ . W ~
O O _ _ _ D
~.~73;~
- 5Z -
( Test Examele 8
Seeds of wheat, ca~chweed bedstraw, common chic~-
weed, persian speedwell and common l~mbsquarters were sowed
in the field as previously laid up in ridges and divided
into plots of 4 m~. A designed amount of the test compound
formulated into a wettable powder according ~o Formulation
Example 1 was diluted with water and sprayed onto the soil
surface by means of a small hand sprayer at a spray volume
of 7.5 liters per are. The application was made with three
replications. A~ter cultivation for 39 days, the herbicidal
activity on the weeds as well as the phytotoxicity on wheat
were evaluated as follows: the aerial parts of the test
plants were cut off and weighed (fresh weight) and growth
inhibition rate was calculated based thereon according to
the following equation:
Growth Fresh weight of test plants in
inhibition = ( 1 - treated plot _ ) x 100
rate (~) Fresh weight of test plants in
untreated plot
The results are shown in Table 14.
- 53 -
Table _14
_ -- ,
Compound Dosage Growth inhibition rate (%)
No~. (q/are)
Wheat Catch- Com~on Persian Common 1,
weed chick- speed- lambs- . j
bstraw weed well quartsrs
_
lS 2 8 9S 95 100 95
1 2 82 87 98 92
16 4 S 97 g7 100 100
2 1 89 83 10~ 95
2 9 98 100 100 100
1 4 93 98 100 9S
2 8 97 100 100 100
1 3 91 93 97 94
C 120 2 27 1 97 87 63
Test Exam~le 9
Seeds of soybean, peanut, redroot pigweed, velvet-
leaf, black nightshade and prickly sida were sowed in the
field as previously laid up in ridges and divided into plots
of 3 m2. A designed amount of the test compound formulated
into an emulsifiable concentrate according to Formulation
Example 2 or 5 was diluted with water and sprayed onto the
soil surface by means of a small hand sprayer at a spray
volume of 10 liters per are. The application was made with
two replications. After cultivation for 40 days, the growth
inhibition rate of the test plants was measured in the same
manner as in Test Example 8.
The resuLts are shown in Table 15.
1 ~7;~;~3~3
- 54 -
Table 15
Compound Dosage Growth inhibition rate
No. (g/are)
Soy- Peanut Redroo`t Velvet- Black Prickly
bean pigweed leaf night- sida
shade
I _ __.
2 3 7100 95 100 100
1 0 0100 75 100 100
0.5 0 0 90 26 93 65
2 3 8100 100 100 lOC
1 O O100 100 100 100
1 0.5 0 0 96 78 100 85
D 2 0 0 0 0 0 0
1 O O O O O O
O . S OO O O O O