Note: Descriptions are shown in the official language in which they were submitted.
~7~3'~;~
01 --1-
PHENOLPHTHALEIN POLYARYLATE POLYMERS AND AI,LOYS
05 BACKGROUND OE THE INVENTION
The present invention relates to polyarylate
polymers and alloys. More particularly, this invention
relates to phenolphthalein polyarylate polymers and alloys
having superior thermal properties.
Polyarylates are defined as aromatic polyester
polymers derived from dihydroxy aromatic compounds
(diphenols) and aromatic dicarboxylic acids.
In general, aromatic polyesters prepared from
bisphenols or functional derivatives thereof and a
terephtha]ic acid-isophthalic acid mixture or a mixture of
the functional derivatives thereof, i.e., bisphenol
terephthalate-bisphenol isophthalate polyesters, have
excellen' mechanical properties, such as tensile strength,
bending strength, bending recovery or impact strength,
~o excellent thermal properties, such as deflection temper-
ature under load or degradation temperature, excellent
electrical properties, such as resistivity, electric
breakdown endurance, arc resistance, dielectric constant
or dielectric loss and low flammability, good dimensional
2~ stability, and the like.
These aromatic polyesters are thus useful in may
fields. Aromatic polyesters find special use as plastics
for injection molding, extrusion molding, press molding,
and the like, as monofilaments, fibers, films and coatings.
U.S. Patent No. 3,216,970 describes polyarylates
which include polymers oE bisphenol A and isophthalic acid
or a mixture oE isophthalic acid and tereph-thalic acid.
These polyarylates are prepared by converting the p'nthalic
acid component to the diacid chloride which is then
reacted with the bisphenol A or its sodium salt.
U.S~ Patent No. 3,88~,990 describes a blend of
various bisphenol polyarylates and poly(ethylene oxybenzo-
ate), which is useful for producing molded articles having
improved cracking and crazin~ resistance. Similarly,
~0
,
~ ~:7~3
61936-17~5
U.S. Patent No. 3,946,091 describes a blend of bisphenol
polyarylates and poly(ethylene terephthalate) which provides
molded articles of reduced crazin~.
U.S. Patent No. 3,792,118 describes a styrene resin
composition resistant to heat deformation which comprises a
blend of polyarylene esters and various styrene resins.
SU~ItIARY OE' TH~ INV~N'l'lON
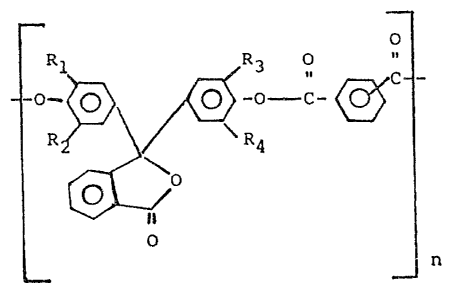
The present invention provldes a polyarylate of the
formula
O
~~ ~n
wherein R1, R2, R3 and R4 are independently lower alkyl of 1 to
4 carbon atomss n is the degree of polymerization and wherein
~0
O ..
_c_~
is an lsophthalic or terephthalic acid moiety present in a
molar ratlo ol 9-1 to 1,9, respectively.
The present lnvention addltionally provides a
polyarylate copolymer derived ~rom
~A) a mixture of a phenolphthalein compound of the
formula
~ '
~7~
61936-1745
Rl ~,R3
HO~ )\R4 H
(~
o
wherein Rl, R2, R3 and R4 are independently lower alkyl of 1 to
4 carbon atoms; and a bisphenol compound of the formula
R5 CH3 /~7
~~ ~ C~ ~ -OH
CH3
R6 R8
whereln R5, R6, R7 and R~ are independently hydrogen, lower
alkyl of 1 to 4 carbon atoms or phenyl; and wherein the molar
ratio of phenolphthalein to bisphenol is from 20:1 to 1:20; and
~ B) a mixture of isophthalic and terephthalic acid in a
molar ratio of 9:1 to 1:9, respectively.
The present invention is further concerned with
polyarylate alloy compositions. In the polyarylate alloy
composition the radicals Rl, R2, R3 and R4, in addition to
being lower alkyl of 1 to 4 carbon atoms, can also be hydrogen
or phenyl, provided that Rl, R2, R3 and R4 may not all be
hydrogen. Thus the invention provides polyarylate compositions
comprising
(A) 10 to 90~ by welght of a polyarylate o~ the formula
~ R3 o ''
_ ~0~ 0--C- @5' -
~ ~0
O
l n
IB
01 _4_
wherein Rl, R~, R3 and R4 are independently hydrogen,
lower alkyl of 1 to 4 carbon atoms or phenyl; provided
05 that Rl, R2, R3 and R4 may not all be hydrogen; n is the
degree of polymerization; and wherein
-C-~
is an isophthalic or terephthalic acid moiety present in a
molar ratio of 9:1 to 1:9, respectively; and
(B) 10 to 90% by weight of a polymer res.in selected
from the group consisting of polybisphenol A carbonate and
polystyrene.
Also contemplated by the present invention is a
polyarylate alloy composition comprising
(A) 10 to 90~ by weight of a polyarylate copolymer
derived from
~U (1) a mixture of a phenolphthalein compound of
the formula
Rl R3
HO~ ~ ~o~ -ROH
wherein Rl, R2, R3 and R4 are independently hydrogen,
lower alkyl of 1 to 4 carbon atoms or phenyl; provided
that Rl, R~, R3 and R4 may not all be hydrogen;
~nd a bisphenol compound o~ the formula
R5 R7
/ CH3 ~
R6 R8
~0
~L~7~3'~
01 _5_
wherein R5, R6, R7 and R8 are independently hydrogen,
lower alkyl o-f 1 to 4 carbon atoms or phenyl; and wherein
05 the molar ratio of phenolphthalein to bisphenol is from
20:1 to 1:20; and
(2) a mixture of isophthalic and terephthalic
acid in a molar ratio of 9 1 to 1:9, respectively; and
(B) 10 to 90% by weight of a polymer resin selected
from the ~roup consisting of polybisphenol A carbonate and
polystyrene.
Amon~ other factors, the present invention is
based on the discovery that certain polyarylate polymers,
deri~ed from substituted phenolphthalein compounds or
mixtures of these compounds with other bisphenols, have
been found to possess superior thermal properties. In
addition, it has been ~ound that the above-described
phenolphthalein polyarylates provide alloy compositions
with polystyrene and polybisphenol A carbonate which also
exhibit excellent thermal properties.
DETAILED DESCRIPTION OF THE INVENTION
For purposes of the present invention, those
polyarylates derived from a single phenolphthalein
compound shall be referred to as "homopolymers" and those
polyarylates derived from a mixture of phenolphthalein and
other bisphenol compounds shall be referred ta as
"copolymers". It is, of course, understood that phenolph-
thalein may be characterized as a type of bisphenol
compound. Furthermore, -the term "alloy" as used herein is
meant to define an intimate physical mixture or blend oE
two or more polymers.
The substituted phenolphthalein compounds which
are useful for conversion into the instant polyarylate
homopolymer5 and copolymers may be represented by the
~ollowin~ ~ormula
~7~3
Ol 6-
Rl /R3
OS HO- ~ ~ -OH
2 ~ / \R~
~/
wherein Rl, R2, R3 and R4 are independently hydrogen,
lower alkyl of 1 to 4 carbon atoms or phenyl; provided
that Rl, R~, R3 and R4 may not all be hydro~en.
Preferably, the phenolphthalein will have from 2 to 4
lS substituents in positions ortho to -the hydroxy ~roups.
Preferred examples of substituted phenolph-
thalein include the tetraalkyl derivatives, that is,
wherein Rl, R2, R3 and R4 are independently lower alkyl o~
1 to 4 carbon atoms. A particularly preferred compound is
that wherein Rl, R2, R3 and R4 are methyl, that is, 1,1-
di-(3',5'-dimethyl-4'-hydroxyphenyl)phthalide or,
commonly, tetramethylphenolphthalein.
The phenolphthalein compounds used to form the
polyarylates of the invention are prepared by reacting an
appropriately ortho-substituted phanol with phthalic
anhydride in the presence of a Friedel-Crafts catalyst. A
typical ortho-substituted phenol is 2,6-dimethylphenol~
Suitable Friedel-Crafts catalysts include zinc chloride,
aluminum chloride, ferric chloride, stannic chloride,
boron triEluoride, hydrogen Eluoride, hydrogen chloride,
sulfuric acid, phosphoric acid, and the like.
The polyarylate homopolymers and copolymers of
the invention are prepared Erom the above substituted
phenolphthaleins or from rnixtures of these compounds and
other bisphenols. The instant polyarylate homopolymers
are prepared Erom a single phenolphthalein compound or a
functional derivative thereof. In a similar fashion, the
instant polyarylate copolymers are prepared from a mixture
of a phenolphthalein compound or functional derivative
thereoE and a bisphenol compound of the formula
~ ~7~)4~
~1 -7-
HO- ~ C ~ -OH
R6 . R8
or functional derivative thereof; wherein R5, R6, R7 and
R8 are independently hydrogen, lower alkyl of 1 to
4 carbon atoms or phenyl. When R5, R6, R7 and R8 are all
hydrogen, the compound obtained, 2,2-bis(4-hydroxyphenyl)-
propane, is generally referred to as bisphenol A. When
R5, R6, R7 and R8 are not all hydrogen, the compound
obtained will herein be referred to as a substituted
bisphenol A. Preferred examples of substituted
bisphenol A include tetraalkyl bisphenol A and diphenyl
bisphenol A. A particularly preferred substituted
bisphenol A is tetramethyl bisphenol A or 2,2-bis(4-
hydroxy-3,5-dimethylphenyl)propane. The various
bisphenol A compounds are prepared by reacting an
appropriately substituted phenoL, such as 2,6-
dimethylphenol, with acetone in the presence of a
Friedel-Crafts catalyst.
Typical functional derivatives of the
above-described phenolphthaleins and bisphenols include
the metal salts and the diesters with monocarboxylic acids
haviny 1 to 3 carbon atoms. Preferred functional
derivatives are the sodium sal-ts, potassium salts and
diace~ate esters.
For the polyarylate copolymers of the present
invention, the mixture oE phenolphthalein and bisphenol
will have a molar ratlo o~ phenolphthalein to bisphenol of
about 20:1 to 1:2~. Preferably, the molar ratio of
3S phenolphthalein to ~isphenol will be about 9:1 to 1:9,
more preEerably, about ~:1 to 1:4.
The acid component which is reacted with the
phenolphthalein or phenolphthalein-bisphenol mixture to
prepare the polyarylates of the invention is a mixture oE
~L2~
Ol -8-
isophthalic and terephthalic acid or functional deriva-
tives thereof in a molar ratio of about 9:1 to 1:9,
oS respectively. Preferably, the molar ratio of isophthalic
to terephthalic acid will be about 3:1 to 1:3, more
preferably, about l:l.
Preferred functional derivatives oE isophthalic
or terephthalic acid include acid halides, such as isoph-
thaloyl or terephthaloyl dichloride and isophthaloyl or
terephthaloyl dibromide, and diesters, such as dialkyl
esters or diaryl esters, having from 1 to ~ carbon atoms
per ester group. ~xamples of suitable diesters include
diphenyl isophthalate and diphenyl terephthalate.
The polyarylate homopolymers of the present
invention can be generally represented by the formula
~ 3 _ C_ ~ I
~I
_ _ n
wherein Rl, R2, R3 and R4 are independently hydrogen,lower alkyl of l to 4 carbon atoms or phenyl; provided
that Rl, R2, R3 and R4 may not all be hydrogen; and n is
the degree of polymerization. Generally, n will be
adjusted to provide a polymer having an average molecular
weight greater than about 15,000.
In the case of the polyarylate copolymers
derived ~rQm a phenolphthalein-bisphenol mixture, the
phen~lphthalein and bisphenol tnoieties will normally occur
in random order throughout the polyarylata.
The polyarylates o this invention can be
prepared by several methods. For example, an interfacial
polycondensation process can be used. In this case an
aqueous alkaline solution of a bisphenol or mixture of
01 _9_
bisphenols and a terephthaloyl dihalide-isophthaloyl
dihalide mixture dissolved in an organic solvent which is
05 immiscible with water are mixed and reacted. Suitable
interfacial polycondensAtion processes which can be used
are disclosed, for example, in W. M. Eareckson, J. Polymer
Sci., XL 399 (1959) and Japanese Patent Publication
No. 1959/65.
The following is a typical polycondensation
process. An aqueous alkali solution of a bisphenol or
mixture of bisphenols is added to a terephthaloyl
dihalide-isophthaloyl dihalide mixture, more preferably, a
terephthaloyl dichloride-isophthaloyl dichloride mixture,
dissolved in an organic solvent, or an organic solvent
solution of a terephthaloyl dihalide-isophthaloyl dihalide
mixture is added to an aqueous alkaline solution o a
bisphenol or mixture of bisphenols. Alternatively, an
aqueous alkaline solution of a bisphenol or mi~ture of
~o bisphenols and an organic solvent solution o~ a terephtha-
loyl dihalide-isophthaloyl dihalide mixture can be simul-
taneously added to a reaction vessel. Interfacial
polycondensation takes place near the interface of the
aqueous phase and the organic phase. However, since the
aqueous phase and the organic phase essentially are not
miscible, it is necessary to mutually disperse the phases.
For this purpose an agitator or a mixer such as Homo-mixer
can be used.
The concentration of the terephthaloyl
dihalide-i~ophthaloyl dihalide mixture dissolved in the
or~anic solvent is usually from about 2 ~o 25 weight %,
more preferably, from 3 to 15 wei~ht ~. The concentration
of the bisphenol or mixture of bisphenols in the aqueous
alkaline solution ls al.so usually from about 2 to
~5 welgh~ ~, more preeerably, ~rom 3 to 15 weight ~.
The amount of the bisphenol or mixture of
bisphenols and of the terephthaloyl dihalide-isophthaloyl
dihalide mixture used (molar ratio) is preferably main-
tained equivalent. ~n excess o~ the terephthaloyl
~0
7 4 ~) L~L ~D
01 -10-
dihalide-isophthaloyl dihalide mixture is not desirable in
the preparation of the high molqcular weight polyarylate.
05 Preferred alkalis are sodlum hydroxide and
potassium hydroxide. The concentration of the alkali in
the aqueous solution can vary widely depending upon the
reac~ion conditions, but is usually in the range from
about 0.5 to 10 weight ~. It is advantageous if the
quantity of alkali is nearly equivalent to the hydroxy
groups of the bisphenol or bisphenols used or is present
in a slight excess. The preferred molar ratio of the
alkali to the hydroxy group of the bisphenol or bisphenols
is from 1:1 to 2:1, most preferably, from 1:1 to 1.1:1.
As organic solvents which can be used for
dissolvin~ the terephthaloyl dihalide isophthaloyl
dihalide mixture, hydrocarbons or halogenated hydro-
carbons are used. For example, methylene dichloride,
chloroform, tetrachloromethane, 1,2-dichloroethane,
1,1,2-trichloroethane, tetrachloroe~hane, benzene and
methylbenzene can be employed. Especially preferred are
those solvents which also dissolve the aromatic copoly-
esters produced. The mos~ preferred solvent is 1,1,2-
trichloroethane.
The reaction temperature is not strictly
llmited, and depends on the solvent used. For example, in
the case o~ methylene dichloride, the reaction temperature
is usually preferably below 40C, with from 5 to 30C
being especially preferred.
Interfacial polymerization is usually conducted
at normal pressure and is completed in about 1 to 4 hours.
Antioxidants, dispersing agents, catalysts and
viscosity stabilizers can be added to the aqueous alkaline
solution or to the reaction mixture, iE desired. Typical
~S examples o~ ~UCtl a~ents are as follows. As antioxidants,
sodium hydrosulfite or sodium bisulfite can be used. As
dispersing agents, anionic surface-active agents, such as
sodium lauryl sulEate and octadecyl benzene sulfonate,
cationic surface-active agents, such as cetyl trimethyl
ammonium chloride, and nonionic surface-active agents such
~7~3~
~1 -1 1-
as poly(ethylene oxide) adducts can be used. As catalysts,quaternary ammonium compounds, such as trimethyl benzyl
OS ammonium hydroxide, trimethyl benzyl ammonium chloride and
triethyl benzyl ammonium chloride, tertiary sulfonium
compounds, such as dimethyl-2-hydroxyphenyl sulfonium
chloride, quaternary phosphonium compounds, such as
triphenyl methyl phosphonium iodide and trimethyl octyl
arsonium iodide can be used. Tertiary ammonium compounds,
such as trimethyl amine, triethyl amine and benzyl
dimethyl amine can also be used as catalysts. As
viscosity stabilizers, mono-valent compounds, especially
mono-valent phenol compounds, such as p-cumyl phenol,
o-phenyl phenol, p-phenyl phenol, m-cresol and ~-naphthol
can be used, if desired.
Another useful method for forming the
polyarylates is melt polymerization, as disclosed, for
example, in A. Conix, Ind. Eng. Chem., 51 147 (1959), in
Japanese Patent Publication 15,247/63 and in U.S. Patent
No. 3,395,119.
Melt polymerization can be conductsd, ~or
example, by heating and reacting an aliphatic carboxylic
acid diester of a bisphenol or mixture of bisphenols and a
terephthalic acid-isophthalic acid mixture at reduced
pressure. A preEerred diester of a bisphenol is the
diacetate. Melt polymerization can also be conducted by
heating and reacting a bisphenol or mixture of bisphenols
and a mixture of a diaryl ester of terephthalic acid and
isophthalic acid. A typical diaryl ester is the diphenyl
ester. The reaction temperature employed is in the range
of from about 150 to 350C, more preferably, from 180 to
320C. The reaction pressure is usually varied in the
course of the reaction from atmospheric pressure at the
early part of the reaction to reduced pressure, such as
below about 0.02 mmHg, at the end of the reaction.
In melt polymerization, the molar ratio of the
bisphenol or mixture of bisphenols and the mixture of
terephthalic acid-isophthalic acid components to prepare a
~1 -12-
high molecular weight polyarylate must be maintained
exactly equivalent.
S A nu~ber of catalysts can be used. Catalysts
which are preferably used are titanium compounds, such as
butyl orthotitanate and titanium dioxide. Other
catalysts, such as zinc oxide, lead oxide and antimony
dioxide can also be used.
Still another method for forming the
polyarylates is solution polymerization, in which the
polyarylates are prepared by reacting a bisphenol or
mixture of bisphenols with terephthaloyl dihalide and
isophthaloyl dihalide in an organic solvent solvent.
Solution polymerizations which can be used are disclosed,
for example, in A. Conix, Ind. 5ng. Chem., 51 1~7 (1959),
and in U.S. Patent No. 3,133,898.
In solution polymerization, the bisphenol or
mixture of bisphenols and the mixture of terephthaloyl
~ dihalide and isophthaloyl dihalide, e.g., terephthaloyl
dichloride and isophthaloyl dichloride, are usually mixed
in e~uimolar proportions in an organic solvent, and the
mixture is warmed gradually to high temperatures, such as
about 220C. ~s the organic solvent used, those solvents
which also dissolve the polyarylates produced, such as
dichloroethyl benzene, are preferred. Usually, the
reaction is carried out in the presence of a base to
neutralize the hydrogen halide, e.g., hydrogen chloride,
formed.
The polyarylate alloy compositions of the
present invention are obtained by mixing the above-
described polyarylate homopolymers and copolymers with a
polymer resin selected from the group consisting of poly~
bisphenol ~ carbonate and polystyrene. In general, the
3S alloy composition will contain about 10 to 90~ by wei~ht
of polyarylate and about 90 to 10~ by weight of polybis-
phenol A carbonate or polystyrene. Preferably, the alloy
composition will contain about 20 to 80~ by weight of
polyarylate and abo-lt 80 to 20~ by weight of polybis-
phenol A carbonate or polystyrene. The polystyrene will
~7~
Ol -13-
normally have an average molecular weight of about 100,000
to 1,000,000, preferably about 300,000. The polybis-
oS phenol A carbonate will normally have an average molecularweight of about 20,000 to 50,000, preferably about 30,000.
To add polybisphenol A carbonate or polystyrene
to the polyarylates of this invention, any well known
mixing technique can be used. For example, grains or
powders of these two components can be mixed and blended
with a V-blender, Henschel mixer, Super mixer or Kneader,
and then the mixture immediataly molded. Alternatively,
the mixture can be formed into pellets after melting with
an extruder, a co-kneader, an intensive mixer, or the
like, and then molded. The pelletizing or molding
temperature is generally in the range of from about 250
to 350C, more preferably, 260 to 320C.
Another addition method comprises adding the
polybisphenol A carbonate or polystyrene to a solution of
the polyarylate and then evaporating off the solvent. As
the solvent, those which dissolve the polyarylate can be
used, such as methylene dichloride, tetrachloroethane and
chloro~orm. The preferred solvent is tetrachloroethane.
The solution of polymers in a solvent may be poured into a
nonsolvent to precipitate the polymer and the precipitated
alloy can be removed by filtration. Suitable nonsolvents
are the lower alcohols, such as methanol, ethanol,
propanol, butanol, and the like. An especially preferred
nonsolvent is ethanol.
The most suitable method for any particular
system can be chosen according to the composition and the
desired shape and properties of the molded articles to be
produced there~rom.
In order to improve the heat resistance, light
3S stability~ weatherability or oxidation resistance of the
composition or articles produced according to this
invention, agents preventing thermal degradation, antioxi-
dants, ultraviolet absorbants, and the like, can be added
thereto, i~ desired~ For example, benzotriazole, amino-
phenyl benzotriazole, benzophenone, trialkyl phosphates,
0 1
such as trioctyl phosphate and tributyl phosphate,trialkyl phosphites, such as trioctyl phosphite, and
05 triaryl phosphites, such as triphenyl phosphite, can be
used. These materials are conveniently added to the
polyarylate copolymers and alloys of this invention at any
time prior to moldin~. Known plasticizers, such as
phthalate esters, e.g., dioctyl terephthalate, dioctyl
orthophthalate and dioctyl isophthalate, and colorants,
such as carbon black and titanium dioxide, may also be
added if desired, in commonly used amounts as are known in
this art.
The polyarylate polymers and alloys of this
invention can be used to form many useful articles using
generally known molding methods, such as injection
molding, extrusion molding, press molding, and the like.
Typical examples of final products produced therefrom are
films, monofilaments, fibers, injection molded materials,
such as machine parts, automobile parts, electrical parts,
vessels and springs. The polyarylate polymers and alloys
of this invention find s~ecial use as engineering plastics
for various uses which re~uire good properties.
The following examples are provided to
illustrate the invention in accordance with the principles
of this invention but are not to be construed as limiting
the invention in any way except as indicated by the
appended claims. In the examples, the term "polycarbonate"
refers to polybisphenol ~ carbonate.
EXAMPLES
Example 1
Preparation of 1,1-di-(3',5'-dimethyl-4'-
hydroxyphenyl) Phthalide
A 500-ml round-bottom, three-necked flask
eq~lipped with mechanical .stirrer, thermometer, water
condenser and nitrot~en gas inlet tube was connected to a
nitrogen supply lin~a. In the Flask was placed 56.0 g
(0.46 mole) of 2,6-dimethylphenol, 28.0 g (0.20 mole) oE
phthalic anhydride, and 50.0 9 (0.36 mole) of zinc
chloride. The mixture was stirred and heated at 125 to
130C by an oil bath. It was then stirred and maintained
01 -15-
at a temperature between 125 to 130C over a period of 10
hours. After 10 hours, the reaction mixture was a reddish
05 slurry.
The product was poured out from the flask into a
2-liter beaker. The crude product was washed with
3 liters of hot water and turned a golden-yellow color.
It was then dissolved in a 10~ NaOH solution and acidified
with carbon dioxide until the pH reached 1. The light
yellowish solid product was collected by suction
filtration and washed with a generous amount of water.
The product was recrystallized three times from a mixed
solvent of 200-proof ethanol and distilled water. The
IS residual solvent was removed by drying the product in a
vacuum oven at 100C in a nitrogen atmosphere. The final
product was a light yellowish powder. The yield was 57 g,
81% of theory. The product had a melting point of 274 to
277C and was found to be of 99.8% purity by liquid
~0 chromatography. The product was analyzed for the percent
of carbon and hydrogen. Analytical calculated for
C22H22O~: C, 75.41; H, 6.33. Found: C, 75.77;
H, 5.83. NMR(acetone-d6): ~7.7 (m, 4, ArH~, 6.95 (s, 4,
ArH), 1.8 (s, 12, ArCH3).
Example 2
Preparation of 1,1-Di-(3',5'-dimethyl-4'-
hydrox~phertyl) Phthalide Iso/Terephthalate Polymer
A 500-ml three-necked flask equipped with
mechanical stirrer, thermometer, and nitrogen gas inlet
and outlet was charged with 10.5 g (0.03 mole) of l,l-di-
(3',5'-dimethyl-4'-hydroxyphenyl) phthalide, 0.20 g
(0~0009 mole) of triethylbenzyl ammonium chloride, 0.02 g
of sodium bisulfite, 2.6~ 9 (0.066 mole) of sodium
hydroxide, 135 ml o~ distilled water, and 30 ml of 1,1,2-
trichloroethane. rrhe reaction mixture wa5 stirred at a
motor speed of 1000 rprn under nitrogen atmosphere at a
temperature not exceeding 10C maintained by an ice water
bath. A mixed solution of terephthaloyl dichloride,
3.05 g (0.015 mole), and isophthaloyl dichloricle, 3.05 g
(0.015 mole)~ in 40 ml of 1,1,2-trichloroethane was added
01 -16-
over a period of 30 minutes. At the same time the mixturewas vigorously stirred. The ice water bath was then
~5 removed and replaced with a room temperature water bath.
Stirring was continued ~or an additional four hours.
Subsequently, the upper layer was decanted and replaced by
100 ml of distilled water and 30 ml o~ 1,1,2-trichloro-
ethane. The mixture was again stirred for 30 minutes.
The resulting aqueous layer was decanted and removed. The
organic layer was poured into 500 ml of 200-proo~ ethanol.
A white polymer was precipitated and collected by suction
filtration, The polymer was washed four times with 200 ml
of ethanol. The product was placed in a vacuum oven at
100C overnight. The yield of the polymer was 13~6n g.
This was a 84.5~ yield. The polymer was dissolved for
Gardner viscosity in a mixed solvent of 40/60 phenol and
1,1,2,2-tetrachloroethane by rotating it overnight. The
Gardner viscosity of a 10% polymer solution was 0.65
poises at 25C. Reduced viscosity was measured at
0.25 ~/100 ml in 1,1,2,2-tetrachloroethane. Reduced
viscosity was 0.36 dl/g at 25C. The glass transition
temperature, rneasured by differential ~canning calorimetry
(DSC), was 277C.
Example 3
Preparation of 1,1-Di-~3',5'-dimethyl-4'-
hydroxyphenyl) Phthalide ~isphenol A
Iso/Terephthalate Copolymer
A 500-ml three-necked flask equipped with
mechanical stirrer, thermometer, and nitrogen gas inlet
and outlet was charged with 6.30 g (0.018 mole, 60 mole ~)
of l,l-di-(3',5'-dimethyl-4'-hydroxyphenyl) phthalide,
2.74 g (0.012 mole, 40 mole %) Oe bisphenol A, 0.20 g
(0-0009 Inole) of triethylbenæy~ ammoniunn chloride, 0.02 g
o~ sodium bisulfite, 2.64 9 ~0.066 mole) oE sodium
hydroxide, 135 ml of water, and 30 ml oE 1,1,2-trichloro-
ethane. The reaction mixture was stirred at a motor speed
of 1000 rpm under a nitrogen atmosphere at a temperature
not exceeding 10C maintained by an ice water bath. A
mixed solution of terephthaloyl dichloride, 3.05 g (0.015
rnole), and isophthaloyl dichloride, 3.05 g (0.015 mole),
~L~7~ 3
Ol -17-
in 40 ml of 1,1,2-trichloroethane was added over a period
of 30 minutes. At the same time the mixture was
05 vigorously stirred. Ihe ice water bath was then removed
and replaced with a room temperature water bath. Stirring ;
was continued for an additional four hours. Subsequently,
the upper layer was decanted and replaced by 100 ml of
distilled water and 30 ml of 1,1,2-trichloroethane. The
mixture was again stirred for 30 minutes. The resulting
aqueous layer was decanted and removed. The organic layer
was poured into 500 ml of 200-proof ethanol. A white
polymer was precipitated which was collected by suction
filtration. The polymer was washed four times with 200 ml
of ethanol. The product was placed in a vacuum oven at
100C overnight. The yield of the polymer was 12.2 g.
This was a 81~ yield. The polyrner was dissolved for
~ardner viscosity in a mixed solvent of 40/60 phenol and
1,1,2,2-tetrachloroethane by rotating it overnight. The
Gardner viscosity of a 10% polymer solution was 3.40
poises at 25C. Reduced viscosity was measured at
0.25 g/100 ml in 1,1,2,2-tetrachloroethane. Reduced
viscosity was 0.88 dl/g at 25C. The glass transition
temperature, Tg, measured by differential scanning
calorimetry, was 266C.
Following the above procedure, various
copolymers were prepared having different mole ratios of
bisphenols. The glass transi-tion temperature, Tg, of
copolymers having various mole ratios o~ bisphenols is
30 shown in Table 1.
Table 1
1,1-Di-t3',5'-dimethyl-
~'-hydroxyphenyl) Phthalide,Bisphenol A,
Mol~ ~ Mole ~ Tg, C
280
~0 266
~0 60 222
225
~2~
01 -18-
Exam~le 4
Preparation of 2,2-Bis-(4'-hydroxy-3',5'-
05 dimethylphenyl) Propane and 1,1-Di-(3l,5'-
dimethyl-4'-hydroxyphenyl) Phthalide
Iso/Tereph-thalate Copolymer
A 500-ml three-necked flask equipped with
mechanical stirrer, thermometer, and nitrogen gas inlet
and outlet was charged with 3.41 9 (0.012 mole, 60 mole ~) --
of 2,2-bis-(4'-hydroxy-3',5'-dimethylphenyl) propane,
2.80 g (0.008 mole, 40 mole ~) of 1,1-di-(3',5'-dimethyl-
4'-hydroxyphenyl) phthalide, 0.14 g (0.0006 mole) of
triethylbenzyl ammonium chloride, 0.02 g of sodium
bisulfite, 1.76 g (0.044 mole) of sodium hydroxide, 135 ml
of distill~d wa~er, and 30 ml of 1,1,2-trichloroethane.
The reaction mixture was stirred at a motor speed of
1000 rpm under nitrogen atmosphere at a temperature not
e~ceeding 10C maintained by an ice water bath. A mixed
solution of terephthaloyl dichloride, 2.03 g (0.01 mole),
~ and isophthaloyl dichloride, 2.03 g (0.01 mole), in 40 ml
of 1,1,2-trichloroethane was added over a period of 30
minutes. At the same time the mixture was vigorously
stirred. The ice water bath was then removed and replaced
with a room temperature water bath. Stirring was con-
tinued for an additional four hours. Subsequently, the
upper layer was decanted and replaced by 100 ml of
dis~illed water and 30 ml of 1,1,2-trichloroethane. The
mixture was again stirred for 30 minutes. The resulting
aqueous layer was decanted and removed. The organic layer
was poured into 500 ml of 200-proof ethanol. A white
polymer was precipitated which was collected by suction
filtration, The copolymer was washed four times with
200 ml of ethanol. The product was placed in a vacuum
ov~n at 100C overnight. The yield of the copolymer was
3S ~.42 ~. This was a 95.6~ yield~ The copolymer was
dissolved for Gardner viscosity in a mixed solvent o
40/60 phenol and 1,1,2,2-tetrachloroethane by rotating it
overni~ht. The Garclner viscosity of a 10~ polymer
solution was 1.65 poises at 25C. Reduced viscosity was
measured at 0.25 g/100 ml in 1,1,~,2-tetrachloroethane.
~L~ 3
o 1 - 1 9 -
Reduced viscosity was 0.08 dl/g at 25C~ The copolymer
had a ~lass transition temperature of 253C.
05 Following the above procedure, various
copolymers were prepared having different mole ratios of
bisphenols. The glass transition temperature, Tg, of
copolymers having various mole ratios of bisphenols is
shown in Table 2.
Table 2
2,2-Bis-(4'-hydroxy~ Di-(3',5'-dimethyl-
3l,5'-dimethylphenyl) 4'-hydroxyphenyl)
Propane, Mole ~ Phthalide, Mole ~ Tg, C
234
253
296
~0 60 288
294
Example 5
Preparation of Alloy of lpl-~i-(3',5'-dimeth~yl-
~U 4'-hydroxyphenyl) Phthalide Bisphenol A
Iso/Terephthalate With Polycarbonate
In a 20-ml vial was placed 1.0 g of bisphenol A
(40 mole %)/1,1-di-(3',5'-dimethyl-4'-hydroxyphenyl)
phthalide (60 mole ~) iso/terephthalate, 1.0 9 of poly-
carbonate (Lexan 141), and 18.0 g of 1,1,2,2-tetrachloro-
ethane. The vial was placed on a rotator and rotated
until the mixture was completely dissolved. This was now
a 1:1 solution by weight of the two polymers. Two ml of
the above polymer solution was placed on a 2.5 in. x 5 in.
glass plate. A film was cast with an 0.02-in. thickness
doctor blade. The cast film was first dried at room
temperature in the hood until most of the solvent had
evaporated. The glass plate with film was trans~erred to
a forced air oven at 40C for four hours and 75C ~or an
additi~nal Eour hours. I`he compatlbillty of the film was
examined after it was removed from -the oven. The
remainder ~f tha solution was poured into 150 ml of
methanol. A white polymer was precipitated which was
collected by suction filtration. The polymer was washed
7~ 3
01 -20
four times with 50 ml of methanol. The product was then
placed in a vacuum oven at 100C until the weight was
05 constant.
Following the above procedure, various alloys
were prepared having different weight ratios of polymers.
The glass transition temperaturel Tg, for these alloys is
shown in Table 3.
Table 3
Polyarylate~ Wt % Polycarbonate, Wt ~ Tg, C
155
203
269
Example 6
Preparation of the Alloy of 2,2-Bis~
hydroxy-3',5'-dimethylphenyl) Propane and
1,1-Di-(3',5'-dimethyl-4'-hydroxyphenyl)
Phthalide Iso/Terephthalate With Polycarbonate
In a 20-ml vial was placed 1.0 g of 2,2-bis-(4'~
hydroxy-3',5'-dimethylphenyl) propane (50 mole %)/l,l-di-
(3',5'-dimethyl-4'-hydroxyphenyl) phthalide (50 mole ~)
iso/terephthalate, 1.0 g of polycarbonate (GE Lexan 141),
and 18.0 g of 1,1,2,2-tetrachloroethana. The vial was
placed on a rotator and rotated until the mixture was
completely dissolved. This was now a 1:1 solutionO Two
ml of the above polymer solution was placed on a 2.5 in. x
5 in. glass plate. A film was cast with an 0.02-in.
thickness doctor blade. The cast film was first dried at
room temperature in the hood until most of the solvent had
evaporated~ The glass plate with film was transferred to
a forced air oven at 40C for four hours and at 75C for
an additional four hours. The compatibility of the film
was examined after it was removed from the oven. The
3C remainder o~ the polymer solution was poured into 150 ml
of methanol. A white polymer was precipitated which was
collected by suction filtration. The polymer was washed
four times with 50 ml oE methanol. The product was then
placed in a vacuum oven at 100C until the weight was
constant.
01 -21-
Following the above procedure r various alloys
were prepared having different weight ratios of polymers.
05 The glass transition temperature, Tg, for these alloys is
shown in Table 4.
Table 4
Polyarylate, Wt ~ Polycarbonate, Wt % Tg, C
1~20 80 1~4
191
282
3S