Note: Descriptions are shown in the official language in which they were submitted.
COMBINED FLUID FILTER AND DELIVERY TUBING
BACXGROUND OF THE INVENTION
This invention relates to an inline final filter
and more particularly to such a filter which can be
combinsd, and form a part of, fluid tubiny for
administration of fluids such as intravenous fluids.
The invention also relates to a method of manufacturing
such a filter.
It has been convent.ional practics to filter
intravenous as well as other parenteral solutions prior
to the administration of such solutions to a patient~
The purpose of such filtration has been to remove
particulate matter and bacteria that may be present.
In recent years, there ha~ been an attempt to place
filtration units inline in the administration apparatus
so that the fluids are filtered as they are being
administered. Example of such inline units include
those disclosed in U.S. Patent 4,06S,556 to
Vaillancourt. It has been found however that these
inline filtration units present problems in that they
are bulky and expensive to manufacture.
According to this invention, there is provided a
combined inline final filter and/or tubing unit for the
filtration and administration of fluids which overcome
the difficulties of the prior art and represents an
advancement in the state of art by efficiently providing
filtration in an inline filter that may be no bulkier
than the administration tubing itself, will be easy to
prime, and will prevent air blockaye irrespective of
position.
SUMMARY
According to this invention, there has been
provided an inline final filter unit for the filtration
and administration of intravenous fluids or the like
wherein a flexible, nonporous intravenous tubing member
contains microporous hollow fibres arranged within the
tubular member parallel to the longitudinal direction of
such tubing. The fibres are closed at one end and open
';;~b,"'`~
-- ; ~
at the opposite end. A flow blocking material is
arranged around the fibres for blocking fluid ~low
through the flexible tubular member, other than through
the material of said hollow fibres.
At least one gas permeable hydrophobic fibre is
arranged to extPnd into the tubing member and proximity
to the microporous hollow filter fibres. One end of the
hydrophobic fibre(s) is in communication with the
atmosphere. In a preferred form of the invention, the
hydrophobic fibre is potted in one arm of an "Y"
connection in the tubing assembly.
It was an object of this invention to provide an
inline final filter that would be simple to manufacture,
~lexible, utilize standard parts, and be capable of
easy packaging and use.
It was a further object of this invention to
provide a means of filtration which could utilize
existing intravenous administration tubing.
It was a further object of this invention to
provide a filter which would allow all entrained air to
be easily vented thereby having a very short primary
time and being easy to prime.
It was a still further object of this invention to
provide a filter from which entrained air could be
removed independent of position.
Further objects of this invention will be evident
from the following description.
DESCRIPTION OF THE DRAWINGS
The lnvention will be more fully understood and
described with re~erence to the drawings wherein:
Fig. 1 is a view in perspective of an intravenous
administration set incorporating the filter of the
invention and showing the administration of fluids to a
patient;
Fig. 2 is a cross-sectional view taken on line 2-2
of Fig. 1;
Fig. 3 is a view in cross-section taken on line 3-3
of Fig. 2; and,
Fig. 4 and 4A are enlarged fragmentary views
similar to Fig. 2 showing details of the invention.
DETAILED DESCRIPTION OF THE INVENTION
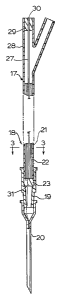
As shown in Fig. 1, an administration set which
comprises the inline fina~l filter according to the
invention includes an I.V. fluid container 10 which
contains an I.V. fluid 11 and has a closure 12. A spike
13 runs through closure 12 to provide an outlet for the
I.V. fluid into a drip chamber 14, of conventional
design. A flexible administration tube 15 is attached
to drip chamber 14. Clamp 16 can be provided for
controlling the flow of the I.V. fluid ll through tube
15. At its other end, tube 15 is connected by means of
a "Y" fitting 17 the final filter unit 18 according to
the invention and as will hereinafter be more fully
described. Filter unit 18 in turn is connected by a
second coupling 19 to injection needl~ 20 which is
inserted into the vein of a patient. Alternatively,
additional tubing, and/or appliances including "Y"
filterings may be connected downstream from filter unit
18.
Referring to Figs. 2 through 4, it will be seen
that filter 18 comprises a flexible nonporous tubing
member 21 into which are arranged microporous hollow
filter fibres 22 with the longitudinal axis of said
fibres being arranged parallel to the longitudinal axis
of tubing 21. A flow blocking material 23 is arranged
around fibres 22 for positioning said fibres in their
longitudinal direction and for blocking flow through
tubular member 21 other than through said hollow fibres
21.
As shown in Figs. 4 and 4A, the hollow fibres 22
have an opened end 2A and closed ends 25 and 26
respectively. In the embodiment o~ Fig. 4, the
.~
78~;
individual hollow ~ibres 22 are all heat sealed together
to provide a closed end 25. In Fiy. 4A, the hollow
fibres are looped back upon themselves to provide a
closed loop end 26.
In accordance with the invention, there is provided
at least one gas hydrophobic permeable fibre 27 as shown
in Fig. 2, which extends into tubing member 21 in the
proximity of the fibres 22. one end of hydrophobic
fibre 27 i5 in communication with the atmosphere, being
held in an arm 28 of the "Y" fitting 17 be potting
material 29 which can be the same material as used for
potting plug 23 for holding microporous fibre~ 22. An
open snd 30 of fibre 17 extends through potting material
29 and is open to the atmosphere. The opposite end 31
of fibre 27 is sealed shut to prevent liquid passage
into the interior of the fibre. Since the fibre 27 is
hydrophobic, no fluid, other than gaseous materials,
will pass through the material of the fibre. In this
manner, gas is vented from the filter unit in the
proximity of the microporous fibres 22 thus eliminating
air blockage.
The tubing 21 for filter unit 18 is preferably the
same type of tubing as used in I.V. administration sets,
i.e., the same tubing as tubing 15. Tubing 21 can be of
any length; however, the practical range would appear to
be from about 3 inches to about 60 inches. Below 3
inches in length, it is believed that there would not be
su~ficient effective length of fibres 22 to provide
adequate filtration and over 60 inches in length goes
beyond the practical length of tubing extending between
drip chamber 14 and needle 18. In practice, the total
length of the tubing including both tubing 15 and filter
18 between drip chamber 14 and needle 18 in a typical
administration set will be from about 24 inches to about
60 inches. The preferrecl length of the filter 18 in
~1 2~86
this arrangement, for ease of use and optimum ~iltration
efficiency, is from about 5 inches to about 15 inches.
The effective filtration area of the microporous
hollow fibres 22 is in the range of about 5 cm~ to about
100 cm2 with a preferred range being from about 12 cm2
to about 60 cm2. The ef~ective filtration area is
measured on the lumen of the fibres.
In both the arrangements of Figs. 4 and 4A
respectively, the flow o~ fluid can be from either
direction, passing from inside to the outside of the
hollow fibres in one direction and from outside to the
inside of the hollow fibres in the opposite direction.
Outside in flow is shown by the arrow in both Figs. 4
and 4A.
The microporous hollow fibres 22 can be of any
length up to and including the length of tube 21 with
allowance being made for fittings and placement of flow
blocking potting material 23. The fibres can also be
relatively short in length compared to the length of
tube 21 provided that sufficient filtration surface is
present for the particular application. For
administrations with tubing 21 of the range of lengths
indicated above, fibre lengths of about 1 inch to about
30 inches and preferably of about 5 inches to about 15
inches are useful provided that said fibres do not
exceed the length of tubing 21.
The porosity of fibres 22 is in the microporous
range of about .05 to about 1 microns. A preferred
range is about 0.1 to about 0.45 microns.
The outside diameter for fibres 22 can be from
about .008 inches to about 0.1 inches with a preferred
outside diameter being in the range of about .012 inches
to ahout .05 inches.
Flow blocking potting material 23 can be selected
from the group of silicon, polyurethane, epoxy resins
,;
8~
or other availablQ adhesive with polyurethane beiny the
currently preferred potting makerial.
The number of fibres 22 for standard I.V. tubing 21
is from about 2 to about 20 fibres.
The porosity of fibres 22 should be greater than
about 50% with a prefsrred range being about 65% to 90%
with the upper range of porosity being limited at a
point where the fibres have no structural integrity.
Packing density of fibres 22 in tube 21 as
expressed in a ratio of cross-sectional areas of fibres
to cross-sectional area of the lumen of tube 21 should
be less than about 60%.
While a single fibre 27 is used in a preferred form
of the invention a number of such fibres can be used.
The fibres can be manufactured from any gas permeable
hydrophobic material of which polypropylene is
preferred. The dimensions of the hydrophobic fibres ~7
can be generally those of fibres 22 with a preferred
inside diameter in the range of about .007 inches to
about .012 inches.
It is preferred that fibre 27 extends substantially
the entire length of fibres 22 to effect removal of Plow
blocking gas at any point along the length of such
fibres 22.
While no induced pressure, other than gravitational
pressure, is required for filtration of intravenous
solutions in the prePerred arrangement according to the
invention, either positive or negative pressure can be
used if desired.
In one alternate embodiment of the invention,
instead of using a separate filter 18, the filter can be
an integral part of the administration tube 15 itself,
thus eliminating the added couplings required to place a
filter inline.
The invention may be embodied in other specific
forms without departing from the spirit or essential
7477~
characteristics thereof. The present embodiments are
therefore to be considered in all respectæ as
illustrative and not restrictive, the scope o~ the
invention being indicated by the appended alaims rather
than by the foregoing description; and all changes which
come within the meaning and range of Pquivalency of the
claims are therefor interlded to be embraced therein.
;~