Note: Descriptions are shown in the official language in which they were submitted.
2~;205-6~8
Oxidation reaction
This invention relates to an industrially advan-
tageous process of oxidation reaction of an oxidizable
organic compound, which comprises a reaction using an
alkali hypohalite as an oxidizing agent, wherein an
5 alkali halogenide by-produced is reused and recycled
without being discharged.
Alkali hypohalites, such as sodium hypochlorite,
are fre~uently used as a reagent for oxidizing an
oxidizable organic compound, and examples of such alkali
hypohalite oxidation reactions include a reaction
involving oxidation of diacetone-L-sorbose to form
diacetone-2~keto-L-gulonic acid which is useful as a
starting material for the production of vitamin C [Kogyo
~ Zasshi (Gazette of Chemical Society of Japan), 64
(10), 1729 tl961)].
The alkali hypohalites, after oxidation reaction,
yield the corresponding alkali halogenide as by-product,
and such by-products have been discharged as being
contained in the waste fluid left after the objective
product of the alkali hypohalite oxidation reaction is
separatedj polluting the environment.
: As describèd in the above, the conventional art
allows the alkali hal~ogenide by-produced through the
oxidation reaction to be~discharged as being contained in
the waste fluid, but according to the present invention
r~ ~
:: :
.
''' ~ - " .'~' ' '' ' ` ' ~ ' ` ' ' '
. ~ ' ~ : ' ' . .
,
. ' ' ' ~, , .
~ ~7~
-- 2 --
alkali halogenide by~produced is reused and recycled
without being discharged~
The waste fluid in the conventional method
contains additionally high concentration of organic
by-products originated from the starting organic
compound to be oxidized. From the standpoint of
environmental hygiene, it is necessary to reduce the
chemical oxygen demand (C~D) component in the waste
fluid as far as possible. Also in the alkali hypohalite
oxidation reaction, it is necessary to realize the
reduction o~ the amount of the above-mentioned organic
by-products in order to keep the environment clean
because such organic by-products are the main con-
stituent of COD component. According to the present
invention, the waste fluid is subjected to electrolysis
as detailedly mentioned below, whereby COD value is
remarkably lowered. The present inventors, in view of
the industrial problems involved in the known method
using an alkali hypohalite as an oxidiæing agent,
carried out extensive investigation for solving such
problems of the hitherto known method, and as a result,
they have found that the problems involved in the known
method are solved by the present invention.
Thus, this invention is concerned with a metllod
for oxidation of an oxidizable organic compound, which
comprises (1) oxidizing the oxidizable organic compound
with alkali hypohalite in an aqueous medium, (2) separat-
ing the oxidized organic compound from waste fluid
containing alkali halogenide, (3) subjecting the waste
fluid to electrolysis to produce alkali hydroxide and
halogen, (4) reacting the alkali hydroxide with halogen
to produce alkali hypohalite, and (S) recycling the
alkali hypohalite as the oxidizing agent.
In the process o~ this invention, the ~irst step
is the oxidation reaction of an oxidizable organic
.
~ . , ~..... . .
.
.
. : -: - . . , ~ '
- . ..
. .
, , ; .
.... . ~ ' ' .
.. . .
-
3 24205-6~8
compound with an alkali hypohalite to produce an oxidized organic
compound.
The organic compound oxidizable wi~h alkali hypohalite
includes a sugar having 3 ko 8 carbon atoms, whereby the sugar
having -CHO group ls oxidized to the aorresponding carboxylic
acidr the sugar having -CH2OH group is oxidized to the
corresponding aldehyde and the sugar having -C~l(OH)CONH2, -
CH(NH2)CHO group or -CH(OH)COOH group is oxidized ~o an aldehyde
with less carbon atom by one. When resulting aldehyde is further
1~ reacted with alkali hypohalite, the compound is further oxidized
into the corresponding carboxylic acid. This is already known
before the present invention. Therefore, any one of the aldehyde
and the carboxylic acid can be produced according to the reaction
condition of the known prior technique.
.
, : . ` . ''" ;.
.
~ ~7~
4 24205-648
The alkali hypohalite oxidation of a sugar having 3 to 8
carbon atoms to the oxidized compound is concretely exemplified by
oxidation of adonitol to ribose, glyceraldehyde to glyceric acid,
glucosamine to arabinose,
~ ~7~
gluconamide to arabinose, arabinose to arabonic acid,
ribose to ribonic acid, glucose to gluconic acid,
threonic acid 3,4-acetonide to glyceraldehyde acetonide,
etc.
The oxidation reaction of the present invention is
most preferable for oxidizing the above-mentioned sugar
having 3 to 8 carbon atoms to produce a carboxylic acid
h~ving 3 to 8 carbon atoms, representatively exemplified
by the above-mentioned oxidation of diacetone-L-sorbose
to diacetone-2-keto-L-yulonic acid or a salt thereof
which is useful as an intermediate for synthesis of
vitamin C.
The alakli hypohalite as referred to in the
process of this invention is not specifically limited,
only if it can be normally used as an oxidizing agent,
and is represented by the formula MOX ~wherein M is an
alkali metal and X is a halogen atom]. Preferable is
sodium or potassium for M and also preferable is
chlorine or bromine for X. Therefore, the alkali hypo-
halite MOX is exemplified by sodium hypochlorite,potassium hypochlorite, sodium hypobromite, potassium
hypobromite, etc. Among others, sodium hypochlorite is
the frequently used oxidizing agent and can therefore be
applied most favorably to the method of this inven-
~5 tion.
An alkali hypohalite MOX, through ~he oxidation
reaction by the following equation, is changed into an
alkali halogenide MX.
MOX ) MX + [O~
Alkali hypohalite is used in the oxidation reac~
tion usually in an amount of 1.1 to 3.0 times the
theorotial amount. The initial concentration o~ MOX in
the reaction mixture is preferably S to 13 ~(w/w).
This alkali hypohalite oxidation is advantageously
conducted in an aqueous medium, e.g. in water, aqueous
` ,, " . ' '; ; ` ' ' : ~ '' ' ' ,: .' ;: ' '. ' ~
- : . :
:
.
~ ~t7~
methanol, aqueous ethanol, aqueous tetrahydrofuran,
aqueous diethyl ether, etc., and is carried out in the
presence or absence of a catalyst usually at a temper-
ature of about 20 to 100C for 5 minutes to 48 hours.
The catalyst usable in this step is usually metal
halide, exemplified by magnesium chloride, magnesium
bromide, zinc chloride, ferric chloride, nickel chloride,
etc, The amount of the catalyst is usually 10 mg to 1 g
per l ~ of ~he reaction mixture.
For example, the oxidation reaction of diacetone-L-
sorbose with sodium hypochlorite is carried out in an
aqueous medium, preferably in water, using as a catalyst
50 mg to 500 mg of nickel chloride per 1 Q of ~he
reaction mixture, at a reaction temperature of about 20
15 to 100C, preferably about 50 to 80C for 10 minutes to
5 hours. The reaction is conducted with or without
addition of sodium hydroxide and advantageously carried
out with addition of sodium hydroxide in order to avoid
decomposition of the hypochlorite, maintaining the
reaction mixture alkaline, e,g. pH 10 to 13.
The second step is separation of the oxidized
organic compound from waste fluid containing alkali
halogenide. Separation is carried out by the conven-
tional method. Separation of the oxidized organic
compound is usually conducted after neutralization or
acidification of the reaction mixture using an acid,
preferably aqueous hydrogen halogenide (e.g. HCl, HBr).
After neutralization or acidification, the pH of the
mixture is preferably 1 to 4.5, most preferably 1 to 3.
The present invention is advantageously applicable
to the case in which the oxidized organic compound is an
acid compound, because such an acid compound is easily
obtained after neutralization or acidification of the
reaction mixture with such an acid as HCl or HBr and the
alkali halogenide produced in this neutralization or
~ : . ~ . ,
.
. ' '
- ` : :. . . :. : :
. . . . . . . .
- ~ - . :
~.2~
acidification step is also usable in the following
electrolysis step.
~ eparation or recovery of the objective organic
compound is conducted using usual method (e.g. solvent
extraction, precipitation, filtration, centrifugation,
crystallization, recrystallization, chromatography,
etc.). In case the product is diacetone-2-keto-L-gulonic
acid, the reaction mixture is neutralized or acidified with
hydrochloric acid whereby the objective compound is
precipitated, and the resulting diacetone-2-keto-~-gulonic
acid is separated by centrifugation or filtration,
whereby there is formed a waste fluid containing sodium
chloride.
Diacetone-2-keto-L-gulonic acid may be obtained as
a salt. Such salts include an inorganic salt (e.g.
sodium salt, potassium salt, calcium salt, etc.) and an
organic salt. These salts can be produced
through reacting diacetone-2-keto-L-gulonic acid with
the corresponding base (e.g. sodium hydroxide, potassium
hydroxide, calcium hydroxide,etc.) according to the conventional
method. It is preferable to remove nickel chloride
used, in advance of separation of the objective product
as crystals, so that it can be reused. The removal of
nickel chloride is advantageous in terms of protection
of electrodes during the below-mentioned electrolysis,
and is usually and advantageously conducted by filtra-
tion or centrifugation.
The third step is electrolysis of the waste fluid
containing alkali halogenide to produce alkali hydroxide
and and halogen, whicn is represented by the following
equatlon.
MX ~ MOH + X2
H20
:`
. . .
: . ~. : .: . . .
- . ~ ' :. : ~ ' : .' ` .. :
,, : : . .
. .
The conventional method of electrolysis can be
applied to the present electrolysis. As the example of
electrolysis, electrolysis of the waste fluid containing
sodium chloride is mentioned below.
As is well known, electrolysis of sodium chloride
produces chlorine on the side of anode and sodium
hydroxide and hydrogen on the side of cathode. With
reference to the mèthod of electrolysis, the per se
known non-diaphragm and diaphragm electrolytic processes
can be adopted,but it is preferable to adopt the diaphragm
process.
The electrolysis, in which the anode is a titanium
or carbon based electrode and the cathode is a stainless
steel or iron based electrode, is carried out for
example by passing an electric current through the waste
fluid containing sodium chloride formed by the above-
described oxidation reaction to thereby produce readily
chlorine as well assodium hydroxide and hydrogen. The
electrolysis produces the products in such amounts as may
be proportionate to the quantity of electricity passed,
irrespective of the presence of diaphragm. The reaction
temperature does not constitute any great problem in
electrolysis, but in view of the fact that this electroly-
sis is an exothermic reaction or for the purpose of
improving the unit consumption of electric power through
increased current efficiency, the reaction temperature
of about 75 to 85C is desirable.
The concentration of sodium chloride is a factor
involved in the current efficiency, and it is desirable
to prepare an aqueous solution of sodium chloride to
about 15 to 25% tw/w) of the final concentratlon to
subject to electrolysis.
In the present invention, ion exchang0 membrane is
advantageously used as a diaphragm. The type of the ion
exchange membranes is selected, depending upon the kinds
. . . . . . . ..
. . ' . ~ , . . .
.
.
:- .. : ~',. ...
., . ~ -,, ~ . . `
~ ;Z'7~
of alkali halogenide and alkali hydroxide, the nature
and concentration of impurities, etc., and their general-
ly suitable examples include NafionO membrane (produced
by Du Pont de ~emours), Flemion~ (produced by Asahi
Glass Co. of Japan), Aciplex (produced by Asahi Chemical
Industry Co. of Japan), etc.
These ion exchange membranes can be used effi-
ciently and continuously without being polluted by the
CQD component normally contained in not less than about 900
ld ppm in the raw electrolytic solution.
This is because a nascent halogen and a small
amount of oxygen generated from the surface of the anode
and a hypohalous acid produced in the neighborhood of
the electrode oxidize the COD component partially or
lS wholly, resulting in releasing out of the system for
example as a carbon dioxide gas; the so-called self-
cleansing or self-purifying action of them can permit
electrolysis to persist, while preventing the progress
of pollution of the diaphragm, which is one of the
effects provided by this invention. The electrolysis
can reduce the COD component in the solution to not more
than about 500 ppm.
As the diaphragm in the electrolysis, there can
also be used, in addition to the ion exchange membranes,
~5 synthetic or natural conventional type of materials such
as asbesto~s memrbanès.s
Electrolysis of other alkali halogenides other
than sodium chloride can also be conducted in the same
way.
The fourth step is reaction of alkali hydroxide
with halogen to produce alkali hypohalite. The
procedure of producing alkali hypohalite from alkali
hydroxide and halogen can be carried out in accordance
with the known process, advantageously using counter-
current contact process in a tower and batch absorption
-
. .
. ~ . . .
- . . ~ :
- . ' , :
, - . , ,. :.
. ` ~ '- ' ` ' ' . .
-- 10 --
process in a reaction vessel.
In this reaction the alkali hydroxide produced in
the above electrolysis is charged in advance into a
reaction vessel, and the halogen generated in the
electrolytic system is allowed to react with it to
thereby produce readily an alkali hypohalite quantita-
tively. This reaction is an exothermic reaction, and it
is therefore desirable to maintain the reaction system
at not higher than about 30C by cooling with cold
water, etc. In order to prevent the decomposition the
alkali hypohalite thus produced, it is preferable to
allow the alkali hydroxide to remain throughout the
reaction at a level of more than 0.2% (w/w). For the
purpose of this, the reaction solution is normally
checked for the concentration of the alkali hydroxide
continuously by means of the oxidation-reduction
potential measurement method, alkali measurement method,
etc. The concentration of the alkali hypohalite and
alkali hydroxide is adjusted to the calculated levels to
20 be used recyclingly as an oxidizing agent in the first
step.
In the process of this invention, the alkali
hypohalite as produced b~ the above fourth step is
recycled as an oxidizing agent,which is to be used in
~5 the above-mentioned first step. A specific example of
the steps of the above-mentioned process of this inven~
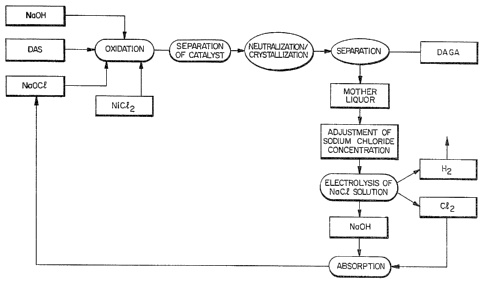
tion is shown in Fig. 1.
Thus, (1) diacetone-L-surbose (DAS) is oxidized
with sodium hypochlorite in the presence of sodium
hydroxide in an aqueous medium under the catalysis of
nickel chloride, (2) nickel chloride is separated,
(3) the reaction mixture is neutralized with hydro-
chloric acid to produce diacetone-2-keto-L-gulonic acid
(DAGA) as crystals, (4~ DAGA is isolated, (5) sodium
chloride concentration in mother liquor is adjusted at a
. . `. '~. ',. ' : '', .' ' . ~`.' ,' ' . :
-
:
~ ~7~
-- 11 --
concentration from 15 to 25% (w/v), (6) the sodium
chloride solution is electrolyzed to produce hydrogen,
chlorine and sodium hydroxide, (7) reacting chlorine and
sodium hydroxide to produce sodium hypochlorite,and
(8) sodium hypochloride is reused recyclingly. This
invention provides an industrially advantageous process
for the oxidation reaction using an alkali hypchalite as
an oxidizing agent. Thus~ this invention offers the
characteristic feature that an alkali halogenide by-
produced in the oxidation reaction is subjected toelectrolysis to convert into an alkali hypohalite, which
is recycled. This permits the effective utilization of
alkali halogenide, such as sodium chloride, which have
been so far discarded in the waste reaction fluid, and
is advantageous from the standpoint of saving natural
resources. At the same time, this, through electroly-
sis, can reduce the COD component in the waste reaction
1uid, and is considered advantageous in the aspect of
prevention of environmental pollution, as well. Further-
more, although the alkali hypohalites are unstablecompounds and must be subjected to an oxidation reaction
before their oxidation capacity is deteriorated, this
invention, permitting the production of alkali hypo-
halites in the continuous process involving the oxida~
tion reaction, is outstandingly advantageous in that the
objective reaction can be always carried out with use of
the alkali hypohalite in the state o~ its enhanced
oxidation capability.
Example
Example 1
1) An aqueous sodium-chloride waste fluid containing
the COD component as produced in preparing diacetone-2-
keto-L-gulonic acid from diacetone-L-sorbose with sodium
hypochlorite used as an oxidizing agent was electrolyzed
in an electrolytic bath (containing the anode made of
.. .. . : .. ,
, ~ ,
- ~ ' ~ ' ' ' ' ,
,
,
,
~ 12 -
titanium and the cathode made of stainless steel) with
an electrode surface area of 0.38 m2 being partitioned
by the ion exchange membrane (Nafion~ membrane, produced
by Du Pont de Nemours Co.) to allow chlorine, and sodium
hydroxide and hydrogen, to be formed at the anode and
cathode, respectively, followed by synthesis of sodium
hypochlorite.
Conditions:
a) Supplied amount of the raw solution (the aqueous
sodium chloride waste fluid having average sodium
chloride concentration of 293 g/); 6.0 Q/hour
b) COD in the raw solution (by the manganese method
in accordance with JIS K-0102); 920 ppm
c) Reaction temperature; 70 to 80C
d) Current density; 12 A/dm
e) Supply of pure water to the cathode
. side; 0.6 Q/hour
Results:
a) Produced amount of sodium hydroxide; 1.4 ~/hour
(average concentration of 417 g/Q)
b) Amount of carbon dioxide gas in
chlorine; 6% (w/w)
c) Sodium chloride concentration of the
waste fluid after electrolysis; 216 g/~
(average)
d) COD in the waste fluid after electrolysis
(by the manganese method in accordance with
JIS K-0102); 420 ppm
e) Bath voltage 3.2 V
2) To 100 kg of about 30~ (w/w) aqueous solution of
diacetone-L-sorbose was added the aqueous sodium hypo-
chlorite solution (89 kg as sodium hypochlorite) as
obtained previously under 1) in an amount 1.55 times the
theoretical amount (about 89 kg as hypochlorous acid),
and the oxidation reaction was allowed to proceed in the
::
- . ,. ~ .
,: ' .
':
- ' ' ' '
.
- . :: :
' ~' ~ ' '' ,
presence of nickel chloride (0.24 g/~) used as a catalyst
at 60C for 2 hours. This reaction solution showed
initially an alkalinity as high as a pH of not less than
13, but a pH of 10 to 11 at the conclusion of the
reaction. The reaction solution, when treated with
hydrochloric acid, crystallized out the product, and the
resulting crystals were separated to give 105 kg (yield
of 93.5%) of white diacetone-2-keto-L-gulonic acidO
Example 2
The sodium hypochlorite solution (82 kg as sodium
hypochlorite) as obtained by the procedure described
under the item 1) of Example 1 was added continuously to
100 kg of about 40% (w/w) aqueous solution of diacetone-
L-sorbose over the period of about 2 hours, and a bath
reaction was carried out at 60 to 70C. By the addition
of hydrochloric acid, diacetone-2-keto-L-gulonic acid
was allowed to crystallize out of the resulting reaction
solution and separated by filtration to give 104 kg
(yield of 92.6%) of white diacetone-2-keto-L-gulonic
~0 acid.
Example 3
The sodium hypochlorite solution (74.5 kg as
sodium hypochlorite) as obtained by the procedure
described under the item 1) of Example 1 was added to
100 kg of about 40% (w/w) aqueous solution of diacetone-
L-sorbose, and nickel chloride (0.36 g/~) was added to
the mixture as a catalyst, followed by reaction at 60 to
70C for 2 hours. From the resulting reaction solution,
diacetone-2-keto-L-gulonic acid was allowed to crystallize
out and separated by filtration.
The separated mother liquor containing sodium
chloride was used as a raw solution for electrolysis in
the production of the sodium hypochlorite solution in
accordance with Example 1. While the resulting sodium
hypochlorite solution was used and recycled as an
- ' ' . ' .- ' '
. . '' . ,: ' . : '
.' . ,. ~: ' ' ' '~ '
~ , ~ . , .
- . .
~ ~t7~
- 14 -
oxidizing agent, the above reaction was continued. This
reaction was repeated continuously ten times, and there
were obtained the results as shown in the ~ollowing
table.
5 ~ time 2 4 6 8 10
_
Yield (%~ of diacetone- 94 1 95 1 93 994 2 94 2
2-keto gulonic acid
CUrrent efficiency (~) 92 5 91 6 94 892.9 94.2
in electrolysis
Change in voltage of
electrolytic bath 1.1 1.0 1.0 1.0 1.0
(initial value = 1.0)
:
~ :
.
. ~ :
... . - . .. .
: .. . . . . . .
.. ~ - - . : - . . . . :
. . ,,:.,, ;:; ~` . ' :
' - . ' ~
: - : . .
.
. .