Note: Descriptions are shown in the official language in which they were submitted.
~2''7~6
EL~clr~cr~ L~
FIELD ~F TH~ VENrIVI~l
Tne invention relates to electroci~elnic~l
apparatus for sensing the presence of a species ln
fluid material. More particularly the invelltion
relates to an improved apparatus for yeneratillg a
electric siynal in response the the presellce of a
predetermined species in a fluid mdterial.
BACKJ~OUND OF THE INVENTION
Electrocl~emical sensors for the detection ~f tne
presence of a species in a fluid material have
existed for quite some tirne. Sucn sensors include
the Clark cell described in ~.S. Patent No. 2,913,38~
issued Noveln~er 17, 19590 The apparatus disclosed in
tha~ patent utilizes a dual electrode structure
immersed in an electrolyte and encase~ at least in
part in a membrane whicn is permeable to a
predeter~nined species. In operation such a device
allows the permeation of tne species to be detected
tl~rough the memDrane and reduces said species at tne
cathode. At the same time the anode is oxidized as a
result of the electrical and ionic connections
between the anode and cathode. These oxidation an~
reduction reactions generate a current Wi~iCh is
measurable and is proportional to the concentration
of the species 3eing detected. The Clark cell is a
large bulky apparatus and rnust include a liquid
electrolytic medium in wnicn the electrodes are
i~nrnersed. The Clark apparatus suffers ~roln sever~L
disadvantages including consumption of the species
being detected during detection, slow response tilnes
and alterdtion of the electrolyte duriny detection.
_._
- 2 - 22770-7
Some of the above-mentioned disadvantages of the
Clark-type electrode cell are avoided by apparatus of the type
described in UOS. Patent No. 3,260,656 issued on July 12, 1966
to James W. Ross, Jr. The Ross apparatus utilizes a sandwich
comprising a cathode and an anode with a spacer therebetween.
This sandwich is immersed in an electrolyte and is geometrically
oriented so that the electrodes are parallel to a membrane whlch
is permeable to the species being measured. The membrane
combines with a housing to enclose the cathode-anode combination
in an electrolyte. In the Ross-type cell the species being
measured is consumed at one electrode and regenerated at the
other electrode such that no net consumption of the species
being detected oc~curs. Therefore -the Ross sensor does not
consume the species being measured as a result of the electro-
chemical reaction of that species with the electrodes. rihereas
the Ross cell effectively overcomes the problems of alteration
of the electrodes and/or electrolyte, depletion of the species
from the test fluid, and extension of the depletion layer into
the test fluid causing stirring and fouling dependence, certain
other shortcomlngs are still evident. Among them is the fact
that readings with the Ross-type cell, obtained by measuring the
current flow between the electrodes, tend to stabilize within a
maximum of one minute in accordance with the Ross patent. It
has been found that response times of this order are not suit-
able for many applications. A further disadvantage is that the
diffusion layer thickness in the Ross cell is determined by the
interelectrode distance which is subject to variation as the
assembly is stressed by forces arising from temperature and/or
pressure variations. Yet another disadvantage is the cumbersome
- 3 - 22770-7
nature of the layered structure making reliable fabrication of
Ross-type devices difficult.
Yet another apparatus for electrolytically detecting a
species in a fluid is described in ~.S. Patent 4,076,596 issued
to Connery et al. on February 28, 1978. The apparatus of
Connery et alO inc]udes an insulating substrate and a plurality
of fingerlike electrodes deposited on the surface of the sub-
strate in a closely spaced interleaved geometric pattern. The
electrodes are covered with a -thin film of electrolyte and a
permeable membrane. The electrolyte is selected so that the
species being measured is generated at one electrode and con-
sumed at the other with no net consumption of the species being
detected. The Connery et al. apparatus may include a solid
electrolyte deposited on the electrodes. While the Connery et
al. apparatus eliminates some of the problems of the Ross-type
cell it has several disadvantages of its own. One primary dis-
advantage of the Connery et al. apparatus is that the solid
electrolyte is deposited on top of the electrodes. The elec-
trodes form an irregular surface having high points where the
electrodes are present and valleys at the spaces between the
electrodes. This makes it difficult to deposit a solid electro-
lyte coating which will be smooth, consistent, homogeneous and
adhere to the electrodes. In addi-tion, the coating of electro-
lyte will be distorted by changes in numidity and temperature
because of the irregular surface upon which it is coated.
Another problem with the Connery et al. apparatus is that its
response times may be too slow for some applications. This
results because of the electrolytic resistance of the electro-
lyte which forms a barrier between the electrodes and the test
~2798~
--4--
fluid. As a result, the species must ~if~use throu~h
the electrolyte prior to contacting tne electtodes.
Since the Connery et al. electrolyte is coate~ onto
an irregular surface tne electrolyte Inust De thicker
than if it were coated on a flat suLface to
accomplish d complete coating. Accordingly the
electrolytic resistance ~ e lo~er ~ut diffusioo
will be slower and can significantly slow response
times.
It is tne primary ooject of tne present invention
to provide an apparatus for electrolytically
detecting a species in a fluid materidl~ whici) nas
smooth electrolyte coatings with yood repeatability.
It is a further object of tne uresent invention
to provi~e a solid electrolyte layer having excellent
adherence to the electrodes.
It is a still further object of t~le present
invention to provide a tninner electrolyte layer to
thereby reduce the diffusion resistance of the
ap~?aratus.
It is a still furtner object of the present
invention to provide an apparatus having a structure
which ~ninimizes stresses on the electrolyte and
tnereby decreases distortion of the electrolyte as a
result of temperature and/or humi~ity variations.
It is a still further object of the present
invention to provide an a~paratus for
electrolytically detecting a species in a fluid
material with a response time which is fast enough
for use in application~ requiring a very fast
response.
Tnese and other ob~ects of the present invention
will be apparent to one of ordinary skill in the art
from the summary an~ detailed descriptions whicl
follow.
~LZ~g~9~
SU~MA~Y OF T~E INVENTION
The present invention relates to a solid
electrochemical sensor for generatin~ an electcical
signal in response to contact witll a predetermined
species pr~sent in a fluid materidl comp~ising a
substrate naving at least one surface, a solid
electrolytic medium having first and second surfaces,
said first surface of said medium being in contact
with dnd adhering to said at least one surface of
said substrate, a working electrode means in contact
with and adhering to said second surface of said
medium, an electrical power source connected for
biasiny said workiny electrode means at a potential
at which said species will ~e consulned at said
wor~ing electrode, and a counter electrode means in
contact with and adhering to said second surface of
said medium and being connected to said ~ower source
for completing a circuit in which a current is
ca~a~le of flo~ing tnrou~h Dotn of ~ai~ elec~r~ e
means as a result of the electrochemical reaction
occurring at said working electrode means.
A second embodiment of the invention relates to a
solid electrochemical sensor for generating an
electrical signal in response to contact with a
predeterlnined species present in a rluid Inaterial
comprising a substrate having at least one surface, a
first layer of a solid electrolytic ~nedium having
first and second surfaces, said first surface of said
first layer of mediuln being in contact with and
adhering to said at least one surface of said
substrate, a counter electrode ~nean.s in contact ~ith
and adhering to said second surface of said first
layer of electrolytic ~nedium, a second layer of a
solid electrolytic ~ediuln having first and secon~3
surfaces, said first surface of .~aid second layer of
__
~2~9~96
medium Deing in contact with and adilering to said
counter electrode means, a workiny electrvde means in
contact with and adhering to said second surface o~
said second layer of electrolytic me(liu~n, an
electrical power source connected for biasing said
wor~iny electrode means at a pote~ ial at ~ icl~ r;ai~
species will De consumed at said working electro~D,
and means for connecting said counter electrode means
to said power source for completing a circuit in
w~lich a curren~ is capa~le of flowing through ~oth of
said electrode means as a result of the
electrocllelnical reaction occurring at said workiny
electrode means.
BRIEF DESCRIPTION OF T~2 DRAWI~GS
Fig. 1 is a cross-sectional view of tMe
amperometric electrochemical apparatus o~ the present
invention.
Fig. 2 is a top plan view of tllP ~perol,letric
electrochemical sensing apparatus of the present
invention.
Fig. 3 is a cross sectional view of an alternate
embodiment of the amperometric electrochemical
sensing apparatus of the present invention.
Fig. 4 is a cross-sectional view of a sandwich-
type amperometric electrochemical sensing apparatus
in accordance with the present invention.
Fig. 5 is a cross-sectional view of a cylindrical
amperometric electrochemical sensing apparatus in
accordance with the present invention.
DETAILED DESCRIPTION ~F TIIE P~FER ED EI~ODIMENT~
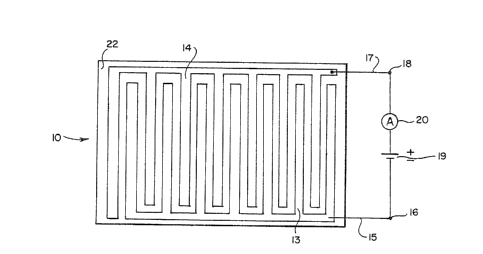
Referring now to Fig. 1 there is showll an
electrochemical sensing apparatus 1~ inc~udin~ a
~279~
--7--
su~strate 11, an electrolyce 22, a counter electrode
means 13 and a working electrode mearls 14. The
sensor depicted in Fig. 1 is tne simplest, least
expensive, as well as one of the most efficient
sensors in accordance with the present invention.
Referring now to Fig. 2 which is a top plan view
of the apparatus of Fig. 1 showing the fingerlike
projections of the electrodes 13 and 14. Courlter
electrode 13 is connected ~y way of line 15 to
terminal 16 and tne working electrode 14 is connected
by line J7 to the terminal 18. The electrical
circuit also includes a series connected electrical
power source 19 for Diasing the working electrode
means 14 at a desired potential and an ammeter 20.
Referring now to Fig. 3 there is shown an
alternate emDodiment of the electrochemical sensor of
the present invention. Tne sensor depicted in Fig. 3
includes substrate 11 having an oxiae layer 21 on the
surface thereof. Deposited on the oxide layer 21 and
aaheriny to the oxide lay~r 21 is a first laye~ 25 o~
electrolytic rnedium. Deposited on the first layer 25
of electrolytic medium are tne counter electrode
means 13 and the working electrvde means 14. Also
deposited on the first layer 25 of electrolytic
medium is a reference electrode 23 having a
protective coating 24 tnereon~ Deposited on top o
tne electrodes 13, 14 and the protective coating 24
is a second layer 27 of electrolytic medium.
Finally, on top of the second layer 27 of
electrolytic medium is snown a selectively permea~)le
memDrane 26.
Referring no~ to Fig. 4 there is depicted anotl~er
alternative embodiment of the present invelltioll
wherein the electrochemical sensing means is forlned
in a sand~ich-type structure. This sarldwich-type
structure is Duilt on a layer ~f sllDstra~e 11. rne
7g~
layer of suDstrate 11 includes an oxide layer 21 on
the surface tnereof. Deposited on top of the oxide
layer 21 is a first layer 25 of electrolytic medium.
Deposited on the first layer 25 of electro1ytic
medium is the counter electrode mealls 13 and the
reference electrode 23. ~rne referellce electrode 2~
is coated vy a protective coating 24. ~eposited on
top of the counter electrode 13 and protective
coating 24 is a second layer 27 of electro1ytic
medium. Then, deposited on the second layer 27 of
electrolytic medium is the working electrode 14 of
the electrochemical sensor. Deposited on top of the
working electrode means 14 is a third layer 28 of
electrolytic medium which includes a selectiv~ly
permeable membrane 26 thereon.
Refe~ring now to Fig. 5 there is shown yet
another alternate embodiment of the present
invention. Fig. 5 depicts d cross-sectional view of
a cylindrical electrochemical sensor in accordance
with the present invention. Tne cyli~drical
electrochemical sensor includes a su~strate 31 having
an oxide layer 32 on the surface thereof. On top of
the oxide layer 32 is deposited a first layer 33 of
electrolytic medium. On the first layer 33 of
electrolytic medium is deposited a cbunter electrode
means 13, a workin~ electrode means 14 and a
reference electrode 23. The reference electrode 23
is coated with a protective coating 24. It will be
understood that any of the alternate embodiments
shown in Figs. 1-4 may be adapted to the cylindrical-
shaped electrochemical sensor as well as other
possible shapes such as spherical. These alternate
shapes may be desirable fvr specific app1ications of
the sensing device.
The substrate 11 may be made of any suitable
materials to which the electrolytic medium can be
` ~2~9~
_9_
adnered. The substrate 11 is preferably an
insulating Inaterial such as glass, ~uaetz, ceralllics
such as alulnina, etc. and silicon. rlle su~stra~e lL
should have a thickness sufficient to assure the
structural integrity o~ the sensor. Another
important feature of the su~strdte 11 is that it ~--
capable of adhering or being made to adhere to a
coating Inaterial such as those used to fabricate
electrodes and electrolytes. This is ilnportan~
because the electrodes and electrolytes must adllece
to the substrate llo There are several ways to
promote adl~erence of a coatin~ material to the
substrate ]1. One Inethod involves oxidation of the
surface of the substrate 11 to forln an oxide layer
thereon. Many electrolytic materials adhere well to
oxides. An additional oxide layer ~nay also be coated
on the suriace of tne substrate 11 to prolnote
adherence of an electrolyte thereto. Also, adhesio
promotors for improviny adhesion of Nafion to glass
and other silacious substrates rnay be used. Such
adhesion promoters include but are not lilnited to
N-(trimethoxysilylpropyl)-N,N,N-trimethyl-a~nlnoniul,
chloride, octadecyltrichlorosilane, and
8-hydroxy-1,3,6-pyrenetrisulfonic acid trisodium
salt. These adhesion promoters chemically bond the
electrolyte to the substrate 11 to give additional
bonding strengtn. The adhesion promoters are appLied
to the substrate 11 just prior to spin ooating of the
electrolyte onto the substrate. Such promoters are
described in Szentirmay, M.N., Calnpbell, L.F., and
Martin, C.R., Silane Coupling Agents for Attaching
Nafion to Glass and Silica, Anal. Chem., Vol. 5B pp.
661-66~, Mdrch 1986.
As mentioned previously, the suL~strate 11
preferably includes an oxide layer on 21 oll the
~2~7~8'~
surface tilereof to promote the adherence vf the
electrolytic metiiu~n to tt~e su~strat~ ucl~ ~n
oxide layer 21 Inay be treated by simple oxidation of
the surface of tne suDstrate 11. For instance, a
substrate 11 such as silicon can be surface oxidi~ed
to produce a silicon dioxide surface COdting.
Alternatively, the oxide layer 21 may be deposited on
or attached to the surface of tlle substrate 11 in any
suitable manner.
The substrate 11 should have a smooth surface
before and after oxidation. Such a smooth surface
will prolnote smootn coatings of electrolytic medium
on the substrate 11. ~oreover, a smooth surface will
lead to c:onsistent and repeata~le coatings of
electrolytic medium enaoling mass production of
consistent sensors. Further, the smooth surface of
tlle subs~.rate 11 promotes adhesion of the
electrolytic mediuln to the substrate and tllereby
prevents tne electrolytic medium from peeling of~ tt~e
substrate 11. ~inally, the existence of a slnoott~
surface on the substrate 11 minimizes the stresses
applied to the electrolytic mediuln by the substrate
11 upon exposure to varying temperature and/or
humidity conditions. Tnis, in turn, will minimize
the distortion of the electrolytic Inediurn as a result
of these temperature and/or humidity variations
The electrolytic medium of the present invention
is preferably a solid material. The electrolytic
medium must be capable of allowing diffusion of all
reactants and products between the cathodes and
anodes as well as allowing exchant~e of the measured
spc~cies with the test fluid. The electrolytic mediuln
must also nave satisfactory chemical, thermal and
dimensional stability. Such polymer electrolytes
such as poly-sulfonic acids, typically polystyrene
sulfonic acid or perfluoro linear polymers sucll as
--ll--
those marketed under ~^a~ie-l~rk ~Nafion~ ~y Du Pont are
suitable for use as the electrolytic medium of the
present invelltion. In ad~ition, the electrolytic
medium must be capable of a~hering not only to the
substrate 11, but also to tne electrodes 13, 14 oe
the electrolytic medium must be capable of Deing
adhered to the substrate 11 and the electrodes 13, 14
by adhesives, adnesion prolnoters or the like.
Electrolytes of the ty~e employed in the
electrolytic medium of present invention dernollstrate
excellent electrolytic an~ electronic colnpati~ility
with oxides such as silicon dioxide, As a result ! i t
is preferable to coat SUCII electrolytes onto an oxide
covered surface, The oxide layer 21 can be obtained
by thermal oxidation of the semiconductor wafer
substrate 11. Other substrates 11 that are already
oxides may also be used, such as alumina,.sapphire,
glass and polymers. In order to o~tain smooth,
repeatable layers of electrolytic mediuln, the
electrolyte may be spin coated using a plloto-resist
spinner, onto the surface of the sunstrate 1~. Tllis
process is described in Unl-t~ S-tates Patent No.
4,795,543. This
spin coating technique produces a very thin, smootn
and homo~eneous coating of the electrolytic mediuln on
the substrate 11. Other Inethods of coating the
electrolytic Inedium onto the substrate 1l may be used
if they produce a coating having the desired
properties of ~moothness~ homogeneity, thickness and
structural stabilityO
Tlle electrodes of the present invention are
preferably Inetal. These electrodes rnay ~e deposited
on the surface of the electrolytic Inediuln through the
use of thick film, or thin film techni~lues. Sucll
methods include sputteriny and/or evaporation onto
the electrolytic surface ~f a thin filln o~ metal to
~Z7~
-12-
form the electrodes witn tne definition of tlle
surface areas ~eing accomp1ished by photo-etclling
processes. Otner thin film tecnni~lues such as
deposition of a metal layer and pnoto-etching of tnat
layer are also accepta~le.
The metals used to fa~ricate the e1ec~rodes oE
the present invention rnay inc1ude one or more of the
following: platinum, palladium, rhodium, lead,
silver, gold and iridium. It will ~e understood that
other ,naterials may ~e used as long ~s they satisy
the re~uirements of the present invention. Tnese
other materials must be capable of reacting with the
species to be detectedl as well as adhering oe bein~
adhered to the electrolytic medium. Selection of the
proper electrode materia' for a particular reaction
will depe.1d on tne species which is to De detected,
as well as t~e ability to adhere t~e electrode
material ~o tne electrolytic medium.
Tne analysis or identification of a gas using
tnese electro~es m~y De aCCui;lpiisi~ed ill al~y of a
number of ways. ~or instance, sucn electronic
variables as resistance, impedance, electrolytic
reactions, oxidation-reductioll reactions and
polarization may be monitored duriny exposure of the
sensor to a gas. Data ootained by monitoring any of
these electronic variables can be used to analyze or
identify a gas or components thereof.
Tne eiectrodes may be characterized as a working
electrode 14, a counter electrode 13 and a reference
electrode 23. The wor~ing electrode 14 is the
electrode at which the species is consumed by an
electroche~nical reaction. The counter electrod~ 13
is the electrode at whicil the species being detected
is preferably regenerated by an electroc~lemical
reaction. However, counter electrodes 13 wtlich do
not regenerate the species being detected, such as
~2~g89~
-13-
those of a Clark cell may also be used though t~ey
are not preferred. The reference electrode 23 ~o~s
not participate in the chemical reactions but does
serve to provide a potential teference for the
working electrode 14. Normally, a potential is
applied between the reference electrode 23 and the
working electrode 14.
Since it is often desirable to prevent
electrochemical reaction from occurring at the
reference electrode 23, the reference electrode 23 is
often coated to prevent exposure o the reference
electrod~ 23 to tne species. Such coatings may
include epoxies and any other coatinys which do not
allow the diffusion of the species to the surface of
the reference electrode 23. Alternatively, the
reference electrode 23 may be left uncoated and
thereby be exposed to the species. In this instance
it is necessary to include a correction factor in tne
syste~n monitoring means in order to colnpensate for
the electrvchemical reaction occurring at the
reference electrode 23. The reaction vccurring at
the reference electrode 23 will cause a change in
potential between the reference electrode 23 and the
working electrode 14. This ~otential change can be
accounted for through the use of tne Nernst
equation. Therefore, the reference electrode 23 may
be left exposed to the species if the monitoring
means is programmed to compensate for the change in
potential by calculating such change using the Nernst
e~uation.
A preferred embodiment of the yresent invention
also includes a selectively permeahle membrane 26
Wllich may ~e deposited over the top of the electrvdes
13, 14 or over the top of a second layer of
electrolytic Inedium. This selectively permeable
membrane 26 serves to allow the diffusion of the
lZ*9B96
~14-
species to be detected through to ti)e ~orking
electrode 14 and the electrolytic Inedium. However,
it does not allow diffusion of certain other
materials which .nay De present in the fluid materia1
being sensed. Therefore, the mern~rane 26 can be use~
to improve species s~ecificity of the sensing
apparatus. The membrane 26 can also be used to
prevent harlnful components of the ~luid material froln
reaching the electrodes 13,14 and the electrolytic
medium and altering tneir ~roperties in solne way.
~he mem~rane 26 may be composed of any material Wt)i
is selectively permeable to the species ~eing
detected Such materials include ru~bers and
synthetic polymers among other materials.
Figs. 1-3 depict a planar sensor structure in
accordance with the present invention. Such a planar
structure is tne most ~referred embodiment since it
requires the least number of components, minimizes
the electrolytic interference and simplifies the
construction. ~urtl;er, the planar sensor allo~s for
smoother and more homogeneous coatings of the
electrolytic medium since these coatings, with the
exception of the second layer of electrolytic
material in Fig. 3, are being applied to smooth
surfaces. This type of sensor geometry gives
excellent results because of its simplicity of
design, ease o manufacture, and consistency.
The device of ~igs. 1 and 2 offers many
advantages over prior art devices. In this
embodinnent the electrodes 13 and 14 are in direct
contact with the fluid material thereby eliminating
the need for tne fluid material to di~use across
meMoranes or electrolytes. This direct contact
results in a shorter response time beca~se of the
elimination of the diffusion resistance of
-15-
electrolytic or membrane layers. Anotner important
advantage of this embodiment results from the coating
of the electrolytic medium directly onto the
su~strate 11 rather than onto tne electrodes 13 and
14. Since the substrate 11 has a smoot~ sur~ace t~e
electrolytic medium will form a smootn, thil),
homogeneous coating on tne substrate 11. Prior art
devices coated the electrolytic medium over the
electrodes 13,14 thus forming a non-holno~elleous
coating due to the rougnness of ti~e surface onto
wnich the electrolytic medium had to be coated. T~e
coating of the invention also minirnizes tne stresses
placed on the electrolyte by the surface onto which
it is coated since the electrolytic mediun is coated
onto a smooth surface.
Another embodiment of the present invention is
shown in Fig. 4. This sensor has a sandwich-type
structure. Again, there is a thin coating of a first
layer 25 electrolytic medium between tne su~strate 11
and the counter electrode means 13. ~o~ er, in the
sandwich-type structure there is also a second layer
27 of electrolytic medium coated atop the counter
electrode means 13. The sandwich-type structure nas
several advantages over the planar structure. The
main advantage of the sandwich-type structure is tne
increased rigidity of the sensor structure due to the
extra layers of material applied thereto. This
increased rigidity will minimize the distortion of
the electrodes 13 and 14 and electrolytic mediuln
whici~ usually results froln thermal and physical
stresses placed on the sensor apparatus. Another
advantage of the sandwich-type structure is that the
counter electrode 13 and reference electrode 23 are
partially shielded from the fluid material by an
additional layer of electrolytic medium. This will
minimize undesirable reactions at tl~e counter
-16-
electrode 13 and the reference electr~de 2~.
Excellent sandwich structures are possible as a
result o~ the coating tect~ni~ues deve1l~ped in
United States Patent No. 4,795 543.
These coating tec}lni~lles a1lo~ foe
smooth, relatively nomogeneous coatings o~ tlle
electrolytic r,ledium to ~e dpp1ied over the el~ctrodes
13, 140 '
The preferred ernbodiment of the present inventio
will maximize the perimeter of tile working electrode
14 with respect to the area of contact of the working
electrode l4 with the electrolytic medium. This
maximization of the perimeter to area ratio results
in a corresponding maximization of the sigllal to
noise ratio of the sensor. The theoretica1 ~asis for
this result is that the area of contact between the
working electrode 14 and the electro1ytic medium
appears to be responsible for the noise in the
sensor. Whereas, the electrochemica1 reactioll
between the working electrode 14 and the species
appears to ~e catalyzed ~y the electrolytic medium.
Therefore, tne triple-phase boundary between the
working electrode 14, electrolytic mediuln, and the
species is the preferred location for the
electrochemical reaction between the species and tlle
working electrode 14. As a result, the signal
generated by the electrochemical reaction appears to
be directly proportional co the perimeter of the
working electrode 14 since the peri~neter is a medsuee
of the triple-phase boundary.
It is important to note that the sensillg
apparatus of the present lnvention, if it uses a
Nafion electrolyte, rnust be operated in an
environment having at least some hulrlidity. Tl~e
absence of water in the environment ~ill prevellt the
7~396
--1 1
successful operation of tn- appdratus by hit~dering
the role of the electrolytic mediuln. rnis is ~ca~l;e
the yer fl~oro melnbrane re~3ires water to activate
free protons. Ocher solid electrolytQs, s~3ch as
polyvinylalcohol and pol~ethylene oxid~, may not
re~uire the presence of nulnidity.
In operation, the assembly is contacted with a
fluid material including the species to be detected.
the species will diffuse to the working electrode 14
and there an electrochemical reaction will take place
generating a measurable signal. The signal is
measured by the ammeter 20 and the measured signal is
preferably fed to a Microcomputer for normalization
of the signal as well as any other mathe3natica1
manipulations SUCh as calibration Whicll may be
necessary. The response time for the sensor is
usually less than five seconds.
The following examples are provided to illustrate
certain embodiments of the present invention.
Example 1
A 2~ silicon wafer wa~ oxidized to provide an
insulating silicon dioxide surface. Then the wafer
was spin-coated with a 5% Nafion solution (Aldrich
Chemical Co., Milwaukee, WI) to Inake a planar
electrolytic structure. A two-step evaporation
procedure was used to create grid electrode patterns
r on the surface of the Nafion layer. An evaporation
system containing both e-bea3n and therlnal evaporation
capability was used to deposit the electrode
structures. A photolitho~raphically etched
evaporation mask was prepared from thin copper foiL
in which a number of parallel rectangles ~.~ere etcl)ed,
each 6 mln long and 125 microns wide. After the first
evaporation deposition the mask was rotated 90 and a
second evaporation was performed. Gold wire (99.9~,
Engelhard Minerals anc Chemicals ~o., NJ) was us~d as
~79~96
-l8-
the evaporation source. The electrodes were
electrically connected to a power source.
~xample 2
A sensor fa~ricated as in ~xalnple 1 was exposed
to various gas mixtures ~ith tne ~ollo~ing results.
The sensor was operated at a constant potential of
+300 millivolts versus the Platinuln/air reference
electrode. S/N is the signal to noise ratio of the
sensor. The signals are glven as normalized values
with the signal for H2S being taken as 1.0 and all
other responses being scaled accordingly.
_ensor Sigrlal to Various Gas !~ixtures
Gas Mi~ture Day _gnal(S) Noi~e(N) S/N
92 ppm NC)/NO2 1 2.380.28 8.5
83 ppi~ H~S/N2 1 27.10.28 27.1
92 ppm NO/NO221 0.24 0.0212.0
83 ppm H2S/N221 2.72 0.0213.6
4~ pp.~ 2~ir 21 0.02 O.U2 l.U
80 ppm SO2/air 21 0.036 U.02 1.8
200 ppm C0/air21 0.00 O.U2 0.0
100 ppm HCN/air 21 spike0.02 spike
;
__