Note: Descriptions are shown in the official language in which they were submitted.
FI~L~-OF TH~ INVEN~IO~
The present invention relates to a process for
entrapment, preservation and\or growth of
anchorage-dependent cells and tissues in an artificial
environment. More particularly, the present invention
deals with methods and related products Eor entrapping
anchorage-dependent cells and tissues in a permeable
gel-like material, nurturlng and growing such cells
within the gel-like mini-environment while supplying
needed nutrients and other materials through the
permeable gel from a macro-environment, and harvesting
the metabolic and~or other products or by-products.
The present invention permits in vitro cell culture or
growth of anchorage-dependent cells and tissues to high
densities, increased yields of biologically produced
products and many other benefits. Similarly, the
present invention permits the entrapment and
preservation of anchorage-dependent cells ~or long
periods of time.
There are molecules of great investigative,
clinical and perhaps commercial value that can best be
produced by growing in culture, anchorage-dependent
cells that synthesize them. The problem is that it is
no simple matter to grow large quantities of
anchorage-dependent cells in an artificial medium. The
well-developed technology of industrial microbiology is
adapted to the requirements of bacteria, yeasts and
,. ..
-.~,
-2~ 8~38~L
molds. Each single cell, encased in a tough cell wall,
is an independent metabolic factory with fairly simple
nutritional requirements; for bacteria, glucose and
some simple salts w~l often suffice. Microorganisms
grow well floating free in a liquid culture medium in
tanks with a capacity of as much as 50,000 gallons,
resisting damage even when they have proliferated to
form a thick suspension and even when the suspension is
stirred vigorously with a mechanical agitator.
Mammalian cells are different. They are larger
than most microorganisms, more fragile and more
complex. The delicate plasma membrane that encloses an
animal cell is not encased in a tough cell wall. The
mammalian cell's nutritional requirements are more
stringent than those of most microorganisms and indeed
have not yet been fully defined. Rather than being a
free-living organism, a mammalian cell is adapted to a
specialized l~e as part of an organized tissue,
dependent on the specialized functions of many other
cells and on a circulatory system that ensures a
precisely adjusted and stable environment for each
cell. Such a cell resists being separated from its
tissue and grown in an artificial medium. Most animal
cells will not grow at all in suspension; they grow
only when they can attach themselves to a surface, thus
the name anchorage-dependent. Over the years
techniques have been developed for growing
anchorage-dependent cells on a small scale ~n the
laboratory. However, it has proved to be much more
difficult to grow them efficiently on even a moderatPly
larger scale.
, .. ;., ...... ..... . ~...... .. .
~3q33~
--3--
Techniques for moderate- and large-scale production
of a~chorage-dependent animal cells have not changed
significantly since their development in the early
1960s. Large-scale growth of anchorage-independent
cells ~suspension cultures) has been achieved by
applying the techniques of submerged cultivation of
microbial cells. However, the surface requirements of
anchorage-dependent cell types has tended to preclude
an analogous development.
Current techniques for the propagation of
anchorage-dependent cells are based on a multiplicity
of small-volume, low-productivity reactors, such as
roller bottles. Since it is common for a
moderate-sized facility to operate hundreds of these
growth vessels for a single production run, even a
simple manipulation such as medium supplementation
requires hundreds of operations. ~ore complex
adjustments requiring multiple operations per bottle,
such as cell harvest, compound the problem
accordingly. Costs of equipment, space, and manpower
are high for this mode of cell production.
In an attempt to overcome these problems and to
increase process scale and productivity, alternative
methods for the propagation of anchorage-dependent
cells have been suggested. These techniques include:
plastic bags or tubes, stacked plates, modified roller
bottles, packed-bed propagators, artificial
capillaries, microcarriers, and encapsulatian. Such
techniques have been reviewed previously by Litwin,
Proc. Biochem., 6:15 (1971), Maroudous, "New Techniques
in Biophysics and Cell Biologyn, R.H. Pain and B.~.
Smith, Eds. ~Wiley, New York (1973)), and Levine et al.
~Cell Culture and its Applicationsn, R. Acton, Ed.
~Academic, New York ~lg77)).
,,., . . , ~ . , ~ . , . . . . : .
_4~ 3B~
A more recen~ innovation in the propagation of
anchorage-dependent cells is the microcarrier syste~.
The potentlally high surface-to~volume ratio (S/~ in a
well-mixed microcarrier system allows a single
high-productivity vessel to substitute for many
low-productivity vessels, reducing the number of
operations required per cell, making practical the
application of better environmental controls, and
providing a homogenous growth environment and cell
yield. A single reactor vessel also reduces laboratory
space and manpower costs.
The microcarrier system is not without its
problems, however. The potentially high S/V, and hence
high cellular prod~ctivity, of the system has not been
realized due to so-called "toxic effects" of the
microcarriers on the growth of certain cell types.
These effects are manifested at low carrier
concentrations (1 g A50/liter) as an initial loss of 50
to 75~ of the cell inoculum, and at higher carrier
concentrations (> 2 g A50/liter) as greater degrees of
cell loss and a general suppression of culture growth.
Various strategies have been employèd to alleviate the
"toxic effectsn, including: pretreating the beads with
serum or nitrocellulose, increasing cell inoculum, and
adding spent culture medium or additives to the growth
medium. It has been proposed that the observed A50
"toxicityn may be the result of the adsorption of
certain critical nutrients by the beads. Others have
suggested that microenvironmental effects are critical
for cell propagation on microcarriers. Additional
disadvantages include: ~1) the cells attached to the
carrier are exposed to an external environment, and as
such subject to collision, shearing, etc.; (2) recovery
_5_ ~ 3 ~
of the cells depending on the degree of attachment -
strongly attached cells are often damaged or killed
upon ~reatment with trypsin and sim;lar enzymes; (3)
growth of cells typically involves the use of
DEAE-chloride, a suspected carcinogen which may be
deleterious to the cells if there is leaching; (4)
bridging between microcarriers resulting in mixing
problems; and (5) recoverability o the microcarriers
for re-use which has proved impractical in industrial
applications.
Yet another innovation in the propagation of
anchorage-dependent cells is microencapsulation. Over
the years, there has been considerable interest in the
encapsulation or immobilization of living cells. See
generally, K. Mosbach, Ed., M~t~ods in-Enzymolo~y, Vol.
44, Academic Press, New York, 1976; B.J. Abbott, ~n~.
Rpt~ Perm. Pr~c., 2:91 (1980); R.A. Messing, ~rih~J~
~erm. Proc" 4:105 (1980~; Shovers, e~ al. U.S. Patent
No. 3,733,205 (1973).
More recently, efforts have been concentrated in
processes for encapsulating tissue and individual
cells, particularly mammalian cells, so that they
remain viable and in a protec~ed state within a
membrane which is permeable to the plethora of
nutrients and other materials required ~or normal
metabolic functions.
One such technique is described in U.S. Patent No.
4,391,909, wherein tissue cells such as Islet of
Langerhans cells are encapsulated within a spherical
semipermeable membrane comprising a polysaccharide
having acidic groups which have been cross~linked with
acid reactive groups of a crosslinking polymer for
permanence of the protective membrane. The
semipermeable membrane has a selected limit of
~ 3
--6--
permeability of no greater than about 200,000 daltons,
so that serum proteins and other high molecular weight
materials necessary for growth can be sealed with the
living cells within the semipermeable membrane, while
other, smaller molecular weight metabolites and
nutrients can traverse the membrane wall and be
interchanged with the outside media. The process
therein disclosed comprises suspending the tissue to be
encapsulated (and the high molecular weight nutrients)
in a physiologically compatible medium containing a
water soluble substance that can be made insoluble in
water (i.e., gelled), to provide a temporary pro~ective
environment for the tissue. The medium containing the
tissue is next formed into droplets by forcing the
tissue-medium-nutrient suspension through a teflon
coated hypodermic syringe, the tip of which is
subjected to laminar air flow which acts as an air
knife. See also U.S. Patent No. 4,352,883, wherein the
spheres are formed by forcing the materials through a
capillary tube into the center of a vortex created by
rapidly stirring a solution of Ca++ cation. The
medium, e.g. a polysaccharide gel, is temporariIy
gelled in a generally spherical shape by contact with
the calcium solution. Thereafter, these "temporary
capsules", are provided with permanent polymeric
semipermeable membranes at their outer layer, formed by
permanently Cross-linking or polymerizing the capsules
with polymers containing reactive groups which can
react with specific constituen~s of the
polysaccharides.
This technique has most recently been applied to a
method of growing anchorage-dependent cells as
disclosed in V.S. Patent No. 4,495,288, wherein the
-7- ~ 3~
cell to be encapsulated is suspended in a medium
containiny an anchoring subs~rate material and other
high molecular weight components needed to maintain
viability and to support mitosis prior ~o
encapsulation.
Such complex prior art processes are not without
limitations. For instance, with mammalian
anchorage-dependent cells, although it has been
possible to encapsulate viable and metabolically active
cells within hardened semipermeable membranes,
promotion of growth therein has not been satisfactory~
Moreover, cell densities thus far achievable within
such membranes has been less than about 106 cells per
milliliter of culture media. Both of these limitations
affect the amount and recovery of useful and desirable
cell products produced by the encapsulated material.
The ability to grow anchorage-dependent cells to hiyher
cell densities within a protected ènvironment (capsule)
would provide a means for achieving greater output of
desirable cell products.
A further disadvantage of prior art methods of
entrapping such cells is the inability to maintain cell
viability at desirable higher cell densities. In
addition, the restricted permeability of the capsular
membrane prevents access of the encapsulated cells to
high molecular weight inducer compounds. This
restriction necessitates the release of the cells from
capsules prior to induction of product synthesis~ The
added steps required to release the encapsulated cells
may effect cell viability and/or product formation in
response to the inducer.
: .........
- 8- ~8~38~
.~LJM M ARY . OF TF ~ ~E~CI~
In accordance with the present invention, there is
provided a novel approach to the entrapment,
preservation and/or propa~ation of anchorage-dependent
cells and tissues and to the recovery of products and
by-products provided therefrom~ More specifically,
there is provided methods of entrapping
anchorage-dependent cells and tissues within an
artificial gel-like environment so as to permit growth
of such cells in in -vi~ro tissue culture media to
greater than normal cell densities, maintenance of high
cell viability and the harvesting of cell products and
by-products produced in the entrapped state.
The basic approach to the entrapment/preservation
and/or propagation of anchorage-dependent cells in
accordance with the present invention involves
suspending the anchorage-dependent cells in a solution
containing an anchoring substrate and a polysaccharide
gum such as alkali metal alginate. The suspension is
thereafter formed into droplets which are gelled in a
calcium chloride solution, washed and grown in culture
media to preserve andior proliferate
anchorage-dependent cells entrapped therein. As noted
above, it has been difficult to grow
anchorage-dependent cells efficiently on even a
moderately large scale wh~e maintaining greater cell
densities and higher cell viabilities. Previous
approaches to solving such problems have not been
entirely successful, i.e. the toxicoids and other
problems of microcarrier systems and the inability of
traditional encapsulation techniques to provide
desirable cell densities and viability. In this
regard, in contrast to the overcoating methods of U.S.
Patent Nos. 4,391,909 (Lim) and 4,495,288 (Jarvis3~ it
- .. - - . - . . ~
33~l
g
is important in practicing the present invention that
no semipermeable membrane be formed on the outside of
the hydogel beads, either by crosslinking of the
hydrogel or by coating with a further polymer, for a
number of reasons. Such coatings may interfere with the
free diffusion into and out of the hydrogel beads. The
added steps required to form ~he semipermeable membrane
will have a negative effect on cell viabillties and
make recovery of cells from capsules more difficult.
Also these "temporary capsules" must be nearly perfect
spheres to insure formation of a non-leaking capsule.
The shape of the hydrogel bead in practicing ~he
present invention is of less importance and has no
direct bearing on the usefulness of the resultant
hydrogel beads. Another advantage of entrapment of
anchorage-dependent cells in accordance with the
present invention is that it permits recycling and
re-use of the cells contained thereinr simply by
dissolution of the hydrogel, which leaves the cells
intact, and free from any non-cellular materials. This
cannot be easily achieved with microcarrier systems nor
with other encapsulation techniques where the cells are
enveloped in an insoluble polymer coating. The present
invention overcomes such obstacles in that it allows
for entrapment, preservation and/or propagation of
anchorage-dependent cells at viabilities in excess of
90% and at cell densities where desirable cell products
or by-products can be economically harvested for
commercial use. The absence of any semipermeable
membrane on the outside of the hydrogel bead permits
diffusion of molecules greater than or equal to one
million daltons in size. This eliminates the need for
any additional steps necessary to release gel-entrapped
-10~ 3 ~
cells prior to induction of product using high
molecular weight inducers. Elimination of added steps
will improve the subsequent cell viabllities and/or
product formation.
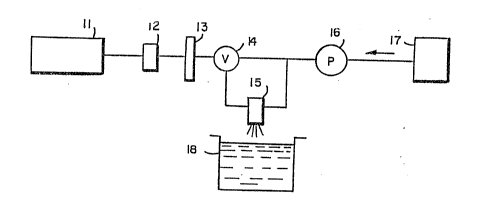
BRIEF DE~cRI~-T-Io~ ~11 G~rlUlL~a
Figure 1 illustrates one apparatus for entrapping
anchorage-dependent cells.
Figure 2A depicts the growth and viability of
entrapped murine epithelial cells designated Cl270
Figure 2B depicts secretion of hepatitis-B virus
surface antigen from gel entrapped murine epithelial
cells designated Cl?7.
Figure 3 depicts the growth and viability of
entrapped murine fibroblast cells designated SV-3T3.
Figure 4 depicts the growth and viability of
entrapped human epitheloid carcinoma cells designated
HeLa S3.
Figure 5 depicts the growth of muri~e mammary tumor
cells in alginate~entrapped gelatin microcarrier
cultures.
Figure 6 depicts the growth of Chinese hamster
ovary cells in alginate-entrapped gelatin microcarrier
cultures.
~ETAII,ED DE~IPTION - QF _~Z~eIQ~
The present invention provides a novel approach for
the entrapment, preservation and/or propagation of
anchorage-dependent cells in---v~tr~ and harvesting
products produced thereby. More spec~ically, it has
now been discovered that anchorage-dependen~ cells can
be entrapped in hydrophilic gels by a process which is
much simpler than those previously used; that such
entrapped cells can be grown to large cell densities
and maintained for substantial periods of time, without
the need for an additional selectively permeable
membrane surrounding the entrapped cells; that such
entrapped cells can be used to produce high levels of
metabolic or other cellular products, such as hormones,
vaccines, interferons7 and that, after a suitable
period whereln the production of the desired
material(s) is maximized, the used, but viable cells,
can be recovered for re-use by resolubilizing the
hydrophilic gel to release the entrapped cells,
followed by re-entrapment using the same procedure, as
described above.
The process described herein by which
anchorag~-dependent cells are entrapped and propagated
and their products harvested therefrom typically
include the following steps:
A~ ~agQn~: (filter sterilized)
1. 1.0~ sodium alginate (Kelco-HV) in 0.
NaCl
2. 0.9~ NaC1
3. 1.2~ CaC12
4. Trypsin-EDTA solution (Flow Labs)
5. 1% EDTA/0.5% NaCl, pH 7.1
6. Complete culture media
7. Vitrogen-100 (Collagen Corp. Palo Alto,
CA)
B. ~ (standard sterile technique employed)
1. Anchorage-dependent cell stocks are
maintained in 850 cm2 plastic
disposable roller bottles or standard
tissue culture flasks under conditions
necessary to maximize cell viability (eg.
150 ml complete media per bot~le, 37C
incubation at a rotation rate of 0.25
rpm).
* Trade Mark
. , :
C. ~xperimental Pr~t~ol
(Standard sterile technique is employed
throughout)
l~ Cells are harvested from roller bottle
cultures by removing the culture medium and
adding 25 ml trypsin-EDTA solution. Roller
bottles are then laid on their side and rolled
to spread the trypsin-EDTA over the entire
area of the cell monolayer. The trypsin-EDTA
is then removed and the process repeated once
again. The roller bottle is incubated at
37C and rotated at 0.25 rpm in a
conventional roller apparatus.
2. After 10-20 minutes cells will begin ~o
slough-off the surface of the roller bottle.
50 ml complete culture media is added and the
roller bottle is tightly capped and agitated
to wash the cells from ~he surface. The
suspended cells are then counted in a
hemocytometer. Typically a maximum of l-3 x
108 cells/roller bottle is obtained.
3. 1-5 x 108 cells (typically 2 x 108) are
then centrifuged at 800 rpm for 5 minutes~
The media is aspirated off and the cell pellet
is loosened by gently flickiny the centrif~ge
tube. The cells are then resuspended in 20 ml
of a collagen solution (Vitrogen-10 ~ which
has been neutralized to pH SoO~7~0 by the
addition of 1.0 N NaOH. The final collagen
concentration can be 0.1-1.0 mg~ml.
Alternatively, collagen may be replaced by
histones, fibronectin, poly L-lysine,
crosslinked gelatin microcarriers and other
~, . . .
-13~ 8~
microcarrier particles or other such
materials or combinations thereof depending on
the requirements of the cell being entrapped.
If crosslinked gelatin microcarriers are to be
used, it is necessary to preincubate cells
with the gelatin particles for a perlod o~
time sufficient to permit cell attachment.
4, 80 ml of 1.0% Na algina~e is then added and
the cells are mixed to form an even
suspension. The final alginate concentration
is 0.8%, although final concentration of
0.6-1.2~ can be used.
50 The cell suspension is then delivered to a
conventional two phase spray head using a
peristaltic pump. Sterile air is also
delivered to the spray head at 3.0-4.0 SCFH.
The alginate/cell droplets are propelled out
of the spray head into 0.5-l.OL 1.2~ CaC12
solution to form shape-retaining gel beads.
Flow conditions are adjusted so that the gel
beads are left in CaCl~ for no more than 15
minutes.
6. The gel beads are then washed twice with 009%
NaCl solution and once with complete media.
7. Cultures are best established by resuspending
the gel beads in complete culture media to
20-30% beads (v~v) and incubating a~ 37C
with mixing. Cultures are refed as neededO
Preservation of entrapped anchorage-dependent
cells is accomplished by modifying the culture
media, i.e. reducing the serum and/or glucose
concentration to decelerate the growth o the
entrapped cells.
'.' .', ' ' ' . ' , ' ' , ' ' , ~
.
-14~
8. Cells are counted by washing a 0.5 ml aliquot
of beads with 10 volumes of 0.9% NaCl and
dissolving the bead~ in loS ml 1~ EDTA/0.5%
NaCl, pH 7.1. After a 10-20 min. incubation
at room temperature 7.0 ml trypsin-EDTA is
added and the sample is incubated at 37C
for 15-30 minutes with occasional shaking.
1.0 ml 0.4% trypan blue solution i5 added and
the cells are counted in a hemocytometer.
9. Metabolic and other cell products may be
harvested from the media where said products
diffuse i~to the media. Entrapped cells may
be released from the hydogel beads for final
harvesting by adding 2-5 volumes of E~TA
buffer and incubating ~or 20 minutes at room
temperature. Cell agregrates may be dispersed
trypsinization.
Although the above-outlined steps represent the
preferred mode ~or practlcing the present invention, it
w~l be apparent to those skilled in the art that the
above-described approach can vary in accoxdance with
techniques known in the ar~.
The hydrophilic gel used for entrapment is
preferably an alginate, which is a natural hydrocolloid
derived from seaweed, although other hydrophilic
materials such as agarose, agar, carrageenan, chitosan,
xanthan gum, poly HEMA, and others known in the art can
be used to advantage in particular environmen~s.
Highly preferred are clari~ied long-chain sodium
alginates, such as Kelco-Gel HV and Kelco-Gel LV, sold
by Kelco Company (San Diego). These are sodium
alginates which are fibrous in nature, are supplied at
a neutral pH, (typically~ about 7.2) and contain
approximately 80~ carbohydrates, 9.4% sodium, 0.2%
* Trade Mark
,. ~ .
., ~ ' ''.
:~ .
-15-
calcium, 0.01% magnesium, and 0.1~ potass~um~
Relco-Gel HV has the higher molecular weight, having a
srookfield viscosity of about 400 ~1% solution) to
about 250 (2% solution). Of these products, the Kelco~
Gel HV is highly preferred. Preferably, the
hydrocolloid is further clarified by sequential
f~tration through filters having pore sizes of 2.5
1.2 and 0.6 micxons, respectivelyl and s~erilized
before use by passage through a ster~e filter having a
pore slze of 0.45 microns or smaller.
The concentration of hydrocolloid in the mixture
should range from about 0.5 to about 1.4%, preferably
about 0.6 to 1.2%, most preferably about 0.7-0.9%.
This is considerably below percentages previously used~
and is believed to resul~ in higher porosity of the gel
beads to nutrients and other factors. Attempts at
making beads below 0.5mm in diameter have met with
difficulty, even with the fairly viscous Relco Gel HV~
and especially with Relco Gel LV.
The particular anchoring substrate used for
propa~ation of anchorage-dependent cells w~l depend on
the requirements of the cell being entrapped.
Exemplary water soluble anchoring substrates include
collagen, a natural protein which is the chief
constituent o~ connective tissue in animals, collagen
plus fibronectin, histones, poly L-lysine, gelatin and
the like. water insoluble anchoring substrates (e.g~
crosslinked gelatin particles or commercial
microcarriers such as dextran and glass particles) may
also be used to advantage. The anchoring substrate
solution is pr~ferably neutralized to a pH between
6.0-7.0 prior to suspension of anchorage dependent
cells therein. The final concentration of the water
16
soluble anchoring substrate may range between about
.1-1.0 mg/ml of alginate. Wa~er insoluble anchoring
substrates may comprise up to 50% (V/~ of the final
bead volume.
Preferably, the micro-environments which contain
the anchorage-dependent cells, the hydrophilic gelling
agent, the anchoring substrate and various nutrients
and accessory materials, are formed into discrete
particles, preferably generally spherically-shaped
particles. Preferably, the gelled particles are mobile
and thus can be arranged for convenient culturing,
treatment and product extrac~ion. Thus, for example,
the entrapment beads can be arranged, nurtured, or
extracted in packed beds, fluidized beds, in stirred
containers, in continuous reactors or treatment units,
which themselves are known in the art, e~g. similar to
those used for treating ion exchange resinsl etc. The
conditions of treatment, including temperature,
pressure, solvent, and physical treatment should be
chosen so that the entrapment beads retain their
particulate nature.
The condition of treatment of the ~entrapped cells
should also be chosen to maintain viability and growth
of the cells contained therein. Thus, the entrapped
cells should not be exposed to extremes of temperature~
pH, or to toxic chemicals, or amounts of time which
would cause loss of viability of the desired cells.
Temperature may range broadly from about 5C to about
45C, preferably between about 15C and about
40C. For many cell systems, growth is optimized at
temperatures around 37C~ The pH at which the
entrapment gels are maintained may also range broadly
between about 5 and 9, preferably between about 6 and
,
,; .
:- .
-17- ~ 38~
8. Various steps in treatment of the en~rapped cells
may require different pH's, and pH values outside of
the broad ranges can often be tolerated by the cells
for limited periods of time without deleterious effect.
Viability and growth of anchorage-dependent cells
normally require, in addition to an anchoring
substrate, access to a source of oxygen for
respiration, as well as various nutrients, vitamins~
amino acids, salts, and other components, known per se
for such cell types. Normally some of these nutrients
and other factors will be entrapped within the gel bead
along with the cells, so that continuous growth for
some periods of time can be maintained without further
additions of such factors. However, culture of such
cells for production of proteins or other metabolites
or products require considerable time, and such
production is normally optimized by providing the cells
with ready access to the required nutrients and other
ingredients. Thus, the entrapped cells are preferably
suspended in or otherwise contacted with a fluid
containing oxygen, nutrients, vitamins, minerals, etc.t
which can diffuse through the hydrophilic gel to the
cells and thus maintain viability and growth. It may
also be desirable to include an anchoring substrate in
the media to optimize attachment and propagation of the
entrapped anchorage-dependent cells. Such substrates
(e.g. fibronectin) are constituents of serum
supplements normally used in culture fluids.
Figure 1 illustrates one apparatus which may be
utilized in entrapping anchorage-dependent cells in
accordance with the present invention. The apparatus
comprises a controlled cource of sterile air, means for
admixing the cells to be grown with the anchoring
substrate/hydrophilic gel-forming material while such
material is in liquid form, means for feeding the
,
. ~ :
-18~
sterile air and admixed cells/hydrocolloid to a
standard gas/liquid atomizing spray head, and a
reservoir of material which receives and gels the
droplets formed by the spray head.
Thus, as shown schematically in Figure 1, the
~aratus used in the preferred embodiment comprises a
compressor or other
source of compressed air 11, an air flow meter 12, an
air filter 13, which has an effective pore size of 0.22
um (micron) or less, so as to sterilize the air used.
The ster~ized air then proceeds through a control
valve 14, to a conventional two-phase spray head 15,
where it mixes with the liquid cel~hydrocolloid
mixture.
The liquid cel~hydrocolloid mixture is preferably
formed in a tank 17, and is fed to spray head 15
through a pump 16, which is preferably a controlled
constant volume, peristaltic pump as is known in the
art.
In the spray head 15, the liquid is forced out a
small diameter (0~006-OolOO m~) cylindrical top, which
is surrounded by an annular air passageway. The air
contacting the droplets formed at the end of the top
frees the droplets from the tipso The droplets are
then propelled out into the atmosphere in the form of
fine spherical droplets. The droplets then contact the
liquid in container 18, which contains a divalent
cation gelling agent, which gels the liquid droplets,
such as a calcium chloride solution, where the
hydrocolloid used is sodium alginate. Other divalent
cation gelling agents include th~ other alkaline earth
metals ~except magnesium}, other divalent metals, and
divalent organic cations, such as ethylene disamineO
Preferably, tank 17 and container 18 are both stirred
~: r ^, 1 l " f `~ ?
,' -
-19~ 38'~
during the process at slow speed, in order to keep the
solids from settling out and to maintain constant
concentratlon.
Preferably, the flow rates of gas and liquid are
adjusted so that the size of the particles or droplets
formed ranges from about 0.4 to about 2mm in diameter.
The flow rates depend to some extent on the viscosity
of the liquid hydrocolloid, which in turn depends on
the type and concentration of the hydrocollo~d used.
The provision of from about 0.4 to 2 millimeter
particles~ preferably about 0.6-1.5 millimeter
particles, permits sufficient diffusion of nutrients
and accessory growth factors into the particles to
provide for cell growth.
The spray head or nozzle utilized in connection
with this invention need not be the modified hypodermic
syrinyes used in previous process. Rather, standard
off-the-shelf biphasic spray heads can be utilized to
advantage in making the desired beads. Suitable spray
heads include those sold by Spraying Systems, Inc.,
such as products sold under the designations 1/8 and
JACN, lf8 JACN 1/8 JBg. Other suitable nozzles are
available in the art. Preferably, the nozzles used in
this invention are beveled at the outside of this tip
to form a conical tip, the sides are sloped at 15 or
30 to the longitudinal axis of the top, to direct
the air flow at more of an angle to the droplets
formed. Such an angle can be simply ground into the
liquid t~,p orifice. Preferred inner diameters for the
liquid spray tip include 0.006n, 0.010n, 0.016n, and
range in size to a maximum of 0.100" with the smaller
sizes preferred, to produce smaller droplets.
-20~ 8~
The following examples are given to additionally
~llustrate embodiments of ~he present invention as it
is presently preferred to practice. It w;ll be
understood that these examples are illustrative, and
that the lnvention is not to be considered as
restricted thereto except as indicated in the appended
claims.
E*k M PFJ, ~
~ ntrapment---of - ~net~c~ nqineereds~-Mu~ine
1. Murine epithelial cells (clone C127
derivatives) were grown as monolayer cultures in 850
cm2 plastic roller bottles using media composed of
Iscove's modification of DMEM supplemented with 10~
fetal bovine serum (FBS), 6mM L-glutamine, 50 units
penicillin per ml and 50 micrograms streptomycin per ml
~complete media). 150 ml complete media per bottle was
used and bottles were maintained at 37C a~ a
rotation rate of 0.25 rpm.
2. Cells were harvested from roller bottles by
trypsinization and counted.
3. 1.1 x 108 viable cells were centrifuged at
800 rpm for 5 min and the cell pellet was resuspended
in 6.0 ml Vitrogen-10 ~ (pH6.0) collagen solution. The
final concentration of collagen was 0.50 mg/ml sodium
alginate.
4. Kelc ~ HV sodium alginate was added to a final
concentration of 0.8% sodium alginate (i.e. 24 ml of 1%
HV sodium alginate). The final concen~ration of cells
was 3.67 x 106 cells/ml alginate.
5. ~ydrogel~cell beads were delivered at 10 m~ min
to a two-phase spray head (1650 head, 64SS air cap)
with an air flow of 3.0 SCFH.
~-r~ k
. ........ . ....... . ..... .
,:
6~ Hydrogel/cell beads were ~ ~ 0.50L 1.3~
CaC12, washed twice with normal saline and once wlth
complete media.
7. Cultures were established at ratio of 20:80
tbeads: complete media~ in a T-flask and incubated at
37C in a humidified atmosphere containing 5% C02O
8. Cultures were fed as needed by replacing 50% of
the spent culture fluid with fresh complete media. ~'h~
spent media was stored at -20C until assayed for
antigen. Antigen was measured by radioimmunoassay~
9. Entrapped cells were counted by dissolving 1.0
ml of washed beads in 9 ml 1% EDTA/0.5% NaCl~
centr~uging the released cells at 800 rpm for 5 min
and resuspending the cell pellet in 4~5 ml trypsin-EDTA
solution.
10. After 20-30 min at 37C, 0.5 ml 0.4~ trypan
blue solution was added and the cells counted in a
hemocytometer.
The growth, viability and antigen production of
entrapped murine epithelial cells (C127) over a two
week period is illustrated in Figs. 2A and 2B with and
without the use of Vitrogen-lOO~as the anchoring
substrate.
E-X*MP~E--II
~ ntr~pment ~f-Murine-Fib~ob~ast-~e~s
1. Murine fibroblast cells (clone SV-3T3; ATCC CCL
163.1) were grown as monolayer ~ultures in media
composed of DMEM supplemented with 10% FBS, 50 units
penicillin/ml and 50 micrograms streptomycin/ml
(complete media).
2. Fibroblasts were harvested from bottles by
trypsinization, counted and 6 x 107 viable cells were
centrifuged.
~ f ~,e, -,~ ,
. . . , . ......... . ,. . ......... . , , . ... , .. ., . .. . . ~ .. .
: ' - ., . : ' '
-22~
3. Cell pellet was resuspended in 6.0 ml histone
II-A calf thymus (lmg/m~ (pH 7.0) and mixed w~th 24 ml
1% Kelco~HV sodium alginate. Final concentration of
histone was 0.20 mg/ml alginate.
4~ All subsequent steps were as descr~bed in
~xample I, steps 5-10, with the exception of the RIA
quantitation of antigen in media.
The growth and viability of entrapped murine
fibroblast cells (SV-3T3) is illustrated in Fig. 3.
~-XAMP~
~ ntr~ment~ um~-E-Ei~h~oi~-~ar~inoma-c~l~s
1. ~uman epitheloid carcinoma cells ~HeLa S3; ATCC
CCL 2.2) were grown as monolayer cultures in T-flasks
in media composed of DMEM supplemented with 10~ FBS, 50
units pennicillin/ml and 50 micrograms streptomycin/ml.
2. Cells were harvested by trypSinizatiOn, counted
and 1.2 x 108 viable cells were centrifuged.
3. The cell pellet was resuspended in 60ml 0.8%
sodium alginate (HV) and further processed as described
in Example I, steps 5-10 with the exception of the RIA
quantitation of antigen in media.
The growth and viability of entrapped human
epitheloid carcinoma cells (HeLa S3) is illustrated in
Fig. 4.
E-X-kMP~
~ntrapment-of Murine-M~mmary-~mo~ ell~m
~-lalnate-entrappe~-~h~h~i~L-~L~rQ~ao~icL~
1. Mouse mammary tumor cells were maintained in
850cm2 sterile disposable roller bo~tles in media
composed of Iscove's modified DMEM (IM) plus 10% fetal
bovine serum (FBS) r 6 mM L-glutamine, 50 units
penicillin/ml and 50 mcg. streptomycin~ml (complete
~ v~
', ,
-23 ~ 3~
IM). Cell passages were carried out by incubation of
monolayers with trypsin-EDTA solution.
2. Gelatin microcarriers (K.C. Biological, Lenexa,
Ransas, catalogue ~MC-540) were prepared as described
in the manufactures Procedures Bulletin #38. Gelatin
microcarriers were swollen and hydrated overnight in
phosphate buffered saline (PBS, pH 7.4, Ca2+, Mg2
free). The microcarriers were then washed twice in PBS
and mixed with 1 vol. PBS. Sterili~ation wa~ by
autoclaving for 15-30 min. at 120C, 15 psi.
Microcarr~ers were stored at 4C ~n the dark until
time of use. Prior to use, the microcarriers were
washed overnight in complete media. Alternatively,
gelatin microcarries may be prepared in accordance with
the protocol set ~orth in Example VI below. Mouse
mammary tumor cells were trypsini~ed, washed in
complete media and counted. Cells were preincubated
overnight with 30 ml microcarriers (0.5-2~0 x 10
cells/ml settled microcarriers) in order to allow for
cell attachment. Af~er 15-18 hours at 37C the
culture was divided into 2 equal aliquots and
centrifuged. One pellet was resuspended in 1~5 ml
complete media and used as unentrapped control
culture. The other was entrapped as described
hereinbelowO
3. Microcarriers were centrifuged at 80Drpm for 5
min. at room temperature and the supernatant was
discarded. The pellet was resuspended in 1 3 volumes
of sterile 0.8% sodium alqinate and the mixture was
entrapped as previously described using a 20~100
spraying head. Microcarrier/alginate droplets were
dropped into a pre-warmed solution of 1.2% calcium
chloride. Alginate gel beads were then washed twice in
sterile saline and once in complete IM. Alginate gel
8~
beads were-added to 3 volumes of complete IM in a
spinner flask and incubated at 37C with gentle
stirrlng. Cultures were fed as needed.
4. In preparation for periodic sampling, alginate
gel beads and/or unentrapped microcarrier control
cultures were resuspended in 10 volumes 1% EDTA/D.5%
NaCl and incubated at room temperature until the
alginate resolubilization was complete. Samples were
spun at 1000 rpm for 5 min. and supernatants were
discarded. Pellets were resuspended up to 9 ml in
trypsin-EDTA and incubated at 37C until gelatin
microcarriers were completely solubilized. One
milliliter 0.1% trypan blue was added and cells counted
in a hemocytometer.
The growth of alginate-entrapped gelatin
microcarrier cultures of murine mammary tumor cells is
illustrated in Fig. 5~
- ~*~MPL~-V
~ nt~pment-of-Chinese- ~mster-~ary~~eHO~ ~1-ls- i~
~ lginate-~nt~-a~pe-d ~e~ati~ ca~ç~rr-i-er~.
Genetically engineered CH0 cells were seeded onto
10ml of gelatin microcarriers (2x106 cells/ml
gelatin) in a total culture volume of 125 ml. After 24
hours at 37C the culture was divided into control
(unentrapped) and experimental cultures (entrapped).
Gelatin microcarriers were mixed with 3 volumes of 1%
alginate and entrapped as described in Example IV.
The growth of alginate-entrapped gelatin
microcarrier culture of CH0 cells is illustrated in
Fig. 6 as compared with unentrapped microcarrier
control cultures.
,, - ., _ . , . . :
. ' ', ` , . _ , : ' ~ ,, ' , ' ' 1 ~
.' " ;.. '.. `.. ' ' . ' ' . . .'' , ` (
28~33~L
~-~kMP~
~ o~uc~on- ~f- ~-ros~link~- ~el~in- ~artic~e~
I. Reagents
1. gelatin-Type ~ porcine, 225 Bloom
2. glut~raldehyde-25% solution
3. distilled/deionized water
4. phosphate buffered saline, pH 7.4 (PBS Ca2~7
Mg2+-free).
II. Protocol
1. 100 gm gelatin was slowly added to 800 ml of
rapidly mixing water preheated to 60 70C. When all
the gelatin was in solution the volume was adjusted to
1000 ml with water.
2. 20 ml glutaraldehyde was quickly added to the
rapidly mixing gelatin solution. Gelation was complete
within 5 min.
3. The crosslinked gelatin was broken into large
pieces, rinsed 3 times with ~ volumes of water and then
mixed with 1 volume of water.
4. The 50% suspension was transferred to a kitchen
blender and liquefied for 30 sec.
5. Gelatin particles were washed 5 times with water
by centrifuging the gel slurry for 5 min. at 3000 rpm
and resuspended the pellet in 3-5 volumes of fresh
water.
6. The gel particles were then resuspended in 2 3
volumes of a 0.1% gelatin solution and mixed overnight
at room temp.
7. Gel particles were then ~entrifuged as above and
washed 3 times in 2-3 volumes of PBS.
8. Gelatin particles were then resuspended in one
volume PBS and transferred to storage bottles.
9. Gelatin par~icles were ster~ized by autoclaving
for 30 min. at 120C~
-
-26- 1Z~i~3 ~3f~
10. Crosslinked gelatin particles wQre stored in the
dark at 4C and washed overnight with complete media
just prior to use.