Note: Descriptions are shown in the official language in which they were submitted.
This invention is concerned with a process for produc-
ing polymethyl methacrylate.
The present invention will be illustrated by way of the
accompanying drawlngs, in which:-
Fig. 1 is a graph comparing the PMMA polymerizationcharacteristics according to the present invention with those by
a conventional process; and
Fig. 2 is a schematic view of arrangements for embody-
ing the process of the invention.
Polymethyl methacrylate (hereinafter called "PMMA"), a
resin with the best transparency among plastics, has long been
used as windshields and in other similar applications. Since it
is a material of a long history, various production methods
depending on polymerization have been proposed. For the manufac-
ture of the resin as an optical link material, however, none of
them have proved satisfactory.
Among the proposed methods, first comes batch suspen-
sion polymerization that consists of polymerizing monomer part-
icles batchwise in an aqueous dispersion and suspension contain-
ing a polymerization initiator. The process is still in wideuse, but the batch operation usually requires a polymerization
initiator (catalyst) which is eventually left unremoved in the
product polymer, together with a suspending agent and the like,
and the molecular weight distribution broadens. Also, the pro-
cess necessarily involves such open stages as dehydration anddrying which allow intrusion of impurities to mar the trans-
parency of the product.
The second method comprises first preparing a syrupy
intermediate polymer by batch bulk polymerization, placing the
syrup
4~3
in between two sheets of glass and effecting polymeriza-tion,
and, following the conclusion of the polymerization, peeling
off the glass sheets to ob-tain a sheet of PMMA. The process
again needs a polymerization initiator and, because the poly-
merization is carried out while the resulting polymer is allowed
to cool naturally between the glass sheets, the polymeriza-tion
temperature becomes ununiform and the polymerization degree
distribution spreads objectionably. The result is poor trans-
parency of the product.
The third is continuous bulk polymerization that can theo-
retically afford t~e most transparent product. According to
the process disclosed, for example, by Japanese Patent Applica-
tion Publication No. 32665/1977, a polymerization initiator
is used and a sirupy intermediate polymer is formed in a pre-
polymerization vessel, and then in a second-stage polymerization
vessel the intermediate is fur-ther polymerized to a final
polymerization ratio ~ > 0.5. The two polymerization vessels
use different polymerization temperatures, and this results
in molecular weight distribution and compositional distribution
as will be described later. Moreover, at ~ ` 0.5, a gel effect
accelerates the polymerization, and the rapid progress of
.,
polymerization causes partial temperature distribution and hence
widened molecular weight distribution. Consequently, the
product will not attain adequate transparency as an optical
link material.
- 2 -
xa~4~
Generally, the molecular weight of PM~ is a function of
the polymerization temperature and the amounts of the polymeri-
zation initiator, chain transfer agent such as ethylbenzene,
and molecular weight modifier such as mercaptan contained in
the feedstock. Together with proper amounts of these additives,
the monomeric material is passed through a high-precision filter
to a particle-free state. The mixture is then fed to a reactor
and polymerization is carried out with stirring. In the case
of batch or continuous [plug (piston) flow type] bulk polymeri-
zation, the reaction is effected at relatively low temperatures
because the initial polymerization velocity is so high that
the heat of polymerization is difficult to dissipate. The
sirupy intermediate polymerization product thus obtained is
transferred to a polymerization reactor, where the rest of
polymerization reaction is performed.
FIG. 1 is a graphic representation of changes in the rates
of reaction with time at different temperatures Tl, T2, and
T3. The experiments showed that, with the same polymerization
reactor and second~stage reactor, the polymerization reaction
proceeded with sequential shifts of the temperature from the
curve Tl to T2 and thence to T3. Toward the end of reaction
a gel effect accelerated the reaction, raising the rate of
reaction to excess. Thus, the molecular weight distribution
in the product PMMA spreads out to impair the transparency.
The initiator and other additives partly left unremoved are
~`
~ 3
"~
~L28~L498
also responsible for the low transparency of the product of ordi-
nary bulk polymerization.
As described above, the conventional PMMA resins have
not been satisfactory for high-precision applications such as
optical fibres for optical links.
The present invention provides a process for producing
PMMA free from impurities such as polymerization initiator or
minute foreign particles and having narrow molecular weight dis-
tribution.
The present invention provides a high-transparency PMMA
suitable as an optical link material in the form of optical fiber
or the like.
In accordance with the invention, there is provided a
continuous proc~ss for thermally producing polymethyl methacry-
late (PMMA) or a copolymer in which, methyl methacrylate (MMA) is
a monomer thereof, which comprises precooling a monomeric feed~
stock comprising MMA or a mixture of MMA and up to 10 mol% of a
monomer highly capable of forming thermal radicals, forcing the
feedstock into a reactor wherein a charge consisting of the
monomers and polymerization product thereof is being circulated
under pressure while being cooled externally, instantaneously
mixing the feedstock and the charge while cooling the charge by
dint of the sensible heat of the feedstock, thoroughly mixing the
charge, continuously taking out from the reactor a portion of the
charge consisting essentially of unreacted feedstock and the
polymerization product at a polymerization product proportion of
50% by weight or less, conducting the portion, while preheating
the same, into a vacuum vessel, removing therein remaining
monomer or monomers by evaporation, and thereafter recovering the
polymerization product. Suitably the highly thermal radical-
forming monomer is styrene. Desirably the polymerization pro-
duct-unreacted monomer mixture in the reactor is circulated at a
- 4 -
~L~1498
velocity of 200 to 300 times as high as the velocity of the
monomeric feedstock being forced thereinto. Preferably the mix-
ing is done by vigorous stirring. Suitably the reactor ls cooled
at all times from the outside during polymerization.
In the invention, rapid temperature rise in the initial
polymerization reaction is avoided, and therefore the initial
polymerization at low temperature, as in the usual process, is
not necessary. MMA alone or together with a thermal-radical-
forming monomer can be instantaneously heated to a predeterminedtemperature and then kept at a substantially constant level for
reaction. Accordingly, the rate of reaction is constant through-
out the reaction period, and the molecular weight distribution of
the resultant polymer is very narrow, giving PMMA of great trans-
parency. The lnvention uses no addltive such as polymerizationinitiator (catalyst)~ and hence the absence of foreign particle
adds to the clarity of the product.
Referring once more to the accompanying drawings, Fig.
1 illustrates the basic difference between this invention and the
prior art. The process of the invention is represented by a
broken line extended past the point C. As soon
2 !3~4~8
as the material monomer or monomeric mixture is forced into
a reactor, it is mixed with the surrounding monomer-polymeriza-
tion product mixture being reacted therein with circulation
and is instantaneously heated to a given temperature. The
monomer-polymer mixture in circulation, on the other hand, is
kept from further temperature rise by the sensible heat of the
precooled monomeric feedstock. After the initial mixing, the
whole mixture is kept at a substantially constant reaction
temperature since it is circulated in the reaction vessel being
cooled from the outside. Accordingly, the molecular weight
distribution too is kept constant.
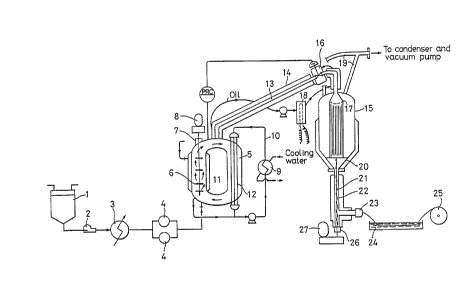
FIG. 2 schematically shows a typical production system
embodying the process of the invention. Referring specifically
to the figure, the present invention will now be described in
detail.
As the material monomer, MMA alone or a mixture of MMA and
up to 10 mol% of a monomer highly capable of thermal radical
formation t especially styrene, is charged into a tank 1. One
of the important features of the invention is that no additive
such as a polymerization initiator (catalyst) that can impair
the light transmission or reduce the purity of the product is
employed. Thermal radical formation by MMA alone is rather
insufficient and is preferably aided by the addition of styrene.
The polymerization reaction in the early stage progresses
rapidly to form abundant hot radicals. Then, styrene is no
- 6 -
~Z~L9~
longer needed and i-ts proportion may be gradually decreased.
The upper space of the tank 1 is filled up with nitrogen to
keep off air.
The monomeric feedstock is forced by a pump 2 into a brine
precooler 3, where it ls precooled, for example, to 15C or
downwards. The precooling temperature is dictated by the
reaction temperature in the polymerization reactor, the tempera-
ture of the monomer-polymerization product mixture being circu-
lated under pressure in the reactor with external cooling, the~
circulation velocity, and other factors.
The feedstock is passed through a super-precision filter
4 to a particle-free condition. Whether the preheating should
precede the filtration or not is of little significance, and
where a sufficiently refined monomer or monomers are used, the
filter 4 may be omitted.
Next, the feedstock is forced into a polymerization reactor
5. As will be explained later, the reactor 5 is sealed by a
monomer-product polymer mixture filling up the space. The
~. _
monomeric feed being pumped into the vessel, therefore, is at
a pressure higher than the internal pressure of the reactor.
For example, when the vapor pressure of the reactant solution
is 35 kg/cm2G, the feed pressure should be equal to or higher
than the value.
The feedstock thus led into the reactor 5 is polymerized
at higher temperatures than usual, or over a relatively high
'` ~ .
" . .... . . ~ ~
8~L~L9~3 -
predetermined temperature range, for example, of 140-170C.
The initial polymerization reaction progresses rapidly and
generates a large amount of heat, necessitating uniform removal
of it to maintain a constant reaction temperature. This
requirement is met in accordance with the invention by the
following construction. As shown in FIG. 2, the reactor 5 is
built in the form of a circulating draft tube, and a high-effi-
ciency propeller-type agitator 6 is accommodated in one leg
of -the tubular structure and is driven for rotation by an
external motor 8 through shaft and seal means 7. The agitator
6 comprises, for example, three high-efficiency marine-type
propellers held in series on a shaft within the draft tube.
It is driven with sufficient power input to effect circulation
of a volume 200 to 300 times the feedstock supply. Thus, the
agitator performs instantaneous mixing of the feedstock with
the mixture of unreacted monomer and polymeriæation product.
The sensible heat of the feedstock is diffused and taken up
by the reactant solution as quickly as the complete mixing.
Cooling oil from a cooler 9, supplied via cooling oil line 10,
is circulated through a jacket 11 around the draft tube section
of the reactor 5 accommodating the agitator 6. Through the
other vertical tube sec-tion on the opposite side, a plurality
of cooling pipes 12 extend and circulates cooling oil therein
to prevent temperature rise of the unreacted monomer-polymeri-
zation product mixture to maintain a predetermined temperature
-- 8
~Z8149~3
level. The tempera-ture inside the reactor is thus kept uniform
by the precooling of -the monomeric feedstock, external cooling
by the jacket 11 and pipes 12, and stirring and circulation
by the agitator 6. Maintenance of the relatively high constant
temperature ensures relatively speedy reaction and enhanced
production efficiency.
It has already been explained in conjunction with FIG. 1
that the polymerization of MMA is accelerated toward the end
of reaction by the gel effect. This must be avoided by reducing
the proportion of the polymerization product in the liquid
reaction mixture to 50% or less and thereby terminating the
polymerization midway. The gel effect can be dramatically
suppressed by the addition of a small percentage of styrene
to MMA. The region of polymerization reaction occupies the
entire reactor space that is filled up with the reactant solu-
tion, the feed being introduced at the bottom with a correspond-
ing overflow at the top. In other words, the reaction region
is all under pressure high enough to keep off minute impurities
through the propeller shaft part or elsewhere. Also, in the
absence of gas phase within the reactor, there is no possibility
of the reactant solution polymerizing on any wall portion
between gas and liquid phases to form solid deposits thereon.
Generally, in copolymerization with the addition of a
comonomer or comonomers, the difference in polymerization
reactlon velocity amcng the participant moncmers prcduces an
_ g _
' '
~:81~!~8
adverse effect. In a batch or con-tinuous piston-flow reaction,
for example, along a full-line curve in FIG. 1, the copolymer
produced at point A is dissimilar in composition to the copoly-
mer at point B, even though the same polymerization temperature
is used. The compositional ratios of such polymerization
reaction products, after all, scatter around the average value.
Strictly speaking, this leads to a slight difference in refrac-
tive index and to a decrease in transparency, though to a slight
degree. In the process of the invention, by contrast, the
addition of a comonomer does not cause the uneven compositional
distribution, but brings uniform copolymer composition through-
out. This is ascribable to the fact that, as described above,
the feedstock is forced into the polymerization react~on solu-
tion being circulated at a rate 200 to 300 times as high as
the feed rate to effect complete mixing, and the heat of poly-
merization reaction is absorbed to a large measure by dint of
the sensible heat of the feedstock, so that the polymer propor-
tion in the polymerization product is kept constant as at level
C in FIG. 1. Thus, the refractive index is constant throughout
the product to a great advantage from the standpoint of light
transmission.
The polymerization product, polymerized to an extent such
that the objective polymer accounts for nearly 50/0 of the total
amount, is forced out of the reactor at an outlet at the top
formed opposite to the bottom inlet, in a volume equivalent
- 10 -
1 2~ 9~3
to that of the incoming feedstock. The outgoing liquid then
flows through the inner pipe 13 of a double-pipe duct toward
a vacuum evaporator 15. The stream of polymerization product
passing through the inner pipe 13 is heated from outside by
oil heated under proper temperature control by a heater 18 and
being circulated through the outer pipe 14. Because the outlet
of the double-pipe duct is connected to the vacuum evaporator
15, the stream is quickly drawn by suction into the latter.
The flow rate is regulated by an automatic pressure control
valve 16 installed at the end of the duct. The polymerization
product flowing out of the polymerization reactor 5 undergoes
partial, additional polymerization on the way, forming some
low-molecular-weight polymer. As the fluid approaches the
vacuum evaporator with a gradual pressure drop, bubbling takes
place. The specific volume (the reciprocal of the density)
is maximized, the flow rate boosted, and the residence time
minimized. Consequently, the formation of the low-molecular=
weight polymer is limited to a minimum. An inlet nozzle 17
of the vacuum evaporator 15 has a number of nozzle openings
as shown in FIG. 2, and the incoming fluid falls in the form
of strings while foaming, expanding its surface area oP contact
with the vacuum of about 1 to 10 Torr. Active evaporation from
the surface removes the volatile contents almost completely
before the fluid reaches the bottom of the vessel. The volatile
matter is extracted through line 19 to a condenser not shown.
-- 11 --
.`"'''' ' ,, ~ ~
The polymeriza-tion product that has fallen to the bottom
20 of the vacuum evaporator, almost completely free from the
volatile matter, is difficult to discharge because it is very
viscous and, moreover, is in a vacuum. Conventionally, the
discharging in such a case is done by the use of a gear pump
or by gear-pumping followed by a pressure increase and extrusion
by an extruder. Neither is appropriate, however, for the
obtainment of high-purity PM~IA of the optical-link or optical=
device grade to which the present invention is directed. The
gear pump necessarily involves friction between the teeth in
mesh, and the friction between the side faces of the teeth and
the side plates of the casing can scarcely be eliminated. In
addition, the upper half of the center shaft seal is exposed
to vacuum, and even a high-grade mechanical seal is unable to
avoid completely the intrusion of the sealing fluid. Thus,
a trace of metal particles that results from abrasion of the
gear teeth and a slight leak of sealing fluid can cause irregu-
lar reflection or refraction of light, eventually reducing the
transparency of the product.
According to the process of the invention, the polymeriza-
-- tion product is discharged downward from the bottom 20 of the
vacuum vessel by means of a vertical screw 21 partly inserted
into the bottom par-t, like starch sirup drawn out of a jar with
the aid of a stick being twisted by hand. By the motion of
the vertical screw with respect to a vertical duct 22, the
;
- 12 -
98
polymerization product is subjected to increasing pressure
as it is carried downward away from the evaporator bottom.
Subsequently, its direction is shifted sideways, and the polymer
is extruded through a nozzle 23 into a filament form. The
filament in turn is passed through a warm water trough 24 and
is wound up on a reel 25.
The vertical screw 21 too requires shaft seal means. To
this end there is provided a shaft seal 26, as shown in FIG. 2,
at a point (the lowermost end) where the polymer drawn out of
the bottom 20 of the vacuum vessel has attained sufficient
pressure by the screwing downward within the duct to overcome
the nozzle resistance. The shaft seal might allow the highly
viscous polymer -to leak out but, on the other hand, precludes
any possibility of intrusion from the outside of oxygen that
discolors the polymer or of fine dust or the like that can
objectionably scatter the light through the product. The
numeral 27 indicates a motor that drives the screw 21 through
transmission means not shown.
The present invention thus establishes a process for produc-
ing transparent, pure PM~A-base polymer, polystyrene or the
like, with little molecular weight distribution and no composi-
tional distribution, by combining all the countermeasures
described above, thereby preventing the intrusion of whatever
fine dust that can cause objectionable scatter of light or of
oxygen that can discolor the resulting polymer, and also avoid-
- 13 -
,.
9~3
ing the formation of metal particles due to abrasion of metal
parts.
Advantages of the invention over the prior art will be
illustrated by a few examples thereof as follows.
Preparatory experiments
In order to evaluate the effect of the addition of styrene,
four different monomeric feedstocks were prepared by adding
O, 10, 5, and 0 mol,S styrene to ~A and they were polymerized
separately in the following manner. The experiments were
numbered 1, 2, 3, and 4, respectively (Experiment Nos. l and 4
differing in the date of experiment).
In each experiment the polymerization was started with oil
bath heating, while condensing the monomeric vapor by Dimroth
condensers (two arranged in tandem) having adequate condensation
capacity, in a reactor of glass with a capacity of 500 mQ~
The reaction was initially effected at a constant boiling-point
temperature. The boiling point rose with the formation of
polymer. In the latter stage of polymerization where the
polymer production increased, the reaction was carried out at
140C, keeping the temperature constant by cooling from the
outside.
Before the start of the reaction, nitrogen gas was conti-
nuously introduced into the reactor to replace air. The monomer
or monomers were fed and the reaction continued while maintain-
ing the above atmosphere. Throughout the experiments a small
:
~ - 14 -
;
:;
lZ~
amount of nitrogen was supplied to the system.
The agitation impeller used was of the crescent (paddle)
type. It was driven at a constant speed of 300 rpm.
Following the conclusion of the reaction, the polymer was
separated from the unreacted monomer or monomers by precipita-
tion in methanol, dried by a hot air drier at 60C, and weighed.
The polymerization rate was determined, after the treatment
of the entire quantity of the reaction product, by dividing
the weight of the resultant polymer by the total weight of the
reactant solution. The experimental results are summarized
in Table 1.
T a b 1 e
Molecular wei~ht
Reac. Polymn _ _ ~
Exp. time MMA Styrene Styrene rateMn 4 Mw 4
No. (m~) (m~) (mol%) (10 ) (10
-
1 10 250 0 0 0.196 75.8 144.1
2 10 250 25 10 0.352 56.1 105.6
3 10 237.5 12.5 5 0.44527.7 82.3
4 6 250 0 0 0.099 46.6 105.0
-
The results indicate the following: ~
; (1) The polymeriæation rates attained in Experiment Nos.
2 and 3 in which styrene was added were by far the greater than
; , in Experiment Nos. 1 and 4 where MMA alone was polymerized,
showing that the additional monomer promoted the reactions in
- 15 -
,
1L~98
)cnown processes including Japanese Patent Application Publica-
tion No. 32665/1977 will clearly show the superiority of the
present invention. According to the invention, a highly trans-
parent MMA-base polymer suitable for fabrication into optical
links, optical devices and the like can be manufactured without
the addition of any catalyst or additive, while avoiding the
intrusion of objectionable shaft-sealing fluid, metal particles,
or dirt into the product.
- 18 -
98
the supply of the monomeric feedstock, so that the dispersion
of the sensible heat of the monomeric material and the exchange
of heat could be effected instantaneously. When the proportion
of the polymerization product approached about 50% by weight,
the polymerization product-unreacted monomer mixture was drawn
out of the outlet. The amount drawn out was the same as the
amount of the material fed into the vessel. The polymer amount
at the outlet i9 governed by controlling the relations among
the capacity of the reactor, the amount of discharge (or the
residence time), reaction temperature, and circulation velocity.
The polymerization product-unreacted monomer mixture thus drawn
out was separated in a vacuum vessel, and the polymer content
and molecular weight were determined. The results are also
given in Table 2. The molecular weight distribution was very
narrow, and the dispersion index (Mw/Mn) was 1.76.
T a b 1 e 2
Polymn Resi- Mol. wt. of pro-
Exp. Feedstock reac. Feed rate dence Outlet duct polymer as
Notemp. time polymer PS, Mn
55 mol% ST 155~C 3.39 ~/hr 5.9 h 49.5% 130,000
bal. MMA
6" 160C 4.29 ~/hr 4.66h 49.0% 116,000
7100% ~MA 155C 3.05 e/hr 6.55h 49.5% 138,000
~ Comparisons of these experimental results with those of
:
~ .
- 17 -
''`" ~
L498
known processes including Japanese Patent Application Publica-
tion No. 3Z665/1977 will clearly show the superiority of the
present invention. According to the invention, a highly trans-
parent ~A-base polymer suitable for fabrication into optical
links, optical devices and the like can be manufactured without
the addition of any catalyst or additive, while avoiding the
intrusion of objectionable shaft-sealing fluid, metal particles,
or dirt into the product.
~ ...
- 18 -