Note: Descriptions are shown in the official language in which they were submitted.
~2~30~4
1391 BANDAGE ~OR TRANSDERMAL DELIVERY OF
SYSTEMICA~LY-ACTIVE DRUG
-
The present inventlon is generally directed to
a device for the adminlstration of a drug and, more
particularly, directed to a medical bandage and
system ior controlled transdermal delivery o~ a
topical and/or systemically-active drug or agent.
In the treatment o~ many diseases andlor body
conditions, metered, or controlled, medication is
most desirable. Unfortunately, intravenouæ and oral
adminis-tration o~ drugs typically results in a
non-uniiorm introduction of the drug into the body's
system as a function o~ time. To overcome this dis-
advantage, transdermal drug delivery systems have
been developed, such as bandages, or the like, which
include topically active or systemically-active drugs.
In these systems, the drug is absorbed into the
body via its dermal surface in a more uni~orm manner,
the drug released by the transdermal system being
regulated by both the structure o~ the system and
the area o~ dermal surface contacted.
Topically active drugs include one or more agents
which cause a pharmacolog~cal or physiological response
at or near the site o~ the application on a dermal
suriace. The term "dermal sur~ace", as it is used
throughout this specification, is meant to be any
skin or mucosa surface.
Systemically-active drugs, on the other hand,
include one or more agents which are absorbed through
the body via a dermal surface and thereafter dis-
tributed throughout the patient's circulatory or
~283014
lymphatic system to cause a pharmacologic or physiologic
response in the patient. A topical and/or systemically-
active drug may also include a penetration enhancer
if the topical or systemically-active agent does
not penetrate the dermal sur~ace in eifective
; quantities. As the name implies, the penetration
enhancer acts in concert with the topical or
systemically-active agent to racilitate the passage
thereof through the dermal surface.
Transdermal systems that have been developed for
the delivery of drugs generally may be classified as
either a matrix system or a membrane system.
In a matrix system, the drug is suspended in a
permeable microporous substrate to iorm a monolithic
body which, when held ~gainst a dermal sur~ace by an
adhesive, enables di~fusion of the drug out oi' the
substrate and into the dermal surfaceO In this system,
the release rate and the amount of drug released is
dependent upon the permeability o~ the microporous
structure. In a membrane system, the drug, in a
liquid or semi-solid ~orm, is disposed on one side of
a membrane which is held to a dermal surface by an
adhesive. The membrane ~unctions to regulate, or
meter, the drug into the dermal surface.
In each of these systems, the amount of surface
area exposed to the dermal surface controls the amount
of migration of the drug into the patient's body;
that is, each system has a delivery rate over time
which is dependent upon the amount of surface contact
3~ area between either the monolithic body or the membrane
and the dermal surface.
128301A
3.
Heretofore, the adhesive in each o~ these bandage
systems has usually been disposed uni~ormly on the contact
sur~ace area so that the drug being administered passes
therethrough. While this may be satisiactory with
some drugs, those containing a penetration enhancer
may also e~fect an undesired transfer o~ some component
of the adhesive through the d,ermal ~ur~ace and into
the patient's body.
~ach o~ the transdermal delivery systems herein-
above-described are ~ingle dose systems in which the
drug dose is determined by the contact area between
the drug supporting structure on the dermal sur~ace.
Hence, a di~erent size 6ystem must be provided for
each prescribed drug dose.
Consequently, in order to dispense the bandage
system in accordance with a physician's prescribed
dose, it is necessary to manu~acture and stock an
array o~ bandage systems to supply the needs o~
patients requiring dif~ering doses of medication.
This, o~ course, gives rise to i~creased manufacturing,
dispensing and storage costs associated with the
treatment of any disease or body condition utilizing
a transdermal system.
The present invention provides ~or a bandage
for a transdermal delivery o~ a topical and/or sys-
temically-active drug which enables a pharmacist or
end user of the bandage to control the amount of drug
being dispensed, thereby adjustably controlling the
surface contact area between the topical and/or sys-
temically~active drug and the dermal sur~ace. Thisieature enables the bandage in accordance with the
present invention to provide a varying number o~
;
.
~33~14
doses of particular drug as may be prescribed by a
physician without the neceæsity ior manuiacturing
an array o~ bandages having di~erent doses and the
concomitant storage and dispensing costs.
SUMMARY OF ~HE INVENTION
In accordance with the present invention, a
bandage ~or a transdermal delivery o~ a topical
and/or systemically-active agent includes a supply
o~ topical and/or systemically-active agent and
adhesive means ~or releasably securing the bandage
to a dermal sur~ace. Control means are provided
which are separate ~rom the supply of topical and/or
systemically-active agent for adjustably controlling
sur~ace contact area between the topical and/or
systemically-active agent and the dermal sur~ace
when the bandage is held thereagainst. By controlling
the surface contact area, the control means effectively
controls the amount of migration o~ the toplcal and/or
systemically-active agent into the dermal sur~ace.
~ore specifically, the control means includes
manual means, operable by a user o~ the bandage ~or
adjustably controlling the surface contact area
between the topical and/or systemically-active agent
and the dermal sur~ace. The manual means may include
at least one selectably removable cover segment
disposed adjacent the supply o~ topical and/or sys-
temically-active agents. Pre~erably, the manual means
includes a plurality of selectably removable cover
segments which together ~orm a cover sheet. The cover
sheet pre~erably completely covers the supply o~
':
:.,
.
~L2~33~
5.
topical and/or systemically-active agent, with the
selectably removable cover segments being deiined
by per~orations in the cover sheet. In this manner,
the periorations enable manual ~eparation o~ one or
more o~ the removable cover æegments ~rom the cover
sheet by tearing along the per~orations.
This ~eature o~ the present invention enableæ
the user to select a dose to be delivered ~rom any
given bandage. Be~ause the dose may be selected at
the point o~ use, storage and dispensing costs can
be signi~icantly reduced. Such cost savings result
~rom the elimination o~ the manu~acture o~ a plurality
o~ bandages, each having a di~erent dispensing dose.
As hereinaiter summarized in greater detail, selection
o~ the dose may be by the user or by a pharmacist
dispensing the bandage.
In accordance with the present invention, the
supply of topical and/or systemically-active agent
may comprise a microporous material containing a
topical and/or systemically-active agent which is
permeable to the passage thereof. In this instance,
the supply of topical and/or systemically-active
agent may be considered a matrix system.
Alternatively, the supply o~ topical and/or
systemically-active agents may comprise a liquid
or a gel containing the agent which is separated
~rom the cover sheet by a membrane permeable to the
passage o~ the agent. In this instance, the source
oi topical and/or systemically-active agent may be
considered a membrane~type source.
~. ..... .
~2830~
6.
Preferably, the bandage in accordance with the
present invention ~urther comprises a backing sheet
with the supply o~ topical and/or systemically-active
agent and adhesive means being disposed on
one side of the backing sheet. In instances where
the supply o~ topical and/or systemically-active
agents includes a penetration e1lhancer, lt is pre-
~erred that the adhesive means :Ls disposed on the
backing sheet in a spaced-apart relationship with the
supply o~ topical andtor systemically-active agents.
In this manner, the control means can adjustably
control, or meter, migration o~ the topical and/or
systemically-active agent into the dermal sur~ace
without contact with the adhesive means, thereby
eliminating or substantially reducing the possibility
of the penetration enhancer causing unwanted migration
of components o~ the adhesive through the dermal surface.
To ~acilitate use by a patient, the bandage in
accordance ~ith the present invention may also include
indicia means which are associated with each covered
segment ior displaying the dosage o~ topical and/or
systemically-active agent delivered into the dermal
surface when the bandage is secured in an operational
position on the dermal sur~ace with a preselected
color segment removed. Pre~erably, the plurality o~
selectably removable color segments comprises a set
of elongate strips disposed in a parallel relation-
ship with one another over the supply o~ topical and/or
systemically-active agent, with each strip having
tab means thereon ~or manually separating each strip
:.
`
~2~330~
7.
~rom the cover sheet.
As hereinbefore noted, the bandage in accordance
with the present invention may include means ior
enabling preselective limiting of the operation o~ the
control means to providing a preselected maximum
surface contact between the topical and/or sys-
temically-active agent and the clermal surface. In
this instance, the tabs on the ~;et of elongate strips
are severable, and each tab has a length su~ficient to
extend beyond a periphery of the bandage to enable
preselecting severing thereo~.
When the adhesive means of the present invention
is disposed along the periphery of the backing sheet
and the tab means extend thereover, the adhesive means
operates ~or preventing separation of a tab ~rom the
backing sheet and corresponding cover segment from the
cover sheet when an end portion means thereon is
severed; that is, the adhesive means securely grips the
remaining portion of the tab, thereby preventing in-
advertent removal of a cover segment. The remainingend portions and tabs are easily gripped in comparison
thereto thereby facilitating the removal of cover
segments appropriate ~or the dose described.
As earlier and briefly mentioned, the present
invention includes a bandage system for providing
multiple doses of a topical and/or systemically-active
agent in selectively equal or differing amounts. In
this instance, the system comprises a plurality of
individual bandage means ~or transdermal delivery of
the topical and/or systemically-active agent, with
each of the individual bandage means comprising
elements hereinbefore recited.
.
83~
8.
This system has the advantage o~ enabling the
production oi' a plurality o~ ~andages, each having a
selectable medica$ion dose which eliminates the need
~or manu~acturing, storing and dispensing o~ an
array of bandages having diii'ering doses which
otherwi~e would be necessary.
BRIEF DESCRIPTION OF THE DRAWINGS
The advantages and ~eatures oi the present in-
vention will appear ~rom the ~ollowing descriptionconsidered in con~unction with the accompanying
drawings in which:
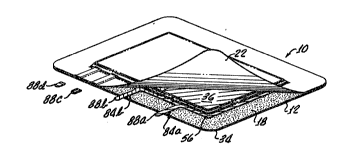
Figure 1 is a perspective view showing a bandage
in accordance with the present invention having a
supply o~ topical and/or systemically-active agent,
adhesive means, control means, as well as a backing
sheet and a releasable liner;
Figure 2 is a perspective view o~ the bandage
shown in Figure 1 showing the releasable llner
partially removed and a plurality o~ tabs, inter-
connected with cover segments, with end portions
thereof extending beyond a periphery o~ the bandage;
Figure 3 is a perspective view o~ the bandage
shown in Figures 1 and 2 showing the release liner
removed and a cover segment partially removed;
Figure 4 is a cross-sectional view of the
present invention;
Figure 5 is an enlarged cross-sectional view o~
the present invention generally showing the supply o~
topical and/or systemically-active agent incorporating
a matrix-type delivery system; and
'
,
.
~ZB~Ol~
9.
Figure 6 is an enlarged cross-sectional view of
an alternative embodiment o~ the present invention
showing the supply o~ topical and/or systemically-
active agent incorporating a membrane-type delivery
system.
DETAILED DESCRIPTION OF THE INVhNTION
Turning now to Figures 1, 2 and 3, there is shown
a bandage, or drug delivery system, 10 in accordance
with the present invention which, taken alone, provides
~or delivery oY a topical and/or systemically-active
agent, and taken in combination with identical bandages
encompasses a system for providing multiple doses o~
topical and/or systemically-active agent in selectively
equal or dif~ering amounts to a dermal surface. (Not
shown).
Generally, the bandage 10 includes a backing
sheet 12, a supply 16 oi topical and/or ~ystemically-
active agent, an adhesive 18 disposed on the backing
sheet 12, a release sheet 22 and a periorated cover
sheet 26 which provides means, separate from the
supply 16 o~ topical and/or systemically-active agent,
for adjustably controlling the suriace contact area
between the supply 16 of topical and/or systemically-
active agent and a dermal suriace.
It is to be appreciated that it is not necessaryto utilize the adhesive 18 ~or releasably securing the
bandage 10 to a dermal surface. For example, a
separate elastic band or cloth (not shown) may be
utilized to hold the bandage 10 against a person's
arm or leg. An adhesive is preferred, however, so that
the bandage can be more easily applied to any dermal
surface on a person's body.
,
`\
330~
10 .
The backing sheet 12 may be ~ormed of any suitable
~lexible material, such as mylar or the like. Simi-
larly, the release ~heet 22 may be ~ormed of any
suitable material and may have a silicone-type
coating thereon, not shown, ~or ensuring adequate
release ~rom the adhesive 18, when it ls ~eparated
there~ore to prepare the bandage 10 ~or application
to a dermal ~ur~ace.
Typlcal systemically_active agents ~hich may be
suitable ~or use in the present invention are thera-
peutic agents which are suf~iciently potent such that
they can be delivered through the skin or other
membrane to the bloodstream in su~icient quantities
to produce the desired therapeutic ei~ect. In general,
this includes therapeutic agents in all o~ the major
therapeutic areas including, but not limited to,
anti-in~ectives, such as antibiotice and antiviral
agents, analgesics a~d analgesic combinations,
anorexics, anthelmintics, antiarthritics, antiasthma
agents, anticonvulsants, antidepressants, antidiabetic
agents, antidiarrheals, antihistamines, anti-in~lammatory
agents, antimigraine preparations, antimotion sickness,
antinauseants, antineoplastics, antiparkinsonism drugs,
antipruritics, antipsychotics, antipyretics, anti-
spasmodics, including gastrointestinal and urinary;anticholinergics, sympathomimetics, xanthine deriva-
tives, cardiovascular preparations including calcium
channel blockers, beta-blockers, antiarrhythmics,
antihypertensives, diuretics, vasodilators including
general, coronary, peripheral and cerebral; central
nervous system stimulants, cough and cold preparations,
decongestants, diagnostics, hormones, hypnotics, im-
munosuppressives, muscle relaxants, parasympatholytics,
... .
-
o~
parasympathomimetics, psychostimulants, sedatlves
and tranquilizers.
The active ingredients o~ systemically-active
agents may include, but are not limited to:
Isosorbide Dinitrate, Nitroglycerin, ~stradiol,
Clonidine, Propranolol, Indomethacine, Ni~edipine,
Nicardipine, Dicloro~enac, ~etaproterenol.
Alternatively, a combination oi a suitable agent
and a penetration enhancer, such as Azone, may be
used. When an enhancer is utilized, it is pre
~erred that the adhesive 18 be disposed on the backing
sheet 12 along a periphery 34 thereoi while the supply
16 is disposed in a center portion 36 thereo~ to
establish a ~paced-apart relationshlp between the
adhesive 18 and the supply 16 in order to ensure that
the penetration agent does not act to conduct components
of the adhesive through the dermal æurPace, which may
be undesirable.
The supply 16 may be o~ the matrix-type, shown in
Figures 4 and 5, or membrane-type, shown ln Figure 6.
As shown in Figures 4 and 5, the supply 16 includes
any suitable matrix 42 permeable to the agent disposed
ln microporous 46 o~ the matrix. The matrix 42 may be
disposed on the backing sheet 12 dlrectly, or on a
separate layer 50 attached to the backing sheet by
any suitable means. A ~ront sheet 54 may also be
provided ~or supporting the matrix 42 which has an
aperture 56 therein to expose the matrix to a dermal
surface when the release sheet 22 is removed and the
cover sheet 26 partially removed as hereina~ter
described. The layer 50 and ~ront sheet 54 may be
~Z830~
formed ~rom any suitable material, such as mylar or
the like
Alternately, as shown in F:igure 6, the supply of
16 may include a liquid or gel 64 o~ agent disposed
between an i~pervious layer 68, such asMylar*, and
a membrane 70, the impervious layer 68 being attached
to the backing sheet 12 in any conventional manner.
The membrane acts in a conventional manner to limit
the migration o~ the agent therethrough and out an
aperture 72 formed in a front sheet 74 when the
release sheet 22 is removed and the per~orated cover
sheet removed, all or in part, as hereinafter described.
- Turning now to Figures 2 and 3, operation of the
bandage ls shown in conjunction with the description
of the perforated cover sheet 26.
The perforated cover sheet 26 may comprise a set
of spaced-apart patterns, each defining cover segments
80a, 80b, 80c, 80d, and it is preferable that the cover
segments are ~ormed into elongated strips disposed in
a parallel relationship over the supply 16, with each
strip 80a, 80b, 80c, 80d, having a tab 84a, 84b, 84c,
84d, thereon, which provides means for manually
separating each strip 80a, 80b, 80c, 80d, ~rom the
cover sheet 26.
While any number o~ elongated strips 80a, 80b,
80c, 80d having tabs 84a, 84b, 84c, 84d, interconnected
therewith, may be provlded, only ~our are shown in
the Figures 1, 2 and 3 ~or the sake o~ clarity of
presentation. Each o~ the tabs 84a, 84b, 84c, 84d
has an end portion 88a, 88b, 88c, 88d, thereon to
~acilitate manual pulling thereof by the fingers of a
user to separate the corresponding tab 84a, 84b, 84c,
84d, from the adhesive 18 and tear the corre-
*Mylax trademark
.. . . .... . .... .
1~3301~
13.
sponding segment 80a, 80b, 80c, 80d ~rom the per-
forated cover sheet 26, leaving the remaining elongated
strips in place.
A removed strip 80a (Figure 33 then exposes a
preselected contact area which occurs between the
supply 16 and a dermal sur~ace (not shown) when the
bandage 10 is disposed in an operational relationship
with the dermal surface by the adhesive 18. It should
be appreciated that the cover segments 80a, 80b, 80c,
80d, may be o~ varying widths as shown in Figure 3 to
enable a wider selection o~ doses available ~rom a
single bandage 10.
It can be seen irom Figures 1, 2 and 3 that the
tabs 84a, 84b, 84c, 84d, with end portions 88a, 88b,
88c, 88d provide means for enabling preselective
limiting o~ the operation of the control means. Each
of the tab means has a su~iicient length to allow the
end portion 88a, 8~b, 88c, 88d to extend beyond the
periphery 34 oi the backing sheet. Hence, before the
release sheet 22 is removed, one or more o~ the end
portions, ior example, 88c, 88d, may be clipped flush
with the periphery 34 (~ee Figures 2 and 3) to limit
the number o~ end portions 88a, 88b, which may be
pulled by a patient. In this manner, the tabs 84c,
84d remaining are secured to the backing sheet 12 by
the adhesive 18 thereon and not easily separated there-
from. This limits the dose which the patient may
select by pulling the tabs 88a, 88b, and removing the
cover segments 80a, 80b. This feature of the invention
; 30 may have particular importance in the administration
o~ speci~ic drugs in which it is important to ensure
that the patient does not inadvertently select a greater
dose than tha- prescribed by the physician.
~2133(~1~
The severing or cutting of the end portions 80a,
80b, 80c, 80d, may be done on a one-by-one basis, or
a jig, not shown, may be provided so that a pharmacist
may stack a plurality oi the bandages on one another
and cut all selected tabs, ~or c~xample, BOc, 80d, with
one operation.
Each of the end portions 88a, 88b, 88c, 88d, as
well as the elongated segments 80a, 80b, 80c, 80d,
may have various colors (as indicated by di~ferent
cross hatching in Figure 3) to provide indicia means
ior displaying the dosage of topical and/or sys-
temically-active agent deli~ered to the dermal sur~ace
when the bandage is secured in operation position on
the dermal sur~ace, with preselected cover segments
80a, 80b removed.
It is to be appreciated that any number o~ bandages
and/or systems may be constructed in accordance with
the method oi the present invention and, although
there has been described hereinabove a number of
speci~ic bandage embodiments in accordance with the
present in~ention, ior the purpose of illustrating the
manner in ~hich the invention may be used to advantage,
it should be appreciated that the ~nvention is not
limited thereto.
Accordingly, any and all modi~ications, variations
or equivalent arrangements which may occur to those
skilled in the art, should be considered to be within
the scope o~ the invention as defined in the appended
claims.