Note: Descriptions are shown in the official language in which they were submitted.
~3~
This invention relates to a ~ie for railway track.
More specifically, the in~ention relates to a resilient coat
for a direct-connection type ~ie composed of a concrete tie
body or supporting rail (~Danchoku~ tie) with its lower
portion coated by a layer of a microcellular polyure hane
elastomer which is adhered to ~he former to form an integral
body, said Danchoku tie achieving easier maintenance and
reduction in railway vibration and noise at the same timel
and furthermore allowing laying of railway track with high
precision and easy operation.
Due to mainly the demand for elimination or reduc-
tion of labor, the recent trend is that the conventional
ballasted tie track is being replaced by direct-connection
type track system such as one using track slab, which is
easier of maintenance. In this direct-connection type track
system, however, concrete slabs were directly laid on solid
roadbed without using ballast and ballast-mat, and consequ-
ently there may be such drawbacks as increased railway
vibration and noise compared with the case of using resi-
lient ballasted track with ballast-mat.
Accordingly the development of a new system which
is easier of maintenance and furthermore causes less railway
vibration and noise has been demanded. To meet this demand,
so-called n resilient coated tie", i.e., larger size concrete
tie (namely, a track slab of reduced size) with its bottom
and side surfaces coated with an elastomeric material, was
proposed, and the so-called direct connection type resilient
-- 2 --
coa~ed tie system was studied~ with the view to reduce the
vibration transmission and occurrence of noisey by laying
such ties on concre~e roadbed ~hrouyh a grout (for example,
synthetic resin, grouting concrete~ etc.~.
AS the elastomeric material to be used for the
resilient coated tie, it was proposed in ~he past to use the
product obtained through the steps of mixing the pulverized
rubber obtained from used automobile tyres with a poly-
urethane adhesive, filling a mold with the mixture, pressing
the latter with a compresser and aging the same under heat-
ing. However, preparation of such a resilient material
requires much labor (production efficiency: one piece of the
product per mold and per day) as well as large scale eyuip-
ments, and besides, the adh~sion of the resili~nt coating to
concrete tie bodies is apt to become incomplete, occasion
ally causing peeling off. Furthermore, in case of laying
the conventional resilient coated ties on concrete roadbed
and burying and fixing them with grouting concrete in situ,
the resilient material is almost completely bound to the
concrete and prevented of free deformation. Hence, the
spring constant increases, the vibration-isolating effect is
reduced and the meeit of using the resilient coat is lost.
Thus, in the system of burying and fixing ties in
and on concrete roadbed using, for example, grouting con-
crete (~Danchokuq track) for reduGing vibration and n`oise,the vibration-isolator coat is firmly confined by the
grouting concrete and inhibited of free deformation, and
~33~
-- 3
for this reason the vibration isolator coat is required to
still fully exhibit its vibration transmission-reducing
effect and noise-reducing effect even under the free de-
formation-restricted condition, as strongly adhered to the
tie bodies and without an increase in tbe spring constant.
The property requirements on the resilient-coating
material for Danchoku ties to be used in railway track, on
which many-coach trains run at high speeds, are still more
rigorous, e.g., the following property ratings are required:
Permanent compression set: 15% or less
Spring constant: 0.2-2 tons/cm/100 cm2
Tensile strength: 5 kg f/cm2 or higher
Tensile elongation: 100% or more
Waterproofness (variation of tensile strength)
: within +20%
(variation of tensile elongation)
: within 20%
Alkali resistance (variation of tensile strength)
: within +20%
(variation of tensile elongation)
: within +20~
Fatigue resistance: amount of permanent deformation
1.0 mm or less.
It is required tbat the material should satisfy all of those
re~uirements at the same time.
Furthermore, there is another important require-
ment that the resilient or elastomeric coating material must
strongly adhere to the concrete tie bodiest never peeling
off under the repetitive compression exerted by intermittent
train running.
The present inventors discovered and proposed,
as an elastomeric coating material fully satis~ying these
requirements, a microcellular urethane elastomer having a
bulk density of 0.3-0.9 g/cm3, which is prepared from a raw
foaming liquid of urethane elastomer composed of a poly-
hydric alcohol having an average number of functional group
Of 2.5-3.5 and a number average molecular weight of about
4500 - about 8500, an organic polyisocyanate~ a chain-ex-
tending agent, a urethanation catalyst and a foaming agent,
said raw liguid containing the chain-extending agent at a
concentration of 0.2 X 10 ~ to 1.0 X 10 3 mol/g per the unit
weight of the urethane elastomer (Laid-open Gazette, Kokai
No. 130754/1980).
This previously proposed microcellular urethane
elastomer satisfies the foregoing ratings, and is actually
used at the straight portions of high-speed railway track.
It is only logical, however, that even with such elastomeric
coating material composed of the microcellular urethane
elastomer, it is preferred to reduce the cost of raw mate~
rials by further lowering the bulk density. Low bulk den-
sity however inevitably reduces ~he spring constant. At
the curved portions of railway track, furthermore, the track
is subject to particularly high centrifugal force and the
elastomeric coating material is severely compressed.
~3~
-- 5
Consequently spaces are formed between the elastomeric
coating material and the grouting concre~e, and water and
dust tend to go in~o said spaces. Therefore, it i8 inauf-
ficient to simply raise the degree of foaming o said mi ro-
cellular urethane elastomer ~o reduce its bulk density.
For example, there is a problem that if bulk density of the
microcellular urethane elastomer is kept at not higher than
0.7 g/cm3 and still the spring constant is to be maintained
at around 1.0 tf/ cm/100 cm2, the permanen~ compr~ssion set
is increased.
Under the circumstances, an object of the present
invention is to provide Danchoku ties of which elastomeric
coating material is made of a microcellular polyurethane
elastomer which, in spite of its low bulk density, shows no
substantial increase in its permanent compression set.
Another object of the present invention is to
provide Danchoku ties which enable very accurate and easy
laying of Vanchoku railway track, by boring plural through-
holes for bolting which vertically pierce through the
Danchoku ties inclusive of the elastomeric coating.
Still other object and advantages of the present
invention will become apparent from the following detailed
explanation of the invention.
According to the present invention, a resilient
coated, direct connection-type tie (Danchoku tie) which is
composed of a concrete tie body for supporting rail and
a microcellular polyurethane elastomer coating layer which
adheres to and coats the lower portion of ~he tie body to
form an integral body therewith, and is provided with two or
more through-holes for bolting which vertically pierce
through said tie body and coating layer, said ~ie body
having buried in its through-holes the nu~s screw-fittable
with the bolts, and said microcelluar polyurethane elastomer
having urethane bonds and a bulk density of 0.4~0.75 g/cm3
and being prepared from the starting foamable liquid of
urethane elastomer composed substantially of
(a) a polyether polyol having an average number
of functional groups of 2.5-4.5 and a number
average molecular weight of 2000-8500,
(b) a vinyl monomer-grafted polyol having an
average number of functional groups of 2.5-4~0,
and the graft ratio of 4-20% by weight,
~c) a liquid polybutadiene polyol having hydroxyl
terminal group(s), an average number of functional
groups of 2.0-3.0 and a number average molecular
weight of 2000-7000,
~o (d) an organic polyisocyanate,
(e) a chain extender,
(f) a blowing agent, and
(g) a urethanation catalyst,
at such ratios that the NCO index i5 within the range of
90-110~ and the concentration of the chain extender, based
on the total amount of the five components of ~a)~ (b), (c),
(d) and (e), being 0.3xlO 3 to 1.5xlO 3 mol/g.
--7--
Hereinafter the tie involving present invention will
be explained in further details, referring to the specific
embodiments shown in -the attached drawings.
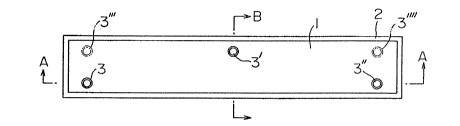
Figure 1 is a plan view of one embodiment of the -tie
involving presen-t invention,
Figure 2 is a section of the embodimen-t of Figure 1,
cut along the line A-A,
Figure 3 is a section of the embodiment of Figure 1,
cut along the line B-B,
Figure 4 is a vertical section illustrating the
Danchoku tie of this inven-tion as temporarily laid on the under-
structure of railway -track,
Figure 5 is a vertical section illustrating the
Danchoku tie of this invention as laid under the railway track,
Figure 6 is a perspective view illustrating the mold
fit on the concrete tie body in accordance with the present
invention,
Figure 7 is a section of the embodiment of Figure 6,
cut along the line C-C,
~0 Figure 8 is a flow sheet showing production steps of
the~Danchoku tie in accordance with the present invention,
Figure 9 is an enlarged section of a portion of the
mold around its deaeration holes, and
Figures lOA and lOB are a side elevation and a sec-tion
view, respectively, of a test -track laid with Danchoku ties.
As shown in Figures 1-3, the -tie involving present
. invention is composed basically of the structure of a concrete
tie body 1 for supporting rail, wi~h its lower portion
adhered and coa~ed with a microcellular polyurethane elas-
tomer coating layer 2, together forming an integral body~
In throughout the present specification and
claims, the surface of the tie on which the rail i5 laid
will be referred to as ~top", the surface opposite thereto,
as "bottomN, and all other surfaces, as "sides~
The coating layer 2 coats the entire bottom and
the lower portions of the sides of the concrete tie body, as
shown in FigsO 2 and 3. In that occasion, the height h of
the coating layers on the sides is not particularly limited,
but can be varied over a wide range depending on the in-
tended utility of the Danchoku tie (for high-speed railway~
ordinary railway, subway, etc.). Normally, however, the
height h is advantageously from 1/20 to 1/1, preferably 1/4
- 3/4, inter alia, 1/2 3/5, of the height H of the con-
crete tie body. And, for the ordinary concrete tie body
having an H of 8-30 cm, recommendably the height h of coat-
ing is 4-18 cm.
The thickness as w, w' of the coating layer 2
neither are limited, but are variable over a wide range
depending on such factors as the intended utility of
Danchoku tie. It is generally desirablet however, that it
should be at least 8 mm. The upper limit of the thickness
is not critical~ but generally that of 50 mm or less is
sufficient, as too thick a coating layer is expensive and
does not show advantages to justify the cost increase.
- 9 -
Thus, the coa~ing layer 2 normally can have a thickness of
10-35 mm, preferably 15-30 mmO Within said range, the
thickness of coating layer 2 may be same at the bottom and
at the sides, but the thickness w' at the sides subject to
less load may be less than the thicknes~ w of the coating at
the bottom of the tie, e.g., w may range 15-50 mm, prefer-
ably 20-30 mm, w' 5-50 mm, pre~erably ~ -29 mm, and w - w',
l-lû mmO
The size of concrete tie body to be coated with
lo such a coating layer is variable depending on he intended
use of the tie, but normally it is 50-1000 cm in width,
200-280 cm in length and 10-30 cm in height.
The most characteristic feature of the tle involv-
ing present inven~ion resides in the provision of at least
two through-holes 3, 3', 3" ... for bolting bored vertically
through the concrete tie body 1 and coating layer 2 of
Danchoku tie as described above, and of nuts 4 fittable with
the bolts, which are buried in the through-hole portions of
the concrete tie body 1.
As illustrated in Fig 4, into each of the through-
holes 3, 3', 3" ... for insertion of bolts, holding bolts 6,
6', 6'' ... are screwed, to pierce through the tie. By the
adjustment of these holding bolts, the three-dimensional
position of the tie can be determined to lay the tie at the
desired height with desired inclination of its top, with
high precision and easy operationO
Such through-holes may be bored, as illustrated in
3~
-- 10 --
Fig. 1, one ~ach in the vicinity of the two ends of one edge
of concrete tie body 1 as 3 and 3n~ and one at the center~
in the ViCillity of the opposite edge, as 3', three in total.
Or, as shown with dotted line in Fig. 1~ instead of boring
one hole 3' at the center of the portion near the other
edge, two holes 3In~ 3~n may be bored at the corresponding
positions to those of the holes 3, 3~ on said edge, making
the total number of through-holes four~
The size of the through-holes is determined
depending on the thickness of holding bolt to be inserted
thereinto, while advantageously the diameter of 10-40 mm,
preferably 20-30 mm is normally selected.
It is permissible to form a depression at the
center of downmost portion of the bottom of the coating
lS layer 2 composed of the microcellular polyurethane elastomer
which is subject to less load, as shown in Figs. 2 and 3,
and to fit into said depression, a soft synthetic resin foam
5 which is cheaper than the microcellular polyurethane
elastomer. This enables to avoid the central reaction which
poses a problem in the strength property of tie body, and to
save the consumption of expensive microcellular polyurethane
elastomer As synthetic resin foams useful for this
purpose, closed cell type crosslinked polyethylene foams
having an apparent density of normally 0.01-0.1, preerably
0.02-0.05 are particularly suitable.
The length of said depression 5t in Fig. 2) nor-
mally ranges 1/4 - 1/2 of the length L of conrete tie body,
~3~
preferably 1/4 - 1/3. Within said range the length t is
suitably variable. Also the depth (d in Fig. 2~ of the
depression may range lJ10 - 1/1, particularly 1/2 - 7J10, of
the thickness w o the coating layer 2 at the bottom. It is
advantageous that the depression should be formed over the
entire width direction of coating layer 2~ as fihown in Fig.
3.
The Danchoku tie involving this invention having
the above-described structure can be laid under railway
tracks in the manner described hereinbelow, as illustrated
in Figs. 4 and 5.
Namely, holding bolts 6, 6', 6" ... are fitted
with the nuts 4 buried in the through-holes 3, 3', 3" ~..
vertically piercing through the Danchoku tie involving this
invention9 as bored in the concrete tie body 1, in such a
manner that thP lower ends of these bolts should project out
of the coating layer 2 of the tie at the bottom. The lower
ends of the bolts are placed in the concrete crib 7 con-
structed on the under-structure of the railway track, and by
adjusting each bolt, the level and inclination of the top of
tie body 1 can be determined (see Fig. 4).
Then on the top of concrete body 1, rails 8, 8'
are fastened in accordance with the accepted practice, and
into the spaces between the concrete crib 7 on the under-
structure and the coating layer 2 at the lower portion ofthe concrete tie, grouting concrete is poured to form the
solid bed 9. Before this concrete is ~ully cured, the
~3~
- 12 -
holding bolts 6, 6', 6" ... are removed~ and onto the tops
of the through-holes 3, 3 ~ ~ 3n, . ~ . CapB made of an elas-
tomer, 10, 10', lQ" ... are fitted (see Fiy. 5).
The tie involving this inven~ion thus having the
holding bolts 6, 6', 6" ..~ screwed into and piercing
through the through-holes 3, 3', 3" ... vertically extending
therethrough, by adjusting said bol~s, the three dimensional
position of the tie as will give the desired level and
inclination of top surface thereof can be determined.
Accordingly it becomes possible to lay the railway track
very precisely through easy operations.
In pouring the concrete, care should be taken
that the concrete should not flow into the through-holes
to directly connect the concrete tie body 1 with the bed 9.
Because, should they be directly connected, the vibration
of concrete tie body 1 is directly transmitted to the bed 9,
and cannot be absorbed and buffered at the coating layer 2.
Therefore~ it is important to keep the hole size, at least
at the lower coating layer portion, at about the same or
only slightly greater than the thickness of the bolt to be
inserted thereinto, to substantially prevent the concrete
from flowing into the hole.
From the same reason, the concrete for making the
bed should not rise over the upper edges of the coating
layer 2 at the sides of the tie, to be directly connected
with the concrete tie body 1 ~see Fig~ S).
In short, it is very important that the concrete
~ ~ ~3
- 13 -
tie body 1 be substantial]y completely isolated from the
concrete bed 9.
One of the characteris~ic features of Danchoku tie
involving this invention is the use of specific micro-
cellular polyurethane foam as the material for making thecoating layer.
That is, the present inventors discoYered that
microcellular polyurethane elastomers are well suited as
the coating material for Danchoku tie, which can exhibit
vibration-isolating effect at the position intervening the
concrete tie body and roadbed as well as the groutiny con-
crete serving as the solid bed, because of the energy loss
effect, etc~ based on their viscoelasticity characteristics.
Whereupon the present invention was completed. According to
the present invention, the spring constant of Danchoku tie
can be reduced to equal or even below that of the ballasted
track, on rigid roadbed such as high level bridge, by a
suitably selecting soft polyurethane elastomer, and whereby
the vibration and noise caused by train running can be
effectively isolated~ Furthermore, by the use of a micro-
cellular polyurethane elastomer, it is made possible that
even when the elastomeric coating material is confined by
the grouting concrete serving as the solid bed, the marked
reduction in the vibration-isolating effect due to the
increase in spring constant can be prevented, as such an
elastomer can be internally freely deformed.
The microcellular polyurethane elastomer to be
~g~:~3~
- 14 -
used in the present invention normally has a bulk density
within the range of 0.4-0.7S g/cm3, preferably 0.55-0.7
g/cm3.
The physical and chemical properties of poly-
urethane elas~omers other than the bulk density can bevaried over broad ranges by the selection of constituents~
but for the specific purpose of isolation of vibration as
in the present invention, naturally the optimum constituent
must be selected so as to attain the desired durability and
vibration-isolating characteristics. Hereinafter the com-
position of the polyurethane elastomer suitable for the
purpose of this invention will be explained in detail.
The microcellular polyurethane elastomer to be
used in this invention is formed from a specific starting
foamable liquid of urethane elastomer composed of (a) poly-
ether polyol, (b) vinyl monomer-grafted polyol, (c~ liquid
polybutadiene polyol, (d) organic polyisocyanate, (e) chain-
extender, (f) blowing agent and (g) urethanation catalyst.
The polyether polyol (a) to be used as one of the
polyol components in the preparation of the polyurethane
elastomer according to the present invention has an average
number of functional groups of 2.5-4.5, and a number average
molecular weight of 2000-8500. When the average numb~r of
functional groups in the employed polyether polyol is less
than 2.5, the foamed urethane elastomer obtained therefrom
shows increased permanent compression set. Conversely when
the average number of functional groups exceeds 4.5, the
~ 3
- 15 -
resulting elastomer shows a tendency to become harder, and
furthermore the possibility of its rupture increases when
it is exposed to the vibratory compression. Thus, the
preferred average number of functional groups is 2.5-4.5,
particularly 2.8-4Ø
Again, when the number average molecular weight of
the polyether polyol (a) is less than 2,000, a foamed poly-
urethane elastomer having a high vibration energy-absorbing
characteristics can hardly be obtained. Conversely, when it
exceeds 8,500, the resulting polyurethane elastomer shows
degradation in its elastic properties, tends tu produce
plastic deformation, and shows a strong tendency particu-
larly for increased permanent compression set. Thus it is
desirable for the polyether polyol to be used in ~he present
invention to have the number average molecular weight nor-
mally ranging from 2000-8500, particularly 3000-6500.
As such polyether polyol (a), those normally used
in the preparation of polyurethane elastomers can be option-
ally used. More specifically, such polyether polyols ob-
tained by addition-polymerizing C2-C4 lower alkylene oxides,
such as ethylene oxide, propylene oxide, etc. to C2-C6
aliphatic polyhydric alcohols such as glycerin, trimethylol-
propane, etc. or to active hydrogen-containing compounds
having active hydrogen atoms such as ethylenediamine, di-
aminodiphenylmethane, etc. may be named. Typical examplesof such polyether polyols (a) include glycèrin/propylene
oxide/ethylene oxide copolymerized adduct (average number
- 16 -
of unctional groups = 3.0, number average molecular weight
= 3000), propylene glycol/propylene oxide/ethylene oxide
copolymerized adduct ~average number of functional gr~ups
= 2~0, number average molecular weight = 4800), glycerin/
pentaerythritol/propylene oxide/ethylene oxide copolymeriæed
adduct (average number of functional groups = 3.7, number
average molecular weight = 5700), etc.
One of the characteristic features of the
present invention resides in that, in combination with
th~ above polyether polyol (a), a vinyl monomer-grafted
polyol ~b) having an average number of functional groups
of 2.5-4.0 and the graft ratio of 4-20% by weight, and a
liquid polybutadiene polyol (c) having an average number
of functional groups of 2.0-3.0, a number average molecu-
lar weight of 2000-7000, and hydroxyl terminal group(s),
are used as the polyol component for composing the foamed
polyurethane elastomer.
The "vinyl monomer-grafted polyols" to be used in
the present invention (hereinafter may be referred to as the
graft polyols) (b) signifies modified polyols prepared by
in situ radical polymerization of vinyl monomers in the
presence of ordinary polyols, which ~er se are known as the
polyol component for producing high elasticity urethane
foams (e.g., Japanese Patent No. 447628, U.S. Patent No.
3033841, U. K. Patent No. 874130, German Patents Nos.
1077430, 1105179, 1081917, and 1111394, Laid-open Japanese
Patent Publication No. 93729~81). According to the
- 17 -
invention, of such graft polyols, particularly those spe-
cific graft polyols having an average numher of functional
groups of 2~5-4.0 and a graft ra~io of 4-20% by weight are
usedO
When the average number of functional groups of
the graf~ polyol employed is less than 2OS, the resulting
microcellular polyurethane elastomer ~bows exc~ssively great
permanent CQmpresSion set, and therefore is not appropriate.
Conversely9 when it exceeds 4.0, the product urethane elas-
tomer shows a tendency to be hardened. The preferred range
of the average number of functional groups of the graft
polyol is 2.5-3Ø Again, when the graft ratio of the graft
polyol is less than 4% by weight, permanent compression set
is aggravated. Conversely, when it exceeds 20% by weight,
the viscosity of the liquid rises to markedly deteriorate
the moldability. Thus it is convenient that the graft ratio
of qraft polyol ranges 4-20% by weight, particularly 5-15%
by weight. The term "graft ratio" used herein means, of the
total vinyl monomer added, the ratio of the vinyl monomer
graft polymerized to the polyol, to the weight of said
polyol.
As the polyols to serve as the trunks of such
graft polyols (b), those having a number average molecular
weight of 2500-8S00, preferably 4000-7000, and a hydroxyl
value of 20-67, preferably 24-50, are advantageously used.
For instance, polyalkyleneether glycol having a number
average molecular weight of 4800v which is obtained by
3~
- 18 -
addition polymerizing ethylene oxide and/or propylene oxide
to glycerin~ may be used~
As the vinyl monomers to be grafted to these
polyols, the following may be named for example: olefins
such as s~yrene, vinyltoluene, l-butene, 2-hexene, 1,4-
hexadiene, lt3-butadiene and 3-pentene; vinyl halides such
as vinyl chloride and vinylidene chloride; ethylenic un-
saturated carboxylic acids, such as acrylic acid and meth-
acrylic acid, or their derivatives (e.g., alkyl esters);
vinyl acetate; acrylonitrile; etc. They may b~ used either
singly or in combination of more than one kind of the mono-
mers.
The grafting of the above vinyl monomer or mono-
mers to the above polyol can be achieved by radical poly-
merizing the vinyl monomer~s) in the presence of the polyolaccording to the method known ~ se. As the useful radical
polymeriza~ion catalyst, for example peroxide-type, azo-type
or redox-type polymerization initiators or metal compound
catalysts, etc.~ may be named. Thus obtained graft polyols
can normally have the number average molecular weight of
2500-8500, preferably 4000-7000.
As the particularly preferred graft polyols for
the present invention, for example, that obtained by graft
polymerizing acrylonitrile and styrene to the polypropyl-
eneether glycol having a number average molecular weight ofabout 5100 and an average number of functional groups oE
about 3, in an autoclave at 120C for 8 hours, using as the
~3~
-- 19 --
polymerization initiator azobisisobutyronitrile, may be
named.
"Liquid polybutadiene polyol" (c~ signifies liquid
butadiene homopolymers or copolymers having terminal re-
active hydroxyl groups, particularly allyl-type primary
hydroxyl groups, which per se have been known (e.g., see
U. S. Patents Nos. 3427366 and 3674743). They can be pre-
pared by, for example, radical addition polymerizing 1,3-
butadiene alone or 1,3-butadiene and no more than 75% by
weight of the total monomer of C2-C12 ethylenically un-
saturated monomers such as styrene, acrylonitrile, vinyl
acetate, etc., in the presence of hydrogen peroxide as the
polymerization catalyst.
According to the present invention, of such liquid
polybutadiene polyolst particularly those having an average
number of functional groups of 2.0-3.0 and a number average
molecular weight of 2000-7000 are used. When the average
number of functional groups in the liquid polybutadiene
polyol employed is less than 2.0, product of high spring
constant is difficult to be obtained. The product further-
more shows a tendency to have larger permanent compression
set. Also the miscibility thereof with the polymer polyol
(a) and graft polyol (b) to be used as mixed therewith is
impaired, and the moldability becomes markedly poor. Con-
versely, when it exceeds 3.0, the product lacks elasticity,becomes brittle, is void of improvement in waterproofness
and alkali resistance, and shows markedly depressed fatigue
resistance.
- 20 -
Thus the convenient average number of functional
groups of the liquid polybutadiene polyol (c) i within the
range of 2.0-3.0t particularly 2.1-2.8. Again, when the
number average molecular weight of the liquid polybutadiene
polyol is less than 2,000, the variations in strength and
elongation used as the norms of waterproofness and alkali
resistance are markedly increased, and the fatiyue
resistance and permanent compression set show strong tend-
ency for marked degradation, and the closed cell-forming
ability is reduced. On the other hand, when it exceeds
7,000, the viscosity of the liquid becomes excessiYely
high, impairing its blendability with polyisocyanate (d).
Thus the product elastomer exhibits not only low tensile
strength, bu~ fails to have a high spring constant, and
shows poor closed-cell-forming ability. Thus it is ap-
propriate for the liquid polybutadiene polyol to have a
number average molecular weight of 2000-7000~ preferably
2400-5000.
Furthermore, it is desirable that the liquid
polybutadiene polyol (c) to normally have a hydroxyl content
of 0.5-1.0 milliequivalent/gram~ and an iodine value of
400-500.
As the particularly preferred liquid polybutadiene
polyol, for example, a hydroxyl-terminated butadiene homo-
polymer havin~ an average number of functional groups of2.2-2.4 and a number average molecular weight of about 2~800
(e.g., poly bd R-45 HT manufactured by ARCO Chemical Co.~,
~ ~ ~3
- 21 -
a hydroxyl-terminated butadiene/styrene copolymer having an
average number of functional groups of 2~2-2.4 and a number
average molecular weight of about 3,500 (e.g., poly bd CS-15
manufactured by ARCO Chemical Co.), and a hydroxyl-termi-
nated butadiene/acrylonitrile copolymer having an averagenumber of functional groups of 2.5-2.8 and a number average
molecular weight of about 4450 (e.g~, poly bd CN-15 manu-
factured by ARCO Chemical Co.) may be named.
The blend eatio of the above-mentioned three types
of polyol components (a), (b) and (c) is variable over a
wide range, according to the physical properties required
for the ultimately produced urethane elastomer. Normally7
it is convenient to select the blend ratio from the below-
specified ranges, based on the total weight of the three
components (a), (b) and (c).
Normal Preferred Optimum
Polyol component range range range
(wt%) (wt~)(wt%)
(a) 15-95 20-95 50-90
(b) 1-60 1.5-40 2-30
(c) 1-50 2-40 3-30
Also the mixing ratio of the graft polyol (b) to
the polybutadiene polyol (c), (~)/(c) by weight, is normally
from 1/0.5 to 1/1.5, preferably from 1/0.8 to 1/1.2. The
mixing ratio of the polyether polyol (a) to the polybuta-
diene polyol, (a)/~c) by weight, is advantageously within
the range of 3/1-15/1, preferably 4/1-10/1.
... .
~3~
- 22 -
The urethane elastomer obtained by the concurrent
use of vinyl monomer-graf~ed polyol tb) and liquid polybuta-
diene polyol (c) in accordance with the present invention
is found to achieve the novel effects unattainable with con-
ventional elastomers, i~e., it gives a high spring con~tant,showing no degradation in tensile strength due to decrease
in bulk d~nsity, even under the condi~ions of high loads and
restricted deformation such as in the use for Danchoku ties,
and furthermore its permanent compression set is small, and
its variations in strength and elongation shown in the
waterproofness and alkali resistance tests are small.
Preferred combination of the graft polyol (b) and
the liquid polybutadiene polyol (c) for achieving the high
quality closed cells, low variations in s~rength and elon-
gation in the waterproofness and alkali resistance tests,excellent vibration-absorbing ability and appropriate spring
constant and elongation, which are obtained as the novel,
synergistic effect characteristic to the present invention,
is that of the graft polyol having a graft ratio of 10-15%,
a number average molecular weight of 5000-7000 and an aver-
age number of functional groups of 3.0-3.8, with the liquid
polybutadiene polyol having a number average molecular
weight of 2500-4800 and an average number of functional
group~ of 2.2-2.8, at a blend ratio of (b) to (c) within
l:O.S to 1:1.5, particularly 1:0.8 to 1:1.2. Furthermore,
the best synergistic effect is obtained when the above
liquid polybutadiene polyol i5 blended in an amount of 3-30%
3~
23 -
by weight based on the total weight of the three types of
polyol components (a), ~b) and (c).
As the organic polyisocyanate (d) to be r~acted
with the above polyol components la), (b) and (c), any of
those normally used for the production of urethane elas-
tomers can be used. Examples are such polyisocyanates as
4,~'-diphenylmethanediisocyanate (M.D.I.), naphthylenedi-
isocyanate, tolylenediisocyanate and hexamethylenediiso~
cyanate, which may be used either alone or in combination~
The ~olyisocyanate (d) may also be used in the form of a
precursor obtained by ~dvance condensation with aforesaid
polyhydric alcohol, i~e., a pre-polymer or a semi-pre-
polymer.
In either case, the amount of the organic poly-
isocyanate (d) i~ variable within the range around stoi-
chiometric equivalent to the total active hydrogen-con-
taining components (polyol components, chain extender, etc.)
which are to react with the isocyanate residual groups
(-NCO) present in the foamable starting liquid of urethane
elastomer, +10%, i.e., in terms of NCO index, within th
range of 90-110, preferably 9S-105.
The chain extender (e) to be used for the Eorma-
tion of polyurethane elastomer in the present invention
reacts with the organic polyisocyanate (d) to ~orm, by means
Of a urethane bond or a urea bond, a hard segment that is
principally an inter-hydrogen bond. It is thus an important
factor controlling the elasticity characteristics of the
- 24 -
product polyurethane elastomer. According to the invention,
relatively low molecular weight, substantially difunctional
active hydrogen-containing compounds are advantageously used
as the chain extender. Examples of such a chain extender
(e) includes C2-C10 diols such as ethylene glycol, propyl~ne
glycol, propanediol, butanediol, hydroquinone and hydroxy-
ethylquinone ether; and amines such as methylenebis(o-
chloroaniline~, quadrol, ethylenediamine and triethanol-
amine. They may be used ei~her alone or in combination.
According to our studies, in the combined use of
the chain extender (e~ with aforesaid polyol components (a),
(b) and (c), it is found appropriate to use the chain ex-
tender ~e) at a concentration within the range of 0.3 X 10 3
mol/g to 1.5 X 10 3 mol/g, based on the total amount of the
five components of (a), (b), (c), ~d) and (e). At a con-
centration lo~er than thatp the chain-extending effect is
insufficient, and the resulting foamed polyurethane elas-
tomer generally shows the tendency to have low strength.
Conversely, at the chain extender concentration higher than
1.5 X 10 3 mol/g, inter-hydrogen bonds increases exces-
sively, and as the result the resulting elastomer tends to
become very hard, although is improved in strength. Such
is rather undesirable for the product's utility as in the
present invention, wherein the product is exposed to per-
manent compression set and repetitive compression stress.The preferred concentration range of the chain extender is
thus from 0.5 X 10 3 mol/g to 1~2 X 10 3 mol/g.
~33~
- 25 -
As the urethanation catalyst (g), any of those
normally used in urethanation reaction, for exa~ple,
tertiary amine compounds, organometal compounds, etc. may
be used. Specific examples include triethylenediamine,
diazabicycloundecene, n-methylmorpholine, N,N-dimethyl-
ethanolamine; tin octylate and dibutyl tin laurate. The
amount of the catalyst is not critical, which is variable
over a wide range depending on the desired reaction rate.
It needs be suitably adjusted, however, according to the
degree of foaming in the urethane elastomer and ambient
conditions (temperature, humidity, etc.). Adjustment of
the amount of catalyst has been a routine practice in the
art, and the selection of suitable amount should be easy.
According to the present invention, foamed
polyurethane elastomers are used. As the blowing agent
(f) to be used for the production of the foamed bodies,
conventional blowing agents, such as water and halogenated
hydrocarbons (e.g., trichlorofluoromethane, methylene
chloride, etc.) may be used. Although the degree of foaming
of the urethane elastomer desired in the present invention
is not strictly limited, it is important that the product
should be relatively lowly foamed compared with ordinary
urethane foams. Normally it is advantageous to achieve the
degree of foaming, as expressed in terms of bulk density,
2~ ranging from 0.4-0.75 g/cm3, preferably 0.55-0.7 g/cm3. The
amount of the blowing agent (f) and/or the degree of foaming
can be eegulated to make the bulk density of the resulting
~ ~ ~3
- 26 -
urethane elastomez a value within the above-specified range.
Besides the foregoing, the starting foamable
liquid of urethane elastomer in accordance with the present
invention may contain, if required, a foam stabilizer (e.g.,
silicone surfactant), pigment(s) (e.g., carbon black,
titanium oxide, etc.), dyes (e.g., Indanthrene dyes), other
fillers (e.g., coal tar, inorganic or organic staple fibers
such as glass fiber, asbestos fiber, nylon filer, vinyl
chloride fiber, polyes~er fiber, acrylic fiber, natural or
synthesized rubber powder; siliceous sand, etc.).
For the microcellular polyurethane elastomer to be
used in this invention to function as a vibration isvlator;
its spring constant per the unit area is desirably about 0.2
ton/cm/100 cm2 or higher, particularly within the range of
0.7 ton/cm/100 cm2 - 2 tons/cm/100 cm2. The spring constant
within said range can be obtained with the microcellular
polyurethane elastomer having a thickness of 5 to 100 mml
the thickness normally employed for a vibration-isolating
layer, by suitably selecting its composition and bulk den-
sity.
The microcellular polyurethane elastomer coating
material exhibits excellent effects when it is integrally
shaped with the concrete tie body and foamed and intimately
adhered thereto. Or, its vibration-isolating effect can be
effectively exhibited by shaping it separately from the
concrete tie body and then adhering it to said body. That
is, the coating layer may be adhered to the lower portion of
- 27 -
the concrete tie body with an adhesive, or a box-type poly-
urethane elastomer shaped body may be formed in advance and
into which the concrete tie body may be inserted.
According to the presen~ invention, however, it
is found that the most preferred embodiment for forming the
coating layer comprises injecting a raw liquid for making
the polyurethane elastomer around the lower portion of the
concrete tie body in a box of a fixed size, and foaming the
liquid to cause an integral shaping and foaming thereof
with the tie body, to cause the former to adhere and coat
the latter.
And, according to another aspect of the present
invention, a process for manufacturing a Danchoku tie is
provided, which comprises fixing a concre~e tie body in
a mold in such a manner that the bottom and at least the
lower portion of sides thereof are substantially completely
encased in the mold leaving a certain space from the bottom
and each of side walls, injecting into said space a foamable
starting liquid of polyurethane elastomer of the aforesaid
composition, and foaming and curing said starting liquid to
integrally form a Danchoku tie coated with a microcellular
polyurethane elastomer firmly adhered to the lower portion
of the tie body.
According to the process of this invention as
above-described, it is possible to make the Danchoku tie
with ease, using an atmospheric injection type simple mold,
and furthermore the shaped product can be released from the
~ ~ ~3
- 28 -
mold after about 2 hours feom the injection which requires
only about 1 minute. Thus the production efficiency can be
drastically increased. Furthermore because in the Danchoku
tie prepared in accordance wi~h the subject integral shaping
method the concrete tie body and the polyurethane elastomer
coating material strongly adhere to each other, use of an
adhering primer is unnecessary and hardly any peeling takes
place.
According to the process of present invention,
the aforesaid components of the starting foamable liquid
of polyurethane elastomer are intimately mixed immediately
before the injection in accordance with the accepted prac-
tice, and injected into the mold for the integral shaping of
the Danchoku tie. The mold is fixed to ~he concrete tie
body in such a manner that the bottom and at least the lower
portion of the sides tthe portions near the bottom) of the
tie body should be substantially completely encased by the
mold leaving a certain space therebetween, so as to enable
the integral shaping of the Danchoku tie. One specific
embodiment of mixing means is illustrated in Figs. 6 and
7. As shown in Figs. 6 and 7, a box-type mold 13 is at-
tached to the concrete tie body 1 in such a manner that the
bottom 11 and the lower portion of sides 12 of the body 1
can be substantially completely encased thereby. In that
occasion, spaces s of the width w and w' are provided
between respectively the bottom 11 of body 1 and the in-
ternal bottom surface of the mold and between the sides 12
~3~
- 29 -
of body 1 and the Lespective internal sides of the mold, w
and w' corresponding to the required thicknesses of the
coating layer. The mold 13 must be capable of encasing the
concrete ~ie body 1 substantially completely so as to sub-
stantially prevent leakage of the starting polyurethaneelastomer liquid which is to be injected into the space s.
In the mold, projections are formed at the locations cor-
responding to the through-holes 3, 3', 3" ... in the con-
crete tie body so as to form the through-holes also in the
coating layer.
The height _ with which the mold 13 encases the
sides 12 of concrete body 1 is made ~he same with the height
h of the coating layer covering the sides 12 of body 1.
After the mold is set as above, a starting foam-
able liquid of polyurethane elastomer is injected into thespace s through an injection inlet 14 provided at a suitable
position of the mold 13. According to the studies of
present inventors, the injection can be performed most
advantageously when the concrete tie body combined with the
mold 13 is given such a posture that, referring to Fig. 6,
the side of the mold provided with the injection inlet 14
becomes the downside and the side of the mold having the
deaeration holes 15 comes to the top, so that the bottom 11
stands substantially perpendicularly.
Fig. 8 is a flow sheet showing the injection
operation of such a starting foamble liquid of polyurethane
elastomer into the mold 13. The concrete tie body 1 mounted
- 30 -
with the mold 13 is placed with i~s bot~om 11 standing
nearly perpendicularly as aforesaid, and ~he starting-liquid
is injected through the inlet 14 loca~ed at a lower portion
of the mold 13. As the injection progresses, the air in the
space s is driven out of the deaeration holes 15.
This starting foamable liquid of polyurethane
elastomer can be formulated, for example, by separately
feeding a liquid A composed of a polyether polyolt graft
polyol, liquid polybutadiene polyol, chain extender, blowing
agent, urethanation catalyst and a foam stailizer, etc~, and
a liquid B composed of organic polyisocyanate into respec-
tively the tanks 20 and 20', and therefrom supplying them
via measuring pumps 21 and 21'~ respectively, into the
two-liquids blender 22 and whereat intimately mixing the two
li~uids. The liquid mixture is then led to the injection
inlet 14 through the conduit 23 having a terminal valve 24.
The typical compositions of the liquids A and B
conveniently used in the present invention are as follows.
Composition of liquid A Parts by weight
Polyether polyol 100
(glycerin/propylene oxide/
ethylene oxide copolymerized
adduct
average number of functional
groups: 3-4
number average molecular
weight: 2,000-7,000).
~3~
Graft polyol lO-~0
[a polymer polyol obtained by
graft polymerizing acrylonitrile and
styrene to glycerin/propylene oxide/
ethylene oxide copolymerized adduct
(number average molecular weight = 5100),
in the presence of azobisisobutyronitrile
(polymerization initiator);
average number of functional
groups = 3
graft ratio = 10%
number average molecular
weight = 6000]
Liquid hydroxyl-terminated
polybutadine polyol 5-20
Ethylene glycol 5-15
Water l.0-1.5
Triethylenediamine 0.5-2.0
Composition of li~__d BNCO index
Polyisocyanate polyol prepolymer 90-llO
le.9. an isocyanate-terminated
precursory precondensatioll product
of 4,4'-diphenylmethanediisocyanate
with glycerin/propylene oxide/
ethylene oxide copolymerized adduct
(number average molecular weight = 6500,
average number of functional groups = 3);
free NCO content = 16 wt~]
~ ~ 8~
The injection of the foamable starting liquid of
polyurethane elastomer into the mold can be performed at a
rate of normally 1-100 kg/min; preferably 30-60 kg/min. The
amount to be injected may be varied within the range of 1/3
to 9/10 of the total volume of aforesaid space in the mold,
depending on the desired deyree of foaming. The injected
foamable starting liquid of polyurethane elastomer is then
foamed and cured. The foaming and curing can normally be
performed at room temperature, but if necessary it may be
performed under heating to a temperature of up to about
70C. The foaming and curing terminates normally within 1-2
hours, and whereupon the mold is detouched from the concrete
tie body.
Then a Danchoku tie coated with a microcellular
polyurethane elastomer can be obtained. The cells in the
microcellular polyurethane elastomer coating shaped in-
tegrally with the concrete tie body are predominantly closed
cells. The physical property desirably to be had by the
elastomer are as follows:
(1) Bulk density: 0.4-0.75 g/cm3,
preferably 0~55-0.7 g/cm3
(2) Permanent compression set:
not more than 15~,
preferably not more than 5~
(3) Spring constant: at least 0.2 ton/cm/100 cm2,
preferably 0.7-2 tons/cm/100 cm2,
(4) Tensile strength: at least 5.0 kg/cm2,
~3~
- 33 -
preerably at least 10 kg/cm2
(5) Elongation: at least 100~,
(6) Waterproofness: within ~20% in variation of
tensile strength, preferably ~10%;
within ~20% in variation of
elongation, preferably ~10%
(7) Alkali resistance: within ~20% in variation of
tensile strength, preferably +10%;
within ~20% in variation of
elongation, preferably ~10%
(8) Fatigue resistance: the amount of permanent
deformation not more than l.0 mm, prefer-
ably not more than 0.2 mm
(9) Closed cell forming ratio: at least 90%,
preferably 99-100~
The Danchoku tie manufactured by the subject
process as above is composed of a concrete tie body 1 and
a microcellular polyurethane elastomer coating material 2
adhered to the lower portion of said body 1 by the integral
shaping, as illustrated in Figs. 1-3.
According to the studies of present inventors, it
is found very convenient for ready release of the Danchoku
tie from the mold,`~hat the deaeration holes 15 of the mold
13 each is given a cross-sectional shape of an inverse
circular truncated corn spreading outwards as shown in Fig.
9. As the tapering angle ~ of the internal wall of each
deaeration hole, that of 30-60 is normally suitable,
- 3~ -
particularly around 45. The inner diameter x of the de-
aeration hole 15 in the mold 13 can be approximately 1-3 mm,
and the length y of the cylindrical portion of said hole in
the mold is preferably about 0.3-2 mm.
The Danchoku tie involving present invention as
above-described exhibits excellent vibxation-isolating
effect, and can drastically reduce the vibration and noise
when used as the ties for railway track for high-speed
trains, contxibuting to alleviate enviroNmental pollution
caused b~ noise and vibration along railway lines.
Furthermore, the Danchoku ties involving present
invention can be laid with high precision and easy opera-
tions in the track-laying work, leading to marked reduction
in labor cost and work period.
Furthermore, according to the preferred embodiment
of the process of this invention, the microcellular poly-
urethane elastomer coating material is integrally foamed and
shaped with the concrete tie body, which advantageously
brings about the strong adhesion of the coating to the
concrete tie bodyO This high adherability is indeed a great
practical advantage, as normally a vibration isolator is
required to transmit the movements of the vibration source
with certainty, to cut off the vibrations and absorb them
within the isola~or.
Still in addition, according to the present
invention the Danchoku ties (resilient coated ties) can he
easily manufactured using a relatively simple apparatus; and
~ ~3
- 35 -
therefore fore the cost and energy consumption for the
production can be decreased.
Hereinafter the subject Danchoku ties and the
process for their production will be further explained with
reference to the working examples.
Example
A 400 mm x 2,000 mm x 200 mm concrete tie body 1
is set in a mold 13 having deaeration holes 15 of x = 1.5
mm~, in the manner illustrated in Fig. 6. The thickness of
the coating layer (w, w') was 2S mm. Although not shown in
Fig. 6, a partition wall was provided in the mold at the
part suita~le for forming a depression or groove of 300 mm
in width and 15 mm in depth at the central portion of the
bottom plane of the coating layer, as shown in ~ig. 2. Then
the liquid-~ A and B of the below-specified compositions were
mixed in the stirrer 22 at a rotation rate of 6,000 rpm
using the device illustrated in Fig. 8, and the mixture was
injected into the space at the lower portion of the mold.
Leaving the system for the subsequent 2 hours at room tem-
perature, the tie was parted from the mold. The physicalproperties of the polyurethane elastomer coating constitut-
ing the resultant Danchoku tie are shown hereinbelow, to-
gether with the compositions of the liquids A and B.
The physical properties were measured by the
below-specified methods.
(1) Bulk density:
Measured in accordance with JIS Z 8807,
- 36 -
"Method of measurement from volumen.
(2) Permanent compression set:
Measured in accordance with JIS K 6301
"10, Permanent Compression Set ~estn.
(33 Spring constant:
Measured in accordance with JIS K 6385 "5,
Static Spring Constant Testn.
(A 10 cm X 10 cm X 2.5 cm test specimen is
subjectd to a pressure of up to 425 kg, and the
spring constant is determined between 100-400
kg on the load displacement curve.)
(4) Tensile strength and elongation:
Measured in accordance with JIS K-6301,
with Dumbbell test pieces No. 1 by "3. Methods
of Tensile Testsn.
(5) Waterproofness:
The same Dumbbell test piece No. 1 used
in the tensile strength test is immersed in
distilled water or ion-exchange water for 96
hours, lightly wiped, and immediately subjected
to the tensile strength test. The variation
from the value before the aging is thus
determined.
[6) Alkali resistance:
The same test method as that in above
waterproofness is employed except that the
immercing liquid is a 1~ (caustic potash/caustic
~3~
- 37 -
soda - 1:1) aqueous solution, and the immersing
temperature is 50C.
(7) Fatigue resistance:
Measured in accordance with SRIS (Standard
Rating of Japan Rubber Associa~ion) 3502. (Test
conditions are: precompression amount 5 mm,
vibration amplitude 4 mm, vibration frequency 5
Hz, repetition 1 x 106 times, and the size of
test piece, 50 X 50 X 25 mm)
(8) Closed cell foaming property:
Measured in accordance with ~ST~ D 2856-70 A.
Example 1
Composition of liquid A Parts by weight
Polyether polyol (I~ 35
(glycerin/propylene oxide/
ethylene oxide copolymerized adduct
average number of functional
groups = 3,
number average molecular
weight = 3000)
Polyether polyol (II) 40
(glycerin/pentaerythritol/
propylene oxide/ethylene oxide
copolymerized adduct
average number of functional
groups = 3.7
number average molecular
weight = 5,700)
- 38 -
Graft polyol 15
[a polymer polyol obtained by
graft polymerizing acrylonitrile and
styrene to glycerin/propylene
oxide/ethylene oxide copolymerized
adduct (number average molecular
weitht = 5100), in the presence of
azobisisobutyronitrile (polymerization
initiator)
average number of functional
groups = 3
graft ratio = 10%
number average molecular
weight = 6000]
Hydroxyl-terminated liquid poly-
butadiene polyol 15
(average number of functional
groups = 205
number average molecular
weight = 2750)
Ethylene glycol 7
water 0-53
Triethylenediamine 0.7
Composit ~ N O index_
Polyisocyanate/polyol prepolymer 100
lisocyanate-terminated
precursory condensation product of
~ a~ &~
- 39 -
4,4' diphenylmethanediisocyanate and
a copolymerized adduct of glycerin/
propylene oxide/ethylene oxide having
number average molecular weigh~ of 6500
taverage number of functional groups
= 3),
number average molecular weight
= 6500)
free NCO content = 16 wt~]
Physical properties:
Bulk density: 0.63 g/cm3
Spring constant: 1.5 tf/cm/100 cm2
Permanent compression set: 2.0%
Tensile strength: 13.0 kg/cm2
Elongation: 145g
~aterproofness
Tensile strength variation: -0.9
Elongation variation: -0.3
Alkali resistance
Tensile strength variation: -0.3%
Elongation variation: -0.2%
Fatigue resistance: amount of fatigue 0.26 mm
Closed cell foaming property: closed cell formlng
ratio 100%
25 Example 2
Composition of liquid A Parts by weight
Polyether polyol ~II) 52
- 40 -
(glycerin/pentaerythri.tol/
propylene oxide/ethylene oxide
copolymeriæed adduct
average number of functional
groups = 3.7
number average molecular
weight = 5700)
Graft polyol 15
la polymer polyol obtained by
graft polymerizing acrylonitrile and
styrene to glycerin/propylene oxide/
ethylene oxide copolymerized adduct
(number average molecular weight = 5100),
in the presence of azobisisobutyro-
nitrile ~polymerization initiator);
average number of functional
groups = 3
graft ratio = 10~
number average molecular
weight = 6000~
Hydroxyl-terminate~ liquid
polybutadiene homopolyol 12
(average number of functional
groups = 2.3
number average molecular
weight = 4700
hydroxyl content = 0.5
milliequivalent/g
. .
~3~
iodine value = 450)
Ethylene glycol 5~7
Water 0.48
Triethylenediamine 0~7
Composition of liquid B NCO index
Polyisocyanate/polyol prepolymer 100
[an isocyanate-terminated
precursory condensation product
of 4,4'-diphenylmethanediisocyanate
and glycerin/propylene oxide/ethylene
oxide copolymerized adduct (number
average molecular weight = 6500)
free NCO content = 16 wt%]
Physical properties:
Bulk density: 0.69 g/cm
Spring constant: 0.98 tf/cm/100 cm2
Permanent compression set: 3~8%
Tensile strength: 14.9 kg/cm2
Elongation: 210
Waterproofness
Tensile strength variation: -3.7%
Elongation variation: -4.3%
Alkali resistance:
Tensile strength variation: -2.2%
Elongation variation: -4.1%
Fatigue resistance: amount of fatigue, 0.16 mm
Closed-cell foaming property: closed cell forming
ratio 99.9%
~3
- 42 -
The Danchoku ties prepared in the above Example
1 were laid for the test track as illustrated in Figs. lOA
and lOB, and their effects were measured as follows.
A part of the conventional ballasted track was
removed from the test line set on Tohoku Shinkansen before
opened to commercial operation, and Danchoku ties of Example
1 were laid over a length of 160 m. The Shinkansen train
was used for the test, which was run at a speed of 200-210
km/h.
The vibration and noise caused by the train run-
ning on the track were as shown respectively in Fig. lOB, as
measured at the two points Vl and V2 (as to vibration) and
at the three points of A, B and C (as to noise). The vibra-
tion was measured with the baliumtitanate accelerometer and
the noise, with the normal sound-meter.
The vibration- and noise-decreasing effects by the
test track laid on the Danchoku ties of Example 1 as com-
pared with the conventional ballasted track w~re as follows:
The vibration at Vl point (acceleration of rail)
was nearly equal to that with the resilien~ ballas~ed track
with ballast-mat, but at V2 point (acceleration of floor
slab of elevated structure), it was decreased by 7dB. At
point A (under the floor slab by 0.3 m) the noise was de-
creased by 7d~ (A), at point B (under the floor slab by 5~0
m) it was decreased by 5dB(A), and at point (C) (under the
elevated structure, 1.2 m high over the ground), by 4dB(A),
as compared with the resilient ballasted track with ballast-
mat~