Note: Descriptions are shown in the official language in which they were submitted.
1286303
BACKGROUND OF THE INVENTION:
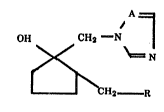
The present invention relates to a derivative of azole
represented by the formula (I):
HO 2 \ _ I
~ CH2 - R (I)
wherein A represents a nitrogen atom or a methine group and R
represents a 3-chlorophenyl group, a 2-chlorophenyl group, a
4-bromophenyl group, a 2,4-difluorophenyl group, a 2,6-
difluorophenyl group, a 3,4-difluorophenyl group, a 2-fluoro-4-
chlorophenyl group or a formula (II):
Xn (II)
wherein X represents an alkyl group, a haloalkyl group, a phenylgroup, a cyano group, a nitro group or a halogen atom and X can
be the same or different atom or group and n represents an
integer of from 1 to 5, provided that when X represents a halogen
atom,n represents an integer from 3 to 5, a process for producing
the derivative of azole and a method for controlling pests in
agriculture and horticulture with its fungicidal activity,
plant glowth regulating activity and herbicidal activity.
Field crops are badly damaged by plant diseases every
year, and agricultural chemicals to control them cause the
serious environmental pollution, which has become people's great
concern. Therefore, there has been a great demand for a new
fungicide for agriculture and horticulture which has a low level
of toxicity to human, animals, birds and fish and also has a low
level of phytotoxicity to useful plants. Such a fungicide should
be highly safe in handling, have very little adverse effect on
the environment, and produce outstanding control effect for a
wide variety of plant diseases.
In an effort to develop such an epoch-making fungicide
for agriculture and horticulture, the present inventors studied
for new compounds instead of mere modifications of existing
compounds. To this end, they synthesized a large number of
derivatives of azole and tested their usefulness in practical
use. As a result, it was found that derivatives of azole
represented by the formula (I):
/ A =
H ~ 2 \ = N~
wherein A represents a nitrogen atom or a methine group and
R represents a 3-chlorophenyl group, a 2-chlorophenyl group,
a 4-bromophenyl group, a 2,4-difluorophenyl group, a 2,6-
difluorophenyl group, a 3,4-difluorophenyl group, a 2-fluoro-4-
chlorophenyl group or a formula (II):
Xn
(II)
~28~;3~'3
wherein X represents an alkyl group, a haloalkyl group, a phenyl
group, a cyano group, a nitro group or a halogen atom and X can
be the same or different atom or group and n represents an
integer of from 1 to 5, provided that when X represents a halogen
atom,n represents an integer from 3 to 5, have the above-mentioned
characteristics.
In addition and surprisingly enough, the present
inventors have found that the derivative of azole exert not only
a fungicidal effect but also a plant growth control effect and
herbicidal effect. These findings led to the present invention.
SUMMARY OF THE INVENTION:
It is an object of the present invention to provide a
novel derivative of azole represented by the formula (I).
It is another object of the present invention to
provide a fungicide for agriculture and horticulture which is
effective agains~ a large variety of plant diseases with a low
toxicity to human, animals, birds and fish and a low phytotoxicity
to useful plant, the fungicide further exerting the plant growth
control effect and herbicidal effect.
It is further object of the present invention to
provide a method for controlling pests in agriculture and
horticulture applying as an active ingredient a derivative of
azole represented by the formula (I), said derivative azole being
superior in handling safely and environmental protection.
1286303
BRIEF EXPLANATION OF THE DRAWINGS:
Figures from 1 to 28 attached hereto are the infrared
absorption spéctra of the derivative of azole pertaining to the
present invention. The figure numbers correspond to the
respective compound numbers shown in Table 1.
DETAILED DESCRIPTION OF THE INVENTION:
The feature of the present invention lies in, (1) a
derivative of azole represented by the formula (I):
/A =
~O ~ 2 \ = N (Il
wherein A represents a nitrogen atom or a methine group and
R represents a 3-chlorophenyl group, a 2-chlorophenyl group,
a 4-bromophen~l group, a 2,4-~ifluorophenyl grGUp, a 2,6-
difluorophenyl group, a 3,4-difluorophenyl group, a 2-fluoro-
4-chlorophenyl group or a formula (II):
Xn (II)
wherein X represents an alkyl group, a haloalkyl group, a phenyl
group, a cyano group, a nitro group or a halogen atom and X can
be the same or different atom or group and n represents an
integer of from 1 to 5, provided that when X represents a halogen
atom,n represents an integer from 3 to 5, (2) a process for
producing the derivative of azole by reacting a derivative of
oxirane represented by the formula (III):
~ CH2 R (III)
wherein R has the same meaning as in the formula (I), with
1,2,4-triazole or imidazole and (3) a method for controlling
pests in agriculture and horticulture with a fungicidal activity,
a plant glowth regulating activity and a herbicidal activity
applying a derivative of azole represented by the formula (I) as
an active ingredient.
A derivative of azole represented by the formula (I)
is a novel compound and some of the derivatives are shown in
Table 1 below with respective melting points.
A detailed description is given below of the process
for producing a derivative of azole represented by the formula
(I) and utilization of the derivative of azole for controlling
pests in agriculture and horticulture.
A derivative of azole represented by the formula (I)
is produced by reacting a derivative of oxirane represented by
the formula (III) with 1,2,4-triazole or imidazole represented
by the formula (IV)
= N (IV)
wherein M represents a hydrogen atom or an alkali metal and
A represents a nitrogen atom or a methine group, in the presence
of a diluent.
6~03
~ :
Table 1
Number Indication in Formula (1) ¦ Melting point¦
compound ¦ R ~ (C) ¦
1 4-t-Butylphenyl N129 - 130
2 4-t-Butylphenyl CH123 - 124
3 4-Biphenylyl N146 - 147
4 4-Biphenylyl CH182 - 183
3-Trifluoromethylphenyl N152 - 153
6 3-Trifluoromethylphenyl CH87 - 88
7 3-Chlorophenyl N152 - 153
8 3-Chlorophenyl CH105 - 106
9 4-Nitrophenyl N131 - 132
2,6-Difluorophenyl N104 - 105
11 2,6-Difluorophenyl CH150 - 151
12 4-Bromophenyl N106 - 107
13 4-Bromophenyl CH119 - 120
14 2-Chlorophenyl N154 - 155
2-Chlorophenyl . CH103 - 104
16 4-Methylphenyl N128 - 129
17 4-Methylphenyl CH122 - 123
18 2,4-Difluorophenyl N118 - 119
19 2,4-Difluorophenyl CH144 - 145
3,4-Difluorophenyl N119 - 121
21 3,4-Difluorophenyl CH103 - 105
22 4-Cyanophenyl N115 - 116
23 4-Cyanophenyl CH103 - 104
24 2-Fluoro-4-chlorophenyl N125 - 127
2-Fluoro-4-chlorophenyl CH141 - 143
26 2,3,4,5,6-Pentafluorophenyl N 118 - 120
27 4-Trifluoromethylphenyl N102 - 103
28 4-Trifluoromethylphenyl CH91 - 92
The infrared spectrum of each compound in the above Table 1 is
shown in attached Figures 1 to 28, respectively.
A derivative of oxirane represented by the formula (III),
which is the starting material of the reaction, is prepared by
reacting a ketone represented by the formula (V):
~ CH2 - R (V)
wherein R has the same meaning as in the formula (I), with, for
example, dimethyloxosulfonium methylide or dimethylsulfonium
methylide in the presence of a diluent. (See Org. Syn. 49, 78
(1969) and J. Amer. Chem. Soc. (1965), 1353).
An example of the compound represented by the formula
(V) is 2-substituted benzylcyclopentanone. It can be prepared
from 2-alkoxycarbonylcyclopentanone and a corresponding substi-
tuted benzyl halide according to the process described in Org.
Syn., 45, 7 (1965) and J. Chem. Soc., (1950), 325. It can also
be prepared from a corresponding substituted benzylhalide and an
enamine of cyclopentanone. (See J. Pharm. Sci., 68, 1501 (1979)).
The diluent used in the process of preparing the
compound represented by the formula (I) includes hydrocarbons
such as benzene, toluene, xylene and hexane; halogenated
hydrocarbons such as methylene chloride, chloroform and carbon
tetrachloride; alcohols such as methanol and ethanol; ethers
such as diethyl ether, diisopropyl ether and tetrahydrofuran;
- 8 -
'''~
~Z863~3
and other compounds such as acetonitrile, acetone, dimethyl-
formamide and dimethylsulfoxide.
The process of the present invention may be carried
out in the presence of the above-mentioned diluent together with
a base. As examples of the base, alkali metal carbonates such
as sodium carbonate and potassium carbonate; alkali metal
hydroxide such as sodium hydroxide and potassium hydroxide; alkali
metal alcoholates such as sodium methylate, sodium ethylate and
potassium t-butylate; alkali metal hydrides such as sodium
hydride and potassium hydride; and triethylamine and pyridine
may be mentioned.
The process of the present invention is carried out in
the following manner. An azole represented by the formula (IV)
is dissolved in a diluent exemplified above, and to the ~esulting
solution is added an oxirane represented by the formula (III) in
an amount of 0.5 to 1.0 equivalent. The solution may contain a
base exemplified above, if necessary. Alternatively, the oxirane
can be dissolved in the diluent first and to the resulting
solution can be added an alkali metal salt of the azole.
The reaction temperature ranges from the freezing point
to the boiling point of the above-mentioned diluent, but the
preferred reaction temperature is from 0C to 100C. The
reaction time is from 1 hour to 3 hours. The reaction is
preferably carried out with stirring.
After the reaction is completed, the reaction mixture
1~8~
is cooled a extracted wi h an organic solvent such as ethyl
acetate, chloroform or benzene in iced water. The organic layer
is separated, washed with water and dried. The solvent is
distilled off under reduced pressure. The residues are purified
to give the desired compound. Purification may be carried out
by recrystallization or sillica gel chromatography.
A derivative of azole (a derivative of azolylcyclo-
pentanol) represented by the formula (I) exerts the following
activities as an active ingredient of agricultural and
horticultural composition.
(1) Fungicidal action on plant diseases
A derivative of azole of the present in~ention exerts
the control effect on a wide variety of plant diseases listed
below.
Rice plant: Pyricularia oryzae, Cochliobolus miyabeanus,
Xanthomonas oryzae, Rhizoctonia solani,
Helminthosporium sigmoideum and Gibberella fujikuroi.
Apple tree: Podosphaera leucotricha, Venturia inaequalis,
Sclerotinia mali, Alternaria mali and Valsa mali.
Pear tree : Alternaria kikuchiana, Phyllactinia ~y~,
Gymnosporangium haraeonum and Venturia nashicola.
Grape-vine: Unccinula necator and Phakospora ampelopsidis.
Barley: Erysiphe graminis f. sp. hordei, Rhynchosporium
secalis, Puccinia graminis and Puccinia triformis.
1;~8630.~
Wheat: Puccinia recondita, ~E~ _ia trlti~i, Puccl~ia
triformis and Erysiphe graminis f. sp. tritici.
Oriental melon plant: Sphaerotheca fuliginea.
Watermelon plant: Fusarium oxYsporum.
Tomato plant: Erysiphe cichoracearum, Alternarla solani.
Egg plant: Erysiphe cichoracearum.
Strawberry plant: Sephaerotheca humuli.
Tobacco plant: Erysiphe cichoracearum and Alternaria longipes.
Sugar beat: Cercospora beticola.
Potato plant: Alternaria solani.
Soybean plant: Cercospora kikuchii and Septoria glycines.
Drupe fruit plant: Sclerotinia cinerea.
Other crop plants: Botrytis cinerea and Sclerotinia sclerotiorum.
A derivative of azole of the present invention produces
not only the preventive effect but also the therapeutic effect
on the some of the above-mentioned plant diseases.
(2) Plant growth regulating effect
Recently, plant growth regulators have come into
general use in agriculture and horticulture, as the mechanism
governing the plant growth regulation by plant hormones has been
elucidated. The usage of plant hormones includes, for e~ample,
the production of seedless grapes by means of gibberellin, the
acceleration of rooting of cuttings by means of ~-naphthalene-
acetic acid and the growth retardation of wheat by means of
2-chloroethyltrimethylammonium chloride (trade name "CCC").
~ZB6303
The utilization of techniques in controlling environment
of plants applying plant growth regulators is now subjected not
only to crops, vegetables and fruit trees but also to ornamental
plant such as with flowers and further to wider range of plants
such as trees. And there is an increasing possibility that the
function of plant growth regulators is going to spread into
rooting acceleration, flowering regulation, fruit bearing and
thickening, growth acceleration, growth retarding and metabolism
regulation. Plant growth regulators are increasing in their
kinds and amount of consumption in recent years, but they are
not so increasing as can be expected from the above possibilities.
The derivative of azole (derivative of azolylcyclo-
pentanol) of the present invention produces the plant growth
regulating effect (including herbicidal effect) on a wide
variety of plants as shown below.
1) Suppression of vegetative growth of plants,
especially suppression of height growth.
2) Increase of available nutrients in plants.
3) Regulation of maturing stage or flowering time of
plants.
The first activity mentioned above is useful for the
suppression of weed growth (weed-killing function), the suppres-
sion of lawn, the prevention of lodging of rice plant and wheat,
the suppression of height of soybean and cotton plants which
make harvesting machine available, the suppression of axillary
buds which promotes the growth of tobacco leaves, the suppression
of hedge plant growth which reduces the frequency of pruning and
the growth retardation of ornamental plants which leads to an
increased commercial value.
The second activity mentioned above contributes to the
improvement of beat sugar, sugar cane, and citrus through the
increase of sugar content and also to the improvement of crops
and soybean through the increase of protein.
The third activity mentioned above makes it possible to
ship fresh fruits and flowers at any time according to demands.
A derivative of azole represented by the formula (I)
can be used as a fungicide, a plant growth regulator or a
herbicide in the form of dust, wettable powder, granule,
emulsifiable concentrate or solution with or without mixing a
carrier (or diluent). It may also be used as such if necessary,
namely, the formulation may contain, in addition to a carrier,
adjuvants such as spreader, emulsifier, wetting agent, and
sticking agent to ensure the effect.
Incidentally, the derivative of azole of the present
invention can be used in the form of inorganic salt, organic
salt or metal complex,because it contains the 1,2,4-triazole
ring or imidazole ring.
The derivative of azole of the present invention has
an azolylmethyl group and a substituted benzyl group at the
l-position and 2-position, respectively, of the cyclopentane ring.
13 -
.
1;~8~
Therefore, it should exist in the form of stereoisomers, e.g.,
geometrical isomer (cis form and trans form) and optical
isomers. In this invention, the derivative of azole may be a
single isomer or a mixture thereof at an arbitrary ratio.
Thus, the agricultural and horticultural chemical pertaining to
the present invention may contain one of the isomers or a
mixture of the isomers as an active ingredient.
The present invention is now illustrated with the
following examples which demonstrate the production of
derivatives of azole and the effect of the agricultural and
horticultural chemical containing a derivative of azole as an
active ingredient.
EXAMPLE 1:
Preparation of 2-(2,4-difluorobenzyl)-1-(lH-imidazol-l-yl-
methyl)cyclopentan-l-ol (Compound No. 19 in Table 1):
Into 10 ml of anhydrous dimethylformamide, 324 mg of
sodium hydride (prepared by washing 60% oily sodium hydride with
dried benzene) were added while stirring under helium atmosphere,
and after adding 910 mg of lH-imidazol into the thus prepared
mixture, the whole mixture was stirred at room temperature until
bubbling was over. A solution of 1.5 g of 4-(2,4-difluorobenzyl)-
l-oxaspiro[2.4]heptane in 10 ml of anhydrous dimethylformamide
was dropped into the thus obtained solution and the whole mixture
was stirred for 2 hours at a room temperature.
After leaving the thus obtained reaction mixture to
1~8~ 03
cool, it was poured into lc-d water, and the mixture was extracted
with ethyl acetate to obtain an organic layer. After washing
the organic layer with water and drying the layer on anhydrous
sodium sulfate, the solvent was distilled off from the layer
under a reduced pressure to obtain a residue. By recrystallizing
the residue with a mixture of hexane and ethyl acetate, 830 mg
of the compound shown in the above title was obtained.
The results of determination of the physical properties
of the thus obtained compound are as follows and in addition,
NMR spectrum of the compound was determlned by using TMS as the
internal standard and the results are shown by the following
marks (the same marks are used in other Examples):
s: singlet
d: doublet
m: multiplet
b: a broad line
Physical Properties
~1) Melting point: 144 - 145C
(2) Infrared absorption spectrum (KBr method) is shown in
Figure 19 in attached Drawings.
Infrared absorption spectrum (KBr method): vmax 3170, 2940,
1610, 1590, 1490 cm 1.
(3) NMR spectrum (CDC13, ppm): ~
1.37-2.13~bs, 7H), 2.33 -2.83(m, 2H), 3.33(s, lH),
3.77(d, lH, J=14Hz), 4.10(d, lH, J=14Hz), 6.57 - 7.60(m, 6H).
~86303
EX~MPLE 2:
Preparation of 2-(4-bromobenzy1)-1-(lH-1,2,4-triazol-1-yl-
methyl)cyclopentan-l-ol (Compound No. 12 in Table 1):
Into 30 ml of anhydrous dimethylformamide, 3.0 g of
4-(4-bromobenzyl)-1-oxaspiro I2.4] heptane was added to be
dissolved while stirring under a helium atmosphere, and into the
thus prepared solution, 1.12 g of sodium salt of lH-1,2,4-
triazole of 90% in purity (commercial product, made by ALDRICH
Co.) was slowly added. The mixture was stirred for 1 hour at 80C
After leaving the thus obtained reaction mixture to
be cooled, the cooled reaction mixture was poured into water and
the mixture was extracted with ethyl acetate to obtain an
organic layer. After washing the layer with water and drying
thereof on anhydrous sodium sulfate, the solvent was distilled
off from the layer under a reduced pressure to obtain a residue.
By purifying the residue through silica gel column
chromatography, 2.7 g of the compound of the above title was
obtained. The physical properties thereof being shown as
follows:
Physical Properties
(1) Melting point: 106 - 107C
(2) Infrared absorption spectrum (XBr method) is shown in
Figure 12 in attached Drawings.
Infrared absorption spectrum (KBr method): vmax
3260, 3100, 2930, 1620, 1270, 1130, 660 cm 1.
lZ86~03
(3) NMR spectrum (CDC13, ppm): ~
1.45 - 3.15(m, lOH), 4.27(s, 2H), 7.20(d, 2H, J=9Hz),
7.60(d, 2H, J=9Hz), 8.13(s, lH), 8.28(s, lH).
The following two examples (Example 3 and 4) are the
examples of the preparation of a fungicide for use in agriculture
and horticulture containing a derivative of azole according to
the present invention as an active ingredient.
EXAMPLE 3:
By pulverizing and mixing 3 parts by weight of one of
the present compounds (Compound No. 5 in Table 1), 40 parts by
weight of clay and 57 parts by weight of talc, a fungicide dust
was prepared.
The thus prepared ungicide dust is used by scattering
on the ob ject.
EXAMPLE 4:
By pulverizing and mixing 50 parts by weight of one of
the present compounds (Compound No. 3 in Table 1), 5 parts by
weight of a salt of ligninsulfonic acid, 3 parts by weight of a
salt of an alkylsulfonic acid and 42 parts by weight of
diatomaceous earth, a fungicide wettable power was prepared.
The thus prepared fungicide wettable powder is used
after diluting with water.
The following five examples (Examples 5 to 9) are the
examples showing the fungicidal effect of the fungicide for use
in agriculture and horticulture according to the present invention
~86~(33
EXAMPLE 5:
Pest controI test against Erysiphe graminis f. sp. tritici
on wheat:
Onto the leave of seedling of wheat (variety: NORIN
No. 64, 16 seedlings per pot) at the second leaf stage cultured
in an unglazed pot, the fungicide wettable powder prepared
according to the method in Example 4 diluted with water to a
predetermined concentration was sprayed at a rate of 5 ml/pot
(control pot was sprayed with water only). After natural drying
of the thus sprayed leave, an aqueous suspension of the spores
of Erysiphe graminis f. sp. tritici collected from the attacked
leaves of wheat was sprayed onto the thus dried leaves of the
potted wheat, and the thus treated seedlings were kept for 24
hours at temperature of from 20 to 25C in a highly humid
atmosphere. Thereafter, the thus treated seedlings were left in
a glass green house. After 10 days of the inoculation, the
morbidity of the seedlings was examined on the basis of the
following standard and the control value was calculated by the
following formula from the avexage morbidity per leaf:
Standard of the examination
Morbidity index Extent of disease infect
0 Not infected
0.5 A l) is less than 10~,
1 A is from 10 to less than 20~,
2 A is from 20 to less than 40%,
Morbidity index Extent of disease infect
3 A is from 40 to less than 60%r
4 A is from 60 to less than 80% and
A is larger than 80%.
ote *11: A is the area rate of disease infect on the surface
of the inoculated leaf.
Control value = (1 ~ morbiditY Onn tcontrteod PoOt ) x 100 (%)
he results are shown in Table 2.
63():~
Table 2
Compound Concentration
number of the sprayedControl value
(as in Table 1) liquid (ppm) (~)
125 95
3 125 95
125 100
7 1255 100
9 1255 100
125 100
100
13 125 655
125 70
6 1225 77o5
18 125 l6o8o
125 100
21 125 100
22 1255 l6o2
1225 1075
26 12255 100
28 125 100
Triadimefon 1 125 100
Control ¦ --
1~863~3
Note: *1: Triadimefon is a commercial fungicide containing
the following compound as the active ingredient:
Cl~ ~
EXAMPLE 6:
Pest control test against Sphaerotheca fuliginea
on cucumber-plant:
Onto the leave of seedling of cucumber (variety:
SAGAMI-HAMPAKU, one seedling per pot, 3 pots in a test of one
compound) at the second leaf stage cultured in an unglazed pot
of 10 cm in diameter, a fungicide wettable powder prepared
according to the method in Example 4 diluted wi.th water to a
predetermined concentration was sprayed at a rate of 5 ml/pot
(control pot was sprayed with water only), and then the spores
of Sphaerotheca fuliginea of cucumber were scattered onto the
thus sprayed leave from the contracted leaf of cucumber plant by
using a brush to inoculate on the leave, and the thus treated
pots were left in a glass green house. After 7 days of the
inoculation,.the morbidity of the leaf of seedlings (one leaf/
pot, three pots/compound) was examined according to the following
standard, and the control value was calculated from the average
morbidity per leaf while utilizing the same formula as in Example
- 21 -
~86~'~
Standard of the examination:
Morbidity index Extent of disease infect
0 Not infected
0.5 A 1) is less than 10%, .
1 A is from 10 to less than 20%,
2 A is from 20 to less than 40%,
3 A is from 40 to less than 60%,
4 A is from 60 to less than 80% and
A is larger than 80%.
ote *1): A is the area rate of disease infect on the surface
of the inoculated leaf.
The results are shown in Table 3.
6:~0~3
Table 3
Compound ConcentrationControl value
number of the sprayed
Table 1 ) liqu d (ppm1 ~ ~
3 62 5 100
62.5 100
6 62.5 60
7 62.5 100
8 62.5 100
_ ,
A commercial 12 5 10 0
funglclde
Control __ O . .
ote *1): A fungicide of quinoxaline series represented by
the following formula:
C~3 ~ ~/X 5 > =
~ Z~3~i3~
EXAMPLE 7:
Pest control test against Puccinia recondita ~. sp.
tritici on wheat:
Onto the leave of seedling of wheat (variety: NORIN
No. 64, 16 seedlings per pot) at the second leaf stage cultured
in an unglazed pot of 10 cm in diameter, a fungicide wettable
powder prepared according to the method in Example 4 diluted with
water at a predetermined concentration was sprayed at a rate of
5 ml/pot (control pot was sprayed with water only). After
natural drying of the thus sprayed leave, an aqueous suspension
of the uredospores of Puccinia recondita f. sp. tritici collected
from the attacked leaves of wheat was sprayed onto the thus
dried leaves of the potted wheat, and the thus treated seedlings
were kept for 24 hours at a temperature of from 20 to 25C in a
highly humid atmosphere. Thereafter, the thus treated seedlings
were left in a glass green house, and after 7 days, the morbidity
was examined on the basis of the following standard. The control
value of each of the fungicide was calculated by the formula
shown in Example 5 from the average morbidity per leaf of the 10
seedlings per pot:
Standards of the examination:
The same as in Example 6.
The results are shown in Table 4.
l 12~30.3
Table 4
Compound ¦ Concentration
number of the sprayed Control value
-s ]n able 1) ¦ 1iquid (ppm)
4 200 883
200 575
7 200 45
8 200 80
9 200 90
200 75
11 200 68
. 12 200 100
4 200 4655
200 75
16 200 75
17 200 83
18 200 88
19 200 65
200 100
21 200 100
22 200 88
23 200 68
24 200 100
200 70
26 200 185
Triadimefon 200 100
Control __
` - 25 -
~8~3()3
EXAMPLE 8:
Pest control test against Cochliobolus miyabeanus
on rice plant:
In the unglazed pots of 10 cm in diameter, the seeds of
rice plant (variety: SASANISHIKI) were sown at a rate of 16
seeds/pot, and at the stage of 4 to 5 leaves, the fungicide
wettable powder prepared according to the method in Example 4
was diluted with water to a predetermined concentration, and the
thus prepared aqueous suspension of the fungicide was sprayed
onto the seedlings of rice plant at a rate of 5 ml/pot. After
natural drying the thus treated leaves of the rice seedlings,
an aqueous suspension of the spores of Cochliobolus miyabeanus
(at a concentration of 15 spores in the visual field of a
microscope of 150 magnification) preliminarily cultured was
sprayed onto the leave of seedlings at a rate of 5 ml/pot for
inoculation. After inoculation, the pots were immediately taken
into an inoculation room of 25C and of saturated humidity, and
after keeping thereof for 2 days, the pots were moved into a
glass green house to be attacked. At the fifth day after the
inoculation, the number of the disease spots on 10 leaves per pot
was enumerated, and the control value of each of the fungicides
was calculated according to the following formula:
Control value (%) = (1 ~ B ) x 100
wherein A is the number of disease spots in the treated pots
and B is that in the control pots (not being sprayed with the
fungicide).
The results are shown in Table 5.
~8~303
Compound Concentration
number of the sprayed Control value
(as in Table 1) liquid (ppm)( )
1 300 100
23 300 100
: 6 300 98
7 300 100
8 300 100
9 300 80
300 9S
11 300 98
12 300 97
13 300 100
14 300 90
300 99
16 300 100
17 300 100
18 300 100
19 300 100
300 100
21 300 100
22 300 198
. 24 300 100
300 100
26 300 100
.
*l)
EDDP 300 88
Control __ _ _
~6303
Note *1): A commercial fungicide containing the following
compound as an active ingredient:
~ 1 ~3
EXAMPLE 9:
In vitro test against several fungal species
Antifungal properties of the present compounds against
several fungal species were examined as follows:
After thoroughly mixing each of the present compounds
with the PSA culture medium at a prescribed concentration, the
thus prepared mixture was poured into dishes of 9 cm in diameter
in an amount of 10 ml. per dish to prepare plate culture medium.
On the other hand, each of the fungal species cultured in a
plate culture medium was punched by a cork borer of 6 mm in
diameter and inoculated on the thus prepared plate culture medium
in the dishes. After inoculating, each of the thus inoculated
fungi was cultured for from one to three days at a temperature
suitable for each fungus, and the growth of the fungus was
determined by the diameter of each fungal colony. The results
were compared to the result on the control (the culture medium
not containing any fungicide), and on the basis of the following
formula, the rate of control of the mycelial growth was obtained:
R = (dc ~ dt) ~
~8630~3
wherein R represents the rate of control of the mycelial growth
of a fungus, dt represents the diameter of the fungal colony on
the culture medium plate containing each of the present compound
and dc represents the diameter of the fungal colony on the
culture medium plate not containing any fungicide (control).
The thus obtained results were evaluated on the basis of
the following standard into 5 ranks and are shown in Table 6.
Standard for evaluation:
Index of growth control Rate of control of mycelial growth
from 100 to 90%
4 from 89 to 70
3 from 69 to 40
2 from 39 to 20
1 less than 20~
I 1286303
r I . I . L L ~ L L ~ L L L ~ L L L L ~ L L L L L
. nnnnn nnnnn nnn n nnnnn Lnnnnn n
~ ~ ~ tnnLn~ LnLn~Ln ~ ~
Z LnLnLnLnLn LnLnLnLnLn LnLnLnLnLn LnLnLnLnLn LnLnLnLnLn n
~ e ~Ln~LnLn ~Ln~ ~ L~ L~ ~ ~ Ln ~ ~ Ln Ln ~ ~ ~ Ln L
Ln~ LnLn~ Ln~Ln~Ln ~Ln~ Ln
OJ :C ~ LnLn~ ~LnLn~ LnLn~Ln Ln~Ln~ Ln
7 t~ = nLnLnLn~ n ~n Ln Ln ~n ~ ~ Ln ~ Ln Ln Ln Ln Ln Ln Ln Ln ~ Ln Ln Ln
~3 - ~ ~ 7 ~ Ln ~
O L~ L~ ~n Ln Ln ~nLn~n Ln Ln ~n Ln LnLn~n LnLnLnLnLn Ln~LnLn~n Ln
S~ ooooo ooooo ooooo ooooo ooooo o
ooooo ooooo ooooo ooooo ooooo o
O L ~ ~
~ ¢ ¢ ~ Ln ~ 7 ~ o ~ Ln ~ 7 ~ o ~ Ln ~7
3()3
Note: The abbreviation in ABLE 6 indicate the fungal species
as follows:
P.o. : Pyricularia oryzae on rice plant
C.m. : Cochliobolus m yabeanus on rice plant
R.s. : Rhizoctonia solani on rice plant
G.f. : Gibberella fuiikuroi on rice plant
He.s.: Helminthosporium sigmoideum on rice plant
Bo.c.: Botrytis cinerea
F.n. : Fusarium oxysporum f. niveum on watermelon
S.c. : Sclerotinia cinerea on peach tree
A.k. : Alternaria kikuchiana on pear tree
V.m. : Valsa mali on apple tree
1~86~30~3
EXAMPLE 10:
Preparation of plant growth regulator composition and
herbicide composition containing a derivative of azole as an
active ingredient.
(1) Wettable powder
The following components were pulverized and mixed to
prepare a wettable powder, which would be applied after dilution
with water.
Compound of the invention 50 parts by weight
(compound No. 3 in Table 1)
Lignin sulfonate5 parts by weight
Alkyl sulfonate3 parts by weight
Diatomaceous earth42 parts by weight
(2) Emulsifiable concentrate
The following components were uniformly mixed to
prepare an emulsifiable concentrate, which would be applied after
dilution with water.
Compound of this invention 25 parts by weight
(compound No. 20 in Table 1)
Xylene 65 parts by weight
Polyoxyethylene alkylaryl ether 10 parts by weight
(3) Granule
The following components were uniformly mixed together
with water and the mixture was granulated by an extrusion
granulator, followed by drying.
Compound of this invention 8 parts by weight
(compound No. 12 in Table 1)
~286303
sentonite40 parts by weight
Clay 45 parts by weight
Lignin sulfonate7 parts by weight
EXAMPLE 11:
Plant lenqth inhibition for wheat
Ten wheat grains were sown in a glass petri dish 8.5 cm
in diameter, containing 5 ml of 50 ppm solution of the compound
under test. (The variety of wheat is AOBA No. 3). After growing
indoors at 27C for 7 days, the plant length was measured.
The results are shown in Table 7.
It i5 noted from Table 7 that all the compounds tested
produced a pronounced effect of inhibiting the growth of plant
length, particularly in the case of triazole-type compounds.
No phytotoxicity was observed.
~ 1286~30;~
Table 7
Compound ¦Inhibition ¦ i
number of plant Phytotoxicity
(as in Table 1) length (~6)
1 3.8 None
2 3.1 None
3 3 5 NNone
20.4 None
7 7 2 NNone
9 45 6 NOnne
81.8 None
11 16.5 None
12 3 7 NNone
14 54.1 None
15.2 None
16 85.4 None
17 38.1 None
18 15.8 None
19 11.8 None
62.6 None
. 21 19.6 None
22 11 2 NNone
24 79.0 None
14.7 None
26 72.5 None
27 35.5 None
28 14.3 None
Average length of wheat in the control group was
80.5 mm.
1 12~ 03
EXAMPLE 12:
Weeds killing test (soil treatment before g~rmination)
Several kinds of weed seeds were sown in sandy loam
filled in a planter (650 x 210 x 220 mm). On the day subsequent
to sowing, the soil surface was sprayed with a properly diluted
liquid of the emulsifiable concentrate prepared according to the
method in Example 10 (2).
The weeds were grown in a greenhouse made of glass,
and the weeding effect was observed on 21st day after treatment.
The weeding effect was rated according to the following standard.
The results are shown in Table 8.
Index Rate of killing
O No weeding effect
1 Weeding effect of 30%
2 Weeding effect of 31 to 50%
3 Weeding effect of 51 to 70%
4 Weeding effect of 71 to 90%
Weeding effect of 91 to 100%
86~30~-~ l
Table 8
Compound ¦ Weeds tested (Refer to Note)
(as in Table 1) ¦ A.r. ¦ B.p. ¦ S.n ¦ E. L . ¦ S .V.
Note: The abbreviations in TABLE 8 indicate the weeds as follows:
A.r. : Amaranthus retyofiexus
B.p. : Bidens pilosa
S.n. : Solanum nigrum
E.f. : Echinochloa frumentaceum
S.v. : Setaria viridis