Note: Descriptions are shown in the official language in which they were submitted.
~28668~3
--1--
J
PROCESS FOR MANUFACTURE OF ALKENE OXIDE
Technical Field:
The present invention is directed to improved
processes for the preparation of alkene oxide from
alkene and oxygen-containing gas employing a sup-
ported silver catalyst. Particular aspects of thepresent invention relate to processes for epoxidizing
alkene in the vapor phase to produce the correspond-
ing alkene oxide at high efficiencies.
Background And Backqround Art:
The production of alkene oxides, or epoxides,
particularly ethylene oxide, by the direct epoxida-
tion of the corresponding alkene in the presence of a
silver-containing catalyst has been known for many
years. One of the earliest disclosures of a process
for the direct epoxidation of ethylene was that of
Lefort, U. S. Patent 1,998,878, issued in 1935 (re-
issued in 1942 as Re. 20,370). Lefort discloses that
ethylene oxide can be formed by reacting ethylene and
oxygen according to the following equation:
15016
121~68~
--2--
2CH2=CH2 + 02~ 2CH2 CH2 (I)
Lefort recognized, however, that some of the
ethylene, when reacted with oxygen, is completely
oxidized to carbon dioxide according to the following
equation:
C2H4 + 32 - -~2H20 ~2C02 (II)
Reaction II, as well as other reactions in which
alkene is converted to products other than alkene
oxide, is undesirable since the alkene reactant is ~
consumed in the formation of undesired products.
Further, the undesired products, e.g., carbon di-
oxide, may adversely affect the reaction system. In
general, the overall effectiveness of an alkene oxide
production system is gauged by the performance char-
acteristics of the system. The most important per-
formance characteristics are the efficiency, theactivity, and the useful life of the catalyst, all of
which are defined and more completely described be-
low.
Percent conversion is defined as the percentage
of the alkene introduced to the reaction system that
undergoes reaction. Of the alkene that reacts, the
percentage that is converted into the corresponding
alkene oxide is referred to as the selectivity or
efficiency of the process. The commercial success of
a reaction system depends in large measure on the
efficiency of the system. At present, maximum effi-
ciencies in commercial production of ethylene oxide
by epoxidation are in the low 80s, e.g., 80 or 81
percent. Even a very small increase in efficiency
will provide substantial cost benefits in large-scale
15016
12~688
--3--
operation. For example, taking 100,000 metric tons
as a typical yearly yield for a conventional ethylene
oxide plant, an increase in efficiency of from 80 to
84 percent, all other things being equal, would re-
sult in a savings of 3790 metric tons of ethylene peryear. In addition, the heat of reaction for Reaction
II ~formation of carbon dioxide) is much greater than
that of Reaction I (formation of ethylene oxide) so
heat-removal problems are more burdensome as the
efficiency decreases. Furthermore, as the efficiency
decreases, there is the potential for a greater
amount of impurities to be present in the reactor
effluent which can complicate separation of the de-
sired alkene oxide product. It would be desirable,
therefore, to develop a process for the epoxidation
of alkene in which the efficiency is greater than
that obtained in conventional commercial processes,
e.g., with ethylene, efficiences of 84 percent or
greater, while maintaining other performance charac-
teristics, particularly the activity, as describedbelow, in a satisfactory range.
The product of the efficiency and the conversion
is equal to the yield, or the percentage of the al-
kene fed that is converted into the corresponding
oxide. The definitions of conversion, efficiency and
yield may be represented as follows:
% Conversion = moles alkene reacted x 100
moles alkene fed
% Efficiency = moles alkene oxide produced x 100
moles alkene reacted
% Yield = moles alkene oxide produced x 100
moles alkene fed
15016
12B6688
--4--
Generally, in a process for the epoxidation of
alkene, a reaction inlet stream containing reactants
and perhaps other additional materials enters the
reactor or reaction zone in which catalytic material
is provided and in which favorable reaction condi-
tions (e.g., temperature and pressure) are maintain-
ed. The reactor effluent is withdrawn or collected
from the reactor. The reactor effluent contains
reaction products, together with unreacted components
- from the reaction inlet stream.
Since at least some alkene is generally not
converted during ;ts initial pass through the reac-
tor, alkene is generally present in the reactor ef-
fluent. To increase the overall yield of the pro-
cess, at least a portion of the alkene in the reactor
effluent is returned to the reactor via a recycle
stream. Means are provided for removing and recover-
ing at least a portion of the alkene oxide from the
reactor effluent, prior to recycling, to form the
product stream. At least a portion of the remaining
reactor effluent (after the product stream has been
withdrawn) becomes the recycle stream. The recycle
stream preferably contains substantially all of the
alkene that was contained in the reactor effluent.
Reactants, i.e., oxygen-containing gas and al-
kene, need to be continuously replaced and are pro-
vided to the reactor by means of a makeup feed-
stream. In general, the makeup feedstream and the
recycle stream are combined to form the reaction
inlet stream which is sent into the reactor or reac-
tion zone. Alternatively, the makeup feedstream and
the recycle stream can be introduced into the reactor
separately.
15016
12~366~38
--5--
The epoxidation of alkene is generally carried
out in the presence of a supported silver catalyst
located within the reactor or reaction zone. The
silver may be supported by a conventional support
material, for example, alpha-alumina. The per-
formance of the catalyst may be affected by the pre-
sence of solid, liquid or gaseous compounds which
may, for example, be incorporated in the catalyst or
provided via the makeup feedstream.
The activity of a reaction system is a measure
of the rate of production of the desired product,
e.g., ethylene oxide, for a particular reaction sys-
tem at a particular temperature. In order to provide
a meaningful comparison of the effectiveness of two
or more reaction systems or of a single reaction
system at different times, factors, such as feed
rate, feed composition, temperature, and pressure,
that affect the rate of production of the desired
alkene oxide must be normalized or accounted for,
preferably by using a standard or fixed set of oper-
ating conditions. Since the rate of production of
alkene oxide is proportional to the volume of cata-
lyst in the reaction system, the activity is usually
expressed in terms of pounds of alkene oxide produced
per hour per cubic foot of catalyst. It should be
noted that this method of measuring activity does not
take into account variations in the densities of the
catalysts since the controlling factor is the volume
of the reaction system available, not the weight of
catalyst which will fit into a given volume. Other
factors that have an effect on the rate of production
of the desired compound include the following:
(1) the composition of the reaction stream;
(2) the gas hourly space velocity of the reac-
tion stream;
15016
12~66~8
--6--
(3) the temperature and pressure within the
reactor or reaction zone.
In order to compare the effectiveness of two or
more reaction systems or of a single reaction system
at different times, differences in factors 1 through
3 above should be minimized and/or factored into the
evaluation of relative effectiveness.
If the activity of a reaction system is low,
then, all other things being equal, the commercial
value of that system will be low. The lower the
activity of a reaction system, the less product pro-
duced in a unit time for a given feed rate, reactor
temperature, catalyst, surface area, etcetera. A low
activity can render even a high efficiency process
commercially impractical. In general, an activity
below 4 pounds of ethylene oxide per hour per cubic
foot of catalyst is unacceptable for commercial prac-
tice. The activity is preferably greater than 8
pounds, and in some instances an activity greater
than 11 pounds of ethylene oxide per hour per cubic
foot of catalyst is desired.
Reaction systems generally deactivate over time,
i.e., the activity of the catalyst begins to decrease
as the process is carried out. Activity may be plot-
ted as a function of time to generate a graph showingthe aging behavior of the catalyst. Experimentation
for the purpose of developing an activity plot is
usually conducted at a set temperature since, in
general, activity can be increased by raising the
reaction temperature. Alternatively, an activity
plot can be a graph of the temperature required to
maintain a given activity versus time. The rate at
which activity decreases, i.e., the rate of deactiva-
tion at a given point in time, can be represented by
the slope of the activity plot, i.e., the derivative
15016
1286688
of activity with respect to time:
deactivation = d[activity~/dt.
The average rate of cleactivation over a period
of time can be represented then by the change in
activity divided by the time period:
average deactivation = ~activity/ ~t.
At some point, the activity decreases to an
unacceptable level, for example, the temperature
required to maintain the activity of the system be-
comes unacceptably high or the cate of production
becomes unacceptably low. At this point, the cata-
lyst must either be regenerated or replaced. The
useful life of a reaction system is the length of
time that reactants can be passed through the reac-
tion system during which acceptable activity is ob-
served. The area under a plot of activity versustime is equal to the number of pounds of alkene oxide
produced during the useful life of the catalyst per
cubic foot of catalyst. The greater the area under
such a plot, the more valuable the process is since
regeneration or replacement of the catalyst involves
a number of expenses, sometimes referred to as turn-
around costs. More specifically, the replacement of
the catalyst generally requires that the reactor be
shut down for an extended period of time, e.g., two
weeks or more, to discharge the catalyst, clean the
reactor tubes, etcetera. This operation requires
extra manpower and the use of special equipment. The
costs involved, which may include replacement cata-
lyst, can mount into the millions of dollars.
15016
12~ti6S8
--8--
As used herein, an activity-reducing compound
refers to a compound which, when present in an acti-
vity-reducing amount, causes a reduction in activity,
some or all of which activity may subsequently be
regained by returning to a situation in which the
concentration of the compound is below the minimum
activity-reducing amount. The minimum activity-
reducing amount varies depending on the particular
system, the feedstream and the activity-reducing
compound.
Conversely, deactivation, as used herein, refers
to a permanent loss of activity, i.e., a decrease in
activity which cannot be recovered. As noted above,
activity can be increased by raising the temperature,
lS but the need to operate at a higher temperature to
maintain a particular activity is representative of
deactivation. Catalysts tend to deactivate more
rapidly when reaction is carried out at higher tem-
peratures.
As previously noted, since the work of Lefort
(U. S. Patent 1,998,878), research efforts have been
directed toward improving the performance character-
istics of reaction systems, i.e., improving the acti-
vity, efficiency and useful life. Research has been
conducted in areas such as feedstream additives,
removal of materials in the recycle stream and
methods of catalyst preparation, including the depos-
ition or impregnation of a particular type or form of
silver. Additionally, research efforts have been di-
rected toward the composition and formation of thesupport, as well as toward additives deposited on or
impregnated in the support.
One of the difficulties in carrying out research
is the necessity of considering the interrelationship
of the various variables. The improvement or en-
15016
128~688
hancement of one performance characteristic must not
be at the expense of, or have too great an adverse
effect on, one of the other performance characteris-
tics. For example, if a reaction system is designed
which has a very short useful life, the system may be
commercially impractical even though the efficiency
and initial activity of the catalyst are outstand-
ing. Accordingly, a system that provides an increase
in the efficiency of the overall catalytic reaction
system, while only minimally affecting the activity
and useful life of the catalyst, would be particular-
ly beneficial.
Diluents have generally been included in the
gaseous mixture to reduce the likelihood of explo-
sion. Diluents are generally supplied via the makeupfeedstream. Such diluent materials have generally
been believed to be inert, i.e., their function is
primarily to act as a heat sink and to dilute the
gaseous mixture. Nitrogen has been found to be a
suitable diluent material. It is well known to use
air to supply both oxygen and nitrogen to the reac-
tion zone. Another material that has been used as a
diluent is carbon dioxide. EPO Patent 3642 discloses
that a diluent, for example, helium, nitrogen, argon,
carbon dioxide, and/or a lower paraffin, for example,
ethane and/or methane, may be present in proportions
of 10-80 percent and preferably 40-70 percent by
volume in total. Similarly, U. K. Patent Application
GB 2 014 133A mentions carbon dioxide as a possible
diluent. Other patents, e.g., U. S. Patents
3,043,854, 4,007,135, and 4,206,128, Japanese Patent
53-39404, and U. K. Patents 676,358 and 1,571,123,
also mention that carbon dioxide is suitable for use
as an additive.
15016
12866~38
--10--
Lefort, in U. S. Patent 1,998,878 (Re. 20,370),
states that carbon dioxide may be introduced into the
reactor to limit the rate of complete oxidation of
ethylene to carbon dioxide. Similar disclosure is
found in V. S. Patent 2,270,780. U. S. Patent
2,615,900 discloses a process for producing ethylene
oxide in which carbon dioxide gas may be added to the
feed gases to act as a "depressant" or "anti-cata-
lytic material". U. S. Patent 4,007,135 discloses a
process in which, according to the patent, carbon
dioxide may be used to raise the selectivity of the
reaction. According to Chem. Abstracts, Vol. 80,
Issue 11, Section 22, Abstract 059195, the presence
of carbon dioxide tends to retard the deactivation of
the silver catalyst.
As mentioned above, any alkene contained in the
reactor effluent stream is preferably returned to the
reaction zone via a recycle stream. It is sometimes
preferred to remove some of the gas contained in the
reactor effluent stream via a purge stream prior to
introducing the recycle stream into the reaction
zone. The purge stream may comprise a straight
purge, i.e., the purge stream can merely draw off a
percentage of the recycle stream. Since a straight
purge stream generally has a composition substantial-
ly similar to that of the stream from which it is
removed, some alkene will generally be purged when a
straight purge is employed. For this reason, means
are sometimes provided to ensure that purge streams
have relatively high concentrations of materials
other than alkene, such as nitrogen and carbon diox-
ide.
U. S. Patent 2,241,019 discloses a process in
which the purge gas is carried through and in contact
with an adsorptive agent which is adapted to adsorb
15016
~2~66~8
--11--
selectively the ethylene content of the purge gas,
while the nitrogen and much of the carbon dioxide
present in the purge gas pass through the adsorption
agent and are discharged to the atmosphere.
U. S. Patent 2,376,987 discloses a process for
the two-stage preparation of butadiene in which, in
the first stage, ethylene is oxidized in a converter
to form ethylene oxide. The converter contains an
oxidizing catalyst which is preferably finely divided
silver on a carrier such as alumina. According to
the patent, if concentrated oxygen is used as the
oxygen source, the ethylene in the stream containing
the oxidation products from the converter may be con-
centrated and recycled to the process by scrubbing to
remove carbon dioxide, etcetera.
U. S. Patent 2,653,952 discloses a process for
the manufacture of ethylene oxide in which the pro-
ducts from the reactor, consisting essentially of
ethylene oxide, ethylene, oxygen, nitrogen, helium
and carbon dioxide, are delivered to an ethylene
oxide absorber. The gases are then passed in contact
with a solvent for carbon dioxide. Ordinarily,
ethanolamine is used as a solvent in this process.
The gas discharged from the carbon dioxide absorber
contains ethylene and is recycled to be again passed
in contact with the catalyst in the reactor. This
patent recognizes that nitrogen tends to build up to
high concentrations when the oxygen is supplied by
air. The process of this patent therefore employs
relatively nitrogen-free oxygen in the feedstream and
dilutes the gaseous mixture with helium.
U. S. Patent 2,799,687 discloses a preferred
embodiment for the oxidation of olefins in which the
reactor effluent may be passed into an ethylene oxide
absorber after which about 70-90 percent of the ef-
15016
12l3t~
-12-
fluent from the ethylene oxide absorber is reeyeled
and the remainder plus adclitional oxygen is diverted
to a second reactor. According to the patent, by the
diversion of a portion of the effluent from the first
reactor to the second reac:tor, the buildup of carbon
dioxide above certain limits, such as above about 5-7
percent, can be prevented. Similarly, U. S. Patent
4,206,128, Netherlands Patent Application 6,414,284,
and V. K. Patent 1 191 983 all disclose processes in
which some carbon dioxide is removed from the recycle
stream.
According to U. K. Patent 1,055,147, one must
remove carbon dioxide from the ethylene oxide produc-
tion system to keep the carbon dioxide concentration
in an acceptable range since, according to the pat-
ent, carbon dioxide acts as an inhibitor and suppres-
ses the reaction of ethylene to form both ethylene
oxide and carbon dioxide.
U. S. Patent 1,998,878, U. S. Patent 3,904,656,
"The Manufaeture Of Ethylene Oxide And Its Deriva-
tives", The Industrial Chemist, February, 1963, Kirk
Othmer, "Ethylene Oxiden, Volume 8, pages 534,545,
"Ethylene Oxide By Direct Oxidation Of Ethylene",
Petroleum Processing, November, 1955, all inelude, as
a process step, the removal of carbon dioxide from
the alkene oxide for the purification of the alkene
oxide produet.
Sinee the early work on the direet eatalytic
oxidation of ethylene to ethylene oxide, it has been
suggested that the addition of eertain eompounds to
the gaseous feedstream or direet incorporation of
metals or eompounds in the eatalyst eould enhanee or
promote the production of ethylene oxide. Sueh
metals or eompounds have been known variously as
"anti-eatalystsn, "promoters" and "inhibitors".
15016
1281~688
-13-
~hese substances, which are not considered catalysts,
are believed to contribute to the overall utility of
the process by inhibiting the formation of carbon
dioxide or by promoting the production of ethylene
oxide.
Various Compounds have been found to provide
some beneficial effects when contained within the
gaseous mixture supplied to the reactor. It is well
known that chlorine-containing compounds, when sup-
plied to an ethylene oxide production process, helpto improve the overall effectiveness of the pro-
cess. For example, Law and Chitwood, in U. S. Patent
2,194,602, disclose that higher yields of olefin
oxide are obtained by retarding the complete oxida-
tion of the olefin through the addition of very smallamounts of deactivating materials (also referred to
by Law and Chitwood as repressants or anti-catalytic
materials) such as ethylene dichloride, chlorine,
sulfur chloride, sulfur trioxide, nitrogen dioxide,
or other halogen-containing or acid-forming mater-
ials. V. S. Patents 2,270,780, 2,279,469, 2,279,470,
2,799,687, 3,144,416, 4,007,135, 4,206,128,
4,368,144, EPO Patent 11 356, U. R. Patents 676,358,
1,055,147 and 1,571,123 also discuss the addition of
halide compounds, such as ethyl chloride, ethylene
dichloride, potassium chloride, vinyl chloride and
alkyl chloride.
U. S. Patent 2,194,602 discloses a method for
the activation of silver catalysts in which the acti-
vation is accomplished by bringing the catalyst incontact with an aqueous solution of barium, strontium
or lithium hydroxide after the catalyst has first
been treated with a "repressant" such as ethylene di-
chloride, nitrogen dioxide, or other halogen-contain-
ing or acid-forming material.
15016
lZ1~68~3
-14-
U. S Patents 2,279,469 and 2,279,470 disclose
processes of making olefin oxides in which very small
amounts, i.e., less than 0.1 percent of the total
volume, of "anti-catalysts" are incorporated with the
reactants. Halogens and compounds containing halo-
gens, e.g., ethylene dichloride, and compounds con-
taining nitrogen, e.g., nitric oxide, can be used as
the anti-catalysts. According to the patents, it is
possible to employ mixtures of the individual anti-
catalyst substances.
U. S. Patent 3,144,416 discloses a method ofmanufacturing silver catalysts to be used for the
oxidation of olefins. According to the patent, in
order to increase the selectivity of the catalyst, a
small quantity of halogen compound or nitrogen com-
pound may be added to the reaction gas or catalyst.
EPO Patent 3642 and U. K. Patent Application GB
2 014 133A disclose processes of producing an olefin
oxide by contacting an olefin with oxygen in the
presence of a silver-containing catalyst and a
chlorine-containing reaction modifier, for example,
dichloroethane, methyl chloride, or vinyl chloride.
According to these references, the catalyst per-
formance is improved, for example, the selectivity is
increased, by contacting the catalyst with a nitrate-
or nitrite-forming substance, for example, a gas
containing dinitrogen tetroxide, nitrogen dioxide
and/or a nitrogen-containing compound, together with
an oxidizing agent, such as nitric oxide and oxy-
gen. The catalyst preferably comprises 3 to 50 per-
cent, more preferably 3 to 30 percent, by weight
silver. According to the references, it is preferred
that the catalyst should contain cations, for exam-
ple, alkali and/or alkaline earth metal cations, as
the corresponding nitrate or nitrite, particularly if
15016
121~88
-15
the catalyst is treated with the nitrate- or nitrite-
forming substance intermittently. According to the
patents, suitable concentrations of the cations may
be, for example, 5 x 10-5 to 2, preferably 5 x 10-4
to 2, more preferably 5 x 10-4 to 0.5, gram equiva-
lents per kilogram of catalyst. Suitably, Mo, K, Sr,
Ca and/or Ba are present in amounts of 2 to 20,000,
preferably 2 to 10,000, more preferably 10 to 3,000,
microgram equivalents per gram of silver. Tn the
processes of these two references, a diluent, for
example, carbon dioxide, may be present and uncon-
verted olefin may be recycled, suitably after removal
of carbon dioxide.
Rumanian Patent No. 53012, published December 2,
1971, discloses a process in which the catalyst is
brought in direct contact with a gas mixture composed
of 5-15 percent oxygen, 8-20 percent carbon dioxide,
60-80 percent nitrogen, completed by 1-5 percent
nitrogen oxides.
V. K. Patent 524,007 discloses a method for
activating catalysts which may be accomplished by
contacting the catalyst with an aqueous solution of a
hydroxide of lithium, after the catalyst has first
been treated with an "anti-catalyst", such as ethy-
lene dichloride or nitrogen dioxide. According to
the patent, the treatment may most advantageously be
conducted simultaneously with the oxidation reaction
of the olefins, inasmuch as the presence of very
small amounts of anti-catalyst (less than 0.1 per-
cent) increases the efficiency by limiting the forma-
tion of carbon dioxide.
There has been some disclosure directed to the
effect of water contained in the gaseous mixture
which is fed to the reactor. Water can be introduced
through the feedstream, or, since it is a by-product
15016
1286i688
-16-
of one of the side reactions in the oxidation of
alkenes, it can be accumulated through recycling.
U. S. Patent 1,998,~78 states that the efficien-
cy of the reaction is increased by introducing a
suitable quantity of water. According to the patent,
if water is introduced in a suitable quantity, the
reaction giving CO2 is checked, owing, as is pro~-
able, to the partial pressure of water.
U. S. Patent 2,367,169 states that in some
cases, for instance, when pure ethylene gas is being
treated, it is advantageous to introduce into the gas
stream traversing the reaction zone small amounts of
steam.
U. S. Patent 2,376,987 discloses a process for
the preparation of butadiene in which ethylene is
subjected to an oxidation reaction in converter.
Water or steam may be supplied to the converter to
assist in controlling the temperature by absorbing
the heat evolved in the reaction. According to the
patent, water in the reaction also tends to increase
the production of ethylene oxide and reduce the for-
mation of other oxidation products in the reaction.
U. S~ Patent 2,615,900 discloses a process for
producing ethylene oxide in which steam may be in-
cluded in the feed gases to act as a depressant oranti-catalytic material.
U. S. Patent 3,959,316 discloses a procedure for
propylene oxide synthesis in which the reaction is
carried out in the presence of water vapor which can
be between 2 and 25 percent with respect to the total
of the feed gas and is preferably between 2 and 15
percent.
U. S. Patent 4,094,889 discloses a process for
restoring the selectivity of unstable alkali metal
promoted silver-containing catalysts for the oxida-
15016
1286688
-17-
tion of olefins to olefin oxides by wetting the cata-
lyst with water and drying it. The catalyst may be
wet with water by contacting it with li~uid water by
wetting it with wet steam or by absorbing water into
the catalyst from steam in the vapor phase.
U. S. Patent 4,125,480 discloses a process for
improving the performance of silver catalysts which
comprises washing the used catalyst with water and
depositing from about 0.00004 to about 0.008 gram
equivalent weights per kilogram of total catalyst of
ions of one or more of the alkali metals, sodium,
potassium, rubidium or cesium. The ions are
deposited by impregnating the washed catalyst with a
solution of one or more compounds of these alkali
metals in a suitable solvent, particularly an organic
solvent. Examples of suitable compounds are the
hydroxides, nitrates, chlorides, iodides, bromides,
bicarbonates, and carbonates. According to the pat-
ent, the process may be carried out within the reac-
tor, for example, water may be passed through thereactor containing the used silver catalyst.
Japanese Patent Publication No. 40051/1976 dis-
closes a process for producing an olefin oxide in
which steam is employed in the starting gas in cer-
tain of the examples given in the patent. In Exam-
ples 1-5, steam comprises 7 percent of the starting
gas and in the Comparative Example it comprises 8
percent of the starting gas.
In the reference, "A Study of the Oxidation of
Ethylene to Ethylene Oxide on a Silver Catalyst", K.
E. Murray, studies indicate that the presence of
water vapor increases the yield of the reaction but
considerably decreases conversion.
15016
12~36688
-18-
Chem. Abstracts, Volume 80, Issue 11, Section
22, Abstract 059195, teaches that ~2 has a retarding
effect on the catalyst deactivation in the oxidation
of ethylene over a silver catalyst.
U. S. Patents 2,270,780, 3,119,837, 4,061,659,
4,248,740, 4,368,144, 4,471,071, and Japanese Patent
53-39404 all discuss the use of water or steam as an
inert material or diluent.
Scientific literature is replete with examples
of the use of alkali metals and alkaline earth metals
and their cations to promote the efficiency of silver
catalysts used in epoxidation reactions. Numerous
examples may be found in literature regarding prefer-
ence for the inclusion or exclusion of one or several
metals or cations in silver catalysts. Although many
reports have indicated that no particular effective-
ness is observed with one alkali metal or alkaline
earth metal cation vis-a-vis another, several have
suggested clear preferences for particular metal
cations.
Potassium is well known as a catalyst promoter
for the epoxidation of alkenes. One of the first
patents to recognize potassium as a suitable promoter
was U. S. Patent 2,177,361. According to this pat-
ent, the catalyst may be promoted by the presence ofvery small proportions of alkali or alkaline earth
metals.
U. R. Patent Application 2,122,913A discloses a
catalyst and a process for oxidation of ethylene in
which an amount of alkali metal is deposited on the
catalyst which removes substantially all activity
from the silver catalyst and then activity and selec-
tivity are recovered by heating the catalyst in a
nitrogen atmosphere.
15016
128~68~3
--19--
When potassium is employed in the catalyst, it
is generally introduced in conjunction with an an-
ion. The choice of the anion has not always been
regarded as significant. For example, U. S. Patents
3,962,136, 4,010,115, 4,012,425, and 4,356,312 state
that no unusual effectiveness is observed with the
use of any particular anion in the alkali metal salts
~sed to prepare the catalysts and suggests that ni-
trates, nitrites, chlorides, iodides, bromates, et-
cetera, may be used. Potassium nitrate was employed
in the silver salt solution of Example 1 in each
patent. According to the patents, from about 4.0 x
10 5 to about 8.0 x 10 3 gram equivalent weights of
ionic higher alkali metal, e.g., rubidium, cesium or
potassium or mixtures thereof, per kilogram of cata-
lyst is deposited on the catalyst support simultan-
eously with the deposit of silver. The amount of
higher alkali metal preferably ranges from about 2.0
x 10-4 to about 6.5 x 10-3 gram equivalent weights
per kilogram of finished catalyst. According to the
patents, the amount of the higher alkali metal (or
metals) present on the catalyst surface is critical
and is a function of the surface area of catalyst.
According to the patents, the alkali metal is present
in final form on the support in the form of its ox-
ide. U. S. Patents 3,962,136, 4,010,115 and
4,012,425 note that the highest level of selectivity
obtainable when potassium is employed typically is
lower than that obtainable when rubidium or cesium is
employed while U. S. Patent 4,356,312 notes that
particularly good results are obtained with potas-
sium.
U. S. Patent 4,066,575 notes that alkali metal
nitrate is suitable for supplying an alkali metal
promoter, but it notes that the anion associated with
15016
12~ ;8~
-20-
the promoter metal is not critical. U. S. Pat~nt
4,207,210 discloses a process for preparing an ethy-
lene oxide catalyst in which higher alkali metals,
such as potassium, rubidium and cesium, are deposited
on a catalyst support prior to the deposition of
silver. According to the patent, the amount of high-
er alkali is a critical function of the surface area
of the support. This patent also notes that no un-
usual effectiveness is observed with the use of any
particular anion in preparing the catalysts and lists
nitrates as one type of salt that may be used. Car-
bon dioxide and steam are listed as diluent mater-
ials.
The use of potassium nitrate, however, to impart
a promoting effect on the catalyst has been widely
described. For example, U. S. Patent 4,007,135 lists
a number of materials, including potassium, which can
be used as promoters. According to the patent, in
general, 1 to 5,000, preferably 1 to 1,000, more
preferably 40 to 500, and particularly 20 to 200,
atoms of potassium are present per 1,000 atoms of
silver. Suitably an aqueous solution of a compound,
such as a chloride, sulfate, nitrate, nitrite, et-
cetera, of the promoter is used for impregnation.
U. S. Patent 4,094,889 discloses a process for re-
storing the selectivity of silver catalysts in which
alkali metal may be introduced as a nitrate and in
which the preferred content of potassium is in the
range of 2 x 10-2 to 3 x 10-5 grams/square meter of
surface area of support. U. S. Patent 4,125,480
discloses a process for reactivating used silver
catalyst comprising (a) washing the used catalyst,
and (b) depositing from 0.00004 to 0.008, preferably
from 0.0001 to 0.002 gram equivalent weights per
kilogram of catalyst of ions of one or more of the
15016
~2~688
-21-
alkali metals, such as sodium, potassium, rubidium,
or cesium. The ions of, e.g., potassium are de-
posited on the catalyst by impregnating it with a
solution of one or more compounds, such as potassium
nitrate. U. S. Patents 4,226,782, 4,235,757,
4,324,699, 4,342,667, 4,368,144, 4,455,392, Japanese
Patent 56/89843, and U. ~. Patent 1,571,123 suggest
the use of potassium nitrate in various amounts.
Potassium nitrate may also be formed in situ when a
carrier material is treated with certain amines in
the presence of potassium ions, for example, when
silver is introduced to a carrier material in a sil-
ver-impregnating solution containing an amine and
potassium ions, followed by roasting.
There has been some disclosure directed to cata-
lysts for use in an ethylene oxide production system
in which silver is present in relatively large pro-
portions, e.g., 35 percent or more. For example,
U. S. Patents 3,565,828 and 3,654,318 disclose cata-
lysts for the synthesis of ethylene oxide from oxygen
and ethylene. According to the patents, the cata-
lysts contain from 60 percent to 70 percent by weight
of silver.
U. S. Patent 2,593,099 discloses a magnesium
oxide-barium oxide silver catalyst support. Accord-
ing to the patent, the conventional amount of silver
is deposited on the support, namely, 2 to 50 percent,
with the best results being obtained between 4 and 20
percent.
U. S. Patent 2,713,5~6 discloses a process for
the oxidation of ethylene to ethylene oxide in which,
according to the patent, the conventional amount of
silver is deposited on the support, namely, 5 to 50
percent, with the best results being obtained between
4 and 20 percent.
15016
12~j688
-22-
U. S. Patent 3,793,231 discloses a process for
the preparation of silver catalysts for the produc-
tion of ethylene oxide in which the silver content of
the catalysts generally range between 15 to 30 per-
cent by weight, preferably 19 to 27 percent byweight. ~
A large body of art directed to various aspects
of alkene oxide production has been developed over
the years since Lefort (U. S. Patent 1,998,878).
Much of it is contradictory and incapable of recon-
ciliation. None of the art is believed to disclose
or suggest a high efficiency process for the produc-
tion of alkene oxide by contacting alkene and oxygen-
containing gas in the presence of at least one gas-
eous efficiency-enhancing member of a redox-half
reaction pair, a supported silver catalyst and an
efficiency- and/or activity-enhancing amount of
water.
Disclosure of_the Invention:
In a process for the manufacture of alkene ox-
ide, by contacting alkene and oxygen-containing gas
under epoxidation conditions in the presence of a
gaseous efficiency-enhancing member of a redox-half
reaction pair and a supported silver catalyst which
includes an efficiency-enhancing amount of at least
one efficiency-enhancing salt of a member of a redox-
half reaction pair, the efficiency and/or activity
can be enhanced by the addition of small amounts of
water, e.g., for ethylene oxide manufacture, less
than about one and one-half percent by volume, to the
reaction inlet stream during the operation of the
process.
15016
12~688
-23-
The present invention provides a high efficiency
process for the epoxidation of alkene to form alkene
oxide comprising contacting alkene, particularly
ethylene and oxygen-containing gas, under epoxidation
conditions and in the presence of at least one gas-
eous efficiency-enhancing member of a redox-half
reaction pair, preferably nitric oxide and/or nitro-
gen dioxide, a supported silver catalyst and an effi-
ciency- and/or activity-enhancing amount of water.
The catalyst comprises a catalytically-effective
amount of silver and an efficiency-enhancing amount
of at least one efficiency-enhancing salt of a member
of a redox-half reaction pair, preferably potassium
nitrate, on a support. The water may be added con-
tinuously or intermittently. The process of thisinvention may be used whether or not carbon dioxide
is present in the reaction inlet stream. However,
the amount of enhancement in efficiency has been
found to be greater in the case where carbon dioxide
is present in the reaction inlet stream. Also, when
carbon dioxide is present, the water should, in gen-
eral, be introduced prior to introduction of carbon
dioxide since it has been observed that the desirable
effect of the water is reduced when carbon dioxide
has been introduced prior to the water.
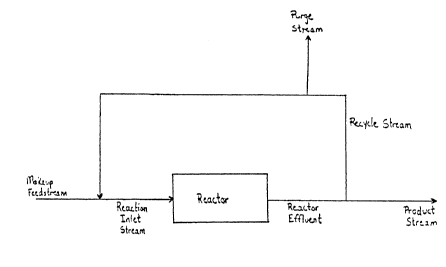
~rief DescriPtion Of The Drawinqs:
Figure 1 is a flow chart of a process in ac-
cordance with the invention.
15016
~2~688
-24-
Detai~ed DescriPtion Of The Invention:
The present invention is directed to high effi-
ciency processes for the epoxidation of alkene to
form alkene oxide by contacting alkene and oxygen-
containing gas in a reaction zone under epoxidation
conditions in the presence of a gaseous efficiency-
enhancing member of a redox-half reaction pair, a
supported silver catalyst and water. The silver
catalyst generally comprises a catalytically-
effective amount of silver and an efficiency-
enhancing amount of at least one salt of a member of
a redox-half reaction pair on a porous support. A
performance-enhancing gaseous halide is preferably
present in the reaction zone. According to the in-
vention, an efficiency- and/or activity-enhancing
amount of water, preferably comprising from about
0.25 to about 1.5, more preferably 0.25 to 1.0, per-
cent by volume when the alkene is ethylene, is con-
tained in the reaction inlet stream.
The following description of the preferred sys-
tem for epoxidation of alkene in accordance with the
present invention may be better understood by refer-
ence to the flow chart in Figure 1.
In steady-state operation of the preferred sys-
tem, a reaction inlet stream containing reactants,
together with other gaseous materials as discussed
below, is fed to a reactor at a controlled gas hourly
space velocity (G~SV). The reactor may take a vari-
ety of forms, but is preferably a collection of ver-
tical tubes containing a supported silver catalyst.
The reaction inlet stream enters the reactor, passes
through the catalyst, and exits the reactor as the
reactor effluent. The desired product, e.g., ethy-
lene oxide, is separated from the other components in
15016
12~
-25-
the reactor effluent, preferably by a scrubbjng oper-
ation. The remainder of the reactor effluent becomes
the recycle stream. It is sometimes preferred to
remove a portion of the material in the recycle
stream in order to, for example, prevent buildup of
certain materials in the system. The removal of
material from the recycle stream may be selective,
i.e., certain compounds may be removed from the re-
cycle stream in greater proportions than other com-
pounds. The remainder of the recycle stream is usu-
ally combined with a makeup feedstream to form the
reaction inlet stream. The reaction system will be
discussed in greater detail below.
Although the present invention can be used with
any size and type of alkene oxide reactor, including
both fixed bed and fluidized bed reactors, it is
contemplated that the present invention will find
most widespread application in standard fixed bed,
multi-tubular reactors. These generally include
wall-cooled as well as adiabatic reactors. Tube
lengths typically range from about 5 to about 60 feet
(1.52 to 18.3 meters), frequently from about 15 to
about 40 feet (1.52 to 12.2 meters). The tubes gen-
erally have internal diameters from about 0.5 to
about 2 inches (1.27 to 5.08 centimeters), typically
- from about 0.8 to about 1.5 inches (2.03 to 3.81
centimeters).
The catalyst generally comprises a support hav-
ing catalyst material or a mixture of catalyst mater-
ial and an efficiency-enhancing material impregnated
or coated on the support. The support can be gener-
ally described as a porous, inorganic substrate which
is not unduly deleterious to the performance of the
system and is preferably substantially inert toward
the other materials in the system, i.e., the catalyst
15016
~21~88
-26-
material, any other components present in the cata-
lyst, e.g., efficiency-enhancing salt, and components
in the reaction inlet stream. In addition, the sup-
port should be able to withstand the temperatures
employed within the reactor, as well as, of course,
the temperatures employed in manufacturing the cata-
lyst, e.g., if the catalyst material is reduced to
its free metallic state by roasting. Suitable sup-
ports for use in accordance with the present inven-
tion include silica, magnesia, silicon carbide, zir-
conia, and alumina, preferably alpha-alumina. The
support preferably has a surface area of at least
about 0.7 m2/g, preferably in the range of from about
0.7 to about 16 m2/g, more preferably about 0.7 to
about 7 m2/g. The surface area is measured by the
B. E. T. nitrogen method described by Brunauer, Emmet
and Teller in J. Am. Chem. Soc. 60, 309-316 (1938).
The support may be composed of a particulate
matrix. In a preferred support, at least about 50
percent of the total number of support particles
having a particle size greater than about 0.1 micro-
meter have at least one substantially flat major
surface. The support particles are preferably formed
into aggregates or "pills~ of such a size and shape
that they are readily usable in commercially operated
tubular reactors. These aggregates or pills general-
ly range in size from about 2 millimeters to about 15
millimeters, preferably about 3 millimeters to about
12 millimeters. The size is chosen to be consistent
with the type of reactor employed. In general, in
fixed bed reactor applications, particle sizes rang-
ing from about 3 millimeters to about 10 millimeters
have been found to be most suitable in the typical
tubular reactors used in commerce.
15016
~2~688
-27-
The shapes of the carrier aggregates useful for
purposes of the present invention can vary widely.
Common shapes include spheres and cylinders, especi-
ally hollow cylinders.
The preferred support particles in accordance
with the present invention have at least one substan-
tially flat major surface and may be characterized as
having a lamellate or platelet-type morphology. Some
of the particles have two, or sometimes more, flat
surfaces. The major dimension of a substantial por-
tion of the particles having platelet-type morphology
is less than about 50 microns, preferably less than
about 20 microns. When alpha-alumina is employed as
the support material, the platelet-type particles
frequently have a morphology which approximates the
shape of hexagonal plates.
The carrier materials of the present invention
may generally be described as porous or microporous
and they generally have median pore diameters of from
about 0.01 to about 100 microns, preferably about 0.5
to about 50 microns, and most preferably about 1 to
about 5 microns. Generally, they have pore volumes
of about 0.6 to about 1.4 cc/g, preferably about 0.8
to about 1.2 cc/g. Pore volumes may be measured by
any conventional technique, such as conventional
mercury porosity or water absorption techniques.
Generally improved results have been demon-
strated when the support material is composition-pure
and also phase-pure. By "composition-pure" is meant
a material which is substantially a single substance,
such as alumina, with only trace impurities being
present. The term "phase-pure" refers to the homo-
geneity of the support with respect to its phase. In
the present invention, alumina, having a high or
exclusive alpha-phase purity (i.e., alpha-alumina) is
15016
12~ 88
-28-
preferred. Most preferred is a material composed of
at least 98 percent, by weight, alpha-alumina.
Under some conditions even small amounts of
leachable sodium can adversely affect the service
life of the catalyst. Notably improved results have
been observed when the support contains less than
about 50 parts per million ~ppm) by weight, prefer-
ably less than 40 ppm, based on the welght of the
total catalyst. ~he term leachable sodium, as used
herein, refers to sodium which can be removed from
the support by immersing the support in a 10 percent
by volume nitric acid solution at 90 degres C for one
hour. Suitable alpha-aluminas having concentrations
of sodium below 50 ppm may be obtained commercially
from suppliers such as the Norton Company. Alterna-
tively, suitable alpha-alumina support materials may
be prepared so as to obtain leachable sodium concen-
trations below 50 ppm by the method described by
Weber et al in U. S.Patent 4,3~9,134.
A particularly preferred support is a high-puri-
ty alpha-alumina support, having platelet morphology,
of the type disclosed in Canadian Patent Appl.
No. 515,864-8, filed August 13, 1986, by
Thomas M. Notermann, entitled "Improved Catalytic
System Por Epoxidation of Alkenesn, attorney's docket
number 15013
The present invention includes in the catalyst
at least one efficiency-enhancing salt of a member of
a redox-half reaction pair. The term ~redox-half
reaction~ ~s defined herein to mean half-reactions
such as those found in equations presented in tables
of standard reduction or oxidation potentials, also
known as standard or single electrode potentials.
These equations are found in, for instance, ~Handbook
`~ 15016
~2l~tj6f~8
-29-
of Chemistryn, N. A. Lange, Editor, McGraw-Hill Book
CGmpany~ Inc., pages 1213-1218 (1961) or "CRC Rand-
book of Chemistry and Physics", 65th Edition, CRC
Press, Inc., Boca ~aton, F]orida, pages D lS5-162
(1984). The term "redox-half reaction pair" refers
to the pairs of atoms, molecules or ions, or mixtures
thereof, which undergo oxidation or reduction in such
half-reaction equations. A member of a redcx-half
reaction pair is, therefore, one of the atoms, mole-
cules or ions that appear in a particular redox-half
reaction equation. The term redox-half reaction pair
is used herein to include those members of the class
of substances which provide the desired performance
enhancement rather than a mechanism of the chemistry
occurring. Preferably, such compounds, when asso-
ciated with the catalyst as salts of members of a
redox-half reaction pair, are salts in which the an-
ions are oxyanions, preferably an oxyanion of a poly-
valent atom, i.e., the atom of the anion to which
oxygen is bonded is capable of existing, when bonded
to a dissimilar atom, in different valence states.
The preferred efficiency-enhancing salts are potas-
sium nitrate and potassium nitrite.
The catalysts of the present invention are pre-
ferably prepared by depositing catalyst material and
at least one efficiency-enhancing salt, sequentially
or simultaneously, on and/or within a solid porous
support. The preferred catalyst material in accord-
ance with the present invention comprises silver,
preferably of a particle size less than about 0.5
micron. Any known method of introducing the catalyst
material and efficiency-enhancing salt into the cata-
lyst support may be employed, but it is preferred
that the support is either impregnated or coated.
The more preferred of these is impregnation wherein,
15016
12~688
-30-
in general, a solution of a soluble salt Gr complex
of silver and/or one or more efficiency-enhancing
salt is dissolved in a suitable solvent or "complex-
ing/solubilizing" agent. This solution may be used
to impregnate a porous catalyst support or carrier by
immersing the carrier in the silver- and/or efficien-
cy-enbancing salt-containing impregnation solution.
Sequential impregnation means that silver is
first deposited within the carrier in one or more
impregnation steps, and then salt is deposited in a
separate impregnation step.
One aspect of the present invention involves the
beneficial effects observed when the catalyst con-
tains high concentrations of silver. In order to
provide such a catalyst by impregnation, it has been
found that it is preferable to deposit the silver via
several impregnation steps. Thus, if a high silver-
content catalyst, e.g., a catalyst containing 30 or
more percent silver, is desired and a sequential
impregnation procedure is to be used, a four-step
process may be employed. Such a process would in-
volve three silver-only impregnation steps followed
by one salt-only impregnation step.
In general, a silver-only impregnation step is
carried out by first immersing the support in a sil-
ver-containing impregnation solution, preferably by
placing the support particles in a vessel, evacuating
the vessel and then adding the impregnation solu-
tion. The excess solution may then be allowed to
drain off or the solvent may be removed by evapora-
tion under reduced pressure at a suitable tempera-
ture. Typically, a silver-containing solution is
prepared by dissolving silver oxide in a suitable
solvent or complexing/solubilizing agent as, for
example, a mixture of water, ethylenediamine, oxalic
15016
12l~ 38
-31-
acid, silver oxide, and monoethanolamine.
After impregnation, the silver-impregnated car-
rier particles are treated to convert silver salt to
silver metal to effect deposition of silver on the
surface of the support. This may be done by treating
the impregnated particles with a reducing agent, such
as oxalic acid, alkanolamine or by roasting at an
elevated temperature on the order of about 100 to
about 900 degrees C, preferably about 200 to about
650 degrees C, to decompose the silver compound and
reduce the silver to its free metallic state. The
duration of roasting is generally for a period of
from about 1 to about 10 minutes, with longer times
for lower temperatures, depending on the temperature
used. As used herein, the term "surfacen, as applied
to the support, not only inclu2es the external sur-
faces of the carrier but also the internal surfaces,
that is, the surfaces defining the pores or internal
portion of the support particles.
The efficiency-enhancing salt may be introduced
into the catalyst in any suitable manner. In gener-
al, the preferred amount of efficiency-enhancing salt
can be deposited in one impregnation step. After
immersion of the silver loaded support in the effi-
ciency-enhancing salt impregnation solution, the
excess solution is generally drained and the silver-
and efficiency-enhancing salt-containing support is
dried, for example by heating to from 80 to 200 de-
grees C. When more than one salt of a member of a
redox-half reaction pair is employed, the salts may
be deposited together or sequentially.
Concurrent or coincidental impregnation means
that generally the final (perhaps the only) impregna-
tion step involves immersion of the support in an
impregnation solution which contains silver as well
15016
~28668~3
-32-
as one or more efficiency-enhancing salts. Such an
impregnation step may or may not be preceded by one
or more silver-only impregnation steps. Thus, to
make a high silver-content catalyst by coincidental
impregnation, several silver-only impregnation steps
might be carried out, followed by a silver- and effi-
ciency-enhancing salt-impregnation step. A low sil-
ver-content catalyst, e.g., from about 2 to about 20
weight percent silver, may be made by a single sil-
ver- and efficiency-enhancing salt-impregnation
step. For the purposes of this invention, these two
sequences are both referred to as concurrent or coin-
cidental impregnation.
The three types of impregnation solutions, name-
ly, silver-containing, efficiency-enhancing salt-
containing, and silver- and efficiency-enhancing
salt-containing, are discussed in more detail below.
There are a large number of suitable solvents or
complexing/solubilizing agents which may be used to
form the silver-containing impregnating solution. A
suitable solvent or complexing/solubilizing agent,
besides adequately dissolving the silver or convert-
ing it to a soluble form, should be capable of being
readily removed in subsequent steps, either by a
washing, volatilizing or oxidizing procedure, or the
like. It is also generally preferred that the sol-
vents or complexing/solubilizing agents be readily
miscible with water since aqueous solutions may fre-
quently be employed.
Among the materials found suitable as solvents
or complexing/solubilizing agents for the preparation
of the silver-containing solutions are alcohols,
including glycols, such as ethylene glycol, ammonia,
amines and aqueous mixtures of amines, such as ethy-
lenediamine and monoethanolamine, and carboxylic
15016
128~6~8
-33-
acids, such as oxalic acid and lactic acid.
The particular silver salt or compound used to
form the silver-containing impregnating solution in a
solvent or a complexing/solubilizing agent is not
particularly critical and any known silver salt or
compound generally known to the art which is soluble
in and does not react with the solvent or complex-
ing/solubilizing agent may be employed. ~hus, the
silver may be introduced into the solvent or complex-
ing/solubilizing agent as an oxide, a salt, such as anitrate or carboxylate, for example, an acetate,
propionate, butyrate, oxalate, lactate, citrate,
phthalate, generally the silver salts of higher fatty
acids, and the like.
Materials which may be employed in the efficien-
cy-enhancing salt-containing impregnation solution to
act as a solvent for the efficiency-enhancing salt
include generally any solvent capable of dissolving
the salt, which solvent will neither react with the
silver nor leach silver from the support. ~queous
solutions are generally preferred but organic li-
quids, such as alcohols, may also be employed.
In order to perform coincidental impregnation,
the efficiency-enhancing salt and the silver catalyst
material must both be soluble in the solvent or com-
plexing/solubilizing liquid used.
Suitable results have been obtained with both
the sequential and coincidental procedures. Some
results have indicated that greater amounts of silver
with more uniform distribution of silver throughout
the pill can be obtained by three or more silver-
impregnation cycles. High silver-containing cata-
lysts prepared by a coincidental impregnation techni-
que generally provide better initial performance than
those prepared by a sequential technique.
15016
12~;~8
-34-
If the catalyst material is to be coated on the
catalyst support rather than impregnated in the sup-
port, the catalyst material, e.g., silver, is pre-
formed or precipitated into a slurry, preferably an
aqueous slurry, such that the silver particles are
deposited on the support and adhere to the support
surface when the carrier or support is heated to
removed the liquids present.
The concentration of silver in the finished
catalyst may vary from about 2 percent to 60 percent
or higher, by weight, based on the total weight of
the catalyst, more preferably from about 8 percent to
about 50 percent, by weight. When a high silver
content catalyst is employed, a silver concentration
range of from about 30 to about 60 percent, by
weight, is preferred. When a lower silver content
catalyst is used, a preferred range is from about 2
to about 20 weight percent. The silver is preferably
distributed relatively evenly over the support sur-
faces. The optimum silver concentration for a par-
ticular catalyst must take into consideration per-
formance characteristics, such as catalyst activity,
system efficiency and rate of catalyst aging, as well
as the increased cost associated with greater concen-
trations of silver in the catalyst material. Theapproximate concentration of silver in the finished
catalyst can be controlled by appropriate selection
of the number of silver-impregnation steps and of the
concentration of silver in the impregnation solution
or solutions.
The amount of the efficiency-enhancing salt of a
member of a redox-half reaction pair present in the
catalyst directly affects the activity and efficiency
of the epoxidation reaction. The most preferable
amount of the salt of a member of a redox-half reac-
15016
~2~
-35-
tion pair varies depending upon the alkene belng
epoxidized, the compound used as the gaseous effi-
ciency-enhancing member of a redox-half reaction
pair, the concentration of components in the reaction
5 inlet stream, particularly the gaseous efficiency-
enhancing compound and carbon dioxide, the amount of
silver contained in the catalyst, the surface area,
morphology and type of support, and the process con-
ditions, e.g., gas hourly space velocity, tempera-
ture, and pressure. The preferred efficiency-
enhancing salt is potassium nitrate.
It has been noted that when conventional
analyses have been conducted with catalysts prepared
by co-impregnation with silver and efficiency-
enh~ncing salt, not all the anion associated with thecation has been accounted for. For example, cata-
lysts prepared by co-impregnation with a potassium
nitrate solution have been analyzed by conventional
techniques and about 3 moles of the nitrate anion
have been observed or every 4 moles of the potassium
cation. This is believed to be due to limitations in
the conventional analytical techniques and does not
necessarily mean that the unaccounted for anions a~e
not nitrate. For this reason, the amount of the
efflciency-enhancing salt in the catalyst is given,
in some instances, in terms of the weight percentage
of the cation of the effLciency-enhancing salt (based
on the weight of the entire catalyst), with the un-
derstanding that the anion associated with the cation
is also present in the catalyst in an amount roughly
proportional (on a molar basis) to the cation.
lt is generally preferable that the efficiency-
enhancing salt be provided in such an amount that the
finished catalyst contaLns from about 0.01 to about
5.0 percent, by weight, of the cation of the salt,
15016
-36-
based on the total weight of the catalyst, more pref-
erably from about 0.02 to about 3.0 weight percent,
most preferably from about 0.03 to about 2.0 weight
percent. The approximate concentration of efficien-
cy-enhancing salt in the finished catalyst can be
controlled by appropriate selection of the concentra-
tion of efficiency-enhancing salt in the salt-impreg-
nation solution.
When more than one salt of a member of a redox-
half reaction pair is employed, the salts may bedeposited together or sequentially. It is preferred,
however, to introduce the salts to the support in a
single solution, rather than to use sequential treat-
ments using more than one solution and a drying step
between impregnation steps, since the latter tech-
nique may result in leaching the first introduced
salt by the solution containing the second salt.
Concurrent or coincidental impregnation may be ac-
complished by forming an impregnating solution which
contains the dissolved efficiency-enhancing salt of a
member of a redox-half reaction pair as well as sil-
ver catalyst material. Silver-first impregnation can
be accomplished by impregnating the support with the
silver-containing solution, drying the silver-con-
taining support, reducing the silver, and impreg-
nating the support with the efficiency-enhancing salt
solution.
Reaction conditions maintained in the reactor
during operation of the process are those typically
used in carrying out epoxidation reactions. Tempera-
tures within the reaction zone of the reactor gener-
ally range from about 180 to about 300 degrees C and
pressures generally range from about 1 to about 30
atmospheres, typically from about 10 to about 25
atmospheres. The gas hourly space velocity (GHSV)
15016
lZB~688
-37-
may vary, but it will generally range from about
1,000 to about 16,000 hr~l.
The product, for example, ethylene oxide, is
recovered from the reactor effluent, e.g., by an
absorption process. One such method comprises sup-
plying the reactor effluent stream to the bottom of
an absorption column while adding a solvent, for
example, water, to the top of the absorption col-
umn. The solvent preferably absorbs the ethylene
oxide and carries it out of the bottom of the ab-
sorption column, while the remainder of the reaction
effluent passes out of the top of the absorption
column to form the recycle stream. The desired pro-
duct is thereafter recovered, for example, by passing
the solvent and absorbed product through a stripper.
As noted above, it may be preferable to remove a
portion of the recycle stream prior to returning the
recycle stream to the reaction zone. It is generally
preferable to selectively remove certain compounds.
An absorption column or other types of separation
means can be used to provide a selective purge.
The recycle stream generally contains the dilu-
ents and inhibitor~ fed to the system, unreacted
alkene and oxygen, together with by-products of the
reaction, such as carbon dioxide and water, and any
minor amount of alkene oxide which is not recovered
as product. After removal of the purge stream, the
recycle stream is returned to the reaction zone,
preeerably being mixed with the makeup feedstream
prior to or as it enters the reaction zone.
The makeup feedstream replaces reactants, i.e.,
alkene and oxygen-containing gas, as well as other
materials not contained in the recycle stream in
sufficient amounts. Alkene, as used herein, refers
15016
128~ 8
-38-
to cyclic and acyclic alkenes which are in a gaseous
state or have significant vapor pressures under epox-
idation conditions. Typically these eompounds are
characterized as having on the order of 12 carbon
atoms or less and which are gaseous under epoxidation
conditions. In addition to ethylene and propylene,
examples of alkenes which may be used in the present
invention include such compounds as butene, dodecene,
cyclohexene, 4-vinylcyclohexene, styrene, and norbor-
nene.
The oxygen-containing gas employed in the reac-
tion may be defined as including pure molecular oxy-
gen, atomic oxygen, any transient radieal species
derived from atomie or molecular oxygen eapable of
existence under epoxidation conditions, mixtures of
another gaseous substance with at least one of the
foregoing, and substances capable of forming one of
the foregoing under epoxidation conditions. Such
oxygen-containing gas is typically oxygen introduced
to the reactor either as air, eommereially pure oxy-
gen or any other gaseous substanee whieh forms oxygen
under epoxidation eonditions.
The makeup feedstream may also contain one or
more additives, for example, a performance-enhancing
gaseous halide, preferably an organie halide, includ-
ing saturated and unsaturated halides, such as 1,2-
dichloroethane, ethyl chloride, vinyl chloride,
methyl ehloride, and methylene ehloride, as well as
aromatic halides. The performance-enhancing gaseous
halide preferably eomprises 1,2-diehloroe~hane and/or
ethyl ehloride. ~n addition, a hydroearbon, sueh as
ethane, ean be included in the makeup feedstream.
The makeup feedstream may also contain a diluent or
ballast, such as nitrogen, as is the case when air is
used as the oxygen-eontaining gas.
15016
12~
-39-
The makeup feedstream generally also includes at
least one gaseous efficiency-enhancing member of a
redox-half reaction pair. The phrase "gase~us effi-
ciency-enhancing compound", as used herein, is an
alternative expression for the expression "at least
one gaseous efficiency-enhancing member of a redox-
half reaction pair. n Both phrases are therefore
meant to include single gaseous efficiency-enhancing
members of redox-half reaction pairs as well as mix-
tures thereof. The term "redox-half reaction pair"
has essentially the same meaning as defined in con-
nection with efficiency-enhancing salts, above. The
preferred gaseous efficiency-enhancing materials are,
preferably, compounds containing oxygen and an ele-
ment capable of existing in more than two valencestates. Examples of preferred gaseous efficiency-
enhancing members of redox-half reaction pairs in-
1 de NO, NO2, N2O4, N2O3, any substance capable offorming gaseous NO and/or NO2 under epoxidation con-
ditions, or mixtures thereof. In addition, mixturesof one of the compounds listed above, particularly
NO, with one or more of PH3, CO, SO3, and SO2 are
suitable. Nitric oxide is particularly preferred.
In some cases it is preferable to employ two
members of a particular half-reaction pair, one in
the efficiency-enhancing salt and the other in the
gaseous efficiency-enhancing compound employed in the
feedstream, as, for example, with a preferred combin-
ation of RNO3 and NO. Other combinations, such as
KNO3/N2O3, KNO3/NO2, and KNO2/N2O4 may also be em-
ployed in the same system. In some instances, the
salt and the gaseous members may be found in half-
reactions which represent the first and last reac-
tions in a series of half-reaction equations of an
overall reaction.
15016
12~688
-40-
The gaseous efficiency-enhancing member of a
redox-half reaction pair is preferably present in an
amount that favorably affects the efficiency and/or
the activity. The precise amount is determined, in
part, by the particular efficiency-enhancing salt
employed and the concentration thereof, as well as
the other factors noted above which influence the
amount of efficiency-enhancing salt. Suitable ranges
of concentration for the gaseous efficiency-enhancing
compound are generally dependent upon the particular
alkene which is being epoxidized, larger amounts of
the gaseous efficiency-enhancing compound generally
being preferable with higher alkenes. For example,
in an ethylene epoxidation system, a suitable range
of concentration for the gaseous efficiency-enhancing
member of a redox-half reaction pair is typically
about 0.1 to about 100 ppm by volume of the reaction
inlet stream. Preferably, the gaseous efficiency-
enhancing compound is present in the reaction inlet
stream in an amount within the range of from 0.1 to
80 ppm, by volume, when about 3 percent, by volume,
carbon dioxide is present in the reaction inlet
stream. When nitric oxide is employed as the gaseous
efficiency-enhancing compound in an ethylene epoxida-
tion system, it is preferably present in an amount offrom about 0.1 to about 60 ppm by volume. When about
3 percent, by volume, carbon dioxide is present in
the reaction inlet stream, nitric oxide, if used as
the gaseous efficiency-enhancing compound, is prefer-
ably present in an amount of from about 1 to about 40ppm. On the other hand, in a propylene or higher
alkene epoxidation system a suitable concentration of
the gaseous efficiency-enhancing compound is typical-
ly higher, e.g., from about 5 to about 2,000 ppm by
volume of the reaction inlet stream when using nitro-
15016
1~8~688
-41-
gen ballast.
Similarly, the concentration of the performance-
enhancing gaseous halide, if one is used, is depen-
dent, inter alia, upon the particular alkene which is
being oxidized. A suitable range of concentration
for gaseous halide in an ethylene epoxidation system
is typically from about 0.1 to about 60 ppm by volume
of the reaction inlet stream. A suitable concentra-
tion for gaseous halide in the reaction inlet stream
in a propylene epoxidation system is typically from
about 5 to about 2,000 ppm by volume when using ni-
trogen ballast. The preferred concentration of per-
formance-enhancing gaseous halide, if one is used,
varies depending on the particular compounds used as
the efficiency-enhancing salt and the gaseous effi-
ciency-enhancing compound and the concentrations
thereof, as well as the other factors noted above
which influence the preferred amount of efficiency-
enhancing salt.
The ranges for the concentration of alkene,
oxygen, hydrocarbon, carbon dioxide and nitrogen or
other ballast gas such as methane, in the reaction
inlet stream, are dependent upon the alkene being
epoxidized. The tables below show typical ranges for
the materials (other than the efficiency-enhancing
compound and the gaseous halide) in the reaction
inlet stream for the epoxidation of ethylene (Table
A) and propylene (Table B).
15016
12~688
-42-
Table A
Component Concentration
5 E.hylene at least about 2,
often about 5
to about 50,
volume percent
10 Oxygen about 2 to about 8
volume percent
Hydrocarbon about 0 to about 5
volume percent
Carbon Dioxide about 0 to about 7
volume percent
Nitrogen or other remainder
20 ballast gas, e.g.,
methane
15016
1286688
-43-
Table B
Component Concentration
5 Propylene about 2 to about 50
volume percent
Oxygen about 2 to about 10
volume percent
Hydrocarbon about 0 to about 5
volume percent
Carbon Dioxide about 0 to about 15
volume percent
Nitrogen or other remainder
ballast gas, e.g.,
methane
The ranges set out in Table B for the concentra-
tion of materials in the reaction inlet stream may be
useful for epoxidation of higher alkenes, e.g., al-
kenes having from 4 to 12 carbon atoms.
In the manufacture of ethylene oxide, the water
is preferably present in the reaction inlet strea~ in
an amount of from about 0.25 to about 1.5, more pref-
erably 0.25 to 1Ø volume percent. For the manufac-
ture of propylene oxide, the water is preferably
present in an amount of from about 0.25 to about 10
volume percent.
As previously noted, water can be added to the
reaction inlet stream continuously or intermittent-
ly. Continuously is preferred. Also, water is pref-
35 erably introduced contemporaneously or prior to the
15016
12lY668~3
-44-
introduction of carbon dioxide since the beneficial
effect of water is greater when it is $ntroauced
before carbon dioxide is present in significant
amounts in the reaction zone.
The ~nvention will be better understood by ref-
erence to the following examples which are offered by
way of illustration and not by way of limitation.
Exam?le 1.
A supported silver catalyst as described below
under the heading Method Of Preparation Of Catalyst
was tested by placing 80 ml of the catalyst in an
autoclave reactor using the feedstream set out in
Table I under the conditions set out in Table II.
The autoclave was a backmixed, bottom-agitated Mag-
nedrive* autoclave as described in Figure 2 of the
paper by J. M. Berty entitled ~Reactor For Vapor
Phase-Catalytic Studies~ in Chemical Enqineerin~
Progress, Volume 70, Number 5, pages 78-84, 1974.
TABLE I
Makeup Feedstream Composition
ComPonentAmount (BY Volume)
ethylene 30 percent
oxygen 8 percent
chloroethane 5 ppm
nitr~c oxide 5 ppm
nitrogen balance.
* - Trade~ark
~5016
12~688
i
-45-
TAsLE I~
GHSV 8,000 hr 1
Temperature 240 degrees C
5 Pressure 275 psLg.
The efficiency and activity of the catalyst
prior to the addition of water using the feedstream
as described in Table 1 were 89 percent and 12.7
pounds of ethylene oxide per cubic foot of catalyst
per hour (calculated based on the 80 milliliters of
catalyst present in the autoclave), respectively.
Water comprising one-half volume percent (based on
the feedstream composition) was then fed into the
autoclave with a Varian TM 8500 liquid chromatography
pump. The activity and efficiency were 89.9 percent
and 13.9 pounds per cubic foot per hour, respective-
ly. Carbon dioxide comprising 1 volume percent of
the makeup feedstream was then introduced into the
autoclave, together with the one-half volume percent
water previously referred to. The efficiency and the
activity of the catalyst were then determined to be
89.0 percent and 6.9 pounds per cubic foot per hour,
respectively. Thereafter, the water feed to the
autoclave was terminated while maintaining the flow
of 1 percent by volume carbon dioxide. The efficien-
cy and activity of the system were then found to be
85.9 percent and 5.7 pounds per cubic foot per hour,
respectively.
15016
128f~688
.
-46-
Method Of PreParation Of Catalyst-.
A silver-containing impregnation solution was
prepared by dissolving 142.5 grams of ethylenediamine
with 200.0 grams of distilLed water and stirring for
a period of 10 minutes. ~o the st~rred solutian were
slowly added 142.5 grams of oxalic acid dihydrate.
The resulting solution was stirred for 10 minutes.
To this solution were added, in portions, 249.6 grams
of silver oxide. The resulting silver-containing
601ution was thereafter stirred for an additional
hour and 49.9 grams of monoethanolamine were then
added to the stirred silver-containing solution.
Stirrlng was continued for an additional 10 min-
utes. This solution was then diluted to a totalvolume of 750 ml by addition of distilled water.
Righ-purity alpha-alumina support pellets (268.2
grams), having platelet morphology of the type disclosed
in Canadian Patent Appl. Ser. No. 515,864-8, filed
August 13, 1986, having a surface area o~ 1.06
meters squarea per gram and a porosity of 0.74 cc.
per gram were placed in a tube which was then evacu-
ated, following which the support pellets were im-
pregnated by immersing them in the silver-containing
impregnation solution, formed as described above, for
1 hour. Excess impregnation solution was then
drained and the resulting pellets were then belt-
roasted at 500 degrees C in a 66.5 SCFH air flow for
2.5 minutes. The catalyst pellets were then immersed
in a solution comprising 4.3 grams potassium nitrate
dissolved in 300 ml distilled water. After standing
in the solution for 15 minutes, the pellets were
removed and dried at 120 degrees C in an oven for 2
hours. The finished catalyst contained 17.0 percent
35 ~ilver ~nd 0.31 percent potassium.
; 15016
2~6813
(
-47-
Example 2 - Coincidental or
Coimpregnation Method Of Preparation
Of A Potassium Nitrate-Containing
Supported Hiqh Sil~er Concentration Catalvst-
A first silver-containing impregnation solution
was prepared by dissolving 1,292.8 grams ethylene-
diamine with 1,281.6 grams of distilled water and
stirring for a period of 10 minutes. To the stirred
solution were slowly added 1~294.8 grams of oxalic
acid dihydrate. The resulting solution was stirred
for 10 minutes. To this solution were added, in
portions, 2,268.4 grams of Ag20. The resulting sil-
ver-containing solution was thereafter stirred for an
additi~nal hour and 453.6 grams of monoethanolamine
were then added to the stirred silver-containing
solution. Stirring was continued for an additional
10 minutes. This solution was then diluted to a
total volume of 5,000 ml by addition of distilled
water.
High-purity alpha-alumina support pellets
(1,925.4 grams) having platelet morphology of the
type disclosed in Ca~adian Pa~ent Application Ser. No.
515,864-8, filed 8/13/~6, hav~ng a sur~ace area of
about 1.2 m~g and a porosity of about 0.8 cc/g were
placed in a tube which was then evacuated, following
which a first impregnation was conducted by immersing
the support particles in the first silver-containing
impregnation solution formed as described above, for
one hour. Excess impregnation solution was then
drained and ehe cesulting pellets were then belt-
roasted at 500 degrees C in a 66 SCF~ air flow for
2.5 minutes. The resulting material contained 24.9
35 percent ~ilver by weight.
n l ~
~B6688
-48-
A co-impregnation solution was prepared by plac-
ing 1,260.5 grams of ethylenediamine into a 5,000 ml
beaker and mixing therewith 1,249.6 grams of dis-
tilled water to form a solution. To the stirred
5 solution were slowly added 1,262.4 grams of oxalic
acid dihydrate and, with continuous stirring, 2,211.7
grams of silver oxide were slowly added. When dis-
solution was complete, 442.3 grams of monoethanol-
amine were added directly to the solution. To the
10 silver-containing solution were added 26.4 grams of
potassium nitrate dissolved in 50 milliliters of dis-
tilled water. To the resulting solution was added
sufficient water to dilute the solution to 4,875
ml. The silver-impregnated catalyst pellets (2,495.6
15 grams) were impregnated in a manner similar to the
first impregnation described above. The resulting
catalyst contained 39.8 weight percent silver and
0.098 weight percent potassium.
Example 3 - Sequential Preparation
Of A Potassium Nitrate-Containing
Supported ~iqh Silver Concentration CatalYst:
A silver-containing impregnation solution was
prepared by dissolving 787.0 grams ethylenediamine
with 780.0 grams of distilled water and stirring for
a per$od of 10 minutes. To the stirred solution were
slowly added 788.3 grams of oxalic acid dihydrate.
The resulting s~lution was stirred for 10 minutes.
To this solution were added, in portions, 1,380.8
grams of Ag2O. The resulting silver-containing solu-
tion was thereafter stirred for an additional hour,
following which 276.3 grams of monoethanolamine were
added to the stirred silver-containing solution.
Stirring was continued for an additional 10 min-
15016
12~ 8
I
-49-
utes. This solution was then diluted to a total
volume of 3,125 ml by addition of distilled water.
High-purity alpha-alumina support pellets
(1,172.5 grams), having platelet morphology, of the
type disclosed in Canadian Patent Application Ser. No.
515,86~-8, filed 8/13/86, having a surface area of
about 1.2 m~/g and a porosity of about 0.~ cc/g were
placed in a tube which was then evacuated, following
which the support pellets were impregnated by im-
mersing them in a portion of the impregnating solu-
tion formed as described above for one hour. The
excess impregnation solution was drained and the
resulting pellets were then belt-roasted at 500 de-
grees C in a 66 SCFH air flow for 2.5 minutes. The
resulting material contained 24.3 percent silver, by
weight.
A second silver-containing impregnation solution
was prepared in a manner similar to the preparation
of the silver-containing impregnation solution de-
scribed above, employing 472.2 grams ethylenediamine,
468.0 grams distilled water, 472.9 grams oxalic acid
dihydrate, 828.5 grams silver nitrate, 165.8 grams
monoethanolamine, and diluted to a total volume of
18.75 milliliters by addition of distilled water.
2S ~igh-purity alpha-alumina support pellets (703.5
grams), similar to those employed above, were then
impregnated with the solution described immediately
above in a manner similar to the first impregnation
described above. The resulting material contained
24.6 precent silver.
~ he two batches of impregnated alpha-alumina
formed as described above were then combined.
A ~econd impregnation cycle was perfor~ed by
impregnating the silver-containing catalyst material
35 of the combined batches with a fresh ilver impregna-
15016
lZ~688
-50-
tion solution. This solution was prepared in a man-
ner similar to the impregnation solutions used in the
first impregnation cycle, employing 1,196.2 grams
ethylenediamine, 1,185.6 grams distilled water,
1,198.1 oxalic acid dihydrate, 2,098.7 grams silver
oxide, 419.9 grams monoethanolamine, and diluted to
4,750 milliliters by addition of distilled water.
The silver-containing catalyst material formed in the
first impregnation cycle was impregnated with the
fresh silver impregnation solution in a manner simi-
lar to the manner in which the first impregnation
cycle was conducted. The silver-containing catalyst
resulting from the second impregnation cycle con-
tained 38.5 weight percent silver.
Potassium nitrate was incorporated into the
catalyst material by immersing 3,040.6 grams of the
silver-impregnated pellets in a solution containing
24.8 grams KNO3 in 4000 ml of distilled water. After
draining, the material was dried at 120 degrees C for
2 hours to yield a catalyst containing 38.5 percent
silver and 0.010 percent potassium, by weight.
The catalysts described above in Examples 2 and
3 may be used in carrying out the process o~ this
invention.
15016