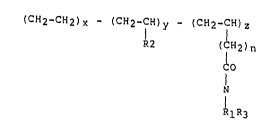Note: Descriptions are shown in the official language in which they were submitted.
` ~87~30
-- 1 `
FIELD OF THE INVENTION
The invention relates to polymeric visco-
sity-improving dispersant additives for petroleum oils,
particularly lubricating oils. These additives
co~prise a terpolymer of ethylene with one or more C3
to C2g alpha olefins, preferably propylene, which have
been co-reacted with an ethylenically unsaturated car-
boxylic ester using a Ziegler-Natta catalyst in a
solvent and then further reacted with a polyamine to
form am amide. The invention includes the use of the
aforementioned terpolymers in oil compositiOnS. The
invention also relates to processes for preparing the
aforementioned terpolymers and especially the use of
aluminum alkyls or aluminum alkyl halides as a complex-
ing agent to shield the carboxylic ester group during
the Ziegler-Natta polymerization and a superior cata-
lyst for amide formation.
BACKGROUND OF THE INVENTION
This invention relates to a multifunctional
polymeric additive for hydrocarbon compositions, parti-
cularly for lubricating oils. The additive is a ter-
polymer of ethylene/propylene/carboxylic amide.
The instant invention exhibits polymers
which are primarily known for their viscosity modifica-
tion. Various polymers have been used as viscosity
modifiers. Terpolymers of vinyl acetate, an alkyl
fumarate and maleic anhydride are taught, for example,
in U.S. Patent 3,087,893 and include copolymers made up
of from 2 to 15 mole percent of maleic anhydride, 25 to
lZ87~
-- 2 --
50 mole percent of an alkyl ester of an alpha, beta-
unsaturated dicarboxylic acid, and from 40 to 70 mole
percent of an alkylene ester of a Cl to C6 monocar-
boxylic acid. Techniques for forming the polymers are
also well-known. For example, a terpolyer of an alkyl
fumarate, vinyl acetate, and maleic anhydride can be
prepared by the process disclosed in the aforementioned
U.S. Patent 3,087,893 or by the improved process
described in U.S. Patent 3,136,7~3.
U.S. Patent 3,637,610 teaches a viscosity
modifier which is an oil soluble polymer having free
carboxylic acid groups which react with amine-contain-
ing polymers.
DESCRIPTION OF THE PRIOR ART
The concept of incorporating acid moieties
into viscosity improving high molecular weight ethylene
copolymers, followed by reaction with an amine to form
a viscosity-improving dispersant oil additive is known
in the art, as indicated by the following patents.
U. S. Patent No. 3,316,177 teaches ethylene
copoly.ners, such as ethylene-propylene, or ethylene-
propylene-diene, which are heated to elevated tempera-
tures in the presence of oxygen so as to oxidize the
polymer and ca~se its eeaction with maleic anhydride,
which is present during the oxidation. The res~lting
polymer can then be reacted with alkylene polyamines.
U. S. Patent No. 3,326,804 teaches reacting
ethylene copolymers with oxygen or ozone to form a
hydroperoxidized polymer, which is grafted with maleic
anhydride, followed by reaction with polyalkylene poly-
amines.
- ~Z1~7~3~
U. S. Patent No. 4,089,794 teaches grafting
the ethylene copolymer with maleic anhydride using
peroxide in a lubricating oil soluiton wherein the
grafting is preferably carried out under nitrogen,
followed by reaction with polyamine.
U. S. Patent No. 4,137,185 teaches reacting
Cl to C30 mono-carboxylic acid anhydrides and dicar-
boxylic anhydrides, such as acetic anhydride, succinic
anhydride, etc., with an ethylene copolymer reacted
with maleic anhydride and a polyalkylene polyamine to
inhibit cross-linking and viscosity increase due to
further reaction of any primary amine groups which were
initially reacted.
U. S. Patent No. ~,144,181 is similar to
U. S. Patent No. 4,137,185 in that it teaches using a
sulfonic acid to inactivate the remaining primary amine
groups when a maleic anhydride grafted ethylene-pro-
pylene copolymer is reacted with a polyamine.
U. S. Patent No. 4,16~,063 reacts an ethy-
lene copolymer in the absence of oxygen with chlorine
at temperatures of 150C to 250C with maleic
anhydride, followed by reaction with polyamine.
A number of prior disclosures teach avoiding
the use of polyamine having two primary amine groups to
thereby eeduce cross-linking problems which become more
of a problem as the number of amine moieties added to
the polymer molecule is increased in order to increase
dispersancy.
1287~30
-- 4
German Published Application No. P3025274.5
teaches an ethylene copolymer reacted with maleic anhy-
dride in oil using a long chain alkyl hetero or oxygen
containing amine.
U. S. Patent No. 4,132,661 grafts ethylene
copolymer-, using peroxide and/or air blowing with male-
ic anhydride and then reacts with a primary-tertiary
diamine.
U. S. Patent No. 4,160,739 teaches an ethy-
lene copolymer which is grafted, using a free radical
technique, with alternating maleic anhydride and a
second polymerizable monomer, such as methacrylic acid,
which materials are reacted with an amine having a
single primary or a single secondary amine group.
U. S. Patent No. 4,171,273 reacts an ethy-
lene copolymer with maleic anhydride in the presence o~
a free radical initiator and then with mixtures of C4
to C12 n-alcohol and amine, such as N-aminopropylmor-
pholine oe dimethylamino propylamine, to form a V.I.-
dispersant-pour depressant additive.
U. S. Patent No. 4,219,432 teaches maleic
anhydride grafted ethylene copolymer reacted with a
mixture of amine having only one primary group, toge-
ther with a second amine having two or more primary
groups.
German Published Application No. 2753569.9
shows an ethylene copolymer reacted with maleic anhy-
dride by a free radical technique and then reacted with
an amine having a single primary group.
3(3
German Published Application No. 284'288
grafts maleic anhydride on an ethylene-propylene
copolymer by thermal grafting at hiqh temperatures and
then reacts with amine having one primary group.
French Published Application No. 2423530
teaches the ther~al reaction of an ethylene copolymer
with maleic anhydride at 150C to 210C, followed by
reaction with an amine having one primary or secondary
group.
U. S. Patent No. 4,518,757 teaches hetero-
geneous catalyzed copolymers of alpha olefins and alpha
olefin ester complexes for frictional drag reduction.
U. S. Patent No. 3,492,277 teaches olefin
copolymers containing functional groups wherein the
polar monomer is reacted with an organoaluminum
compound by heating between 60 to 150??C??.
The early patents, such as U. S. Patent Nos.
3,316,177 and 3,326,804, taught the general concept of
grafting an ethylene-propylene copolymer with maleic
anhydride and then reacting with a polyalkylene poly-
amine, such as polyethylene amines. Subsequently,
U. S. Patent No. 4,089,794 was directed to using an oil
solution for free radical peroxide, grafting the ethy-
lene copolymer with maleic anhydride and then reacting
with the polyamine. This concept had the advantage
that by using oil the entire reaction could be carried
out in an oil solution to form an oil cocncentrate,
which is the commercial form in which such additives
are sold. This was an advantage over using a volatile
solvent for the reactions, which has to be subsequently
removed and replaced by oil to form a concentrate.
Subsequently, in operating at higher polyamine levels
~ ~287~30
-- 6 --
in order to further increase the dispersing effect,
increased problems occurred with the unreacted amine
groups cross-linking and, thereby, causing viscosity
increase of the oil concentrate during storage and
subsequent formation of haze and, in some instances,
gelling. Even though one or more moles of the ethylene
polyamine were used 2er mole of maleic anhydride during
imide formation, cross-linking became more of a problem
as the nitrogen content of the polymers was increased.
One solution was to use the polyamines and then to
react the remaining primary amino groups with an acid
anhydride, preferably acetic anhydride, of 4,137,185 or
the sulfonic acid of ~. S. Patent No. 4,144,181. The
cross-linking could also be minimized by avoidance of
the ethylene polyamines and instead using amines having
one primary group which would react with the maleic
anhydride, while the other amino groups would be ter-
tiary groups which were substantially unreactive.
Patents or published applications showing the use of
such primary-tertiary amines, noted above, are U~ S.
Patent No. 4,219,432, wherein a part of the polyamine
was replaced with a primary-tertiary amine; U. S.
Patent No. 4,132,661; ~. S. Patent No. 4,160,739; U. S.
Patent No. 4,171,273; German No. P2753569.9; German No.
2,845,288; and French No. 2,433,530.
Still another problem which arose when using
free radical initiators with mineral oil as the
grafting medium is that, as the grafting levels were
increased to increase the dispersancy level, a larger
proportion of the oil molecules in turn became grafted
with the maleic anhydride. Then, upon subsequent reac-
tion with amine, these grafted oil article tended to
become insoluble and to form a haze. To avoid using
initiators, such as peroxides, for grafting and to
avoid the use of oil, several of the above-noted
~287430
-- 7 --
patents utilized thermal grafting in solvent, prefer-
ably while using an e~hylene copolymer containing a
diene monomer so as to achieve an "ene" type reaction
between the unsaturation resulting from the diene moi-
ety and the maleic anhydride. However, generally such
"ene" reactions are slower than peroxide grafting
- U. S. Patent No. 4,517,104 represents a
further improvement over the art in that it permits the
utilization of tne generally less expensive polyalky-
lene polyamines having two primary amine groups, while
achieving good dispersancy levels, inhibiting cross-
linkin~ and allowing initiator, e.g.,, peroxide,
grafting in oil.
The present invention represents a further
improvement over the art in that it produces a car-
boxy-containing ethylene terpolymer in a single process
step, whereas the prior art requires both a polymeriza-
tion reaction and a separate graft on "ene" adduction
or process. The present invention has the further
advantage in that no deleterious byproducts are
formed, such as acid functionalized solvent. Such acid
functionalized solvent byproducts are a serious
problem and are overcome in U. S. Patent No. 4,517,10~
only by the addition of a significant quantity of high
molecular weight polyisobutylene succinic anhydride.
The present invention has the further advantage in that
the dispersant functional group, i.e., the amine, is
attached to the polymer backbone via an amide linkage
with much supeeior viscosity stability during storage
compared to the imide linkage of the prior art.
t2~7430
-- 8 --
SUMMARY OF THE INVENTION
The present invention relates to hydrocarbon
solution additives which are terpolymers and have mul-
tifunctional properties including viscosity modifica-
tion and sludge dispersing properties wherein the
instant terpolymers are also viscosity control agents
for nonpolar hydrocarbon liquids.
GENERAL DESCRIPTION OF THE INVENTION
The present invention relates to hydrocarbon
solution additives which are terpolymers of ethylene/
propylene/carboxylic amide and have multifunctional
properties including viscosity modification and sludge
dispersing properties, wherein the ins~ant terpolymers
are also viscosity control agents for nonpolar hydro-
carbon liquids, such as jet fuel.
The terpolymers of the instant invention are
terpolymers of ethylene/propylene/carboxylic amide. The
terpolymers of the instant invention are formed by a
Ziegler-Natta polymerization of the ethylene propylene
and a carboxylic acid or ester to form a terpolymer of
the ethylene, propylene and carboxylic acid or ester.
This terpolymer is subsequently reacted with a poly-
amine or amino alcohol to form the terpolymer of the
ethylene, propylene and a carboxylic amide.
A suitable hydrocarbon soluble, water
insoluble terpolymer of the instant invention has the
formula:
~ 3~
_ 9 _
(CH2-CH2) X - (CH2-cH) y ~ (CH2-CH) Z
R2 (CH~n
~:ONRl R3
or
(CH2-CH2)X --(CH~-CH)y ( ~ )z
R2
~ (CH2) nCONRlR3
wherein x is about 15 to about 75 mole %, more prefer
ably about 30 to about 67 mole ~, and most preferably
about 30 to about 63; y is about ~5 to about 85 mole ~,
more preferably about 33 to abo~t 70 mole ~, and most
preferably about 37 to about 70 mole ~; and z is about
0.1 to about 10 mole ~, more preferably about 0.2 to
about 9, n = O to 12; and Rl and R3 equal H, Cl-Clo
alkyl, alkylamino, alkyl polyamino, alkyl aminoether,
alkylamino alcohol ~roups, and R2 is an alkyl group
having 1-8 carbon atoms.
A preferred monomer is propylene, however,
alpha-olefins suitable in place oE propylene to form
the copolymer, or to be used in combination with ethy-
lene and propylene to form a terpolymer, tetrapolymer,
etc. include l-butene,l-pentene, l-hexane, l-heptane,
l-octene, l-nonene, l-decene, etc.; also branched chain
alpha-olefins, such as 4-methyl-1-pentene, 4-methyl-1-
hexene, S-methylpentene-1,4,4-dimethyl-1-pentene, and
6-methylheptene-1, etc. and mixtures thereoE,
The polymers of this invention generally
will have a number average molecular weight (Mn) of
from about 5,000 to about 500,000, preferably 10,000 to
200,000 and optimally from about 20,000 to 100,000.
The multifunctional viscosity modifiers of this inven-
tion will generally have a narrow range of molecular
~8~
-- 10 --
weight, as determined by the ratio of weight average
molecular weight (Mw~ to number average molecular
weight (Mn) Polymers having a lMW/Mn) of less
than 10, preferably less than 7, and more preferably 4
or less, are most desirable. As used herein, (Mn)
and (Mw) are measured by the well known techniques of
membrane osmometry and gel permeation chromatography.
The ter?olymer of the ethylene/propylene/
carboxylic acid or ester is reacted with a polyamine,
amine, aminoalcohol or amine ether in the presence of a
ca'alyst at a temperature of about 100C to about 260C
for a sufficient period of time to form the terpolymer
of the ethylene/propylene/carboxylic amide.
Suitable polyamines include ethylene dia-
mine, diethylene triamine, triethylene tetramine,
tetraethylene pentamine, N,N-dimethyl ethylene diamine,
N,N diethyl ethylene diamine, N,N dimethyl trimethylene
diamine, N,N-diethyl trimethylene diamine, etc.
Amino alcohols and ethers are also suitable.
Examples include 2-aminoethanol, diethanolamine tri-
ethanolamine, N-aminomethylmorpholine, N-aminoethylmor-
pholine, N-aminopropylmorpholine, tris hydroxymethyl
amainomethane (THAM), Azadioxabicylooctane (DOBO),
aminomethyl pyridine, aminoethylpyridine, aminopropyl-
pyridine, aminothiazoles, piperazines, aminopipera-
zines, hydroxy derivatives thereof and other amines
with similar functional groups.
Suitable catalysts for the amidation process
are tin metal salts, such as stannous octoate (~-ethyl-
hexoate). Other catalysts suitable for the reaction of
carboxylic acid polymer functionality with amines to
produce amides include silica gel tetraalkyl or tetra-
28~3C~
aryl pyrophosphites, trialkyl or triaryl phosphi.es.The triaryl phosphites are preferred phosphite cata-
lysts. Polyphosphoric acid and boric acid are also
catalysts for the formation of the amide polymer deri-
vative.
The cbncentration of the terpolymers of the
instant invention in the hydrocarbon liquid is about
0.001 to about 25 weight percent, wherein the oil com-
position range fro~ gasoline fractions through oils.
The additives of this invention can also be employed,
either alone or in combination, with other hydrocar-
bon-soluble additives in jet fuels and gasolines in
concentrations ranging from about 0.001 to 1.0 weight
percent as detergent and/or rust preventive additives.
In controlling the viscosity of non-polar
hydrocarbon liquids the terpolymer of the instant
invention is added to the non-polar hydro~arbon liquid
at a concentration of about 0.001 to about 25 weight
percent, more preferably about 0.05 to about 15, and
most preferably about 0.7 to about 14.
nESCRIPTION OF THE PREFERRED EMBODIMENTS
The following Examples illustrate the
present invention without, however, limiting the same
hereto.
Example 1
A reactor containing 3,000 ml of dry n-
heptane was fed ethylene at 20 g/hour, propylene at 40
g/hour, methyl undecylenate (7.5 ml methyl undecylenate
+ 6.75 ml diethyl aluminum chloride + 19 ml n-hexane)
at 22 ml/hour, VC14 (10 ml VC14 + 90 ml cyclohexane) at
. . .
lZ~7~3~
12 ml/hour, and diethyl aluminum chloride (25~ DEAC +
75% n-hexane) at 25 ml/hour. The reaction temperature
was 15C. The reaction time was 2 hours~ The polymer
was precipitated in 3.5 gallons of acetone and washed
in acetone + 10 ml concentrated HCl + 90 ml H20 and
then washed again in acetone + 2 g Irganox 101 ~ The
yield of polymer was 80 g. The polymer purified by
- reprecipitation contained 19 mmoles ester/100 g poly-
- mer.
Example 2
A reactor containing 3,000 ml dry n-heptane
was fed ethylene at 20 g/hour, propylene at 40 g/hour,
methyl undecylenate (10 ml ester complexed with 8.5 ml
diethyl aluminum chloride + 25.5 ml hexane) at 28
ml/hour for a total of 42 ml, VC14 (10 ml VC14 + 90 ml
cyclohexane) at 12 ml/hour for a total of 21 ml and
diethyl aluminum chloride (25~ DEACl plus 75~ n-hexane)
at 36 ml/hour for a total of 63 ml~ The reaction tem-
perature was 10C, the total reaction time was 3 hours.
The polymer was precipitated in 3.5 gallons acetone
plus 10 ml HCl and 90 ml H2O. The yield of polymer was
108 g. The inherent viscosity in decalin at 135C was
0.97. The polymer purified ~y reprecipitation
contained 15.7 mmoles ester/100 g polymer.
Example 3
A reactor containing 3,000 ml dry-heptane
was fed ethylene at 20 g~hour, propylene at 40 g/hour,
phenyl undecylenate (ester complexed with ethyl
aluminum sesquichloride) at 18 ml/hour, VC14 (10 ml
VC14 + 90 ml cyclohexane) at 4 ml/hour, and ethyl
aluminum sesquichloride (25~ ET3A12C13 + 75~ hexane) 40
ml/hour. The reaction temperature was 10C, the reac-
. ~ ,
1287~3~
tion time was 4 hours. The polymer was precipitated in3.5 gallons of acetone containing 20 ml concentrated
HCl plus }00 ml H20~ The above procedure was repeated
until the yield of polymer was 770 g. The inherent
viscosity in decalin at 135C is 1.45. The polymer
purified by reprecipitation contained 18.7 mmoles of
ester/100 g of polymer.
Example 4
A reactor containing 3,000 ml dry n-heptane
was fed ethylene at 20 g/hour, propylene at 70 g/hour,
thioethyl undecylenate (7.55 ml ester complexed with 4
ml of diethylaluminum chloride, 16.5 ml hexane) at 18
ml/hour, VC14 (10 ml VC14 + 90 ml cyclohexane) at 8
ml/hour and diethyl aluminum chloride (25% DEAC ~ 7S~
n-hexane) a~ 40 ml/hour. The reaction temperature was
10C. The total eeaction time was 3 hours. The poly-
mer was precipitated in 3.5 gallons acetone plus 30 ml
HCl plus 70 ml H20. The yield of polymer was 126 g~
The inherent viscosity in decalin at 135C was 0.97.
The polymer purified by reprecipitation contained 10
mmoles of ester/100 g polymer.
Example 5
A reactor containing 3,000 ml dry n-heptane
was fed ethylene at 20 g/hour, propylene at 40 g/hour,
trimethyl silyl undecylenate (8.25 ml ester + 4 ml
diethyl aluminum chloride, 16.2 ml hexane) at 52
ml/hour, VC14 (10 ml VC14 + 90 ml cyclohexane) at 8
ml/hour and diethylaluminum chloride (25% DEACl ~ 75%
hexane) at 40 ml/hour. The polymerization temperature
was 10C, the polymerization time was 3 hours. After 3
hours of polymerization 44 g of NAPM (N-aminopropyl-
morpholine) was added. The temperature was raised to
- ~.2lS7~3~
- 14 -
85C for 1/2 hour. The polymer was precipitated in
acetone-isopropanol plus 30 ml HCl once, followed by
reprecipitation from acetone-isopropanol plus 2 g IRG
1010. The yield of polymer was 143 g. The inherent
viscosity in decalin at 135C was 1.15. The nitrogen
content of the polymer was 0.315%. Based on nitrogen
the polymer contained 11.3 mmoles amide/100 grams poly-
mer.
Example 6
g of the polymer of Example 1 was
dissolved in 85 g of SlOON at lubricating oil 170C.
After the polymer dissolved, 0.5 g NAPM (N-aminopropyl-
morpholine) was added and the temperature maintained at
170C for 24 hours. ~nreacted NAPM was removed with N2
stripping. The weight percent nitrogen incorporated
into the polymer was 0.091. On the basis of nitrogen
the polymer contained 3.2 mmoles NAPM/100 grams of
polymer.
Example 7
Same as Example 6, except 0.1 g of stannous
octoate was added with the NAPM. The weight percent
nitrogen incorporated into the polymer was ~.282. on
the basis of nitrogen the polymer contained 10.1 mmoles
NAPM/100 g polymer.
Example 8
g of the polymer of Example 2 was
dissolved in 10 ml of tetrahydrofuran. 0.5 g NAPM was
added to the above solution. The tetrahydrofuran was
carefully removed by evaporation. The remaining
contents of the test tube were heated to 240C for six
- ~2~7430
- 15 -
hours in an oil bath under a nitrogen blanket.
Unreacted NAPM was removed by nitrogen stripping. The
weight percent nitrogen incorporated into the polymer
was 0.123. On the basis of nitrogen the polymer
contained 4.4 mmoles NAPM/100 g polymer.
Example 9
Same as Example 8 except a . 1 g stannous
octoate was added with the NAPM. The weight percent
nitrogen incorporated into the polymer was 0.914. On
the basis of nitrogen the polymer contained 6.9 mmoles
NAPM/100 g polymer.
Example 10
320 g of the terpolymer of Example 3 was
added to 2880 grams of S-lOON lubricating oil. The
reaction was heated to 170C with nitrogen sparging for
5 hours to dissolve the polymer. 21.3 g of N-aminopro-
pylmorpholine (NAPM) were added over 15 minutes after
3.2 grams of stannous octoate were added at 170C with
a nitrogen blanket. The reaction was stirred and
heated at 17~C for 5 hours, heated again to 170C and
6 g of NAPM added. The mix was again heated for 2
hours. The mix was then heated to 180C for 3 hours
while the excess NAPM was stripped off. The reaction
was cooled and the material removed for analysis. The
purified polymer contained 0.416 weight percent N. On
the basis of nitrogen the polymer contained 14.9 mmoles
NAPM/100 g polymer. On the basis of the amide IR peak
at 1670 cm~l the polymer contained 16.2 mmoles NAPM/100
g polymer.
~ ~287~3~
- 16 -
Example 11
An oil solution containing 7.5~ of the poly-
mer of Example 4 was heated to 170C for 24 hours with
stannous octoate catalyst and NAPM. The resulting
polymer contained 11.2 m~oles NAPM/100 g polymer.
Example 12
The ability of a catalyst such as stannous
octoate to markedly improve the conversion of ester to
amide is demonstrated in Table I. The data in Table I
also demonstrated the superiority o phenyl ester
compared to methyl ester in the conversion to amide.
TABLE_I
CONVERSION OF ESTER TQ AMIDE
_
Ester Amide
ContentContent
mmoles/mmoles/
Ester 100 g100 g Amide
Polymer Type polymer Cat. Polymer Polymer
Ex. 1 methyl 19 no 3.2 Ex. 6
Ex. 1 methyl 19 yes 10.1 Ex. 7
Ex. 2 methyl 15.7 no 4.4 Ex. 8
Ex. 2 methyl 15.7 yes 6.9 Ex. 9
Ex. 3 phenyl 18.7 yes 14.9 Ex. 10
Example 13
The outstanding stability o the amide poly-
mer o Example 10 in terms o viscosity growth during
heated storage compared to a typical succinimide multi-
functional viscosity modifier (Comparison A) is demon-
strated in Table II.
~ ~L287~3~
- 17 -
TABL E I I
Viscosity, Cts @ 100C Change, 82C Storage
Viscosity Viscosity Viscosity Viscosity
Initial 2 Weeks 4 Weeks 8 Weeks
~xample 10 891 888 ~77 841
Comparison A 1380 1815 2136 2757
The polymer of Comparison A is a maleic
anhydride peroxide graft of an ethylene-propylene
copolymer reacted with the same amine as Example 4 and
is further described in U.~. P~tent No.
4,780,228, issued October 25, 1983.
Example 14
.
Multifunctional viscosity modifiers where
the amine functionality is bound via succinimide
leakage are known to interact with polar additives
normally found in lubricants. This interaction, which
leads to and is indicated by an increase in viscosity,
is disadvantageous. The amide polymers of this inven-
tion have a markedly reduced interaction with polar
lubricant additives, as shown by the data in Table III.
. ~ . .
s
--" 1287~3~
TABLE III
Viscosit , Cts @ 100C, Change 60S Storage
Y
Viscosity Viscosity
AfterAfter
1 Hour24 Hours
Polymer of Example 101 14.4 19.5
Polymer, Comparison Al~2 15.1 15.8
1. Oil blend, 14.6 wt.% polymer (Example 10 or Com-
_ parison A), 77.7 wt.~ S140N mineral oil, 7.5 wt.~
detergent inhibitor, pl~s zinc dialkyl dithiophos-
phate and 0.2 wt.% pour depressant.
2. Comparison A polymer is same for polymer used in
Table II.
Example 15
The ability of the polymer of Example 10 to
disperse sludge and control varnish is demonstrated in
Table IV.
TABLE IV
SLUDGE (SrB) & VARNISH (VIB~
rNHIBITOR BENCH TEST
POLYMER SIB RESULT VIB RESULT
Example 10 6.0 2
Comparison Al11.2
Paratone 7lS215.0 3
None 14.5 ll
1. Comparison A is the same nitrogen containing poly-
mer used in Table II.
2. Paratone 715 is a non-nitrogen viscosity modifier
commercially available from Exxon Chemical Co.
:~287~3~
-- 19 --
Sludge Inhibition Bench (SIB) Test
The efficacy of the derivatized copolymers
of this invention as dispersants in lubricating oil is
illustrated in a Sludge Inhibition Bench (SIB) Test.
The SIB test has been found, after a large number of
evaluations, to be an excellent test for assessing the
dispersing power of lubricating oil dispersant addi-
tives.
The medium chosen for the SIB test was a
used crankcase mineral lubricatinq oil composition
having an original viscosity of about 325 SUS at 38C,
that had been used in a taxicab that was driven
generally for short trips only, thereby causing a
buildup of a high concentration of sludge precursors~
The oil that was used contained only a refined base
mineral lubricating oil, a viscosity inde~ improver, a
pour point depressant and zinc dialkylthiophosphate
anti-wear additive. The oil contained no sludge dis-
persant. A quantity of such used oil was acquired by
draining and refilling the taxicab crankcase at
1,000-2,000 mile intervals.
The Sludge Inhibition Bench Test is con-
ducted in the following manner: The aforesaid
crankcase oil, which is milky brown in color, is freed
of sludge by centrifuging for one hour at about 39,000
gravities (gs.). The resulting clear, bright red
supernatant oil is then decanted from the insoluble
sludge particles, thereby separated out. However, the
supernatant oil still contains oil-soluble sludge pre-
cursors which on heating under the conditions employed
by this test will tend to form additional oil-insoluble
deposits of sludge. The sludge inhibiting properties
of the additives being tested are determined by adding
1~87~13~
- 20 -
to portions of the supernatant used oil a s~all amount,
such as 1 or 2 weight percent, on an active ingredient
basis, of the particular additive being tested. Ten
grams of each blend being tested are placed in a
stainless steel centrifuge tube and are heated at
138/C for 16 hours in the presence of air. Following
the heating the tube containing the oil being tested is
cooled and then centrifuged for about 30 minutes at
room temperature at about 39,oO0 gs. Any deposits of
new sludge that form in this step are separated from
the oil by decanting the supernatant oil and then care-
fully washing the sludge deposits with 25 ml of heptane
to remove all remaining oil from the sludge and further
centrifuging. The weight of the new solid sludge that
has been formed in the test, in milligrams, is deter-
mined by drying the residue and weighing it. The
results are reported as percent of sludge dispersed by
comparison with a blank not containing any additional
additive. The less new sludge precipitated in the
presence of the additive the larger the value of
percent sludge that is dispersed, and the more effec-
tive is the additive as a sludge dispersant. ln other
words, if the additive is effective, it will hold at
least a portion of the new sludge that forms on
heating and oxidation stably suspended in the oil so it
does not precipitate down during the centrifuging.
Varnish Inhibition Test
-
Each test sample consisted o 10 grams of
lubricating oil and either 1 or 2 weight percent of the
neutralized polymer. The test oil to which the
additive is admixed was a commercial lubricating oil
obtained from a taxi af after about 2,000 miles of
driving with said lubricating oil. Each ten gram
sample was heat soaked overnight at about 140C and
28~43(3
- 21 -
thereafter centrifuged to remove the sludge. The
supernatant fluid of each sample was subjected to heat
cycling from about 150C to room temperature over a
period of 3.5 hours at a frequency of about 2 cycles
per minute. During the heating phase gas which was a
mixture of about 0.7 volume percent So2, 1.4 volume
percent NO and balance air was bubbled through the test
samples. At the end of the test period, which testing
cycle can be any additive, the wall surfaces of the
test flasks in which the samples were contained are
visually evaluated as to the varnish inhibition. The
amount of varnish imposed on the walls was rated 1 to
11, with the higher number being the greater amount of
varnish.