Note: Descriptions are shown in the official language in which they were submitted.
~6~0
Goddin, Ho, Reed
7~7~s~
PROCESS AND APPARATUS FOR LOW TEMPERATURE
AMINE REMOVAL OF ACID GASES
FIELD OF THE INVENTION
The invention relates to a process for acid gas
removal and more particularly to a process for removal of
15 carbon dioxide and/or hydrogen sulfide from gases. In a
further aspect the invention relates to a process for
carbon dioxide removal from hydrocarbon containing gaseous
streams utilizing aqueous alkanolamines.
BACKGROUND OF THE INVENTION
The removal of acid gases from gaseous streams
by absorption in aqueous alkanolamines has long been a
part of industrial technology. In the past, such pro-
cesses have been used in connection with the production of
natural gas to remove, for example, carbon dioxide (CO2)
25 present at relatively low levels typically not exceeding
20-30 mol % CO2 and hydrogen sulfide (H2S) from the pro-
duced gases.
In recent years, the development of CO2-miscible
flooding has created a need for processes capable oE
30 treating gases which contain large quantities of acid
gases including CO2, in the range for example of 15 to
about 90 mol ~ CO2 or even higher from gaseous streams
produced in connection with such CO2 miscible floods. The
gas produced during CO2 miscible flooding can have in
35 addition to CO2, H2S, and significant amounts of methane,
and especially, of ethane and higher hydrocarbons. It is
geneLally desirable to recover the CO2, for example, for
reinjection into the reservoir undergoing CO2 miscible
~ ~7~3~
--2--
flooding or for other uses, and the hydrocarbons for fur-
ther processing.
In addition, low quality natural gas reservoirs
occur ln nature which contain high levels of CO2. Such
5 reservoirs have sometimes not been produced for lack of
processes which can recover salable gases efficiently and
economicallyr while meeting environmental requirements.
A number of processes using aqueous amine solu-
tions have been proposed for the removal of acid gases
10 from gaseous streams containinq high levels of CO2. Some
of these processes have accomplished the removal of CO2 in
two or more stages for efficiency, utilizing, for example~
at least a first stage for bulk removal of CO2, and at
least a second stage for final removal of CO2. One such
15 process is described in U.S. Patent 4,466,946.
A major object of such processes is to accom-
plish removal of CO2 and other acid gases such as hydrogen
sulfide from gas streams containing hydrocarbons with a
low consumption of energy. Significant factors in deter-
20 mining energy consumption are the operating temperaturesmaintained in the CO2 absorption and regeneration stages.
In conventional operation, a rich amine liquid containing
C2 and other acid gases such as H2S is regenerated by
passing the liquid downwardly in generally continuous
25 fashion through a column having a plurality of vapor
liquid contact devices therein. Adjacent to the bottom of
the column, stripping vapor, predominantly water vapor or
steam is generated by heat input typically by a reboiler.
The rising vapor passes upward countercurrently to the
30 descending liquid stripping CO2 and other acid gases from
the descending liquid. Such a system can be considered to
form a disperse system in which the liquid amine forms the
generally continuous phase and the rising bubbles of vapor
comprising steam and stripped gases form the discontinuous
35 or disperse phase. Desorption of the acid gases is from
the generally continuous phase to the dispersed phase.
The heat requirement Eor generating the stripping vapors
in such systems can be considerable. The heat requirement
for regeneration of the rich amine solution, by such steam
stripping, for example, can be reduced greatly when the
5 stripping temperature is reduced below the boiling point
of the amine solution. The heat requirement continues to
decrease as the temperature of stripper operation
decreases, so it is advantageous to operate at the lowest
reasible temperature. Regeneration at lower temperature
10 also facilitates major investment cost savings by elimina-
tion of the amine cooler and lean/rich amine exchanger
which are an integral part of many such acid gas removal
stages operated at higher temperatures.
SUMMARY OF THE INVENTION
During absorption of acid gases from fluid
streams using aqueous alkanolamine solutions a lean alka-
nolamine stream is contacted with the stream undergoing
treating, acid gases such as carbon dioxide and hydrogen
sulfide are absorbed, and a rich alkanolamine stream com-
20 prising absorbed acid gases and a product fluid stream
reduced in acid gas content are produced. During regener-
ation, the acid gases are desorbed from the rich alkanola-
mine solution and a lean alkanolamine solution is pro-
duced. The lean alkanolamine solution is not completely
25 free of absorbed acid gases but contains a residual acid
gas loading which at equilibrium is a function of tempera-
ture, acid gas partial pressure, and alkanolamine concen-
tration in the base of the stripper. It is the difference
between the residual acid gas loading oE the lean alkanc-
30 lamine stream and the acid gas loading of the rich alkano-
lamine stream which determines the acid gas "carrying
capacity" or "net loading capacity" of the alkanolamine
solution. It is therefore highly advantageous that the
residual acid gas loading of the lean alkanolamine stream
35 be substantially the equilibrium acid gas loading. How-
ever, it has been found that equilibrium acid gas loading
values become more diffi_ult to attain at low stripping
temperatures.
3~ 3~
We have found that acid gases can be clesorbed
Erom a rich aq-leous tertiary alkanolamine solution com-
prising absorbed acid gases, by atomizing and flashing the
rich aqueous tertiary alkanolamine solution under condi-
5 tions of droplet size and settling time effective for sub-
stantially attaining equilibrium acid gas loading values
while in the resulting disperse droplet phase, the temper-
ature of the rich tertiary alkanolamine solution being
below about 200F. The resulting lean tertiary alkanola-
10 mine solution droplets can coalesce to form a lean ter-
tiary alkanolamine solution having substantially equili-
brium acid gas loading values which can be returned,
without further acid gas desorption, for further absorbing
acid gases.
In accordance with an aspect of the invention,
an acid gas-rich feed gas can be contacted with an aqueous
tertiary alkanolamine under pressure in a first contacting
zone at a temperature below about 200F. The rich aqueous
tertiary alkanolamine can then be removed from the first
20 contacting zone and then flashed with atomization or dis-
persal into finely divided droplets into one or more
regeneration zones and carbon dioxide and other acid gases
desorbed while in the droplet phase to substantially equi-
librium loading values in the alkanolamine solution.
25 Regenerated lean alkanolamine from the regeneration zones
can be recycled to the first contacting zone. According
to a further aspect of the invention, the acid gas com-
prises carbon dioxide, H2S, and a minor proportion oE
hydrocarbon components.
As indicated, the first contacting zone com-
prises a tertiary alkanolamine absorber operated at a tem-
perature well below the normal boiling point of the amine
solution, ~or example, below about 200F, preferably in
the range of about 190F, to about 140F or even lower.
35 The operating temperatures of the lean alkanolamine stream
leaving regeneration and entering the Eirst contactinq
zone can be about the same, so neither heat exchange
between lean and rich amine streams, nor cooling of the
77~2
lean amine prior to entering the Eirst contacting zone
need be used. Consequently, heat input to the stripper
can be limited primarily to heat leakage from the system
and vaporization of water into the stripper overheacl
5 vapor.
In a further embodiment of the invention, the
gaseous stream from which a first major quantity of CO2
has been removed in the first contacting zone can be
introduced into a second contacting zone in which residual
10 CO2 and other acid gases can be removed. Preferably,
according to this embodiment~ the first contacting zone
uses an aqueous tertiary alkanolamine and the second con-
tacting zone uses aqueous secondary or primary alkanola-
mines to accomplish final cleanup.
The invention will be further understood and
other features, embodiments, and applications of the
invention will be apparent from the following detailed
description of the invention and from the drawings.
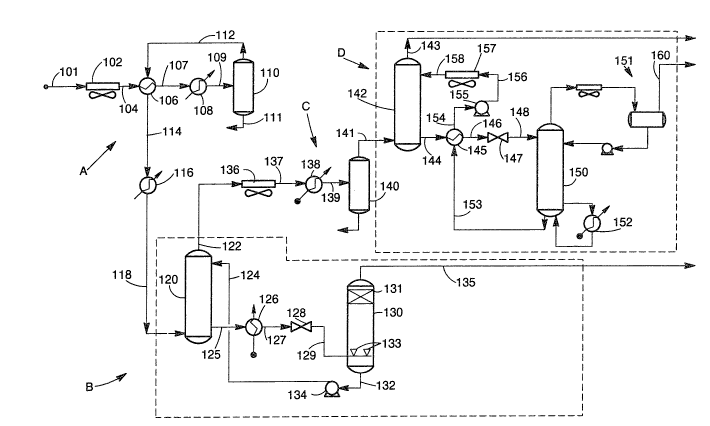
BRIEF DESCRIPTION OF THE DRAWINGS
FIGURE 1 represents schematically a first embod-
iment of the invention.
FIGURE 2 represents schematically a second
embodiment of the invention.
D ILED DESCRIPTION OF THE INVENTION
The present invention is based on the discovery
that carbon dioxide and other acid gases can be effec-
tively removed from a rich tertiary aqueous alkanolamine
solution by flashing accompanied by atomization or dis-
persal of the rich amine into small droplets. This method
30 of desorbing carbon dioxide is particularly advantageous
at low temperatures such as in the range of about 1~0F to
about 200F where conventional desorbing techniques using
a plurality of vapor liquid contact devices were found
incapable of acnieving equilibrium values of acid gas
35 residual loading. As will be described hereafter the
present invention contemplates subjecting the rich alkano-
lamine solution to flashing and atomization in one or more
regeneration stages. The flashing and atomization is
~ ~.3~ 3~
effective for removing carbon dioxide, and other acid
gases if present, substantially to equilibrium, under the
conditions of temperature, and pressure, and alkanolamine
solution employed, during the time that the rich absorp-
5 tion solution is in the atomized dispersed state. A sub-
stantial approach to equilibrium as used in the descrip-
tion of this invention shall mean a residual acid gas
loading in the lean alkanolamine solution within 10% of
equilibrium acid gas loading values, more preferably
10 within 5% of equilibrium acid gas loading values.
By substantially attaining equilibrium acid gas
loading values while in the disperse phase, retention of
liquid for further acid gas desorption is unnecessary;
consequently retention time, vessel size, total volume of
15 alkanolamine solution and the like can be reduced. Fur-
ther, at the temperatures of interest herein, it has been
found that desorption of acid gases from pooled liquid
even at retention times as high as 30 minutes was ineffec-
tive to achieve equilibrium values of acid gas loading.
It will be appreciated that a wide range of dro-
plet sizes can be employed, the range selected being that
which will accomplish mass transfer of carbon dioxide and
other acid gases from the liquid droplet phase to the
vapor phase so that desorption oE the acid gases can occur
25 substantially to equilibrium. The mean droplet size is
determined by the average droplet settling time which in
turn is dependent on the average droplet velocity and the
vessel geometry.
Generally, the lower limit of droplet size can
30 be determined so as to prevent entrainment and loss of
absorption solution droplets in the gaseous phase removed
from the regenerator; the mean-droplet size will be that
necessary to provide the necessary droplet surface; and
the upper limit of droplet size will depend upon the dro-
35 plet size distribution characteristic of the nozzle. Inany specific application, selection of proper droplet
size, _ettlincl time, and equipment configuration can be
readily determined by a skilled person in accordance with
~3~3~
7--
the invention as hereinafter described and set forth. In
the illustrated embodiment described below, mean droplet
diameter of the finely divided droplets can be gerlerally
in the range of about 100 mlcrons to about 5,000 microns,
s preferably in the range of abou~ 1000 microns to about
5000 microns; settling time can be, ~or example, 0.050
seconds or longer, preferably in the range of 0.10 to 0.50
seconds.
According to a preferred embodiment of the
10 invention a gaseous stream comprising a significant quan-
tity of carbon dioxide can be introduced into the base of
a first absorber (first contacting zone) for removal of at
least a major portion of the carbon dioxide from the
gaseous stream. Carbon dioxide can be present in the
15 gaseous stream from about 15 mol % CO2 to 90 mol ~ or
higher. For effective absorption and regeneration
according to the invention, the CO2 should generally have
a partial pressure in the gaseous stream of about 50 psi
to about 500 psi or higher.
The gaseous stream can be derived, for example,
from gases produced during a CO2 miscible flood operation.
Such produced gases from CO2 miscible flood operations,
after CO2 breakthrough, can contain as much as 90 mol % or
even higher levels of CO2, as well as methane, and espe-
25 cially, ethane and higher hydrocarbons. Hydrogen sulfide
will also generally be present~
Such gaseous streams can contain significant
levels of higher hydrocarbons which can cause foaming
problems during acid gas absorption if the gas is not pre-
30 treated to remove at least a portion of the hydrocar-
bons. One method for such hydrocarbon removal is
described in U. S. Patent No. 4,466,946. Briefly, the
hydrocarbon removal can be achieved so that the hydro-
carbon dew point profile as CO2 is removed in the first
35 and subsequent contactors is preferably at least 10F
below the operating temperature profile of the
absorber. One convenient method of such hydrocarbon
3~
--8--
removal is by chilling the feed to condense and remove
heavier hydrocarbons which can cause Eoaming and other
operatlng problems in the contactor.
The gaseous stream containing hydrocarbons and
5 significant amounts of carbon dioxi~e can also be derived
from natural gas reservoirs or from other sources; and in
such instances, of course, the instant invention will also
be useful.
As indicated above, we contemplate that in most
lO cases, the instant invention will find greatest applica-
bility when the CO2 content is above about 15 mol % of the
gaseous feedstream since below about this level conven-
tional absorber systems may be preferred. A typical range
f C2 content can be in the range of about 30 mol % to
15 about 90 mol % CO2, although as indicated, more or less
C2 can be present.
In a preferred embodiment, the first contactor
can be any suitable contactor for contacting the gaseous
feedstream with a lean aqueous tertiary alkanolamine solu-
20 tion introduced in the top of the first absorber and pro-
duced in accordance with the invention for removal of
carbon dioxide and other acid gases present to substan-
tially equilibrium values of acid gas loading. Suitable
contactors can include, for example, towers filled with
25 packing material, the alkanolamine flowing down through
the packing and gas flowing upward in countercur.ent
fashion; spray towers; tray towers containing bubble cap,
sieve or valve trays; stirred vessels, and the like.
Preferably, packed towers or tray towers can be used.
The aqueous tertiary alkanolamine is preferably
selected from the group consisting of triethanolamine
(TEA), methyldiethanolamine (MDEA), and the like. The
alkanolamine can be present in the aqueous solution in the
range of about 20 mol % to about 90 mol %, preferably in
35 the range of about ~0 mol % to about 60 mol %. Activators
can also be present, such as, for example, piperazine and
the like.
_9_
The first contactor can be operated under condi-
tions of temperature and pressure effective for removal of
at least a major portion of the carbon dioxide from the
feed stream. Preferably, the operating temperatures are
5 well below the boiling point of the amine solution
employed. For example, in a preferred embodiment, the
temperature of the lean amine fed to the first contactor
can be broadly in the range of about 1~0F to about 200F,
or even lower. Most preferably, the operating tempera-
10 tures are in the range of about 160F to about 190F.Typically the contactor operating pressures can be in the
range of about 200 psi to about 800 psi, although lower or
much higher pressures can be used depending on the pres-
sure of the inlet gas source. The CO2 preferably has a
15 partial pressure of about 50 psi to about 500 psi.
The rich aqueous tertiary alkanolamine solution
can be removed from the base of the first contactor and
introduced with pressure reduction (flashed) into a flash
regeneration vessel such as a tank or tower. Whereas a
20 regeneration vessel is typically equipped with trays or
packing or other contact devices, we have found that due
to the very low volume of stripping vapor ascending within
the vessel at the low temperatures of interest, the oper-
ating efficiency of the trays or packing is much impaired.
25 Accordingly, trays or packing are not required or desired
when regenerating alkanolamine solutions in accordance
with the present invention. In fact, the presence of
trays or packing can interfere with regeneration. Accord-
ingly, an empty vessel can be used and is preferred.
30 Demisting packing can, of course, be used in such gener-
ally empty vessels to minimize entrainment of liquids
overhead.
The flashing can be accompanied, preceded, or
followed by mechanical dispersal of the rich aqueous ter-
35 tiary alkanolamine solution into droplets, for example, byspraying, atomizing, misting, or nebulizing. Preferably,
the mechanical dispersal is accomplished by spraying the
rich amine solution by atomiæing nozzles into the flash
7~
--10--
vessel. Preferably, the mechanical dispersal ls accom-
plished by upwardly directed atomizing nozzles since such
an arrangement has been found to result in slightly better
stripping than downwardly directed spray nozzles. Other
5 arrangements can, of course, also be used as can other
mechanical devices effective Eor dispersal of the rich
amine into finely divided droplets.
Any suitable atomizing nozzles can be used in
accordance with the invention. Preferably, pressure
10 nozzles such as hollow cones, solid cones, and fan nozzles
can be used. Hollow cone nozzles are preferred for their
high atomization efficiency; most preferably wide angle,
low capacity nozzles operating with moderately high pres-
sure drops are used because such are effective for gener-
15 ating small droplets and increasing the rate of masstransfer.
The nozzles can be oriented within the regenera-
tion vessels to maximize contact between the droplets and
vapor phase. Opposing sprays may be advantageous since
20 collision between finely divided droplets can result in
shattering and further fragmentation of the droplets.
Either vertical or horizontal regeneration vessels can be
used. Use of a plurality of nozzles at low liquid rates
per nozzle facilitates generation of smaller droplets.
25 There also is a turndown advantage since sorne of the
nozzles can be taken out of service, thus maintaining suf-
ficient pressure drop across the remaining in-service
nozæles to give adequate atomization.
The flashing of the rich amine can be accom-
30 plished at an inlet temperature about the same as that atwhich the rich amine is removed from the first contactor,
that is, in the range generally of about 140F to about
200F; and the lean amine after the flashing and mechan-
ical dispersal step(s~ can be returned directly to the
35 first contactor. When operating according to the inven-
tion the lean/rich amine heat exchanger and the lean amine
cooler can be eliminated reducing equipment require-
ments.
32
--11~
The ~lashing can be accomplished in one stage,
or in more than one stage. Typically, if the CO2 product
must be recompressed prior to, for example, reinjection
during CO2 miscible flooding, it ~ill be desirabLe to use
5 more than one stage of flashing, for example, two or more,
to reduce recompression requirements. Thus regeneration
can be achieved by stages of flashing. Optionally a small
amount of heat can be introduced prior to the first ~lash
to maintain the lean amine temperature in the desired
10 range. When two or more stages are used for flashing to
conserve recompression energy, the flash pressures can be
selected to maintain a ratio between stage outlet pres-
sures of about 2.5 to 3.5, i.e., equivalent to the com-
pression ratio for a single stage of compression. Thus,
15 for example, for a two-stage flash with rich amine leaving
the contactor at 300 to 400 psia, and final Elash at about
20 to 25 psia, the first stage Elash can be accomplished
at about 50 to 90 psia.
According to a preferred embodirnent of the
20 invention, the process stream from the first contactor
from which at least a major portion of CO2 has been
removed can be provided to a second stage of carbon
dioxide removal. Preferably, the CO2 content will be
below about 20 mol %, more preferably in the range of
25 about S to about 15 mol % CO2 since these ranges can be
more efficiently handled by primary alkanolamines such as,
for example, monoethanolamine and the like or secondary
alkanolamines such as, for example, diethano]amine (DEA)
and the like than by tertiary alkanolamines~ A primary or
30 secondary amine is preferred for final cleanup according
to the invention because stringent sales gas specifica-
tions are more readily obtained with these more reactive
amines than with tertiary amines. The use of such primary
and/or secondary alkanolamines can be conventional and
35 need not be described here in detail. See also U. S.
Patent 4,466,946. In any event regeneration can be accom-
plished under conventional conditions of high temper-
3773~
-12-
ature and high energy input for the fina:L stage o~ regen-
eration regardless of the amine choice.
The invention will be further understood and
appreciated from the following Examples in which EXAMPI,E I
5 indicates that conventional stripping of tertiary alkano-
lamines is inefficient at the low temperatures of interest
and EXAMPLE II indicates the effectiveness of mechanical
dispersal of rich amine into small droplets during
flashing.
EXAMPLE I
A 60 gpm (gallons per minute) skid mounted amine
unit having a contactor and a stripper was modified for
testing and data retrieval. The contactor and stripper
were each 2.5 feet in diameter with 20 Nutter valve trays.
15 The range of operating conditions is given in Table IA.
TABLE IA
TEA TEST CONDITIONS
AMINE TEA
Rate - GPM 30-100
Net Loading - SCF/gal1.5-4.0
FEED GAS
Rate - MSCFD 400-900
C2 in Feed - mol % 38-68
CONTACTOR
Top Press. - psia 220-570
C2 in Offgas - mol % 2-30
STRIPPER REBOILER
Temp - F 160-235
Press. - psia 21-24
Data collected using the skid mounted amine unit
demonstr~ted operability of the tertiary amine process for
bulk CO2 removal from a natural gas containing C3 and
heavier hydrocarbons. With stripping temperatures above
35 about 190F, performance of the TEA unit conformed to that
predicted, but at lower temperatures, the net CO2 solution
loading fell below predicted values.
3~
The liquid and vapor rates in the contactor were
sufficiently close to the normal operating rates that it
was considered unlikely that the average tray efficiency
would differ greatly from the 15~-30% anticipated for
5 tra~s in such service. On the other hand, vapor rates in
the stripper column were far below the rates desired for
effective tray action in the stripper. Accordingly, the
burden was placed on the stripper as the principal source
of deviation from predicted TEA performance at lower tem-
10 perature levels.
In an attempt to discern whether mass transfer
or reaction kinetics was controlling in the stripper,
reaction rate constants were calculated based on the first
order stripping equation:
kr = ~ ln A0
here: ~ = residence time
Ao = CO2 concentration in rich amine
A = CO2 concentration in lean amine
Since the residence time in the stripper system
is not well defined, a pseudo kr was calculated in which
25 the amine flow rate in GPM was substituted for 1/~.
Logarithms of the pseudo-reaction rate constants
were plotted versus the reciprocal of the reboiler temper-
ature in degrees Kelvin. The straight line representing a
least squares fit to the data had a slope of -3500 which
30 corresponds to E/R in the Arrhenius equation:
kr = Ae
Johnstone (see Johnstone and Thring, Pilot
35 Plants, Models and Scale Up Methods, Ch. 6, McGraw-Hill,
1957) has defined a "temperature coefficient of reaction
rate" which represents the ratio of reaction rates for a
temperature rise of 10C above a base temperature of 15C.
7~3~
In this instance the coefficient amounts to 1.50.
According to Johnstone, the temperature coef~icient for
most chemical reactions lies between 2 and ~. A coeffi-
cient below 1.5 characterizes a dynamic (mass transfer-
5 controlled) regime. The coefficient was determined to beabout 1.5 which indLcated that mass transfer was probably
the controllin~ factor.
Another useful criterion is that if the system
were chemical reaction rate controlled, a change in fluid
10 velocity would not affect the reaction rate constant at a
given temperature. The effect of changing amine flow rate
through the stripper system on the pseudo-reaction rate
constant at constant reboiler temperature was investi-
gated. Results indicated that decreasing the amine rate
15 at constant temperature decreased the reaction rate, which
supported the concept that mass transfer, which is sensi-
tive to velocity, was the controlling mechanism in the
stripper.
It was concluded that decreasing temperatures in
20 the stripper impaired the mass transfer due to decreasing
diffusivity, increasing liquid viscosity, and increasing
partial pressure of CO2 in the stripping vapor; and that
valve trays in the unit stripper were ineEfective due to
very low vapor velocity.
_AMPLE II
As indicated, tests conducted on the skid-
mounted amine unit indicated deterioration in bulk CO2
removal performance when bottoms temperature of the
stripper was decreased below about 190F. Since process
30 energy and investment cost savings can be realized by
operation at lower amine temperature, the decision was
made to conduct additional tests. The primary objective
of these tests was to define the minimum practical oper-
ating temperature with a modified stripper column design.
35 The 20 Nutter valve trays in the stripper column were
removed and a 10-foot Flexipac* high efficiency packing
was installed in the upper section of the stripper column.
Spray nozzles located at four different levels were
* Trademark
3~ 2
-15-
installed, three directed downwards and one upwarcls. A
top nozzle lnstalled above the packing served to dls- tri-
bute liquid over the packing. The remaining three nozzles
were installed at different levels in the empty column
5 below the packing to accomplish spray atomization.
The amine unit was restarted, and ten test runs
were completed. The schedule of run conditions is given
in Table IIA. All tests were conducted with two amine
pumps on line, corresponding to about 65 gpm. TEA concen-
10 tration was 50-53 weight %.
TABLE II
Run CO2-Mol ~ CO2 in Feed Lean Aminel Rich Amine2
Number Feed Offgas (psi) Temp-FNozzle No.
15 1 53 14 17~3 204
2 58 20 186 181
3 59 24 189 180 2
4 58 24 183 181 3
60 23 186 180 4
20 6 58 24 178 160
7 56 19 177 158 4
8 56 18 170 140 4
9 42 8 133 159 4
53 12 181 162 1 & 4
25 NOTES:
1. Lean Amine
(a) The stripper reflux drum was at 24-26 psia.
(b) The temperature of the lean amine to the con-
tractor was held equal to the lean amine leaving
the stripper reboiler (no cooling).
2. Spray Nozzle Arrangement
(a) Nozzle 1 was in the top of the column, directed
downwards; Nozzles 2 and 3 were intermediate in
the column directed downwards; Nozzle 2 was
located below the packing about 25 feet above
liquid in the base; Nozzle 3 about 14 Eeet above
the liquid; No zle 4 was in the base of the
column and directed upwards.
-16-
Regeneration column performance data collected
in these runs showed excellent conformance to predlcted
values, indicating that the nozzles maintairled essentially
single-stage equllibrium flash performance independent of
5 the temperature level, representing a significant improve~
ment over valve tray operation at temperatures below about
190F. It is considered that the high transfer area
created by atomization is much more effective for mass
transfer than the valve tray action at very low vapor
10 rates. The use of Nozzle No. 4 located in the lower por-
tion of the column with the spray directed upwards
appeared to have a slight edge in performance.
The improvement in operation resulting from the
use of nozzles confirms mass transfer by diffusion to have
15 been limiting in the valve tray stripper. The close cor-
relation between the modified stripper and predicted
values based on one equilibrium flash indicated that the
mass transfer rate throughout the temperature range
explored (140 F-200F) was adequate to attain a close
20 approach to e~uilibrium.
From this study it was concluded that perform-
ance of the stripper at lower amine temperatures was much
improved by the use of spray nozzles compared to valve
trays; that high efficiency pac~ing in combination with a
25 distribution spray was comparable to downward directed
atomizing spray nozzles in an open column; and that
slightly better stripping was observed by the use of an
upward-spray nozzle located in the base of the column.
The improved regeneration column performance facilitated a
30 closer evaluation of absorber performance. It was con-
cluded that a decline in absorber efficiency occurred with
decreasing amine temperatures which could be attributed to
reaction kinetics becoming the dominant mechanism at lower
absorption solution temperatures.
DETAILED DESCRIPTION OF THE DRAWINGS
The invention will be further understood and
appreciated from the following description of the draw-
ings.
~ %~3t;~73~
Referring now to the drawings, FIGIJRE 1 repre-
sents schematically a first embodiment of the invention in
which an acid gas feed havlng 20 mol ~ or more CO2 aCl well
as methane, ethane, and higher hydrocarbons is processed
5 in a feed pretreatment zone A to remove hydrocarbons which
might otherwise condense during operation of the amine
towers, a bulk CO2 removal zone B in which CO2 is reduced
to less than about 20 mol ~, preferably in the range of
about 5 to about 15 mol % CO2, a second feed pretreatment
10 zone C and a cleanup CO2 removal zone D in which remainin~
C2 is removed from the process stream.
Thus a gaseous feed stream can be introduced by
line 101 into feed pretreatment zone A where the feed is
chilled and hydrocarbons condensed which can otherwise
15 condense out during CO2 removal. This can be accomplished
by any suitable arrangement of chilling and condensing
equipment. In the illustrated embodiment of FIGURE 1, the
feed can be chilled in air cooler 102, then removed via
line 104, exchanger 106, line 107, chiller 108, and line
20 109 to separation vessel 110 where liquid hydrocarbon can
be removed by line 111. The pretreated vapor can be
removed from vessel 110 by line 112, heated in indirect
exchange with the feedstream in exchanger 106, and then
provided by line 114, heater 116, and line 118 to tertiary
25 amine contactor 120 in bulk CO2 removal zone B.
In the bulk CO2 removal zone B, the stream in
line 118 can introduced at the base of absorber 120 and
contacted therein with lean tertiary amine, such as, for
example, aqueous MDEA or TEA provided to the top of
30 absorber 120 by line 124 and a substantial amount of CO2
removed to produce a first contactor overhead stream in
line 122 having preferably about 5 to about 15 mol ~ CO2.
A rich amine stream can be removed from contactor 120 by
line 125, exchanger 126, line 127, throttle valve 128, and
35 line 129 to flash atornization vessel 130.
In flash atomization vessel 130, the rich amine
can be atomized, as llustrated, by upwardly directed
atomizing nozzles 133, and flashed to regenerate the amine
77~:
-18-
in accordance with the invention. The releasecl CO2 can be
removed by line 13S after removal oE entrained liquids by
demisting packing 131. The lean regenerated amine can be
removed from the base of vessel 130 by line 132 and pump
5 134 and lean amine returned by line 124 to contactor 120.
The process gas stream in line 122 from which a
bulk removal of CO2 has been accomplished can be provided
to a second pretreatment zone C in which the feed can be
chilled and hydrocarbons condensed and removed which can
10 otherwise condense out during CO2 removal in final CO2
removal zone D. Thus, as shown in the illustrated embodi-
ment, the stream in line 122 can be provided via air
cooler 136, line 137, exchanger 138, and line 139 to
vessel 140 from the base of which liquid hydrocarbons can
15 be removed. The treated process stream can be provided by
line 141 to, for example, DEA contactor 142 which can be
operated conventionally and need not be described here in
detail since such operation will be familiar to those
skilled in the separation arts.
In contactor 142, the process stream from which
bulk CO2 has been removed can be contacted with, for
example, lean DEA solution provided by line 158 to remove
remaining CO2 and to provide a sweet hydrocarbon product
stream 143 from which substantially all CO2 and H2S has
25 been removed. The rich amine can be provided to regener-
ator 150 by line 144, exchanger 145, line 146, throttle
valve 147, and line 148. CO2 can be stripped from the
rich amine in regenerator 150 and an acid gas stream can
be removed via an overhead cooler/condenser indicated gen-
30 erally by 151 and by line 160. Lean amine can be removedfrom the base of regenerator 150 and returned by line 153,
exchanger 145, line 154, pump 155, line 156, air cooler
157, and line 158 to contactor 142.
~eferring now to FIGURE 2, FIGURE 2 represents
35 an embodiment of the invention in which regeneration of
rich amine in contact zone B can be accomplished in two
stages. Except for zone 3 described in detail bel~w,
FIGURE 2 iS the same with the same reference numerals as
FIGURE 1 described above.
7~3~
-19-
Referring now to FIGURE 2, the Eeed stream after
pretreatment in zone ~ is provided to bullc CO2 removal
zone B by line 118. In zone B the pretreated feed stream
is contacted in contactor 220 with a tertiary amine pro-
5 vided by line 224, producing a product stream in line 222having preferably 5-15 mol % CO2 therein which can be pro-
vided to zone C as described above for line 122 for
FIGURE 1. Rich amine from contactor 220 can be withdrawn
by line 22S and flash-atomized in two stages in strippers
10 230 and 240, operated respectively, for example, at
75 psia and at 20-25 psia. Thus, rich amine can be with-
drawn by line 225 and provided by exchanger 226, line 227,
throttle valve 22~, line 229, and atomized by nozzles 233
into flash vessel 230 operated at the intermediate pres-
15 sure. Released CO2 after demisting by packing 231 can bewithdrawn by line 235. Semirich amine can be withdrawn
from flash vessel 230 by line 232 and provided by throttle
valve 235, line 236, and by atomizing nozzles 237 to flash
vessel 240. Released CO2 can be removed by line 241
20 after demisting by packing 238; and lean amine can be
returned by line 242, pump 243, and line 224 to contactor
220.
It will be appreciated that the invention can
provide an economical and efficient method for removing
25 acid gases such as CO2 and H2S from gaseous streams at low
temperatures below about 200~F and regenerating the loaded
absorbent solution substantially to equilibrium values.
Other advantages and applications will be apparent to
those skilled in the art from the description herein; how-
30 ever, the invention is not limited thereto by the claimsappended hereto.