Note: Descriptions are shown in the official language in which they were submitted.
27628-lD
SPECIFICATION
FERTILITY DRUG AND METHOD OF PRODUCING THE SAME
This is a divisional application of the application
Serial No. 503,235 filed March 4, 1986. It should be noted
that the term "invention" appearing in the specification
applies to the subject matter of both the parent and the
divisional applications.
Background of the Invention
1. Field oE the Invention
The present invention relates to a fertility drug
or an ovulation-inducing drug and a method of producing the
same, and more particularly -to a fertility drug having an
eEfective componen-t comprising a composition or compositions
involved in Coix lacryma-jobi var. mayuem Stapf L. (herein-
later described simply as "Job's tears"). The parent applica-
tion relates to such a drug which is extracted from Job's
tears. This applica-tion relates to components of the drug
which are synthetically produced in organic reactions.
2. Prior Art Description
Typically known to the world as fertility drugs
are those comprising clomiphene and cyclohexil. These con-
ventional fertility drugs have been used for more than 20
years and their pharmacological effects are recognized in
clinical trials. However, it has been found in practice
that they will often cause extraordinary sex periods, re-
sulting in such troubles as multiple pregnancy and failure
-- 1 --
8~Z~
27628-lD
of pregnancy. They often produce side effects. Under the
circumstances, nevertheless, no other fertility drugs have
yet become available for practical purposes.
Examination and study on novel fertility drugs
have been attempted.
~ ~fi42~
lor exumple, it has been known that leaves of eorn, rye and wheat
conluiil a mQterial whicll will indnce ovulalioll of domestic rabbits
(Nii~ata Medical Society Bulletin; vol.78, puge 305; in 1964; Japull).
Up to the present, however, its ovulation-inducin~ effect on humall-kind
hus noL yet been proved and therefore such u muterial is still not
apl)licable to practical use.
Meanwllile, it llas become apparent Lhat some pharmaeoloKical
effects ~ill inllere in extractunts from Job's tears alld co;x seed, u
fruit of Job's tears prepared by rernoving hulls and peels froln Job's
leurs sced. "Seiyuku~aku" by Ina~uki et ul; publisllcd by Nunkodo in
1975 in Japan; pa~e 162; writes as follows:
1) Tll(! o~xtrucLullt~. of .lob's t~ars ~nd eoix seed lluve d;urel.ic effeclsand tllerefore may be used for the rernedy of tumor, beriberi, nephrolith
and cystolith and hurum~
2) l`hey may be used as puillkillers und crampkillers.
:1) 'I`hey ure 800d for wurts und rou~llncss.
Moreover, jL has now been eonfirmed Lllat proteins exLIueted flom
unLllreshed ~owder of Job's teurs seed will spur securetioll Or humall milk
(Masahiko Shi~ernitsu; Bulletin of Kumarnoto l,ocal Deprlrtment of Japan
Womun Science Society; vol.~, pu~e 191; 19~). An anticullcer rnaterial
can be isolùted from eoix seed (Chemieal and PllurmaeeuLical Bullctill,
.Jul)ull; vol.9, pa~e ~,; 196t). Ilowever, Lhe ovuluLioll- inducin~ effecL
of .lob's tears and/or tlle extructants thereof has not yet been knowll in
lhc prior alt.
Summary of the Invention
It is therefore an object of the invention to provide a novel
fertility dru~ of a typc diffcrent from the conventional one typicslly
comprisin~ clomiphcne and cyclohexyl, capnble of inducing ovulation in
physiologically nutural rnanncr, without causinK ubnormal scx pcriods.
Another object of the invention is to provide a novel metllod of
sylllllelically producillK un ovulution-inducin~ maLeriul, idclllicul to
that contained in Job's tears, with high efficiency, to thereby make it
possible to manufacture on commercial basis a fertility drug having said
ovulution-inducillg material as an effective component.
According to an uspect of the invention there is provided a
fertility drug havin~ an effective component comprisin~ oil-soluble
frùction of a whole or u part of Job's tears sced.
According to another aspect of the invention there is provided a
t5 fertility dru8 baving an effective component comprising ferulyl stanol
derivative and/or phytosterol fatty acid ester, contained in Job's tears.
'I'hc said fertilily dru8 i3 prcpurcd by incorpor~ cthyl uceLa~e into
Job's tears bran to extract an oil-and-fat fraction from the Job's tears
bran and collectin~ the effective component contailled in the oil-and-fat
fractioll.
Accordin~ to still another aspect of the invention there is
providcd a synthetic method of producin8 ferlllyl phytostanol dcrivative
coml)risin~ the steps of acetylatin8 ferulic acid with a Inixture Or
pyridinc alld acetic anllydride, trcatill~ the acctylaled feIulic ucid will
t.hionyl chloride to prepare an ac;d clllorid(, reactillg in thc presellce
of ~yridinc lhe ucid chil)lidc willl pllyl03lullol Lo form a pllylo~Lullol
compoulld, dissolving the phytostanol compound into a mixture of mcthanol
and chloroform, and adding sodium borohydridc to a rcsultin8 solution,
with stirrin8, to thereby deacetylate the phytostanol compound.
Brief Description of Drawin~s
Further objects and advantn~es of the present invention can be
S fully understood from the following detailed description when resd in
conjunction with the accompanying drawin8s, in which;
Figure 1 diagrammutically illustrates tlle steps of the test mcthod
in Test 1 for extracting components froln a whole seed of Job's tears
with extractin~ reaKents of n-hexane, ethullol, 50~ ethullol solution and
water;
~ ;gure 2 is u 8raPh showin8 relationship between dosage of a
purified extructant obtained from Job's teurs seed with n-hexane and
the number of nuLurally produced ova alld the sl,ute of ovaria;
~ ures 3 and ~ are charts showing infra-red spectrum und nuclear
1~ maKIletic resonance spectrum of a fertility drug accordinK to one aspect
ol' Llle inventioll; and
Fi~ures 5 and 6 are charts showin~ infra-red spectrum and nuclear
mabnetic resonunce spectrum of a fertility drug uccording to another
uspect of the invention.
Detailed Description of The invention
'I'llc ~res-~ invclllioll uccordin~ lo onc u;~ccL lllcrcor is conccllcd
with a fertility dru~ or an ovulatioll-inducillK drug havin~ un effective
component comprising un oil-solubie fraction of a whole or u part of
Job's tears seed. The oil-soluble fractio:l may be extracted from powder
whicll is prepared by grindinK the whole of thc Job's tears seed.
~2 ~3~3 4 2 ~L
Alternativeiy, tbe Job's tears seed is threshed and purified in a known
mullllcr to be clu~,iried inlo coix sced, bruu und hulls, floln any Or
wllicll may bc extructed tlle oil-soluble fruction. In view of
extractability, however, the use of bran itself or the whole seed,
inclusive of the bran, is preferred.
In extraction of the oil-soluble fraction from Job's tears s~?ed,
an extractin~ solvent may be n-hexane, ethyl acetate ester or any other
suitable one. In the cùse of n-hexane being used, yield of the oil-
soluble fraction would be decreased but it is easier to be evaporated
after extraction, so that n-hexane is a preferred solvent in a practical
sencc .
The fertility dru~ thus prepared has an effective eomponent of the
oil-soluble fruclion extruclcd from .lob's tcurs sced or u purificd onc
tllercor. A vehiclc, muLcllill~ agcnt, diluent and uny otllcr kind of
udditi VCS muy bc incorl~oruled ulone or in combinulion, ul)on demund.
1'he fcrtility dru~ rnay be in the form of pellets, powder medicil)es,
cul)sules, cyrup or iniection.s, and upplied orully or externally.
I:or better underslulldin~ of the invention some exemplifyin~ tcsts
will l)c Nivcn llercunder.
Test l
In tlli~ tesL, a water-soluble fraction und an oil-soluble fructio
werc cxtructcd rc;l)(?ctively from tlle wholc s(?(?d of .lob s tCUl'S and tlle
ovulution-induce effects of tlle respectivc fractions werc mcusllred and
compul cd l(> cucll otll(!r .
Ille wllole sced of .lo~ s tears w~s Kroulld into powd(?r to plel)ure
5008 s&mple. This sample powder was subjected to extraction w;tll an
_~_
3 2 ~8 4 2 3L
extractill~ reagent of 1.5~ n-hexane at u lemperaturc ranKc of 15~-20~C.
After the extruction the extracting reagent was distilled under pressure
reduccd condil.ions to obtain about 45~ of yellow, oily substallcc
(cxtractabiliLy of about 9~ by weight - the percenta~e i3 giYen by
weiglll throu~llout the Specification unlcss oLllerwise specified). This
yellow, oily extructant wus deterlnined as colnponent A. Then tlle residue
a~Lcl exlractioll of coMponent A was furtller sul)jected to extraction Witl
1.5Q ethanol hut only a ne~ligible ainount of an extractant was obtained.
Thc residuc was d;pped into a Icaching linuid of 50X ethanol solution.
and the leachillg liquid was concelltrated aL a temlerature below ~0C
undcr prcssurc redut:cd conditions, to thcrc~y obtaill a plccipitatt~ and
about 2~ e~tractant. This cxtractant wus deterlnined as colnpon~llt B.
Thcl-, from tlle residuc ufter extractioll of colnponent ~ wa9 CXll~aclCd
witll 1.5Q water, a slnall alnount of allotl~er cXtractant, WhiCIl was
dclcrmined a9 COII)I)OllCllt C. In 5u~nmury, colnpollcnts A, B and C wcrc
extracted in such Inunller as diagralnlnatically illustrated in PiKure 1.
I IIIU';~' COMI)Ol~ ulld C W~'I'C IIIIU I i l.U 1. i V(! I y UllU I i '~.(.'~i i 11 U kllow
nallner to find that cornponent A contains ~Iyceride and such eslels as
~crulic acid cstcr, cotnpollcnL ~ conlaill~i polyulnido alld colnpollcllt C
contains arnino acid and peptidc.
Next, the ovulation-inducing effect or actiYity of the respective
coMponellLs A ,B and C wcle lcstcd in Lhc following Inanller~ Morc
l~articularly, 70 ~olden halnsters in thc a~e of 5 - ~ weeks W(!l'('
dcvidcd into 7 ~roups euch having 10 golden hamsters. Tlle foddcl werc
I)rel)aled by addin~ to tllc basic fodder lZ of coml~ollellls A, h and C, and
KiVèn orally to thc ~roup Nos. 1, 2 and 3, respectively. As thc avera~e
intake of the fodder of a ~olden halnster is ~9~ a day, t90~y of Ihe
~1~2~342~
r(!3pcctivc componellts werc given to Llle respectiYc groul)s. Thcy wcre
fed with such fodder for 3 weeks and then slaughtered. The 8roul~ Nos. 4,
5 and 6 were fcd in the same manner, but after 3 wecks' fecd of lhe
foddcr containillg the coml)onents only the basic fodder werc ~ivcn
Lhcrclo for 5 w(!elts, alld tllcn slaughtcrcd. Whereas, tbc groull No. 7 wus
initi~lly given only the basic fodder for ~ weeks and tllen slaughtered.
As lo ttlt! ~('SI)(!Cl.iV(' KrOUpS, tllC S('X l)('riods WCTC olls(!rv(~l ulld
the number of naturally produced ova was counted beforc they wele
sllu~htcred, and after slau~htered the weight and thc state of tllc ovary
were observed hy mealls of dissection. The results of thcse tests are
shown in the following Table I .
38~2
T A B L E
. _ ._. . . ._
test sample comparison of nlllnber Or¦ dissection test
_ _ ______ ~
~roul~ coml30nell1 scx period natural weight of state of
No. udded sex pcrioc rcmurkc ovulutiol ovary(~) ovary
. ,, .,. . ._ ___ ___ __ .,.,, __ ._, _
1 A ~ days regular 17 1 2 ~.~ ~9.48 + 2.35 (~)
2 B 4 duys regular 12 + 2 1~.01 + 0.3~ (-I)
C 4 days regul~r 12 + 2 17.93 + 0.23 (+)
~ A ~ d~ys regular 17 ~ 3 ¦ 19.59 + 0.2~ (++)
B 4 days regular 12 -t 2 18.20 + 0.2~ (+)
6 C ~ days re~ular 12 l 2 t7.8~ + 0.56 (t)
7 4 days re~ular 12 + 2 18.03 t 0 15 (~)
_ . . . , ,___
Notes: ~9 ~i~urc~ in the column of Scx Peliod show the averu8 e.
G~ tll(- collllnll of State of Ovury Ihe murk~ ) und (l~)
meall rcluLive comprison in the number of corpora lutea found in
cut-outs of the ovary.
~ The murk ~ identified in Lllc columll of Natural Ovulatioll
mcalls lhuL Lherc could bc found a ;i~niricullce witll rc.pccl lo
other 8roul~s ut u sigllificallce Icvel Or 1%.
As iL al~eurs floln Lhc re3ulLs ;howll in `lablc I Lllc compollellt A
extructcd from the whole seed of .loh s teurs with n-llcxal)c will promoLe
natural ovulation witllout disturbing the ;cx periods. As a resull: of
orKanic dissection Llle effective componellL contained in tllc corn!)onent A
wu-; l~roved to uct on tllc sex center so us to promotc formation of
corpora lutea.
Meanwhile T~ble I AISO shows that this eftective compollellt is
colltuilled only in the oil-solu5~1c fruclioll Or tlle .lob -. Lcar; sccd and
is ~.carcely presellt in Lhc extractullts ohtaincd with etllullol 50
eLtlanol solulion und wùter.
~ ~ ~842~ 27628-lD
Test 2
~ This test was carried out to find the optimal dosage of
the ovulation-inducing effective component contained in the
component A extracted in Test l.
First, the component A was dipped into leaching liquids
of ethanol, 50~ ethanol solution and water, step by step, and then
the leaching liquids were removed. Then the residue was purified
by column chromatography with n-hexane. Thus, a purified
extractant was obtained in a yield of 90% of the component A.
This extractant was dissolved in 0.2mQ soybean oil in
quantities varied between 95~J380mg to prepare three fodder
containing different quantities of the extractant, which were
orally applied by injector to three groups each having lO golden
hamsters, once a day, for 3 weeks. Thereafter, the test animals
were slaughtered. These groups were put into the tests for the
natural ovulation and the ovary weight in the same manner as in
Test l, upon which matural relation of the dosage of the extractant
per body weight of a golden hamster was determined with these
parameters. The test results are shown in Figure 2.
As shown, the optimal dosage per body weight of a golden
hamster was found to be in a range of 0.76~1.4mg/g a day.
Accordingly, provided the body weight of a grownup is 60kg,
4.6rJ8.4g/day of the purified n-hexane extractant was determined
to b~ an optimal range when applied to an adult. The medication
term will be dependent upon the condition of a patient and varied
case by case.
Test 3
In this test, extractability of the effective component
;~ _g_
~ 27628-lD
obtained respectively from the whole seed, coix seed, bran and
hull of Job's tears was measured and compared to each other.
Respective 500g powder were prepared by grinding the
coix seed, bran and hull of Job's tears seed, and extractants
corresponding to component A were extracted in the same manner as
in Test 1. Quantities of the extractants thus obtained and
extractabilitv in the respective cases are shown below in Table
II. Data for the whole seed in Table II are the reproduction of
those in Table I. It will be obvious from Table II that the
greatest extractability was gained in the case o~ bran, leading
to the fact that the effective component is in substance contained
in the bran of Job's tears seed. Thus, the effective component
can be extracted with great efficiency from the bran alone or the
whole seed including the bran.
TABI.E II
. _ ,
test sample amount of extractant.(.g). .extractability (%)
_
whole seedA5.0 9.0
coix seed27.0 5.4
bran 80.0 16.0
hull 0.6 0.12
Note: The extractability is calculated by the equation of
amount of extractant (g)/ sample powder (500g) X 100
The inventors have made thorough investigation of the
effective ovulation-inducing material contained in Job's tears
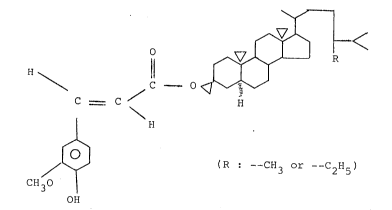
seed and found in the end that ferulyl stanol derivatives expressed
by the following formula (I) and/or phytosterol fatty acid ~ster
--10--
~ 421 27628-lD
expressed by the following formula (II) has significant effect
and activity on induction
-lOa
3l~3~34~L
of nuturul ovulution. 13ascd on this fucl thc inventors have comc to a
~uccessful end that a novel fertility dru~ having Ull effcctivc coml)ollellt
coml)rising ferulyl stnllol derivutivcs und/or l)llytostcrol fatly acid
cster may ~e prel~ared.
~ormula (I)
R ~ <
O
Il
,/ C ~ O ~\\~"'
C = C 11
.. ..
~ ,J
Cl130 ~ (R: CI13 or '-C21k~)
011
I:ormula (Il)
~ R,
R,COO
(R~: remaining radicai of palmitic or stearic acid
R2: - Cll~ or -C211s~
~ 3 8 4~3L
Stanol ferulic ~cid derivatives representcd by the formulù ( I)
nay be, for exu~nple, truns-ferulyl stigmastallol, trans-ferlllyl
cuml)estanol or a mixture thereof.
Phytosterol fatty acid ester represented by the forlnula ( ~) may
bt~, for exu~nl)le, ~ -sito3terol palmitic acid cster, ~ -silosterol
slearic ucid estcr, carnlleslelol pallnitic ucid ester, campeslcrol <;tearic
uci(3 ~Islcl O~ y 011(! of lllc colnbillul.ioll~ cof.
A process for producing the above fertility drug is desclibcd in
detaiI hereundt,?r.
First, ù Job's tears seed is threshed and purified il?~ a known
mallncr to be scl~arated into coix seed, brall and hull. To I part of the
6run is incorl-olutcd ~ ~ 5 purts (l~y wei~lll; Ihc pall i; ~iV~'II by
wei~lll. thlougholll lhe Specification unless olllerwise sl~ecified) of ethyl
acetate, and thc? resullallt is agitated at 15 ~ 20C for 5 -~ 10 hours,
lh~.?reby extracting ùn oil-and-fat fraction. The extract is Lhe
filLered to relnove nn insolllble fraction.
To 1 purt of tlle residue is incorl)()l~Lcd 3 ~ 5 purts of elhan
and lhc solulioll is u~ilatcd at 15 ~ 20(~ for 5 ~ 10 hours. lhe
rcsulLulll cxtract is lllell filtcred Lo rclnovc un insolublc fracLioll.
1'he elhullol-sol~ lt? frucLioll thlls obluillcd is disLilled lo rclnove
etllullol, therel3y obtuinillR all elllallol extracLulll. To 1 pUlt of Llle~
ethanol extractant is added 3 ~- 5 partx of clhyl acetate, and lhe
solulioll is a~ilutcd uL 15 -` 20~C for 5 -` 10 hours, to obLuill un
cLhyl acetate-soluble fraction.
The oil-and-fut fraction and the ethyi acetate-solu~le fraclioll
are Inixcd to~ethcr and cthyl acetate is separatcd froM ihc InixLure. Tht?
resulLunt is supplied to a column char~ed with silicu ~el to effect
~ . .
,
Kradient elution with a mixed eluent of n-bexulle and ethyl acetate
whereby fructions eluted with the Inixed elucl1ts of n-llexalle and ethyl
UC(?lUi(! lluvillg Mixillg lulio~ Or 3~: 1 ~ 10: 1 u~ /ol 100: 0.5 ~ 100: 1
are collected. Thc fractions thus collected are Inixed together and tht
cluents are reinoved from the Inixture Lllcrcby obtainin8 ovulution-
induceill~ Inaterials. The fractions eluted with lhe eluellt of ~0: 1 ^-
10: 1 Inixture of n-llexulle und ethyl ucetatc will contnill ferulyl stunol
derivatives and tl~e fractions eluted with the eluent huvin8 tlle tnixin~
ratio of 100: 0.5 ~ 100: ~ will COlltUill phytosterol fatty acid ester.
Ifrective Inaterials will be contailled in the nespeclive eluates obtailled
with thc cluents havillg different ranges of mi~ing ratio and may be used
alone or in a Inixcd state to Inanufacture llle fertility dru8 according to
thc invelltion. The drug muy be a pellet a powder Incdicine n capsulc
an inièction or in uny other forln and applied orally or externally.
Tllc dosaK(? of the dru6 will vury dcp-!lldillg ulll)ll tllc collditioll of u
paiient an(l the way of upplicutioll but in Inost cases ~0 -~ 80~ once a
~ay Wi ~ (? cf ~CC Li VC Wll(!ll 01'~ 1 I y a~ 1.O ~11 adull.
The fertility drug of the invention having an effective componellt
comprising ferulyl stallol derivativc ulld/or pllytosterol futty acid ester
will be furtllcr dcscribcd l~y waY of exelnplifying tesis.
., ~ . . __
This test was carried out to observe physical and chemical
characteristics of ferulyl stanol derivative. The test sample was
X colnl)onent prepared in tlle saMe Inanner as in Example 3 des(:ribed later.
Illi; tcst <;ulnplc was allali~.ed by tllin luycr chrolnutoKIalllly Lo
- 13 -
~ 42~ 27628-lD
detect a single spot (hereinlater defined as material X) which
manifested a blue-green color with sulfuric acid and was
distinguishable in ultraviolet rays.
The material X was purified by alumina column chroma-
tography and isolated as colorless, needle-like crystals having
melting point of 156C. The material X showed positive adsorption
in the Gibbs' adsorption test and had a M' peak at m/e 592 in the
mass spectrometry. In the infrared spectrophotometry, as shown
in Figure 3j it showed adsorption signals based on hydroxyl group
at 3120r~3500cm 1, conjugated carboxyl group at 1710cm 1, double-
bond of ~,~-unsaturated carbonyl group at 1640cm 1, C-C stretching
~ibration of benzene ring at 1600 and 1510cm 1, and out-of-plane
deformation vibration of benzene ring at 840cm 1, respectively.
In the nuclear magnetic resonance spectrometry (CDCQ3), as shown
by the chart of Figure 4, it manifested a signal pattern peculiar
to phytosterol at 0.62~ 2.Oppm. Further, signals were found based
on methoxy group at 3.88ppm, and the AB type spin-spincoupling
(J - 16Hz) of hydrogen in the double-bond of ~,~-unsaturated
carbonyl group of cinnamic acid derivative and the ABM type spin-
spincoupling of hydrogen in the tri-substi-tuted benzene ring
thereof was found at 6.22ppm and 7.55ppm, respectively. The
singlet of lH at 5.95ppm was disappeared by adding D20. According
to these results, the material X was determined to have hydroxyl
group of a phenol.
The material X was subjected to alkalic hydrolysis to be
separated into an acid and a neutral fractions, to both of which
were applied chemical analysis to pro~e that only ferulic acid
was isolated from the acid fraction (which was identified in
-14-
- .
~ 8'~2~ 27628-lD
comparison with specimen). The neutral fraction was silylated in
a known manner and then quantitatively analyzed by gas chromato-
graphy, whereby it was identified as a mixture of stigmastanol
and campestanol in a ratio of 9 : 1.
Further, the material X was acetylated with a`mixture
of pyridine and acetic acid to form mono acetal in the form of
colorless, platy crystals, having melting point of 155-V156C.
According to nuclear magnetic resonance spectrometry (CDCQ3),
there could be seen signals based on methyl and methylene radicals
of phytosterol at O.6-V2.Oppm, and a signal based on acetyl group
at 2.32ppm. At 3.84ppm, a signal of singlet of 3H was found
based on methyl of methoxy group. The AB type spin-spincoupling
of olefin hydrogen in a,~-unsaturated carboxyl group was noted
at 6.32ppm and 7.60ppm, and the ABM type spin-spincoupling of
hydrogen on the tri-substituted benzene ring appeared at 7.07ppm.
Consequently, the material X was identified as a mixture
of trans-ferulyl stigmastanol and trans-ferulyl campestanol in a
ratio of 9 : 1. By the results of analyses including its melting
point, the trans-ferulyl stigmastanol was found to be the same
material as dihydro-~-sitosterol ferulic acid ester which is
isolated from a corn embryo bud oil (Tamura et al; "Nippon Kagaku
Zasshi (Japan Chemical Magazine)"; vol. 79, page 1011; in 1958).
Test 5
This test was carried out to reveal physical and chemical
characteristics of phytosterol fatty acid ester. Y component,
prepared in the same manner as in Example 3, described later, was
used as a test sample.
-15-
~ 2 ~ 27628-lD
This sample was analyzed by thin-layer chromatography
in a known manner to isolate a material of colorless, needle-like
crystals, having a melting point of 64~v65C, which showed a
spot manifesting a blue-green color with sulfuric acid and being
distinguishable in ultraviolet rays. According to mass spectro-
metry, a peak based on M~ was found at m/e 680. A peak based on
carbonyl group was observed at 1740cm 1 in infrared spectrophoto-
metry, as shown in Figure 5. In nuclear magnetic resonance
spectrometry (CDCQ3), as shown by the chart in Figure 6, signals
were noted based on methyl and methylene protons of phytosterol
at 0.6~v2.0ppm, and -(CH2)- of long chain fatty acid residue at
1.28ppm. A signal of triplet based on methylene group adjacent
carbonyl groups, a signal of multiplet (12 Hz in half width)
based on hydrogen of C3 phase and a signal of multiplet based on
binyl hydrogen were found at 2.25ppm, 4.6ppm and 5.35ppm,
respectively. Then, the material was subjected to alkalic
hydrolysis to be classified into an acid and a neutral fractions.
The neutral fraction was proved to contain sterol, which comprised
~-sitosterol and campesterol. The acid fraction was a mixture
of higher fatty acid comprising in substance stearic acid and
palmitic acid, which was determined by means of methylation and
gas chromatography.
In conclusion, the said Y component was identified as
a mixture of long chain fatty acid esters of phytosterol. A part
of this material was identical to ~-sitosterol derivative
mentioned by Kuksis et al in "Journal of Organic Chemistry",
vol. 25, page 1209; in 1960.
- 16 -
~ 84~ 27628-lD
Test 6
In this test yield of stanol ferulic acid derivative
and phytosterol fatty acid ester from the whole seed, coix seed,
bran and hulls of Job's tears was compared to each other.
The whole seed of Job's tears was ground in a known
manner, threshed and purified, and thus separated into hulls,
bran and coix seed. Among 100 parts of the whole seed, 33 parts
of the hulls, 15 parts of the bran and 52 parts of the coix seed
were obtained. ~o these four star~ing materials were respectively
added n-hexane and ethyl acetate each in a triple quantity
thereof, and the solution was agitated at 15 ~20C for 5 hours,
to thereby effect extraction. After the respective extracting
solvents had been removed, oily components were obtained. The
weight ratios of the respective oilycomponents with the starting
materials are shown in the following Table III, which shows the
fact that the effective components comprising ferulyl stanol
derivative and phytosterol fatty acid ester are both included in
the bran of Job's tears seed.
-17
9.28842~L 27628-lD
_ _ .. _.___ .
U~
a)o ~ ~ a~
S:: h ~1 O ~ r ~1
oa) v ~ ~ ,~ ~
~d o ~ o
O O ~ o ~1 0
4~
O _ _ _, ,
~0
S~ ~
P~ ~ a
u~
,~ O ~ o
~1 ~1 ~ o ~ O
a) ~ ~ rl ~ rl
rl ~1 ~ O ~1 0 ~1
h :
a) c)
4~
H
H
H
~-~ ~
o ~ :
~ a) 0~
O
t) t)
~ ~1 ~ Ou~
O ~a)
.. _
~ rl
o ~;
S~ ~ ~`1
~ ~ ~ ~ ~1 0 ~
0~ ~ . . . .
C) ~ ~ O ~9Ln
X
s~ a~
~1
,~ I
O o ~
_ ~ _ __
~ a)
~ ~ a)
a)
X
O ~1 ~ ~rl
o
~; 3 ~ Q o
. . . _. ,
--18--
~ 421 27628-lD
Test 7
This test was carried out to determine fractions contain-
ing the effective components comprising ferulyl stanol derivative
and phytosterol fatty acid ester, by means of silical gel chroma-
tography.
1) Preparation of Test Samples
According to the method described later in Example 3,
1!250g of an oily fraction was extracted from 5kg of Job's tears
seed bran with ethyl acetate and ethanol.
300g of oily fraction was subjected to column chromato-
graphy using 5kg of silica gel, wherein the eluent was at first
n-hexane and then mixtures of n-hexane and ethyl acetate in mixing
ratios being varied to gradually increase the proportion of ethyl
acetate. The eluates were 4.352g of F-I component eluted with a
mixed eluent of n-hexane and ethyl acetate in a mixing ratio of
100: 1, 283g of F-II component with an eluent of a 20: 1 mixture
and 8.756g of F-III component with ethyl acetate alone. F-I
component was purified by column chromatography using 250g silica
gel and a mixed eluent of n-hexane and ethyl acetate in a ratio
of 100: 1. F-II component was further subjected to alumina
column chromatography and eluted with a 20: 1 mixture of n-hexane
and ethyl acetate to obtain 128mg of F-II-l component, 1.284g of
F-II-2 component, 210mg of F-II-3 component, 210mg of F-II-4
component and 55mg of F-II-5 component. Thus, seven specimens
were prepared.
2) Test Method for Physiological Activities
These seven specimens were dissolved into 0.2mQ of a
soy bean oil to prepare various oil solutions, which were orally
--19--
~" ' .
34~:~
27628-lD
given once a day to respective groups each having 10 golden
hamsters in the age of 5~ 8 weeks. The amounts of the components
respectively contained in such oil solutions were made different
to be 0.2mg and 0.5mg. During the administration for 3 weeks
with such oil solutions, the sex periods and the number of
naturally produced ova were observed, the results of which were
compared with those of reference group which had been treated
with 0.2mg of soy bean oil containing no sample component.
3) Results
The results with respect to the natural ovulation are
shown in Table IV.
TABLE IV
component amount of component addëd
added 0.2mg 0.5mg
. .__ ~ . . . _ _.
(reference) 12 12
F-I 18 15
F-II-l 12 10
F-II-2 11 10
F-II-3 12 12
F-II-4 18 13
F-II-5 12 11
F--III 11 12
The sex periods were 4 days for all groups including the
reference and there could be found no disturbing effect on the sex
period. It will be quite obvious that F-I and F-II-4 components
have significant ovulation induce effect. These components were
identified in a known manner to determine that F-I component was
phytosterol fatty acid ester and F-II-4 component was ferulyl
stanol derivative.
-20-
~ 84~ 27628-lD
Test 3
This test was performed to determine effective dosage
of stanol ferulic acid derivative, an effective ovulation-inducing
component of the fertility drug according to the invention.
l) Preparation of Test Sample
X component was prepared in the same manner as in
E~ample 3, described later, and used as a test sample. X
component was a 9 : l mixture of trans-ferulylstigmastanol and
trans-ferulylcampestanol.
2) Test Method
The test was carried out in the same manner as in Test
7, except the amounts of the component added in the soy bean oil
were O.lmg, 0.2mg and l Omg once a day, respectively.
3) Results
3-1) Sex Period
TABLE V
. . . .
amount of component added sex period (day)
~reference) 4
O~lmg 4
0.2mg 4
l.Omg 4
As shown from Table V, each group showed regular sex
period.
3-2) Number of Natural Ovulation
-21-
3~2~84z~ 27628-lD
TABLE VI
amount of component number of natural
added ovulation
. .. ___ . ._ _
(reference) 11 + 1
O~lmg 16 + 1
0.2mg 18 + 2
l.Omg 13 + 2
Note: The mark ~ shows a significance at a
significance level of 1%.
As shown from Table VI, the groups to which O.lmg and
0.2mg of the effective components were given show significance
with P~O.Ol with respect to the reference group.
As the average bodv weight of a hamster used in this
test was 150g and the body weight of an adult is supposed to be
60kg, the effective dosage to an adult is proved to be 40~'80mg
once a day.
Test 9
This test was performed to determine effective dosage
of phytosterol fatty acid ester, another effective ovulation-
inducing component of the fertility drug according to the
invention.
1) Preparation of Test Sample
Y component was prepared in the same manner as in
Example 3, described later, and used as a test sample.
2) Test ~ethod
The test was carried out in the same manner as in
-22-
,
: `
~ Z ~84~ 27628-lD
Test 7, except the amounts of the component added in the soy bean
oil were 0.lmg, 0.2mg and l.Omg once a day, respectively.
3) Results
3-1) Sex Period
TABLE VI I
... . __ __ _ . _ .,_ _
amount of component added sex period ~day)
(reference) 4
0.lmg 4
0.2mg 4
1.0mg _ _
It will be quite obvious from Table VII that each group
showed regular sex period.
3-2)Number of Natural Ovulation
TABLE VI I I
,
amount of component number of natural
added ovulation
(reference) 12 -~ 2
0.1mg 16 + 1
0.2mg 18 ~ 2
1.0mg 15 + 1
Note: The mark ~ shows a significance at a
- significance level of 1%.
As shown from Table VIII, the groups to which 0.lmg and
0.2mg of the effective components were given show significance
with P<0.01 with respect to the reference group.
-23-
~ '
27628-lD
842~L
As the average body weight of a hamster used in this
test was 150g and the body weight of an adult is supposed to be
60kg, the effective dosage to an adult is proved to be 40 ~ 80mg
once a day.
Ferulyl phytostanol derivative, one of the effective
components having ovulation-inducing effect, may be prepared not
only by extraction from Job's tears seed as described before, but
also by synthetic method. Such a synthetic method of production
of ferulyl phytostanol derivative is characterized by successive
steps of acetylating ferulic acid with a mixture of pyridine and
acetic anhydride, treating the acetylated ferulic acid with
thionyl chloride to form an acid chloride, reacting in the
presence of pyridine the acid chloride and phytostanol to prepare
phytostanol compound, dissolving the phytostanol compound into a
mixture of methanol and chloroform, and adding sodium boron
hydride to a resulting solution, with stirring, to thereby
deacetylate the phytostanol compound.
The respective steps will be described in detail as
follows.
A) Acetylation
Ferulic acid (4-hydroxy-3-methoxycinnamic acid) is
dissolved into a liquid mixture of pyridine and acetic anhydride
(mixing ratio of 3 : 1) in a concentration of 10 ~15 wt.%. After
reflux for 4~5 hours the solvent is distilled to prepare
acetylated ferulic acid.
-24-
~' ` .
27628-lD
8421
CH=CHCOOH CH=CHCOOH
pyridine
acetic anhydride
I OCH3 1 OCH3
OH OH
B) Formation of Acid Chloride
The acetylated ferulic acid is dissolved into chloroform
anhydride in a concentration of 10 ~15 wt.%. Thionyl chloride
(SOCQ2) is added to the solution in a proportion of 1.5 moles per
lg acetylated ferulic acid -to effect the reaction thereof at
20 ~30C for 5~-6 hours. Then, the solvent is distilled in
pressure reduced conditions to prepare acid chloride.
CH=CHCOOH CH=CHCOCQ
thlonyl chloride
\ OCH3 ~ OCH3
OCOCH3 OCOCH3
C) Reaction with Phytostanol
The acid chloride thus prepared is dissolved into
pyridine in a concentration of 10~-15 wt.% to prepare a first
solution, and an equivalent of phytostanol is dissolved into
pyridine to prepare a second solution. The first solution is
dropped into the second solution in an ice-cooled condition to
effect the reaction at 20--30C for 2 ~ 3 hours, and the solvent
is distilled and removed.
-25-
27628-lD
34~1
CH=CHCOCQ ~ ~
~1 Hov~--
3 H
OCOCH3
pyridine
r
\ C =C /
¦ H
~ (R : -CH3 or -C2~5)
CH30
OCOCH3
D) Deacetylation
The reactant thus obtained in the step C) is dissolved
into a liquld mixture of chloroform and methyl alcohol (mixing
ratio of 1 : 1~ in a concentration of 3 ~ 5 wt.%. While this
solution is being agitated in an ice-cooled condition, sodium
borohydride in a quantity of double equivalent is added thereto
by degree. After the foaming has ceased the solution is allowed
-26-
.
. ~
~ 84~ 27628-lD
to stand at 20 ~25C for 1~ 1.5 hours, a small quantity of water
is added and then the solvent is evaporated in pressure reduced
conditions. Thus, ferulyl phytostanol derivative is prepared.
H C--o t7
C - C H
H
~ NaBH4
CH30 1' CH30H + CHCQ3
OCOCEI3
Il-o ~ ~
\ / H
C--C
\ H
~ tR : -CEI3 or -C2H5)
CH30
OH
-27-
~r
~ .
.. .
~ 42~ 27628-lD
Ferulyl phytostanol derivative thus prepared by the
successive steps A) to D) may be further purified by silica gel
column chromatography and then recr~stalized with methyl alcohol,
thereby producing the same in a pure state. Alternatively, the
purification may be carried out each time the intermediate product
is formed in each step.
The followings are exemplifying tests for the method of
producing ferulyl phytostanol derivative according to the
invention.
Test 10
This test was carried out to examine physical and
chemical characteristics of the intermediate product formed by the
acetylation step A).
1) Preparation of Test Sample
Test sample was prepared in the same manner as the
acetylation step in Example 6, described later. The acetylated
product was sub~ected to silica gel column chromatography whereby
a fraction eluted with acetone was obtained, from which acetone
was evaporated to prepare a purified test sample.
23 Test Method
The test sample was put into tests for melting point,
mass spectrometry, infrared spectrophotometry (KBr method) and
nuclear magnetic resonance spectrometry (CD30D * CDCQ3) in known
manner.
3) Results
The test results were as follows.
mp . 195-~196 C , FD-MS m/e; 236 (M ), IR~ (KBr) Cm
3500 (COOH), 1760,1690 (~C=O), 1630 (-COOH=CH-), NMR (CD30D +
- 28 -
:.
~ 2~8421 27628-lD
CDCQ3), ppm: 2.32 (3H, S, COCH3), 3.82 (3H, S, OCH3), 6.32
(lH, d, J=16Hz, ~COCH=CH-), 7.07 (3H, m, aromatic ring), 7.60
(lH, d, J=16Hz, -COCH=CH-).
Conse~uently, the test sample was identified as
4-acetylferulic acid (4-acetoxy-3-methoxycinnamic acid).
Test 11
This test was carried out to observe physical and
chemical characteristics of the intermediate product formed by
the reaction of acetylated ferulic acid chloride and stigmastanol.
1) Preparation of Test Sample
Test samp]e was prepared in the same manner as the step
of formation of acid chloride in Example 6, described later. The
acid chloride compound thus prepared was sub~ected to silica gel
column chromato~raphy with a mixed eluent of n-hexane and ethyl
acetate ester (mixing ratio of 20: 1). The eluate was
recrystalized with methyl alcohol to prepare a purified test
sample.
2) Test Method
The tests applied to the sample were the same as in
Test 10. The elementary analysis was carried out in a known
manner.
3) Results
A melting point was 156C. The results of the elementary
analysis were C: 77.80 and ~I: 9.89~ which are in conformity to
the calculated values of C: 77.60 and H: 9.78 according to the
molecular formula of C41H62O5 of 4-acetylferulylstigmastanol.
The results of mass spectrometry, infrared spectro-
photometry and nuclear magnetic resonance spectrometry were as
-29-
~r
~ 27628-lD
follows.
FD-MS m/e ; 634 IM ), IRv (KBr) : 1770,1710 (>C=O), 1640
(-COOH=CH-), 1600,1510 (benzene ring?, 1180,1080 (-C-O-C-), NMR
(CDCQ3), ppm : 0.6rJ2.0 (-CH3~ -CH2-), 2.32 (3H, S, COCH3),
3.84 (3H, S, OCH3), 4.70 (lH, m, -O-CH<), 6.32 (lH, d, J=16Hz,
-COCH=CH-), 7.07 (3H, m, aromatic ring), 7.60 (lH, d, J=16Hz,
-COCH=CH-).
In accordance with these results the test sample was
identified as ~-acetylferulylstigmastanol.
Test 12
_
This test was performed to test physical and chemical
characteristics of the final product prepared by the method of
the invention.
1) Preparation of Test Sample
Test sample was prepared and purified by the same method
as in Example 6.
2) Test Method
The same tests were applied as in Test 11.
3) Results
A melting point was 152C. The results of the elementary
analysis were C: 79.17 and H: 9.86, which are in good agreement
with those of C: 79.05 and H: 10.14 calculated according to the
molecular formula of C39H60O4.
The results of mass spectrometry, infrared spectrophoto-
metry and nuclear magnetic resonance spectrometry were as follows.
FD-MS m/e; 592 (M ), IRv (KBr) ; Cm : 3200 - 3500
(-OH), 1710 (-CO), 1640 (-COOH=CH-), 1600,1520 (benzene ring3,
-30-
~ '
42~
27628-lD
1180,1080 (-C-O-C-)I NMR (CDCQ3), ppm : 0.6r~2.0 (-CH3, CH2 of
sterol<), 3.88 (3H, S, -OCH3), 4.72 (lH, m, -O-CH<), 5.96 (lH, S,
-OH), 6.22 (lH, d, J-16Hz, -COCH=CH-), 6.98 (3H, m, aromatic
ring), 7.55 (lH, d, J=16Hz, -COCH=CH-).
In conclusion, the final product was identified as trans-
ferulyl stigmastanol.
For better understanding of the invention some preferred
examples thereof will be given here~nder.
Example 1
5kg powder of Job's tears prepared by grinding the whole
seed thereof was subjected to extraction with 15Q n-hexane at a
temperature of 20C and the solvent was evaporated at a tempera-
ture below 40C under reduced pressure, thereby obtaining about
450g of a yellow, oily material at extractability of about 9%.
This extractant was then purified by silica gel chromatography
with n-hexane. The purified extractant was added to basic feed
to prepare fodder which was given to a group having 10 golden
hamsters in the age of 5~ 8 weeks. The quantities of the fodder
given to a golden hamster and the purified n hexane extractant
contained therein were determined l9g and 171mg a day, respect-
ively. While the test animals were fed for 3 weeks the sex
periods were observed, and thereafter they were slaughtered to
measure the number of naturally produced ova and observe the
state of the ovaria. For reference, an equivalent quantity of the
basic fodder not containing the purified n-hexane extractant, was
given to another group also having ten golden hamsters, to which
the same tests were applied.
~ 3842~ 27628-lD
The test results are shown in Table IX, from which it
is confirmed that the oil-soluble fraction of the whole seed of
Job's tears has a favourable effect on natural ovulation and
formation of corpora lutea, without disturbing sex periods.
-32-
3421 27628-lD
_ _
~ ~1 +~+1 ~ .
__ , .
O
~ 4~ ~ ~ ~ O
u o ~ +l +l
~ ri 0 ~ OD ~
3:
_
~ r~ ~ r~
R ~ ~ ~ ~ s~
~ O ~ ~1 ~
H ~. . .
u~ h ~
~H ~ ~ ~ O
E~ O ~a s~ _ .
u~ a~ ~) x
h ~0 u~
Id X ~ ~ td
P~ a) a) ~ ~ 4
O u, ~ u~ O a)
~ X ~r ~ O
~q E~
- ~ ~
a~ u~
a
~ p~ ~
~ o ~, ~
so~ ~ c~ ~ ~
.
u~ u~ c)
rl ~ ~
~ ~ ~ o
~ ~ z
~ ~ --~
-33-
~ .
27628-lD
4Z~
Example 2
In Example l was used the whole seed of Job's tears but
in this example 500g bran of Job's tears was used, and by
extraction with l.S~ n-hexane at 20C was obtained about 80 g of
a yellow oily material at extractability of about 16%. This
material was purified by silica gel chromatography with n-hexane
and the solvent was evaporated at 40C under reduced pressure,
thus preparing about 72g of a purified extractant. This purified
extractant was given to a group of ten golden hamsters in a
proportion of 1.5mg a day per lg of the body weight of a golden
hamster, and the same tests were put into practice as in Example 1,
results of which are shown in Table X. As shown, the oil-soluble
fraction of the bran of Job's tears seed will promote natural
ovulation without disturbing the sex period and bring a
significant effect on formation of corpora lutea.
-34-
~ .
, , .
~ 2 ~38421 2 7 6 2 8 -lD
~= .~ .... _
~o
_
O ~ +l
o ... _ ~ '
~ 4~ ~ ~ ~
~, o ~ .~ ~ ~
u~ ~ _ ,~ o Q
,~ ~ + I + I
3 ~ 0!:~ ~ ~:1
O ~ 1~ 4-(
-
~` U~
O ~ O
~ S-l ~ N ~ ~
~1 ~ ~ ~ oo ~`
~ O ~ ~1
m . . _
,o
~ ~ 1 ~ H
o ~ ~ ~ a)
~ a~ a~ a) d
~: ,0 _~ _~ _ O ~
'S~ ~ ~ _ O '~
~ ~ U~ U~ rl tn
)~ X ~1 ~ ~ ~ tl~
a~ ~ ~ (d
~O~ U~
a) ~r ~r
U~
__ _
S~
. ~ O ~ ~
O ~. ~' E~ ~n
5~
a) D~ ~ O
,1 S~ Z
_
--35--
, .. . .~.. . ~, . .
,
~ 4~ 27628-lD
Example 3
50kg of Job's tears seed was threshed and purified in
known manner to prepare about 6.5kg bran, among which 5kg bran
was subjected to extraction with 5kg ethyl acetate at 20C for
5 hours while being agitated. This extraction procedure was
repeated three times to obtain a first extractant. To 4kg of
the residue was added lOkg ethanol, and the reaction solution
was agitated at 20C for 5 hours. This second extraction
procedure was repeated twice and then the collected extracts
were filtered to remove insoluble fractions. From the ethanol-
soluble fraction thus obtained was evaporated ethanol in a known
manner. To 120g of the resulting component was added 360g ethyl
acetate and the solution was agitated at 20C for 5 hours, to
extract an ethyl acetate soluble fraction, a second extractant.
The first and second extractants were mixed together.
Ethyl acetate was evaporated and removed from the extractant
mixture to obtain 1,310g of an oil-and-fat fraction.
A first portion of 500g of the oil-and-fat fraction was
subjected to column chromatography charged with 8kg silica gel,
whereby 450g eluate was obtained witha mixed eluent of n-hexane
and ethyl acetate (mixing ratio of 20: 1). This eluate was
further introduced to alumina column chromatography (800g)
whereby elution was effected with a mixed eluent of n-hexane and
ethyl acetate (mixing ratio of 20: 1~ and about 540mg of a
fraction showing a spot in ultraviolet rays was obtained by a
fraction collector. This component was subjected to reverse
phase high performance liquid chromatography utilizing Rp-18
-36-
~ Z~384~1
27628-lD
with a mixed eluent of ethyl acetate and methanol (mixing ratio
of 5 : 3), to obtain about 450mg of a component having a U V
absorption. This component was further subjected to column
chromatography using 50g of silica gel with a mixed eluent of
n-hexane and ethyl acetate (mixing ratio of 20: 1), to elute
about 330mg of a component (X component) having a U V absorption.
X component was put into the same tests as in Test 7 so that it
was proved to have ovulation-inducing effect.
On the other hand, another portion of 500g of the oil-
and-fat fraction was subjected to column chromatography using 8kg
silica gel, with a mixed eluent of n-hexane and ethyl acetate
(mixing ratio of 100:0.5), then an eluate (Y component) was
obtained in a quantity of about 6.87g. Y component was also
submitted to the same tests as in Test 7 and its ovulation-
inducing efect was observed.
Example 4
To 3kg o Job's tears bran was added 3kg ethyl acetate
and the solution was agitated at 17C for 8 hours for effecting
extraction. This extraction procedure was repeated four times
and extractants obtained in respective extraction were combined.
Thus, an ethyl acetate extractant was prepared. To 2.2kg of the
residue was added 8kg ethanol and the mixture was agitated at
17C for 8 hours. The extract thus obtained was filtered to
remove insoluble components. By evaporating ethanol from the
ethanol-soluble fraction in a known manner, an extractant with
ethanol was obtained in a quantity of 65g. To this extractant was
added 260g ethyl acetate, and the solution was agitated at 17C
for 8 hours, to extract an ethyl acetate soluble extractant.
27628-lD
~1~8~
These two ethyl acetate extractants were combined, from which
ethyl acetate was evaporated to obtain 790g of an oil-and-fat
fraction.
A first portion of 300g of the oil-and-fat fraction was
subjected to column chromatography using 5kg silica gel, with a
mixed eluent of n-hexane and ethyl acetate (a mixing ratio of
30: 1), to obtain an eluate in a quantity of 269g. This eluate
was subjected to alumina column chromatography(500g), thereby
obtaining 330mg of a fraction having a spot in ultraviolet rays
This fraction was subjected to reverse phase high performance
li~uid chromatography utilizing Rp-18, thereby eluting with a
mixed eluent of ethyl acetate and methanol (a mixing ratio of
5 : 3) to obtain about 248mg of a component having U V
absorption. This component was further subjected to column
chromatography using 30g silica gel with a mixed eluent of
n-hexane and ethyl acetate (a mixing ratio of 30: 1) to obtain
about 190mg of a fraction having a U V absorption. This fraction
was submitted to tests in the same manner as in Test 7 so that it
was proved to have ovulation-inducing effect. On the other hand,
the other portion of 300g of the oil-and-fat fraction was
subjected to column chromatography using 5kg silica gel with a
mixed eluent of n-hexane and ethyl acetate (a mixing ratio of
100:1) to obtain 4.352g of an eluate. This eluate was tested in
the same manner as in Test 7 to find its significant ovulation-
inducing effect.
Example 5
The procedure in Example 1 was repeated several times to
obtain 40g of X and Y components, respectively, to which 150g of
- 37a -
~;
,
~ ~84~ 27628-lD
middle-chain fatty acid triglycerol on the market was added to
make 1,000 soft capsule medicines therefrom, respectively. A
soft capsule medicine made from X component contained 40mg of
the effective component (that is, a 9 : 1 mixture of trans-
ferulylstigmastanol and trans-ferulylcampestanol) and a soft
capsule medicine made from Y component contained 80mg of the
effective component (that is, phytosterol long-chain fatty acid
esters in a mixed state).
Example 6
According to the following steps trans-ferulyl-
stigmastanol was synthetically manufactured.
1) Acetylation
l.Og of ferulic acid (Tokyo Kasei Kougyo K.K., corres-
ponding to 5.15m moles) was dissolved into 8m of a liquid mixture
of pyridine and acetic anhydride in a mixing ratio of 3 : 1, and
after reflux for 4 hours the solvent was evaporated.
2) Formation of Acid Chloride
l.Og of the acetylated ferulic acid (4.24m mol) was
dissolved into lOmQ chloroform anhydride and 0.46 mQ (6.36m moles)
of thionyl chloride (Wako Jun'yaku K.K.) was added to the
solution, to effect the reaction thereof at 25C for 5 hours, and
then the solvent was evaporated under reduced pressure.
3) Reaction with Phytostanol
The acid chloride compound thus obtained was dissolved
into lOmQ pyridine anhydride and the resultant was dropped in an
ice-cooled condition into lOmQ of another pyridine solution
prepared by dissolving thereto 1.77g (4.24m moles) of stigmastanol
(Aldrich Chemical Co.). After the solution had been allowed to
-37b-
~ 84X~ 27628-lD
stand at room temperature for 2 hours, the solvent was evaporated.
The remaining residue was subjected to a silica gel column, and
the column was eluted with a mixed eluent of n-hexane and ethyl
acetate (a mixing ratio of 20: 1). Thus, a reactant was
obtained in a quantity of 1.65g.
4) Deacetylation and Purification
1.6g of the reactant was dissolved into 48mQ of a liquid
mixture of chloroform and methyl alcohol ~a mixing ratio of 1 :
1), and 480mg (12.7m moles) of sodium borohydride (Wako Junlyaku
K.K.) was added thereto little by little. After the foaming had
ceased the reaction mixture was allowed to stand for 1 hour, and
then lmQ water was added thereto. The solvent was then removed
under reduced pressure.
The reaction product was dissolved into 5.OmQ of chloro-
form and the solution was led to a column charged with silica
gel, which was eluted with a mixture of n-hexane and ethyl acetate
(a mixing ratio of 20: 1). The eluate was recrystalized with
methyl alcohol to obtain 1.23g (2.08m moles) of colorless, needle-
like crystals, that is trans-ferulylstigmastanol, in 83% yield.
Example 7
1) Acetylation
Acetylation was carried out in the same manner as in
Example 6.
2) Formation of Acid Chloride
Acid chloride was formed in the same manner as in Example
6.
3) Reaction with Phytostanol
The acid chloride was dissolved into lOmQ pyridine
-37c-
X
~ 2~842~ 27628-lD
anhydride and the resulting solution was dropped into lOmQ of a
separately prepared pyridine solution containing 1.72g (4.2m moles)
campestanol, in an ice-cooled condition. The solution was
concentrated and then led to a silica gel column which was eluted
with a mixture of n-hexane and ethyl acetate (a mixing ratio of
20: 1), thus obtaining 1.45g of a reactant.
4) Deacetylation and Purification
1.4g (2.25m moles) of the reactant was dissolved into
42mQ of a liquid mixture of chloroform and methyl alcohol (a ratio
of 1 : 1), and to the solution was added little by little sodium
borohydride (Wako Jan'yaku K.K.) in a total quantity of 478mg
(12.6m moles). The reaction mixture was allowed to stand at
room temperature for 1.5 hours after the foaming had ceased. The
excessive sodium borohydride was inactivated by adding water and
then the solvent was evaporated under reduced pressure.
The resulting residue was dissolved into 4.5mQ chloroform
and the solution was subjected to a silica gel column which was
eluted with a mixture of n-hexane and ethyl acetate (a mixing
ratio of 20: 1) and recrystalized with methyl alcohol. Thus,
trans-ferulylcampestanol in a quantity of 1.15g (1.73m moles)
was obtained in 77~ yield.
Although this invention has been described in conjunction
with specific exemplifying tests and preferred embodiments thereof,
it is to be understood that many variations may be made without
departing from the spirits and scopes thereof as defined in the
appended claims.
-37d-
~, , .