Note: Descriptions are shown in the official language in which they were submitted.
3~;'Y5
INSTRUMENT AND METHOD FOR
TESTING FOR FLUID CONSTITUENTS
~ACKGROUND OF THE INVENTIOM - - -
This inventlon primarily relates to a method and to
an instrument for use in medical diagnosis, and in particu-
lar, to detecting and determining glucose concentration in
blood.
Diabetes $s a health problem affecting many indivi-
duals and its prevalence is increasing. The usual treatment
for diabetes is single or multiple insulin in~ections
daily. Insulin is available in slowly or rapidly absorbed
- . . : , , ~ , .. ... ..
forms, which may be injected alone or in combination. Such
insulin in~ections have been effectlve in treating the ~
,, ., _ . . .
disease and in prolonging life.
. ,,. ., . . ., , : . , ~ ~:
Presently in order to determine if insul1n is
needed, blood is withdrawn from a patient and is tested for -
,, , ., , ~ . , . , .. . , .. . ,, ..,~ ... . ...
glucose concentration by a litmus-type indicator test. ~`lf- -
indicated, insulin is taken by the patient.
This type of testing has several problems. For
" : , , ,, ~ - - -~ -,
example, the testing is periodLc, and thus the administra-
tion of insulin is periodic, whioh can result in wide -~ -
variations in glucose concentration over time and peaks inthe glucose concentration. Such variations can have
physiological effects which may be adve~se to the patient.
, - , . , ,,- - . ~ - . - , , ,, ~ , . ~,; . . . -
It has been reco~nized that it is desirable to -
, . .
administer insulin periodically on demand and in'response to
changes in glucose levels. One such system ~s disclosed in
, ~ . ;
Albisser A, "Devices for the Control of Diabetes Melletus",
Proc. IEEE 67 No. 9, 1308-1310 (19793 , wherein a servo
., . . ~ - .. . . .. .. .
system is employed which continuously withdraw~ blood from a
patient and analyzes the same for glucose. Using a computer
or microprocessor, calculations are made from the withdrawn
~,,'' ,
, .
sample as to the need for insulin, and in response thereto,
lnsulin is administered. This system has only been used for
short periods and has a disadvantage in that the system is
invasive (i.e., the patient is catheterized continuously for
withdrawing blood samples).
The litmus-type system has the disadvantage in that
it is invasive and the patient is periodically and
repeatedly pricked for blood samples.
It ls therefore an ob~ect of this invention to
provide a glucose testing dsv~ce which can be used to
monitor a patient's glucose level cont$nuously, if desired,
so as to provide a more uniform administration of insulin
and a more uniform glucose concentration in the bloDd.over
time.
It is another ob~ect to provide a glucose moni-
toring system which is noninvasive and does not require-
periodic blood withdrawal to determine glucose levels.
It is sometimes desirable to test body fluids for
other constituents. For example, law enforcement officers
test individuals for alcohol content of their blood using a
breathalyzer. However, breathalyzer tests may be inaccurate
in that non-ingested alcohol, such as in mouthwashes, will
provide false results.
It is another ob;ect of this invention to provide a
noninvasiave diagnosis apparatus for use in determining the
concentratlon of various constituents of body fluids, such
as glucose and alcohol and drugs.
Nuclear magnetlc resonance (NMR) ls a diagnostic
technique which ls used widely for medical imaging and
medical d$agnosis. In NMR, the test ob~ect is sub~ected to
-- 3 --
4'7~i
a first or blasing magnetic field to align previously
randomly oriented 1H protons in the nuclei and a second
field or burst of energy to lncrease the energy of a
selected nuclsus. When the second magnetic field or energy
source ls turned off, th~ return to the flrst alignment
releases energy which is detected and analyzed. This
release is analyzed or processed to form an lmage or
spectrum. From the spectrum, the presence of particular
molecular bonds can be observed and associated with various
molecules or materials from which the concentration of that
molecule or material can be determined.
NMR machines are most frequently used for imaging
sections of a human body and require large magnets, for
example, superconducting ma~nets. The machines are there-
fore quite large and expenslve. Furthermore,-the NMR
testing of fluids has requ$red 1nvasive sample withdrawal
techniques, which sample was then tested in the larger-
machines.
Using such NMR machines, blood serum has been - -
analyzed and a spectra of the lH resonance developed. In
such spectra, identifiable peaks are obtained for water,
glucose and ethanol. In reported tests, blood serum has - -- -
been taken from animals, placed in a contalner and excited
so as to yield the lH spectra, which is then analyzed.
Unfortunately, NMR testings are not common nor oonveniently
available. The reason is believed to be that the equipment
ls generally large, complex and expensive, and is therefore
available only at selected centers, such as hospitals,
universities, and other similar research and test sites.
The equipment therefore is not normally used for blood or
34~75
b~dy fluid analysls as more convenient and less expensiYe
alternatives are available.
Another disadvantage in preser,t N~R tests is that
they are conducted on fluid samples which are withdrawn from
the patient by the usual lnvasive techniques.
It is therefore an ob~ect o* this invention to
provide a more convenient NMR instrument for use in
analyzing body fluld samples.
It is a further object of this $nventlon to provide
an NMR instrument for use ln analyzing body fluid for
.
glucose.
It is yet another ob~ect to provide a portable NMR
instrument for use by a person having diabetes to analyze
his blood for glucose concentration.
It is yet a further ob~ect to provide an NMR
instrument for use by a diabetic in noninvasively analyzing
his blood serum for glucose concentration.
It is a still further ob~ect of the invention to
~,. . . . .
provida an NMR method and apparatus to test for other
substances, for example, alcohol and drugs.
These and other cb~ects of this invention will
become apparent from the followin~ disclosure and appended
claims.
SUMMARY OF THE INVENTION - -
. . : .
This lnvention provides a method and a portable NMR
instrument for use ~n noninvasively analyzing body fluids
for the concentration of various constituents. Specifi-
cally, a diabetic can use the instrument to noninvasively
and substantially instantaneously analyze his blood for
~ ?~3847S
glucose, thereby eliminating the need to invasively obtain a
blood sample which is then tested. Using the device dis-
closed herein, a patient can periodically, frequently if
necessary, and painlessly analyze hls blood for glucose
concentration. This device may also be u~eful in analyzing
body fluids for alcohol or drugs.
In one form, the device is portable and provided
with means for receiving an extremity of the pati~nt, such
as a finger, and exposing the extremity to a first or
biasin~ magnetic field and a second field or ener~y
source. Sensors are provided for sensing the rates of
relaxation or energy release so 85 to develop the
spectrum. Analytical means are coupled to the sensors for
recelving and analyzing the signals emitted, discriminating
between various peaks, comparing the amplitude or hei~ht of
various peaks, such as water and glucose, and normal~zing
the analysis by reference to a standard sample so as to
obtain the concentration of constituents in the tested
materials.
One of the principal components of the NMR
instrument is the first or biasing magnet for providing the
first magnetic field. In this device th~ biasing magnet is
physically much smaller than the magnet~ used in standard
NMR machines. For example, the magnet may be one pound in
weight and may exhibit a field strength of at least five to
six kilogauss. Another component is a coil apparatus for
applying a second field or energy to the test sample and
sens~ng the energy released therefrom. A single coil or
multiple coils can be used. Yet another important element
of this invention is the electron~c clrcuit used for the
347S
analysis. This circuit is controlled by a microprocessor
that is programmed to control the application of the second
field or energy source and cooperates in detectlng and
analyzing the spectra received from the sample when the
field is relaxed. Operation o~ the microprocessor is
disclosed herein.
Other specific features of the instrumen~ are
disclosed herelnafter.
BRIEF DESCRIPTION OF THE DRAWINGS
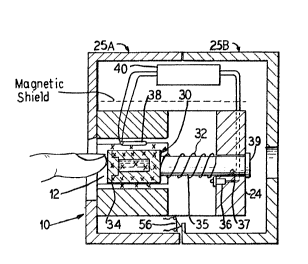
FIGUR~ 1 is a vertical cross-sectional ~iew of an
instrument according to this invention;
FIGURE 2 is a vert~cal cross-sectional view taken
along line 2-2 of Fig. 1 and also showlng a housing and
other components;
FIGURE 3 ~s a block-type schematic diagram for the
circuitry to operate the instrument
FIGURES 4a to 4c are flow charts showing the
operation of the instrument;
FIGURES 5a and 5b are representative NMR spsctrums
showing the water, glucose peaks and alcohol used for
analysis;
FIGURE 6 is a schematic diagram showing a three-
coil system for use in the instrument;
FIGURE 7 is a schematic diagram showing the
electrical connections for the three-coil system of Fig. 6;
FIGURE 8 shows an NMR probe for implantation in a
body;
~ IGURE 9 i5 a schematic block-type diagram of the
electrical circuit for use with the implantable probe of
Fig. 8;
FIGURE lO shows a human arm having a distended vein
for NMR test$ng;
FIGURE ll is a fragmentary and sectional view of a
magnetic probe for use in NMR analysis using a surface blood
vessel; - -,
FIGURE 12 is a schematic representa~ion of an
alternative circuit arrangement for use with separate
energizing and receiving coils; ~-~
FIGURE 13 is a schematic representation of the coil
and magnet relationships which may be used ~n an arrangement .~-,
of the type shown in Fig. 12; -- - ~~
-- FIGURE 14 1s a-schematic reprqsentation of a multl- --
_."-,~
coil arrangement;
FIGURE 15 is a top view of the elements of Fig. 14;
and -
FIGURE 16 is a side view of an alternativ~ C-shap~ed - -a
magnet which ~ay replace the magnetic ~tructure of Figs. 1 --
and ~
.
DESCRIPTION OF THE PREFERRED EMBODIMENT
Referring now to Figs. 1-3, a first embodiment of
the test instrument is shown. Other em~odiments and c~
features will be discussed ~fter consideration of principal - -
features of th~s invention by way o~ the first embodiment. --
The test lnstrument 10 is ~hown as including 3 box- -^
shaped assembly which defines a finger-receiving recess 12 _.
therein. ~he assembly lncludes a body section 14 defined by
the top, bottom and elongated side walls 16, 18, 20 and 22 ~
and the back wall 24. The assembly is enclosed in a two- _
~ ~8475
piece cover or housing 25A and 25B within which the
electronic components discussed hereinafter are also
enclosed. Alternatively, the electronics can be enclosed in
a separate housing connected to the body section,- A p~ir of
first or biasing permanent magnets 26 and 28 form the top
and bottom walls 18 and 22, are positioned opposite one
another and provide the first alignins magnetic field. It
is to be noted that the poles of the respective magnets are
aligned 50 that the field is additive and provide construc-
tive lnterference, and the pole pieces or shoes shape themagnetic fleld in the finger-receiving recess 12. ~his
alignment is shown by the "X" designation, wh~ch indicates
that the magnetic field from the magnets passes through the
secess 12 in the same direction, in Fig. 2, into the paper.
A sample holder or container:34 for a standard sample
starting apparatus 30 is shown positioned in the recess.
The apparatus includes a compression blasing spring 32
pressing at one end against the back wall 24 and against the rear -
wall 30 of sample holder 34 at the other end. The holder 34 is
mounted on a post-like member 35, which is guided through an
aperture 37~ A start switch 36 is mounted to the back wall -~
offset from the member 35 so that when the sample holdRr
34 is pushed against the spring toward the back wall, the
holder will depress the start switch to start operation of
the instrument. Release of the sample holder will
release the switch. The switch may also be mounted outside,
say beneath the head 39, and operated upon movement of the
head 39.
A surface coil 38 is mounted in the hou~ing
ad~acent one of the permanent magnets 26 and 28. The coil
_ g _
47~
produces the second field and acts as a source of energy for
realignment and for sensing purposes. As seen in Fig, 1,
the second field produced by the surface coil is transverse
to the first or permanent magnet field. The surface coil
has been selected for this embodiment because the depth of
magnetization (i.e., extent of penetration of the field) is
related to the diameter of the coll and can thus be
controlled.
The surface coil 38 may be a slngle coil for both
energlzation and sensing. The co~1 can also be an assembly
in which there are multiple coils, each of which are for
energizatlon and 6ensing. Furthermore, the coil may be an,
assembly of at least two coils, where at least one is for
energization and at least one other coil is for sensing.
.
These alternatives are shown in Figs. 13, 14-and 15.
The cover,or housing 25A and 25B for the
electronics is provided with an electronic interlock system
(schematically shown as 56 in F~g.; 3 ) 80 that unauthorized
vpening or removal of the cover will disable the electronics ,-
described herelnafter, thereby prevent~ng unauthorized '--
tampering or repair of the device which could destroy ' '
calibration and result in improper usage. - -'
Physically the test is run by ~he patient inserting
his finger into the instrumen~ and pushing the sample holder
toward the back wall 24 and into engagement with the start
switch 36 to start the analysis as describsd herelnafter.
It will be noted that the finger is positioned so
that the fingernail i8 located adjacent the surface coil.
Thl~ positioning is chosen as the fingerna~l i5 dead t~ssue
but has a bed of active blood vlessels pos~tioned ~ust below
-- 10 --
s
the nail. These vessels are believ~d to provide an accurate
testing site. In many other test sites, llve body tissue or
bone must be penetrated in order to test blood in a vessel,
which means that the tissue or bone will'émlt s~gnals due to
testlng which act as noise and may interfere with analysis
of the blood for glucose concentration. The finger region
is preferable, since the nail is essentially dead material
and produces little, if any, interfering noise, thereby
increasing the signal to noise ratio. It ls believed that
other body extremities can be tested, for example, the ear
of either a human or other'~nimals. ' '''''
- ' The testing circuit 40'includes a battery power
supply 42. In'a permanent installation, such as a doctor's-
office, hosp~tal, etc., a commercial AC power supply and
battery charger may be used to supply energy to the
battery. Depression of the start switch activates the '- - - -
circuit and, thereby the microproces~or 44. The microprocessor
activates an RF generator and cyciically-operated gate 46,
,
which excites the surface coil~38 (or coil assembly) for
applying the second fleld, raising the energy state and -
realigning the nuclei.
At the appropriate time and under control of the' --
microprocessor, the RF generator is deactivated, thereby
' permittin~ the nuclei (dipolesj to relax or return to the
first alignment. The surface coil then detects the energy '' ' --
released duriny relaxation and realignment. Those sisnals
are received by receiver/gate 48, converted from analog
signals to digital signals by the ~/D converter S0 and fed
to the microprocessor 44. A rea~ only memory (ROM) 52 is
provid~d for storing the program for use with the micro-
-- 11 --
~J
~ ~f~47~rj
processor in calibrating the machine and analyzing and
displaying test results. If separate coils are used, then
the circuit ls chang0d so that the RF generator is connected
to the energizing coil and the receiver is connscted to the
sPnsing coil as shown in Fig. 12.
The ROM is continuously energized by the battery
54. A cover interlock switch 56 is provided between the ROM
52 and battery 54 to deenergize the ROM in the event the
electronics cover 25A or 25B is opened, removed or tampered
with. In such an event, the switch 56 ~s opened and the
program in the ROM is erased. In this instance, the ROM may
be selected from the well-known classes of electrically
erasable or alterable ROM's. The specific function of the
ROM-cover interlock arrangement may be selected as desired,
i.e., to generate an error message on the panel display, or
simply to disable the apparatus from operating or exhibiting
any panel display. Various other forms of electronic-type
interlocks are well-known in the computer art.
The testing circuit 40 also ~ncludes a display 58,
preferably digital, which is connectsd to the microprocessor
and a group of status lamps (read 60, calibrate 62, display
64 and error 66), which indicate the status of the system1s
operation.
The ROM 52 includes a program as represented by the
flow chart of Figs. 4a-4c, whereby operation of the tester is
controlled. In general, the operation of the tester is as
follows:
1. A finger is inserted to depress the sample
holder and activate the-start switch.
2. The ~inger ~s tested.
- 12 -
9 ~475
3. The finger test results are stored in the RAM
45.
4. The finger is released and the standard sample
moved to the test position.
5. The standard sample is tested.
6. The standard sample test results are stored in
the RAM 45.
7. The standard sample test results are compared
with predetermined calibration data previously entered in
memory to determine if the standard sample data reading is
still within preset and allowable tolerances.
8. Then the finger test results are compared with
the sample standard test result data and the finger data is
normalized and proportioned to determine glucose
concentration.
Referring now to the flow diagrsm, Figs. 4a through
4c, tha various phases of the microprocessor and ROM are
shown. These phases can considered as follows:
1. Patient reading cycle.
2. - Standard sample reading cycle.
3. Operational system check.
4. Calculation of normalized patient data and
stan~ard sample for equal ~2 peak.
5. ~alculation of glucose level.
Within each one of these broad steps are a series of smaller
steps.
Reerring first to Fig. 4a, the flow chart begins
with depression of the starting switch 36, init~ation of the
- 13 -
~J
4~i
program and activation of the read light 60. Next, a one
second homodecoupling pulse (or a plur~lity of pulses~ to
saturate the water peak is activated. A five microsecond
sample pulse is taken, and the free induction deeay output
from the A/D converter is noted. Next, th~ data points are
stored in the memory 45 and the process is repeated (i.e,,
looped) perhaps one hundred tlmes. In the right-hand
column, there is shown a serles of diagrams representing the
one second homodecoupling pulse, the five microsecond
sampling pulse, the decay, and a Fourier transformation of
the decay data points. The amplitude (Amp.) of the response
is recorded along the Y-axis. After the samplings, the read
lamp ~s deactivated, the accumulated responses are -- -
multiplied by an exponent1al decay to provlde line
broadeniny, a Fourier transformation is run, and a ~pectrum
is stored as the chemical shifts versus the peak height as
patient data.
Turning now to Fig. 4b, the standard sample reading
cycle is next activated. Here the calibrate light is turned
on, and the start switch is released. Once the switch is
released, a one second homodecoupling puls0 (or plurality of
pulses) is provided, a five mlcrosecond ~ampllng pulse is
taken, the free lnduction decay ls recorded, and the data
points are stored ln the memory 45. The system is then
repeated again,-perhaps--one hundred times.~`As `i`n ~e ; `
patient reading cycle, the accumulated responses-are- --
multiplied by an exponential decay to improve lina ~ ~-
broadening, Fourler transforms are run and the spectrum of
chemical sh~fts versus peak helght is stored as-sample~data.
- 14 -
3475
The standard sample initially contains predeter-
mined amounts of the constituent material or materials being
tested for and acts as a reference level. In order to
assure th~t there has been no significant change in these
value(s), the next step is an operational check where the
spectrum of chemical shifts versus peak height data for the
standard sample is recalled and compared to the standard
data previously taken within allowable tolerances. If the
error is not within an ac~aptable tolerance, the~- error-
display lamp 66 is lit and the operator notified. If thedata is with~n an allowable error, the system proceeds to
the next step. lt is noted that on the right-hand side of
Fig. 4c that a comparison is shown between the standard
sample data and standard sample ~pectrum showing the allow-
able shifts, peak height and frequency with amplitude
plotted along the Y-axis. ~
The next step is to normalize the patient data and
standard sample data for e~ual water heights. Here the
patient data 18 recalled and the s~andard sample data is
recalled. Next, the pati~nt data water peak height is
scal~d to match the standard sample data water peak height.
The system then executes the next step whlch is to
calculate the glucose 1PVe1. To do th~s a ratio is obtained
of the patient data glucose peak height and ths standard
sample data peak height. This ratio is then mult$plied by
the known standard sample glucose to water ratio to obtain
the patient reading and multiplied by a concentration factor
(K) from the standard samplP and expressed in milligrams per
-- 15 -
7S
deciliter or some other convenient unit. Then the patient
glucose level is displayed in relation to plasma level.
Normal 91ucose concentration is ninety milligrams per
deciliter.
This relationship is derived as follows:
1. The standard sample is prepared having a known
glucose concentration expressed, ~or example, in milligram
of glucose/deciliter of water (mg/dl) and is referred to as
K.
2. A patient is tested and the water and glucose
peak heights are obtained.
3. The standard sample ~s then tested for water
and glucose peak heights.
4. The patient's water peak height is normalized
by determining the ratio of water standard peak height/water
patient peak height. ~his ratlo can be referred to as gain.
5. The patient's glucose peak height is
normalized by multiply1ng the patient ~lucose peak height by
the gainO The result is the normallzed patient glucose
level. Expressed algebraically:
Glucose = (Water ~tandard) x glucsse pati~nt
normalized (Water patient )
6. In order to obtain the actual p~tient glucose
concentration, expressed in units such as mg/dl, the
normalized glucose now is divided by the glucose standard
and the resulting ratio ls multiplied by the concentration
factor K. In other words:
Patient glucose ~ Glucose normalized x K
concentrat1on Glucose standard
- 16 -
;,'
~ ~3847~
7. The entire expression which combines the 8tep8
of numbers 1-5 above can be stated as:
Patient glucose - ( m~ ) - K ( m~ ) -
concentration ( dl ) ( dl ) -- -
... . . . . .
(Glucose patient) (Water ~tandard)
x ( peak height ) x ( peak height
(Glucose standard) (Water patient ~
( peak height ) ~-peak height ) - - -
In Fig. 5a, a 1~ typical blood spectrum is shown
with the water (H20) and glucose peaks clearly shown. It 1s
the ratio-of the peak heights as determined from the cali-
bration and test samples that permit determination of the
test sample glucose concentration. Fig. 5a shows the work~
of Jay Block, "Analysis of Serum by High Yield NMR", Clin.
Chem. 28/9, 1983, (1982) taksn from normal blood serum. ---- -
Sample volume is 0.4 ml serum to which ~as been added D.l ml - - ---
of 2H20 for field lock. In addltion, 10 mmol/l of TSP was - ~ _
added to the 2H20 to serve as a reference to assign chemical --
shifts and peak area. The work was done on a WM 500 Bruker
spectrometer. Samples were maintained at 30C and a 1
second homodecoupling pulse was applled before the S
mill~second sample pulse (45~ notation angle) to saturate
and reduce the H20 peak. A total of 16k data points was
~eoorded in ~n ac~uisitlon time o~ 1.5 ssconds with B0 ~uch
transients averaged for each spectrum (2 min per spectrum).
~ven with the water peak suppressed, ~t is still the most
prominent feature, however, the glucose peak which is four
orders of magnitude lower i8 still easily identified. The
glucose concentration is in the normal range of 90 mgJdl as
m~asured by the conventional glucose ox$dase procedure.
- 17 -
~,
347~
Lactate was also detectable. It is also interesting to look
at the glucose peak at 5.25 in the otherwise peak free
region.
Fig. 5b is an enlarged portion of the 1H blood
spectrum of Fig. 5a, showing the ethanol and water peaks, as
also reported by Bock and showing the spectrum of serum
obtained 30 minutes after ingesting 30 ml of vodka. The
ethanol concentration measured by routine gaschromatographic
method was only 30 mg/l, while the methyl resonance of
ethanol at 1.20 ppm was detected with better -than 40:1
signal to noise ratio. The methylene resonance is buried in
the glucose region. In addition, a large peak appears at
1.93 ppm, the position of acetate, presumably derived from
the oxidation of ingested ethanol. In serum from
intoxicated patients, the ethanol resonance had a much
greater intensity and dominated the spectra.
Another smbodiment 70 of this lnvention is shown in
Fig. 6. In this embodimentj three coil pairs 72, 74 and 76,
a~e provided, whlch lie ln the same plane and are equally
spaced, that is at egually spaced 60 intervals. The coils
are arranged to provide constructive interference at the `
center of the coils where a sample ~such as a flnger or test
tube) ls to be located. These coil pa$rs act as the
energization ~r realignment coll ~nd as the sensor, in a
- 18 -
4~5
manner similar to the surface coil described hereinbefore.
This arrangement ls believed to provide better signal
discrimination by ~ncreasing the signal-to-noise ratio. The
coils are mounted in a housing similar to that shown in
Figs. 1 and 2 and are controlled by a circuit and in the
manner similar to that described in connection with Fig.
3. Physically, the standard and sample is inssrted into one
of the coils, such as the test tu~e 78 into coil 72. The
portion to be tested is located at the center of the coils
as shown.
The test sample is then tested as described above
with coils first acting as the energization or realignment
magnets and then as sensors or receivers. In other regards,
such as s$gnal pr~cess$ng and concentration analysis, this
system operates in the same manner as above.
In those cases in which it may be desirable to
implant a portion of the instrument, reference is made to
Figs. e and 9.
A third embodiment 80 is shown in Fig. 8, which is
constructed to surround a blood vessel which is internal of
or within a body, for example, a vein or artery in the body.
The test device includes the principal magnet 82,
which in this case is C-shaped and a palr o* RF coils 84.
The vein or artery 86 is positioned between the coil pa~rs
and the poles of the magnet. By so doing, blood in the vein
or artery is subjected to the first magnetic field, and the
energization or realignment field and relaxatlon is ensed
by coils 84.
In a fourth embodiment, the test instrument 90 ls
constructed for surgical implantation as shown in Fig. 9.
-- 19 --
847~
Such a device has two component parts: one part is the
internal or implanted portion 92 and the oth~r part is th~
external or power supply and sensing part 94. The two parts
are electronically coupled by transformer-like members as
described herein.
In the fourth embodiment 90 an external AC power
supply 96 is induct~vely coupled to an internal pow~r supply
98. The internal power supply 98 powers the NMR unit 100,
which is conneeted to probe snd magnet unit 102. Signals
from the probe and magnet are received by the receiver 104,
which is inductively coupled to the microprocessor 106,
through the coil element 108. The microprocessor then
provides an output to the digital display 110 of the glucose
concentration.
The magnet and probe assembly 102 is in the same
form as that in Fig. 8 and is positioned to surround an
artery. The signal processing is performed by the micro-
processor in the same manner as with the other embodiments,
particularly Fig. 3.
In a fifth embodiment, a surface blood vessel,
usually a vein, is distended and used to analyzs for glucose
concentration. Such an embodiment is shown in Figs. 10 and
lI, where a patient's arm 120 is shown surrounded by a
pressurizable cuff 122 for causing a vein 124 to protrude or
distend from the skin surface. In that situation, the NMR
unit is fitted on either side of the protruding vsssel at
the surface of the arm. In this embodiment a C-shaped
permanent magnet 126 is arranged so that its north and south
poles (~ & S) are on opposite sides ~f the vessel. A
surface coil 128, like that ln Figs. 1-2, is employed for
- 20 -
f .
i,~ j,
f347~
en0rgization and realignment and sensing. Testing circuitry
of the type shown in Fig. 3 is also employed in the embodi-
ment of Figs. lO snd 11.
A principal advantage of the test instrument shown
herein is that the device is smaller than the large ~R test
instruments now used at hospitals, etc. The reason is that
the present instruments include a large principal magnet for
surrounding the body of a patient. Here, since the tested
portion is a finger or other extremity, the principal magnet
may be smaller 50 that the instrument may be mounted on a
table top, carried in a brief case, or be even smaller. In
order to achieve such a device, the m~gnet must be small in
size, be of a comparatively light we~ght, such as one pound, - -
~
ana still exhibit an adequate field strength. ~deq~ate - --
strengths ~hould be on the order of at least five to s1x ~:
kilogauss. One particularly suitable material containing .',,7
Neodynium is manufactured by General Motors Corporation.
Fig. 12 shows the generator and gate 46 and the
receiver 46 and gate 48, respectively, connected to separate
transmit and recelve coils 38', 38". - ~--~
Fiy. 13 shows an embodiment of the coils 38' and- ---~
33" along with the field directions, including the bias ~ ~~ r'
field Ho, at 9O with respect to one another.
Figs. 14 and 15 illustrate the u~e of a plurality
of surface coils 38''', which are connected for addltive
fields, as a single transmitJreceive arrangement. --
Fig. 16 shows an alternate bias magnet, similar to
that shown in Fig. 11. The magnet 138 comprises a pair of
spaced pole pieces 132, 134, which define a gap for ---
receiving; in this example, a finger.
- 21 -
Although the invention has been described with
respect to preferred embodiments, it is not to be so
limited, as changes and modiflcations can be made which are
within the full intended scope of the invention as defined
by the appended claims.
: - - - - --
- 22 -