Note: Descriptions are shown in the official language in which they were submitted.
3~
1 --
l The present lnventlon relates to a process for
destroylng organlc products wlth toxlc effects,
preventlng pollutlon of the environment after an
lncldent, accldent or any other case requlrlng these
products to be deactlvated.
The inventlon also relates to the equlpment for
carrying out thls process.
Xore preclsely, use ls currently belng made, ln
certaln flelds of lndustry, of organlc compounds of, for
example, the polychloroblphenyl type (usually called
PCB> on account of thelr favourable characterlstlcs,
especlally thelr good thermal stablllty, thelr good
cbemlcal lnertness hnd thelr hlgh dlelectrlc constant 9
Justifylng thelr lntenslve use ln partlcular as flulds
for transformers and/or condensers.
However, for some years studles have shown that such
products are not blodegradable and that there ls
evldence of thelr conslderable accumulatlon ln the food
chaln. Furthermore, these studles h~ve shown that these
products are hlghly toxlc and exhlbit behavlour whlch
may be carclnogenlc~ In vlew of thls research, the
governments of varlou~ countries concerned have lssued
regulatlons rel~tlng to the use, stor~ge and destruction
of these products. The result has been that varlous
lndustrlal processes have been developed since then.
These processes generally lnvolve an o~eration of
lnclneration wlth an excess of ~lr or oxygen ln devlces
such ~s cement furn~ces or domestlc waste lnclner~tors.
Thus, one can clte:
'~ ~
' ~'' , :
~ . .
334
l - French Patent ~o. 2 323 432 for a c~talytlc
decomposltion process of halo~enated organlc compounds;
- Pntent W0 82/0~509 for a decomposltlon process
uslng a plasma burner operatlng at between 3000 and
4000 C;
- Patent W0 80~022116 for a decomposltion process
uslng a fused salt and an oxygen source;
- European Patent 0 060 089 for a decomposltlon
process wlth the nld of a reagent comprlslng a
polyglycol and an alkallne hydrox~de ln the presence of
oxygen.
Such processes have proven p~rtlally effectlve ln
the sense that when they are carried out they present
the maln risk of causlng the formntlon of hlghly toxlc
products of decomposltlon and restructurlng of the
molecule whlch lt ls wlshed to destroy, and everyone
kno~s the dangers created by a decompositlon product of
the dioxln type ln the case of the PCBs whlch ls
obt~lned by degradation of the chlorinated
molecules ln the presence of oxygen. We should also
mentlon the very slgnlflcant formatlon of A volume of
llberated gas of the types CO2 and N0~, a fact whlch
necesslt~tes approprlate treatment equlpment.
Thus, the present lnventlon alms to prevent these
dlsadvantnges and provldes a process for destroylng
toxlc organlc molecules wlthout the rlsk of the dangers
llsted Above, ln partlcular the formatlon of hlghly
toxic degradatlon products, whlle at the same tlme
reducln~ to a minlmum the volume of llberated gns, thls
process belng characterlzed ln that lt comprlses as lts
maln stage a vapo-cracklng operatlon.
Accordlng to an especlally advnntageous ~ethod to carry
out this ~rQcessthls vapo-cracklng ls effected ln a
reduclng medlu~ ln the presence of hydrogen.
128933~
-- 3 --
l Accordlng to an ~dv~ntngeous method of conducting
the prooess, the quantlty of hydrogen chosen ~s the
reducing medlum ls between 1 nnd 1.5 Nm~/~g of the
product to be tre~ted. The pr~sence of a reduclng medium
of thls type results ln the preventlon of oxld~tlon of
the organlc products le~dlng to hlghly toxlc dloxln-type
by-products ln the case of the destructlon of ~ compound
of the PCB famlly.
The tempernture of the reactlon ls adv~nt~geously
10 between 1000 nnd 1500-C.
Characterlstlcnlly, the process ls effected by an
lnJectlon of superheated steam and oxyhydrogen
combustlon.
Advant~geously, the quantlty of hydrogen needed for
thls oxyhydrogen combustlon corresponds to an excess of
2 to 10 % ln relatlon to the stolchlometrlc condltlons.
Thus, in carrying out of thls process, the
lnventlon covers all equlpment comprlslng R chamber for
combustton and mlxlng wlth the admlsslon of hydrogen,
oxygen and water vnpour; at least one reactor to recelve
the mlxture resultlng from thls combustlon and the
product to be treated and, at the exlt from thls
reactor, statlons for the condensatlon and treatment of
solld, llquld and gaseous reactlon products.
Other characterlstlcs nnd advantnges of the
lnventlon wlll emerge more clearly from the followlng
descrlptlon made wlth reference to the att~ched Flgure
lllustratlng ln a hlghly diagrammntlc ~anner an equipment
for carrying out the process of the lnventlon.
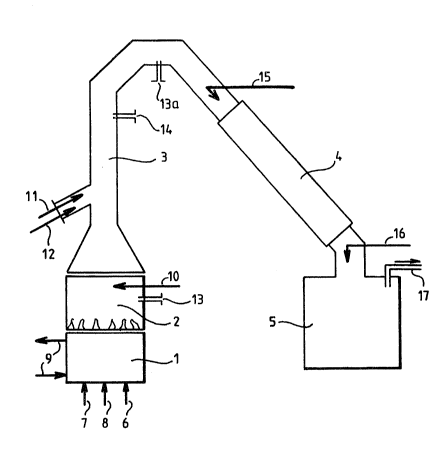
The equlpment shown ln the Flgure comprlses a known
dlffuslon burner l, a combustlon Rnd mlxlng chamber 2,
reactor 3, a condenser 4, ~ tank 5 for storlng the
condensed products ~nd ~ pipe 17 ~or evncuatlng the gas
to a conventlonal treatment devlce whlch 1~ not shown.
~2893:~4
-- 4
l The diffuslon burner 1 comprlses a plpellne 6 for
ln~ectlng hydrogen or nltrogen and a plpellne 7 for
ln~ectlng oxygen or alr. These two plpellnes supply a
plurallty of dlffusers. The dlffusion burner 1 also
comprises ~ plpellne 8 for lnJectlng propane and oxygen
supplylng the pllot llght and a clrcult 9 for supplylng
w~ter to ensure coollng of the burner ~acket.
The combustlon ~nd mlxlng chamber 2, posltloned
above the burner 1 ~nd comprislng a supply of w~ter
vapour 10, ensures the adJustment of the temperature and
the resldence tlme.
In the body of the reactor 3 there ls effected the
lnJectlon of the product 11 to be treated, for example
pyralene, ~nd of water vapour 12 servlng as a carrier
~nd purglng gas. The product 11 ls admltted ln an
ultrasonlc resonance inJector requlrlng an adduct secondary
fluld, for example water vapour 12. Thls allows
pulverlsatlon to be lmproved and thereby the efflclency
of the reactlon. The chamber 2 and the reactor 3
comprise ~pertures 13 and 13a for thermocouples and
manometers for controlllng operatlon.
The condenser 4 comprises ln lts upper part a water
feed plpe 15 ensurlng the condensatlon of the gas-
coollng water and an lnlet 16 for ~ baslc product, for
example dlluted sodlum hydroxide allowlng the
neutralisatlon of the hydrogen chlorlde gAS formed ln
the case of the treatment of chlorlnated products.
The operatlon of the apparatus comprlses the
followlng st~ges:
- start-up:
. purglng of the plpellne 6 of hydrogen wlth an
lnert gas such as nltrogen by dllutlon under pressure,
. establlshment of coollng 9,
.
334
1 . establlshment of flow of compressed ~lr lnto the
dlffusers throu~h the plpellne 7,
. start-up of the pilot llght,
. establlshment of hydro~en flow - at the outset a
S f1fth of the nomlnal flow,
. replacement of the compressed alr by the oxygen ln
the plpellne 7,
. adJustment of the hydrogen and oxygen flows ln a
stoichlometrlc manner durlng a flrst phase,
. introductlon of the water vapour lnto the
chamber 2,
. extlnction of the pllot llght,
. puttlng into thermal operatlon for one hour;
- reactlon:
. the destructlve reactlon occurs in the reactor 3,
whlch ls preferably made of material reslstant at a
temperature of 1500'C, for example refractory stalnless
steel. The product, for example the pyralene, is
lnJected lnto a plpellne 11 wlth the ald of a pump. The
ultrasonlc resonance lnJector used requlres an auxlllary
fluld, ln the present case w~ter vapour superheated to
120-C by a heatlng shaft. An aperture 13 for a
thermocouple measures the ln~ection temperature ln the
reactor, another aperture 13a allows the pressure to be
me~sured. Flnally, an aperture 14 makes posslble the
removal of the gases for analysls.
The hydrogen~oxygen reactlon ensures the calorlflc
supply of the vapo-cr~cklng whlch, by dllution of the
products by an lnert vapour, favours the decomposition
of the molecules;
- recovery of the liquid, solid and gaseous
dlscharges;
. an lnJectlon of water 15 completes the
purlflc~tlon of the gases and the inJectlon of dlluted
33~''L
fi
l sodlum hydroxide 16 ensures the neutrallzatlon of the
hydrogen chlorlde gas resultlng from the reactlon of the
hydro~en wlth the chlorlne llberated by the
destructlon of the chlorlnated or~anic comDounds ;
. a tank 5 permlts the storage of the condensed
llqulds whlle the ~ases are dlrected ~fter debubbllng to
a conventlonal depollutlon system (not shown) whlch
comprlses at lts outlet a propane~air excess gas burner
for burning the excess hydrogen and the light
hydrocarbons.
The followlng Example ls glven by way of
lllustratlon and ln no way llmlts the present lnventlon.
Examp,Le
Tr~al carried out wlth pyr~le~e
200 g of pyralene used ln transformers and
containlng 60% polychlorblphenyl and 40%
trlchlorobenzene were lnJected lnto the reactor.
Chromatographlc me~surements have made lt posslble to
show that 99.9985'~, of the pyralene was destroyed.
The by-products of decomposltlon comprlse notably
naphthalene, blphenyl, phenanthrene, fluoranthene, and
heavy hydrocarbons.
The llberated gases comprlse excess hydrogen and
llght hydrocarbons: fractlon C2/C3; these gases are
burned by the excess gas burner but may advantageously,
ln large units, be used as a source of heat wlth a vlew
to the productlon of water vapour necessary to the
process.
~o tr~ce of dloxln was detected at the analysis
threshold of the method, l.e. 2 ppm, <parts per mllllon~
for the by-products ~nd less than 15 ppt ~parts per
trllllon) for the purlfylng waters.
The descriptlon of the present lnventlon has only
been glven by way of lllustratlon ~nd ls in no way
.
':
12139339~
,7
l llmltlng; ln fact lt is capable of belng varled ln
dlverse ways wlthln lts scope and of allowlng treatment
of all llquld, gaseous and solld organlc products, ln
partlcular:
- pyralene cleanslng solvents, especlally freon,
- products <lubrlcatlng olls and other solvents)
contamlnated radloactlvely by metal salts partlcularly
of the ceslum and cobalt types, for e~ample
sclntlllatlon llqulds such as 1,5-dlphenyloxazole, 1,4-
dl-2- (5-phenylo~azolyl) benzene etc. The metal lons
formlng chelates, thls process decomposes these
molecules, ensures thelr mlneralizatlon and the
dlssolutlon of the radloactlve metal salts whlch, once
ln aqueous phase, may then be easlly concentrated and
treated by conventlonal processes of the nuclear
lndustry.
The appllcatlons envlsaged thus relate to the
treatment of waste from the electrlcal (transformers,
condensers), chemlcal or lndeed nuclear lndustrles.
Moreover, lt wlll be clearly understood that the
sources of hydrogen sultable for carrying out the
process can be any. Thus especlally, thls hydrogen can
be provlded by gas from coklng plants, flue gas or
domestlc ~as, the quantltles of these gases used ln the
process dependlng, of course, on thelr hydrogen content
in order to fulfil the conditions of operation set forth
in the pres`ent description concerning the quantity o~
hydrogen required.