Note: Descriptions are shown in the official language in which they were submitted.
~289S~9
ENER Y-SAVING TypE ZINC _LECTROLYSIS METHOD
Field of Invent_on
I'his invent.:i.on relates to the met.hod of zinc
electrolysis which saves energy by adding sulfur dioxide and
a sma.ll. quantity of iodide ions to the anolyte, that play a
catalytic function in the o~idizing reaction of sulfur
dioxide at the anode instead of o~ygen evolution reaction t SO
that the electrol~sis ma~ proceed under a lower voltage.
Background o _the~ Inyention
There are two kinds ot' known zinc manufacturing
methods the dry process and the wet process. Of these, based
on the electrolytic collection of sulfllric acid-zinc bath,
the wet process is adopted in most countries.
In the known sulfuric acid-zinc bath electrolysis
different electrochemical reactions take place at the cathode
and anode.
Renction formulAs And electrode potentials at
equilibril.lm state are as follows:
Cathode: Zn{ 2 + 2e ) Zn E = -0.76V t1)
Anode : 2H20 > o2 + 4H{ + 4e E = 1.23V (2)
Accordingly, the cell voltage at eq-lilibrium state is
only 0.76 V + 1.23 V = 1.99 V, but the reaction proceeds in
fact with 3.5 V at the current density of 5n mA/cm2 due to
o~gen over-voltage.
`;~'`3
1;~89S09
While the theoretical energy consumption is 1,630
kwh/t at the equilibrium cell voltage of 1.99 V, it is
2,870 kwh/t at the actual voltage of 3.5 V. When the loss
due to electric current efficiency, the loss resulting from
the conversion of alternating current to direct current and
the loss due to conductor resistance between the rectifier
and electrolyzer are put together, the energy of
approximately 3,500 kwh is required per metric ton of zinc
electrolyzed.
The much higher cell voltage required as compared with
the theoretical value, if attributable to the remarkably
high anodic reaction over-voltage of 0.84 V, where as the
cathodic reaction over-voltage is 0.06 V and almost
negligible.
Thus, the greatest disadvantage of the known zinc
electrolysis method lies in the tremendous energy
consumption of the anodic reaction. The huge amount of
oxygen generated from the anode in the electrolytic
production has no particular uses but rather has such side
defects as vaporizing the solution and causing the
corrosion of electrodes.
Such an anode over-voltage can be decreased using
sulfur dioxide which is a known anodic depolarizing medium.
Reference for this may be had to A.J. Appleby and B.J.
Pichon, Electroanalytical Chem., 95, 59 (1979). The
oxidizing reaction of sulfur dioxide and the zinc
electrolyzing reaction in the acidic solution are as
follows:
1289~9
Anode : S02 + 2H20 > HS0~- ~ 3H' + 2e E = 0.12V (3)
Cathode: Zn~ 2 + 2e > Zn E = -0.76V (1)
Consequently, the zinc elect,rol,vtic voltage in the
sulfur dioxide solution is only 0.76 V + 0.12 V = 0.88 V, a
decrease of 1.l V from the cell voltage in the conventional
sulfuric acid-zinc bath, with no oxygen over-voltage
occurring either.
Summary of the Invention
This invention relates, instead of' merely oxidizing
the sulfur dioxide solution which usually proceed.s very
slowly to the method of allowing faster oxidation of sulfur
dioxide at a lower electric potential by adding a small
quantity of iodide ions ~potassium iodide or iodine) which
play a catalytic function in the oxidation of the sulfur
dioxide solution.
Brief Descri~tion of the DrawinRs
Fig. l~a) is a cross-sectional view of cathode and
nnode of an electrolyzer with one separator from among the
zinc electrolyzers of this invention.
Fig. l(b) is a cross-sectional view of cathode and
anode of an electrolyzer with two diaphragms f'rom among the
zinc electrolyzers of this invention.
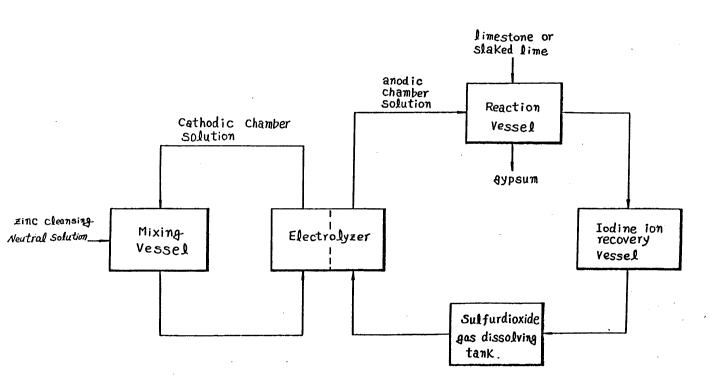
Fig. 2 is the process flow sheet for zinc
electrolysis of this invention.
1289509
Detailed DescriPtion of the_Invention
In accordance with the invention, solutions are
C preferably chosen *~ to be 1.5M su1fllric acid + sulfur
.- dio~ide + iodide ions in the anodic chamber and 1.5M sulfuric
acid + 0.8M zinc in the cnthodic chamber, while the
electrolysis is conducted at 50 mAJcm2 current density by
using a porous graphite anode and an aluminum cathode. The
electrolytic voltage is about 2.0 V, a voltage drop of 1.5 V
as compared with the field operation voltage of the known
method.
When the electrolysis is conducted without the
catalyst II-) ~n*~ the anodic solution, the electrolytic
voltage is about 2.5 V, a voltage drop of approximately 1.0 V
as compared with the field operation voltage of the known
method.
Accordingly, a voltage drop of about 0.5 V can be
expected by adding a small quantity of catalyst instead of
simply usin~ sulfur dio~ide solution.
The oxidizing reaction of sulfur dioxide in the
catalytic solution is attributable to the catalytic reaction
in the oxidation of triiodide ions (I3-) and iodine (I2)
produced by reaction formulas ~I) nnd (5) at the lower
electric potential. (G.S. Calabrese and M.S. Wrighton, J.A.
C.S., 103 121), 6273 (1981)).
1~39S09
3I- > l.~- + 2e E = 0.536 V i4)
2I- -~ I2 + 2e E = 0.621 V (5)
SO2 + 2H20 + I3- ~ H2SO~ + 2H' + 3I- (6)
S(j2 + 2H20 + L2 - > 1~2SO4 + 2H' + 2I- (7)
Preferably condit.ions in the oxidizing reaction of
sulfllr dioxide are G.OOlM-O.1~ iodide ion concentration, -
0.5M-2. OM sulfuric acid concentration, O.lM-2. OM sulfur
dioxide concentration and 20C-40C temperature.
When less expensive iodine per unit weight of iodide
ion is used as the catalyst, instead of potassium iodide, the
iodine oxidizes sulfur dioxide to be reduced to iodide ions,
thus producing the same effect as the method using potassium
iodide, with the catalyst cost also decreased.
The cell voltage in this invention is 2.0 V, while
t.he energy consumption is 1,640 kwh/t. When the loss due to
electric current efficiency, the loss resulting from the
conversion of alternating current to direct current and the
loss due to conductor resistance between i.ts rectifier and
eleet.rol~zer are combined, the energy of about 2,000 kwh is
~0 required per metric ton of zinc electrolyzed.
The present invention requires about 57 percent of
the energy consumption required in prior methods which
represents a saving in energy of approximatelv 1,500 kwh per
metric ton of æinc electrolyzed.
~ 1289509
According to this invention, sulfur dio~ide is
o~idized to produce s~llfuric acid in the ~nodic chamber, thus
increasin~ the concentration. G~psum is produced bv the
reaction of this anodic chamber solution with limestone or
slaked lime. The anodic solution, after filtration, can be
reused thus not only producing gypsum, but also ensuring a
continuous process with no consumption of iodide ions. (M.
, ~ JY~ rk
Grayson and D. Eckroth, ~ t-Othmer encyclopedia of chemical
technology, ~1,443 (1978)).
H2SO4 + CaC03 or Ca~OH)z--~CaSO~ . 2H20 ~8)
Moreover, since sulfur dio~ide in the anodic solution
reacts with slaked lime to produce gypsum, the sulfur dio~ide
in the produced sulfuric acid poses no problem.
SO2 + Ca(OH)2 - -- ~ CaS03 . 1/2 H20 (9)
2CaS03 . 1/2 H20 + 02 + 3H20-- ~ 2CaSOs . 2H20 (10)
The presence of iodide in catholyte for long term
electrolysis, which can be accumulated b~ diffusion throu~h
separator from the anodic chamber, dose not disturb the
cathodic process. but rather it may help to produce a
porefree zinc deposition on cathode. This observation was
confirmed also several times b~ adding a small quantity of
iodide to catholyte.
In this invention, when the current densit~ is raised
to 100 mA/cm2 for operation. double the worlcin~ current
density of the Icnown method. the volta~e is nt the level of
-- 6 --
. . ~ .~..~
. ~
1289509
2.2 V, whereas when the working current density is raised
to 100 mA/cm' in the known sulfuric acid-zinc bath
electrolysis process, the voltage not only rises greatly
(to 3.8 V) but also Pb-Ag anode dissolves due to the high
voltage which causes the electrodeposition of Pb on the
cathode, thus adversely affecting the purity of the
product. Also as the anodic potential becomes higher, more
NnO, electrodeposits on the anode. This lowers the electric
conductivity of the anode thereby causing the voltage to
rise.
On the other hand, since the anodic over-voltage
increase due to an increase in the current density is
relatively small and the anodic potential is lower in this
invention, the dissolution of graphite anode and the
electrodeposition of MnO, do not occur. When the working
current density is doubled by this invention, the
productivity is doubled, thus the equipment installation
and other expenses as compared with the known electrolysis
a~e, h~t~
process ~h~reduced to~ e~.
As mentioned above, this invention has the advantage
of not only drastically reducing the energy required for
zinc electrolysis, but also producing gypsum with sulfuric
acid manufactured at the anode and providing double the
productivity by doubling the working current density.
1289509
Example 1
The electrolysis was conducted for 20 hours at the
current densi.ty of 50 mA/cm2 in the electrol~tic bath (a~ of
Fig. 1 by fixing the interelectrode distance between the
porous ~raphite anode and the aluminum cathode at five
centimeters, while maintaining the anolvte at 1.5M sulfuric
acid + lM sulfur dioxide + O.OlM potassium iodide and the
catholyte at 1.5M sulfuric acid + 0.8M zinc. Here, the
anolyte and the catholyte were continuously recycled in order
to prevent the lowering of the concentration of sulfur
dioxide and zinc, while a battery diaphragm was used for the
separator.
- The electric current efficiency of the obtained
electrolytic zinc was 89 percent, and the cell voltage in the
electrolysis showed 2.0 V.
1289~9
Example 2
The electrolysis was conducted in accordance with the
electrolytic conditions and electrolysis method of Example
1, while the anolyte was maintained at 1.5M sulfuric acid
+ lN sulfur dioxide + O.lM potassium iodide and the
catholyte at 1.5M sulfuric acid + 0.8M zinc.
The electric current efficiency of the obtained
electrolytic zinc was 89 percent, and the cell voltage in
the electrolysis showed 1.9 V.
Exam~L~_~
The electrolysis was conducted in accordance with the
electrolytic conditions and electrolysis process of Example
1, while the anolyte used was maintained at 1.5M sulfuric
acid + lM sulfur dioxide + O.OlM potassium iodide and the
catholyte at 1.5M sulfuric acid + 0.8M zinc + O.OlM
potassium iodide.
The electric current efficiency of obtained
electrolytic zinc was 91 percent, and the presence of
iodide ions in the catholyt did not disturb cathodic zinc
deposition, but helped to form porefree electrolytic zinc.
The cell voltage here also showed 2.0 V.
E~m~le 4
The electrolysis was conducted for 20 hours at the
current density of 50 mA/cm' in the electrolytic cell (b) of
Fig. 1 by fixing the interelectrode distance between the
porous graphite anode and the aluminum cathode at six
centimeters, while maintaining the anolyte at 1.5M sulfuric
acid + sulfur dioxide + O.OlM potassium iodide.
1289509
and the catholyte at 1.5M sulfuric acid + 0.8M zinc.
Here, the anolyte (the anodic chamber solution) and
the catholyte (the cathodic chamber solution) were
continuouæly recycled in order to prevent the lowering of the
concentration of sulfur dioxide and zinc, while a battery
diaphragm was used for the separator.
The electric current efficiency of the obtained
electrolytic zinc was 90 percent, and the electrolytic
voltaqe in the electrolysis showed 2.1 V.
g