Note: Descriptions are shown in the official language in which they were submitted.
s,j~
PROCESS FOR FORMING METAL-CERAMIC COMPOSITES
B kground of the Invention
The present ;nvention comprises a process for the in-situ
precipitation of ceramic material in a metallic matrix, wherein the
ceramic comprises a boride, carbide, oxide, nitride, silicide, etc.,
of one or more metals other than the matrix metal. The matrix
metal, moreover, may constitute an alloy of two or more metals.
For the past several years, extensive research has been devoted
to the development of metal matrix composites, such as aluminum
reinforced with carbon, boron, silicon carbide, silica, or alumina
fibers, whiskers, or particles. Metal matrix composites with
excellent high temperature yield strengths and creep resistance have
been fabricated by the dispersion of very fine (less than 0.1
micron) oxide or carbide particles throughout the metal or alloy
matrix. The production of such dispersion-strengthened composites
is convent;onally accomplished by mechanically mixing metal powders
of approximately 5 micron diameter or less with the oxide or carbide
powder (preferably 0.01 micron to 0.1 micron). High speed blending
techniques or conventional procedures such as ball milling may be
used to mix the powder. Standard powder metallurgy techniques are
then employed to form the final composite. Conventionally, however,
the ceramic component is large, i.e. greater than 1 micron, due to a
lack of availability, and high cost, of such small particle size
materials. Further, in many cases where the particulate materials
are available in the desired size, they are extremely hazardous due
to their pyrophoric nature.
Alternatively, molten metal infiltration of a ceramic mat has
been used to produce composites. In some cases elaborate particle
coating techniques have been developed to protect ceramic particles
from molten metal during molten metal infiltration. Techniques such
as this have resulted in the formation of silicon carbide-aluminum
composites, frequently referred to as SiC/Al, or SiC aluminum. This
approach is suitable for large particulate ceramics (e.g. greater
than 1 micron) and whiskers. The ceramic material, such as silicon
carbide, is pressed to form a compact, and liquid metal is forced
~9~5~7
into the packed bed to fill the intersticies. Such a technique is
illustrated in U.S. Patent 4,444,603, of Yamatsuta et al, issued
April 24, 1984.
The presence of oxygen in ball-milled powders used in the prior
art metallurgy techniques, or in molten metal infiltration, can
result in oxide formation at the interface of the ceramic and the
metal. The presence of such oxides will inhibit interfacial binding
between the ceramic phase and the matrix, thus adversely effecting
ductility of the composite. Such weakened interfacial contact can
also result in reduced strength, loss of elongat;on, and crack
propagation.
Internal oxidation of a metal containing 3 more reactive
component has also been used to produce dispersion strengthened
metals, such as internally oxidized aluminum in copper. For
example, when a copper alloy containing about 3 percent aluminum is
placed in an oxidizing atmosphere, oxygen may diffuse through the
copper matrix to react with the aluminum, precipitating alumina.
This technique, although limited to relatively few systems, has
offered a preferred method for dispersion hardening.
In U.S. Patent 2,852,366, of Jenkins, it is taught that up to
10 percent by weight of a metal complex can be ;ncorporated into a
basis metal or alloy. The patent teaches blending, pressing, and
sintering a mixture of a base metal, a base metal compound of the
non-metallic complexing element, and an alloy of the base metal and
the complexing metal. Thus, for example, the reference teaches
mixing powders of nickel, a nickel-titanium alloy, and a
nickel-boron alloy, pressing, and sintering the mixed powders to
form a coherent body in which a stabilizing unprecipitated "complex"
of titanium and boron is dispersed in a nickel matrix.
Precipitation of the complex phase is specifically avoided.
In recent years, numerous ceramics have been formed using a
process referred to as self-propagating high-temperature synthesis
(SHS), which involves an exothermic, self-sustaining reaction which
propagates through a mixture of compressed powders. Generally the
3~ SHS process takes place at super atmospheric pressures, and is
ignited by electrical impulse, thermite, or spark. The SHS process
s~9~ ~
-- 3 --
involves mixing and compacting powders of the constituent elements,
and igniting the green compact with a suitable heat source. On
igni tion, sufficient heat is released to support a sel f-sustaining
reaction, which permits the use of sudden, low power initiation of
5 high temperatures, rather than bulk heating over long times at lower
temperatures. Exemplary of these techniques are the patents of
Merzhanov et al. In U.S. Patent 3,726,643, there is taught a method
for producing high-melting refractory inorganic compound by mixing
at least one metal selected from groups IV, V, and VI of the
10 Periodic System with a non-metal such as carbon, boron, silicon,
sulfur, or liquid nitrogen, and heating the surface of the mixture
to produce a local temperature adequate to initiate a combustion
process. In U.S. Patent 4,161,512, a process is taught for
preparing titanium carbide by ignition of a mixture consisting of -
80-88 percent titanium and 20-12 percent carbon, resulting in an
exothermic reaction of the mixture under conditions of
layer-by-layer combustion. These references deal with the
preparation of ceramic materials, absent the presence of a binder.
Similarly, U.S. Patent 4,431,448 teaches preparation of a hard
20 alloy by intermixing powders of titanium, boron, carbon, and a
Group I-B binder metal, such as copper or silver, compression of the
mixture, local ignition thereof to initiate the exothermic reaction
of titanium with boron and carbon, and propagation of the ignition,
resul ting in an alloy comprising titanium diboride, titanium
25 carbide, and the binder metal.
This reference, however, is limited to the use of Group I-B
metals such as copper and silver, as binders, and requires ~ocal
ignition. Products made by this method have low density, requiring
subsequent compression and compaction.
30 Summary of the Inventlon
It is an object of the present invention to provide an
inexpensive method for forming composite materials, consisting of
finely dispersed particulate ceramic or intermetallic materials in
metal, metallic alloy, or intermetallic matrices.
~305~7
-- 4 --
It is a further object of this invention to provide d method
for dispersion hardening of metals and alloys. It is a particular
object of this invention to provide a method for the formation of
submicron titanium diboride particulates in an aluminum matrix,
without the necessity for utilizing expensive submicron starting
materials.
The present invention relates to a process for the in-situ
precipitation of up to about 95 percent by volume of a ceramic
material in a metallic matrix, wherein the ceramic comprises a
boride, carbide, oxide, nitride, silicide, aluminide, selenide,
sulfide, or germanide, of a metal other than the matrix metal. It
has been fGund that by mixing the constituents or elements of the
desired ceramic material with a solvent matrix metal, and heating to
a temperature at which substantial diffusion and/or solvation of the
reactive elements in the matrix can occur, typically, close to the
incipient melting point of the solvent matrix metal, a solvent
assisted reaction, which is generally exothermic, can be initiated.
This solvent assisted reaction results in the extremely rapid
formation and dispersion of finely divided particles of the ceramic
material in the metal matrix. ~hile this invention may be
associated with pure metal systems, it is also applicable to alloys
wherein at least two alloying elements react to form a ceramic
precipitate in a matrix of the metal or an alloy thereof.
Brief Description of the Drawings
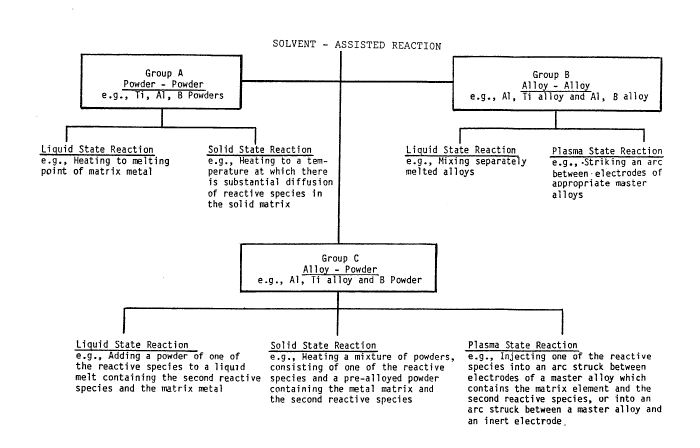
Figure 1 represents a schematic presentation of various
reaction modes and states within the scope of this invention.
Figure 2 represents a schematic presentation of two of the
basic reaction modes of the present invention.
Figure 3 represents a typical time-temperature profile of the
solvent assisted reaction.
Figure 4 constitutes a photomicrograph of a dispersion of
titanium diboride in aluminum, prepared in accordance with the
present invention.
~L~6~V~97
-- 5 --
Description of Preferred Embodiments
The present invention is particularly directed to a novel
process for the in-situ precipitation of fine particulate ceramics,
or intermetallics, such as refractory hard meta7 borides or
alum;nides, within metal and a110y systems to produce a metal matrix
composite having enhanced mechanical properties, such as high
elastic modulus, high-temperature stability, and improved wear
resistance. However, the process described may also be employed for
producing larger particles of the ceramic material in the chosen
matrix, up to the point at which such larger particles result in
component embrittlement, or loss of ductility, etc. Such improved
properties offer weight-savings in stiffness limited applications,
higher operating temperatures and associated energy efficiency
- improvements, and reduced wear in parts subject to erosion. A
specific application of such materials is in the construction of
turbine engine components, such as blades.
Broadly stated, the present invention constitutes a method
whereby ceramic forming elements react in a solvent metal to form a
finely-divided dispersion of the ceramic material in the solvent
metal matrix. Surprisingly, it has been found that the reaction
commences, or is initiated, at a temperature far below the
temperature conventionally associated with the reaction sought. In
accordance with the present invention, the ceramic-forming
constituents most easily combine at or about the melting temperature
of the solvent metal, and the exothermic nature of this reaction
causes a very rapid temperature elevation or spike, which has the
effect of melting additional metal, simultaneously causing the
further reaction of the ceramic constituents.
Alternatively, in systems where the reactive elements have
substantial diffusivity in the solid matrix metal, the reaction may
be initiated at temperatures well below the melting point of the
matrix metal. Thus, a solid state reaction is possible, wherein a
liquid state may or may not be achieved.
Exemplary of suitable precipitates are the borides, carbiaes,
oxides, nitrides, oxynitrides, sulfides, oxysulfides, silicides,
aluminides, selenides, sulfides, and germanides. Suitable elements
~L~ 37
inc1ude all of the elements which are reactive to form ceramics,
including, but not limited to, transition elements of the third to
sixth grGups of the Periodic Table. Particularly useful
ceramic-forming or intermetallic compound forming constituents
include aluminum, titanium, silicon, boron, molybdenum, tungsten,
niobium, vanadium, zirconium, chromium, hafnium, yttrium, cobalt,
nickel, and iron. Additional elements suitable for inclusion as
ceramic constituents include magnesium, carbon, oxygen, nitrogen,
sulfur, selenium, tantalum, gallium, manganese, germanium, zinc,
arsenic, antimony, lithium, beryllium, and thorium.
As the matrix metal, or solvent metal, one may use any metal
capable of dissolving or sparingly dissolving the constituents of
the ceramic phase, and having a lesser capability for dissolving the
ceramic precipitate. Thus, the matrix metal component must act as a
solvent for the reaction species, but not for the desired ceramic
precipitate. It is to be noted that the matrix metal acts primarily
as a solvent in the process of the present invention, and that the
constituents of the ceramic precipitate have a greater affinity for
each other than either has for the solvent metal.
Suitable matrix metals include aluminum, gallium, nickel,
titanium, copper, vanadium, chromium, manganese, cobalt, iron,
silicon, molybdenum, beryllium, zirconium, tungsten, antimony,
germanium, palladium, rhodium, ruthenium, silver, gold, platinum,
magnesium, lead, zinc, tin, niobium, tantalum, hafnium, bismuth, and
alloys of such metals. When alloys are utilized, one may retain the
beneficial properties of said alloys, and increase the modulus of
elasticity, high temperature stability, and wear resistance,
although some loss of ductility may be encountered in certain soft
alloys. For example, 7075 aluminum alloy, containing from about 5
percent to about 40 percent by weight titanium diboride, responds to
age-hardening in tne same fashion that the 707S alloy alone does,
but exhibits a substantial increase in modulus of elasticity, higher
temperature capability, greater high temperature stability, and
extremely high wear resistance. Further, the composites of the
present invention may be fabricated in conventional fashion, by
forging, extruding, rolling, machining, etc.
Varying amounts of ceramic may be incorporated into the
composite material, depending upon the end use and the properties
~L~9~) S~3~
desired in the product. For instance, for dispersion strengthenea
alloys having high moaulus, one may utilize a preferred range of
from about 10 percent by volume to about 25 percent by volume (15
percent to 40 percent by weight, depending on specific ceramic phase
and matrix employed). However, the ceramic volume fraction for
dispersion strengthening may be varied considerably, so as to
produce a composite with the desired combination of properties,
within the range of from about 1 percent by volume up to the point
at which ductility is sacrificea to an unacceptable extent. In
contrast, cermets of up to about 95 percent or more by volume of
ceramic material in the metal matrix may be produced. The primary
determining factors in such utilization as cutting tools will be the
wear and chipping resistances of the composite material produced.
Preferred ranges for cermet materials will, of course, be dependent
upon the desired end use. It is possible to effectively tailor the
composition to achieve any desired properties by controlling the
proportions of the reactant and solvent materials. For instance,
the modulus of elasticity may be approximated by the "Rule of
Mixtures" such that the appropriate proportions of starting
materials are employed.
Utilizing such procedures, it is possible to prepare "Master
Concentrates", containing a ceramic phase, which may be utilized to
introduce the ceramic phase into a diluent metal in controlled
fashion. Thus, one may prepare a master concentrate of a high
percentage of titanium diboride in aluminum, and add metal to
achieve a composite having the desired concentration of ceramic. In
fact, an ingot of unreacted components, such as titanium, boron, and
aluminum, may be utilized as a grain refiner for aluminum. When
such an ingot is introduced into the molten aluminum, the reaction
produces nuclei for grain refining. A wide range of such materials
for different metallurgical applications exists.
A comparison of various properties of composites made in
accordance with the present invention with those of a conventional
SiC/aluminum material is shown in Table I. As can be seen,
considerable improvements in modulus of elasticity and elongation
are achieved.
597
TABLE I
I I I Typical
Materia1 I Hardness I Modulus I Elongation
I Rockwell C I lO~ lbs/in2 1 %
7075 Al 175 1 10.3 1 7
7~91-20 vol% I90-lO0 1 16.7 1 1.8
SiC (Forging)
7075-15 vol% I90-lO0 1 18 1 4.0
TiB2 (Forging)
Three basic reaction modes have been identified in accordance
with the present invention as illustrated in Figure l. In the first
mode, Group A of Figure l, the starting materials constitute
individual powders of each of the soivent metal and the individual
constituents of the ceramic to be formed. For example, one may
react a mixture of aluminum, titanium, and boron, to form a
dispersion of titanium diboride in an aluminum matrix.
In the second mode of the invention, Group B of Figure l, one
may react individual alloys of a common metal, one such alloy
comprising an alloy of the solvent metal with one of the
constituents of the ceramic, and the other comprising an alloy of
the solvent metal and the other constituent of the ceramic. As an
example, one may react a mixture of aluminum-titanium alloy with
aluminum-boron alloy, to form a dispersion of titanium diboride in
aluminum. This alloy-alloy reaction route may, in some cases, be
relatively slower than the elemental route, yet may offer economic
advantages because the alloys utilized can be cheaper than the
elemental powders. In this case, tne preferred form is that of
separately melting master alloys containing the chosen elements and
mixing them in the molten state, forming a mass in which the
dissolved matrix element acts as a liquid solvent for the
constituents of the ceramic.
The third reaction mode, Group C of Figure 1, constitutes a
combination, or intermediate, of the first two modes discussed
~9~59~
g
above. Thus, one may react a prernixed alloy conta;ning one reactive
species and the matrix metal, with an elemental powder of the second
reactive species, such as combining an aluminum-titanium alloy with
elemental boron powder. This reaction mode may be relatively more
expensive than the alloy-alloy reaction mode, but offers a more
rapid reaction, which in turn permits formation of finer grain
precipitates than obtainable by the alloy-alloy route. However, the
a11oy-elemental powder reaction mode could be relatively less
expensive, although slower, than the elemental powder mode, in most
cases.
Moreover, the three reaction modes may occur in different
physical states. While all three reaction modes function, to
varying degrees, in all three physical states, each of the first two
basic modes of the solvent assisted reaction may preferably occur in
either of two states. The elemental powders can preferably react to
form the desired second phase via diffusion of the reactive species
through the liquid solvent or, in cases where diffusion is very
rapid, in a solid state. Similarly, in the case where two alloys
are used, each containing an alloying element constituting a
reactive component, the reaction can preferably occur in the liquid
state, or in a plasma state achieved by striking an arc between
electrodes of the two alloys. The third reaction mode, however, may
function in all three states. That is, the reaction of an alloy
with an elemental powder may be conducted as a liquid state
reaction, a solid state reaction, or in the plasma state. The
preferred range of reaction states is shown in Figure 1.
It is also to be noted that comp1ex compounds, as well as
plural ceramic phases, may be precipitated by these three reaction
modes. Thus, solvent matrix combinations with complex ceramics such
as titanium zirconium boride, may be prepared.
It is particularly to be noted that the prior art teaches that
the combination of elemental powders, particularly of a coarse
particulate size, would yield intermetallic compounds. In fact,
conventional techniques for forming intermetallics involve reacting
a mixture of powders of titanium and aluminum, to form titanium
aluminide, and a mixture of powders of boron and aluminum to form
1~90S9~
-- 10 --
aluminum diboride. Thus, one would anticipate that a mixture
comprising powders of titanium, aluminum, and boron would yield an
aggregate agglomeration of titanium aluminide, boron aluminide, and
possibly, titanium diboride. In contrast, the present invention
provides for the formation of one finely dispersed precipitate in a
matrix of the third component. It is important that the ceramic
precipitate material is not soluble in the solvent metal, while the
constituents of the ceramic, individually, are at least sparingly
soluble in the solvent metal. Thus, the exothermic dispersion
reaction mechanism depends upon a certain amount of each ceramic
forming constituent dissolving and diffusing in the solvent metal,
and while in solution (either liquid or solid state), reacting
exothermically to form the insoluble ceramic, which precipitates
rapidly as a very fine particulate. This is illustrated in
Figure 2. The solvent metal, provides a medium in which the
reactive elements may diffuse and combine. Once the initial
reaction has occurred, the heat released by the exothermic reaction
causes additional diffusion of reactive components in the solvent
metal, and drives the reaction to completion.
~O This is illustrated by Figure 3, which demonstrates a
temperature trace of a mixture constituting 22 percent titanium, 10
percent boron, and 68 percent aluminum powders. The mixture was
placed in a crucible, and placed in a furnace which was heated to
735C to heat more than a localized portion of the mixture to
initiate reaction, unlike known techniques. The temperature
readings were obtained by a thermocouple placed against the
specimen. A plateau exists on the temperature trace, indicating
absorption of energy, attributable to localized melting, and the
initiation of substantial diffusion of the reactive components. The
temperature trace then illustrates the solvent assisted initiation
of the reaction of the reactive constituents, and the very rapid
temperature increase associated therewith. As shown by the
temperature trace illustrated in Figure 3, extremely high
temperatures may be achieved in very short periods of time. During
this time frame, essentially all of the reactive components in the
solvent metal react to form the insoluble ceramic, which immediately
precipitates.
~L~3~)~j9 7
The cool-down period following initiation of the reaction and
consumption of the reactive constituents is believed critical to
achieving very small grain size, and limiting grain growth. It is
known that at high temperatures, it is possible for the ceramic
particles to grow, e.g. by agglomeration. This should also be
avoided, because of the negative effect of large particle sizes on
ductility. The cool-down or quenching of the reaction is, in a
sense, automatic, since once the ceramic forming constituents are
completely reacted, there is no further energy released to maintain
the high temperatures achieved. However, one may control the rate
of cool-down to a certain extent by control of the size and/or
composition of the mass of material reacted. That is, large thermal
masses absorb.energy, and cool down more slowly, thus permitting
growth of larger particles, such as may be desired for greater wear
resistance, e.g. for use in cutting tools. It is recognized that if
it is desired to rapidly cool the reaction mass to an intermediate
temperature, one may achieve this by the introduction of a stream of
cool inert gas, such as helium. Thus, the temperature may be
rapidly reduced from the maximum temperature attained to a
temperature where grain growth is minimal. In terms of temperatures
that cause coarsening of the particle size, temperatures in the
region of 1000C are not generally believed to have substantial
impact on particle growth. However, at temperatures in the region
of 1600C and higher, grain growth can occur over extended time
periods. For example, silicon nitride may begin to grow at 1600C,
over a period of days, whereas titanium diboride will not begin to
exhibit grain growth below about 1~00C. The incidence of particle
growth will depend on the particular ceramic phase being formed.
Further, slowly cooling the reaction product is in some cases
advantageous, since rapid quenching of some metal-ceramic composites
as are formed by this invention may result in a high incidence of
fracturing due to thermal stresses.
The reaction temperature has generally been found to be
relatively close to the melting temperature of the solvent metal
utilized in liquid state reactions. For example, in the production
of titanium diboride in an aluminum matrix, the reaction proceeds at
a temperature around 650C, or very close to the melting point of
~ 9 O 5 ~7
the aluminum solvent. It should be noted that in the absence of a
solvent metal, the reaction of titanium and boron to form titanium
diboride was not observed to proceed below a temperature of about
1200C, and generally will produce large crystallites having a
particle size at least as large as the starting materials. ~hile
one need not actually reach the melting temperature, one must
achieve a temperature where localized or incipient melting occurs,
or a state where substantial diffusion of the reactive species in
the solvent metal can occur. It is observed that, in some cases, as
one increases the temperature it is possible for the starting
constituents to diffuse into the matrix solvent metal, forming an
alloy therewith having a lower melting temperature than the matrix
metal, and thus lowering the reaction initiation temperature.
It is noted that with respect to impurities, the solvent metal
may be alloyed at will, while in the reactive constituents, a
limited amount of alloying element or impurity may be tolerated. It
has been found that an impurity with which a reactive constituent
forms a stable compound may not exceed approximately-10 percent by
volume. For example, the presence of magnesium in boron will
inhibit the formation of titanium diboride in an aluminum matrix by
forming a magnesium-boron complex on the surface of the boron
particles, thus limiting diffusion of the boron in the matrix. The
presence of magnesium in the aluminum, however, does not have this
effect. That is, boride forming materials in the boron itself will
inhibit the desired dissolution or diffusion of the boron and its
subsequent reaction to form titanium diboride.
It is also to be noted that in accordance with the present
invention, one may cause the complex precipitation of a plurality of
systems. There is no real limit on the number of ceramic phases
3Q which can be precipitated. Thus, it is possible to precipitate
complex phases, such as Ti(Bo 5C0 5), or alternatively, to
precipitate a mixture of titanium diboride and zirconium diboride in
an aluminum matrix, in accordance with the reaction:
Ti + Zr + 4B + Al -~ TiB2 + ZrB2 + Al.
It is also possible to achieve a low temperature solvent
assisted reaction in a metal ma~rix which has a high melting
temperature by alloying the high melting solvent metal with a minor
31~9 O'3 9 7
proportion of a lower melting metal. For example, titanium diboride
has been precipitated at very low temperatures, such as 620C, in
cobalt, chromium, and nickel matrices, by including up to 20 percent
by weight aluminum powder. In the absence of the alloying aluminum,
the reaction requires temperatures of about 800C or greater.
It is further possible to prepare ceramic-ceramic composites,
i.e. a dispersion of particulate ceramic material in a ceramic
matrix. This may be accomplished by the in-situ precipitation of a
ceramic phase from a solvent/matrix metal, followed by conversion of
the matrix metal to a ceramic by reacting it with a secondary
gaseous reactant.
In accordance with the present invention it has been found that
the powders need not be compacted prior to firing, but doing so
allows easier diffusion and thus initiation at lower temperatures.
For instance, loose powder mixtures of aluminum, titanium and boron
do not react until approximately 660C whereas highly compacted
powders react at approximately 620C. This is due to localized
incipient melting, and increased diffusion, which are possible when
the powders are in close proximity.
Porosity of the final composite can be minimized by a vacuum
degassing operation prior to initiation of the reaction. The degree
of vacuum applied and temperature of the degassing ste,p is
determined purely by the kinetics of evaporation and diffusion of
any absorbed moisture or other gasses. High vacuum and elevated
temperatures aid the degassing operation. In the case of titanium,
aluminum, and boron mixtures, however, the pre-reacted compact must
not be exposed to temperatures above 300C for prolonged periods of
time as this will induce the volatilization of some components and
induce the formation of titanium aluminide by solid state
diffusion. This is undesirable because it forms as large plates
which are detrimental to mechanical properties, and also reduces the
chemical driving force for the formation of the titanium diboride.
Conversion of titanium aluminide to titanium diboride in the
presence of boron and aluminum can occur if the components are held
at temperatures above the melting point of aluminum.
~905~7
- 14 _
The starting powders must be protected from extensive oxidation
due to exposure to the atmosphere, as this will restrict the
diffusion of the components into the metal matrix, and the reaction
should preferably be carried out under an inert gas to avoid
S oxidation at high temperatures.
The particle size of the powders utilized in the elemental
powder mode does not appear to be critical. It has been found,
however, that particle size of the ceramic reaction product is
dependent upon heat-up rate, reaction temperature, cool-down rate,
and crystallinity and composition of the starting materials.
Appropriate powder sizes may range from less than 5 microns to more
than 200 microns. For economic reasons, one normally may utilize
larger particle size powders. It has been found that the particle
size of the precipitated ceramic phase in the matrix may vary from
less than about 0.01 microns to about 5 microns or larger, dependent
upon the factors cited above.
It has been found that some specific reactant properties have a
greater impact than powder particle size on the particle size of the
ceramic produced. For example, thè use of amorphous boron results
in the precipitation of a finer grain size titanium diboride than
does the use of crystalline boron of otherwise comparable nature.
The precipitation of specific grain size ceramic phase may be
selectively controlled by proper control of starting composition,
temperature of reaction, and cool-down rate.
The production of relatively porous composites, containing high
concentrations of the ceramic phase, provides a technique for the
production of high purity ceramic powders of desired particle
sizes. This may be achieved by selective dissolution of the matrix
metal, yielding a suspension of ceramic powder in the dissolution
medium. If the medium is an appropriate acid or caustic solution,
one may then recover the finely divided ceramic powder. If, on the
other hand, the dissolution medium is another metal, one may obtain
a dispersion of a ceramic phase in a matrix in which it may not be
directly precipitated. For example, titanium can be reinforced by
precipitating titanium diboride in aluminum, and subsequently
dissolving the titanium diboride/aluminum composite in titanium.
i~O ~;~37
- 15 -
Examples 1 and 2 illustrate the precipitation of fine particles
of titanium diboride in aluminum by powder-powder mode reactions, in
the liquid state and in the solid state.
Example l
A mixture of 34 percent by weight of titanium powder, 16
percent by weight of boron and 50 percent by weight of aluminum was
isostatically compacted to 38,000 pounds per square inch. The
compacted artifact was then heated in a furnace set a~ a temperature
of B00C. Upon reaching approximately 670C, a rapid increase in
temperature to approximately 1250C was noted. The rate of increase
in temperature was very rapid (greater than 900C per minute)
followed by a fast cool down rate of approximately 400C per
minute. On subsequent examination the sample was found to contain a
fine dispersion (0.1 - 3 microns) of titanium diboride particles in
an aluminum matrix. A photomicrograph of this composite is shown as
Figure 4.
Example 2
A mixture of 20.5 percent titanium, 9.5 percent boron and 70
percent by weight cobalt was isostatically pressed to 40,000 pounds
per square inch and heated in a furnace. A highly exothermic
reaction occurred at 800C, with a temperature rise to about
1600C. Subsequent x-ray analysis identified the presence of
titanium diboride in a cobalt matrix. It was shown here that if
sufficient diffusion of the reactive species can occur, the
initiation temperature can be below the melting point of the matrix
metal, which in this case is 1495C; and the reaction may occur in
the solid state.
The alloy-alloy reaction, in the liquid state, is exemplified
by Examples 3 and 4, described below.
Example 3
Two separate aluminum alloys, one containing lO percent
titanium, and the other 4 percent boron, by weight, were placed in
35~7
-- 16 -
an alum;na crucible and heated to 1400C for one hour under an argon
atmosphere. Mixing of the alloys occurred through diffusion and
thermal effects. The experiment was performed at 1~00C to ensure
that all of the titanium and boron were dissolved, thereby allowing
the titanium diboride to fully precipitate, being considerably less
soluble than the individual elements. Subsequent SEM/EDS analysis
Ot the metal matrix composite produced identified a submicron TiB2
- dispersion in the aluminum matrix. Whi1e this experiment was
intended to completely dissolve the titanium aluminide and aluminum
boride such that all the titanium and boron were held in solution in
the aluminum, it was recognized that because of its limited
solubility titanium diboride would precipitate at any temperature
above the melting point of the solvent metal, even if not all of the
alloys were dissolved.
Example 4
To support the contention that it was not necessary to fully
dissolve the titanium and boron in the alloys, three equivalent
experiments to Example 3 were performed, except that the maximum
temperatures achieved were limited to 1200C, 1000C and 800C
respectively. As in Example 3 finely dispersed TiB2 particles
~ere observed in the aluminum matrix, in all cases.
The following Example 5 describes the production of
aluminum/titanium diboride composites by alloy-alloy reaction, in
the plasma state.
Example 5
In this example the reaction is achieved by striking an arc
between two electrodes, each containing the metal matrix and one of
the reactive species, in a closed vessel. The relative positions of
the electrodes is adjusted to achieve the passing of the arc. The
said electrodes may also be rotated as to achieve even melting.
Atomizing the homogenized molten metal into powder can be achieved
in air, but is preferably performed in a non-reactive atmosphere
such as an inert gas or a vacuum. Alternatively, the molten metal
~9o~
may be collected in a heated container placed below the arc to
obtain an ingot.
The reaction between the ceramic constituents within the arc
yields a ceramic compound which is mixed with the matrix metal. Due
to the very rapid heat up and cool down rates associated with this
process,-a very fine distribution of ceramic particles in the
metallic matrix is achieved. Striking an arc in the above manner
between two electrodes, one of which contains aluminum and titanium
and the other aluminum and boron, results in the formation of a fine
dispersion of titanium diboride in a molten aluminum droplet which
solidifies as it drops through the inert gas. The powder thus
produced can be subsequently processed by conventional powder
metallurgical techniques. In a different variant of this process,
the molten metal droplets are collected in a heated crucible to
produce an ingot for con~entional metal working operations. In yet
another variant the droplets are collected on a chilled rotating
drum to produce metal-ceramic flakes.
The following example teaches the influence of amorphous boron
on the particle size of titanium diboride precipitated in an
aluminum matrix.
Example 6
An identical mixture (but for the use of amorphous boron
instead of crystalline boron) as that described in Example 1 was
prepared (i.e. approximately 34% by weight of titanium, 16% by
weight of boron and 50~ by weight of aluminum), compacted, and
heated in a furnace. At a temperature of about 620C, a rapid
exotherm was noted. Subsequent examination revealed the presence of
very fine 0.01 - 1.0 micron titanium diboride particles in an
aluminum matrix.
The high concentration composite prepared in either Example 1
or Example 6 is suitable for use as a master concentrate for
subsequent dilution to achieve dispersion hardening of metal/alloy
systems.
'i~3 0~;~37
- 18 -
The following examples teach the use of one matrix solvent to
induce precipitation in a second, higher melting point matrix.
Example 7
A mixture of 16 percent by weight of aluminum, 56 percent by
weight of chromium, 20.6 percent by weight titanium and 9.4 percent
by weight of boron was compacted and subsequently heated in a
furnace. On attainment of a temperature of about 620C, a rapid
reaction occurred, resulting in a temperature increase to over 800C
and melting of the chromium matrix. The temperature-time curve
showed a double peak, ;ndicating an exothermic reaction in aluminum
(which typically occurs between 600-680C) and a subsequent reaction
in the chromium. The lower melting matrix therefore acted as a "low
temperature initiator" for the reaction, which released heat and
induced further reaction in the higher melting compound. Titanium
diboride in a matrix of chromium-aluminum alloy was identified in
the composite produced.
Example 8
A mixture of 2006 percent by weight of titanium, 9.4 percent by
weight boron and 70 percent by weight of chromium was compacted to
40,000 pounds per square inch, and then heated in a furnace. A
rapid exothermic reaction was noted at approximately 880~C. This
temperature is 260C above that at which the same proportions of
titanium and boron react when 20 percent of the matrix is made up of
aluminum. As in the case of Example 7, titanium diboride was
identified by x-ray analysis.
The following examples illustrate various characteristics and
aspects of the invention as discussed hereinabove.
Example 9
An experiment was conducted whereby zirconium diboride was
precipitated in a matrix of copper. A mixture of approximately 24
percent zirconium, ll percent boron, and 65 percent aluminum powders
by weight was compacted, and, then heated in a furnace. On
3Li~9~
19
attainment of a temperature of 830C, rapid reaction occurred to a
temperature maximum of about 970C. Subsequent X-ray and SEM
analysis showed the presence of zirconium diboride in a Cu matrix.
Example lO
An experiment was conducted, whereby molybdenum disilicide was
precipitated in an aluminum matrix. A mixture of approximately 7.5
percent silicon, 12.5 percent molybdenum, and 80 percent aluminum
powders by weight was compacted and subsequently heated in a
furnace. On attainment of a temperature of approximately 640C, a
sudden exotherm was noted. Subsequent X-ray and SEM analyses
confirmed the presence of molybdenum disilicide in an aluminum
matrix.
Example 11
A mixture of 20.4 percent titanium, 9.6 percent boron and 70
percent by weight of lead was compacted to 40,000 pounds per square
inch and then heated to 450C. No exotherm was noted and subsequent
x-ray analysis identified only the unreacted elemental powders.
This behavior illustrates, as others have shown, that there is no
solubility of boron in lead and thus no diffusion of boron in the
20 lead can occur to react with the titanium. In contrast to such
behavior, a silicon, titanium and lead mixture does produce titanium
disilicide in lead, as both silicon and titanium have a finite
solubility in lead wh;ch enables diffusion and reaction to occur.
Example 12
A mixture of nickel, aluminum, titanium and boron in the
stoichiometric proportions for the formation of nickel aluminide
(Ni3Al) and titanium diboride tTiB2), i.e. 10 percent by weight
aluminum, 62 percent by weight nickel, 19 percent by weight titanium
and 9 percent by weight boron, were compacted to 40,000 pounds per
square inch, and then heated in a furnace. Upon reach;ng 620C, a
rapid exotherm was noted which, subsequent analysis by x-ray
diffraction and scanning electron microscopy identified as resulting
from the formation of titanium diboride particles in a nickel
- 20 -
aluminum matrix. It was evident from this experiment that a ceramic
phase, e.g. titanium diboride could be precipitated in a
intermetalllc phase, e.g. nickel aluminide, provided the affinity of
the ceramic-forming species for each other is greater than either
has for the two elements making up the intermetallic matrix.
Additional experiments were conducted to produce a variety of
metal matrix composites, as set forth in Table II which follows.
s~ ~
- 21 -
_ . e:
E
,
~ _ o ~ ~ ~ ~ ~ ~ ~
L 1~ O _ _ _ _ _ _ r O _ O
~_
N O O _ _ O
E o o o
- o ~ ~
E ~ ~ o o u7 o co o o ~s: ~ o ~ Lr) ~ o o
x E ~ ~ co co o 1~ 0 -- O 1`
~ I~ I~ _ ~ ~ ~ ~ N Z 2 t~ ~ O) Z O CD
O l_
C C,~ ._
CO
._
~ a~ o
-- E o o O o o o o '1~ o o o ~: o r~
2 co t:o o a~ c~ a7 a ~ z z
._
C~
~D ~ I ~ ~O
^ I ~ O~
_
C~ ~ o ~
O I
I ~ _ _
I o ~ ~
o o ~ t " ~ ~, ~ o
O O O r~ 0: ^ D 1
Lt~ N et ~D I ^ ~ C~l ^ ^ I
a:l ~ O ~C ~ O C cc C ~: I c c c
a _ ~ ~o ~ ~ C ~ 1~
~Q C ~ _ X
C~ O r ~I~~O I
a~ ~ O ^ I I ~ I I I ~-
C
N O e~ _ ~ ~ ~ et
~J ~J N _ ~ ~ _
x ~3 3 3 3
._ ~ ~ C~~ C C C ~
s. C _ _ _ _ _ _ _ :~: ~ ~ ~ ~ ~ ~ _
:E 2 ~ ~ ~ ~ ~vl ~ ~
-
,,_3 O 1-~ o o o o o c~.l o _ o r~
cn c~ m tD c~ m m a~
~ O u~
)5~g7
- 22
~ .
O ~
a~ ~ _ , , ,~, ",, ~ q
~ L~ ~ O C O-- C~l-- r _ ~
a. ~ o o c o o o o o o o o
~ o
E ~ 'r ~: o~ So o o o o O O o O
x l~i ~ ~ OC~l O
2 2 CO C Z ZZ l~ r~ r
C ~ .
.~00.
~ ~ ~ ~C O ¢ ~ 0 0 0 0 0 0 0 0 0
~ E 2 2Lo 2 2 2 --
_ ~_
O O ~ o o
I I 1~ a~ I o ~ o o C~J O O
r~ I r~ ¢
~ ~ . N e~ O . ~ X
3 I -- I ~ ~n ~ _ 1_, c~l ~ ~ 1
vl I I I I~) ~
~ ~ ~ ^ ^ ^ ~ ~
_ IJ~ ~ 1~N C~.l O~ O o U'~
1~C~J _ C~l _ _ N N 00C~J X CO C~' Lf~
L L L ~ O O C N ~ q
ty ~ ~ ~ S~ ~ ~ 3 ~
¢ ~ 3 3 3 ~
_ ~ O
3 t~ o o
_~D ~ , O C~l 1~ U~ ~ O o
F al I I -- IC`J I I O C~l I O U:~ I I I I
L ~5 m m Lln æO m mO ~ Lm 1~ mC~J m a: m m
.
~0~37
E u~ u~
~_ o
~ ~ I L~ 0~ _ o
L 1~1 N O
O O O O O
V
E O
E ~a: o Oo ~: ~o o o o O ~: O
x E~ 1~ od 0 ~ 0 ~ o o
zo~ _ ~ z z1~ _c~J 0 _ z ~
_ _ _ ~
c~
o o
O o o ~C ~o o o o O ~: o
E ~ I~r~ u:7 N ~ r C~
2 0 01~ 2 =1` o o 1~ 2 _
C _ _ _
_.
r~
O ~ ~O
^ o C~
J
a~t~ 0 ~ _ ~ ~ôC~ e~ ~^ ~ ^ ^ ^
3 ~L~ ~ _I ~_ _ I~ Ic~ ~ cr
_ ~m a! ~ x mI I I e~
~
N ~ 0 0 N~ O O O O
~ ~ N~t _ Lr)1~ _ 0 C`J U~
~_ L L L LC O i t _ ~ _ _
L ~ ~ ~ ~ ~~ ~ ~ ~ ~ L a~ al
L
. ~ ~
_ ~ O
3 O L O O
~ _ ~ ~ I ~ ~~ O ~ O O
E a.u~ I I N ~`J IC~l O D C~J I I I
s ~mL m L L OO
Il'~ O ~n
~9~s~
- 24 -
al E
_, ~ ~ _
._ _ 01
~-~ o o O
E .
'-- C O S o .cl ec o o o O O O'6 0 E O ~
x Et~ ~ ~ '~ ~ I~ o a:) o o ~ ~ o ~; o z
o o
.
a~ ~ O~C O ~ 0o o o o o ~: o o o
.~, E 2 ~Z 2 o C~ o a ~L~o~ Z ~'
_ _
~ u7 In
l l l
. ~ o _
O I et O~^ ~ ~^ O t ~ ~ ~
O I ~ I 1~1 1 1 1~ o ~ O
I~ cn z z ~0 ~ ~1. " C
._
r~. ~D ~ 0 ~ ~) N X
O~ O ~ I~ O ~0 0 0 0 ~0 CO l~ ~ l~ l_
~ I I I I I I I I I I l l l l I ~
qJ ~ 2 3
X
a~ ~ C O O O O O O
Z Z Z I I I ~
~: o
o 2~ 0
3 ~ C~ O O
O _ I O ~ ~ O O G O ~0 O ~0 I
E a~ ~ ~ I O O ~ ~ ~ ~ O , ~ ~ o
1 InN ~_ I N I N N C~
~ O U~
-
)59
- 2s -
E , ., ._ o
_ ~ ~ ~
L N 1~')Lt~
~ ~ .
S~
E O o o
E c, E ~ ¢ ~ C~ o o
x Eao ~ s_ ~ ~ ~ ~ ~ CO
O ~ aJ Z Z Z Z d-
C C~
o o
~ EO ~ o O'~ ~ o O
' O o o o O
O 1-- ~ D O 1~ 1
I I I O 1`
o ~ - , ~ ~ Q
^ ^ ^ ~ ^ N 01:)
O^ ~ ~ 0 . . L
~ ~ ~ . c~l _ _ E
-- - m m a:m ~ -- _ v
~ m m ^ ^ ^
C ^ ~O ~ d-~ et ~ C~l
~ o . . ..^ ~ . .
~ ~ 2~C o æa, ~ ~ - ~c
I I I I I I I I I ~ O
3 i~ .~
a~ ~
+ + ~ cn
X ~ ~ ~ ~ ~ - ~
O N 111 ~-- -- ^ ~ ~ .0 al
E ~
n~ ~ c C ~_ ~ ._
= .~ ._ ._ ~_ C ~ V~
L~ O 0
~O o C V
o a~
, . s ~ n.
.~
~Q + ~ E `~ c 4_
3 + + o o o _ ~
_ O O O O Od' _ ~ ~ ~ a~ ~ _ ~ o
~ ~ ~ ~ ~C~l ~ I I I ~ 3 z ~ 2
E aJ ~ l ~ N C~ N C~J
_ m m m mm m ~ ~ ~
c~ 3 i~ ~ ~ ~ ~ ~ ~ + +
u~ o ~
~05~
- 26 -
It is noted that the present invention has a number of
advantages over methods taught by the prior art. For example, this
invention circumvents the need for submicron, unagglomerated
refractory metal boride starting materials, which materials are not
commercially available, and are often pyrophoric. This invention
also eliminates the technical problems of uniformly dispersing the
ceramic in the solvent metal, and avoids the problem of oxide
formation at the ceramic/metal interface during processing.
Further, the present invention yields a metal matrix composite with
a ceramic phase precipitated therein, having superior hardness and
modulus qualities over currently employed additives such as silicon
carbide. The metal matrix composite also has improved high
temperature stability, in that the ceramic phase is not reactive
with the metal matrix. Further, the metal matrix composite can be
welded without degradation of material properties, and possesses
superior corrosion resistance, when compared to any metal matrix
composites presently available.
It is understood that the above description of the present
invention is susceptible to considerable modification, change, and
adaptation by those skilled in the art, and such modifications,
changes, and adaptations are intended to be considered to be within
the scope of the present invention, which is ,set forth by the
appended claims.