Note: Descriptions are shown in the official language in which they were submitted.
,S d' ~ P,~ ~ c /1 ~
~9080S
Rechargeable Organic Electrolyte Cell
BACKGROUND OF T~E INVENTION
This invention relates to a rechargeable organic
electrolyte cell expected to be used as a power source for a
variety of small sized electronic apparatuses.
So-called organic electrolyte cells, making use of
lithium as the anode active material and an organic
electrolyte as the electrolyte, are low in self-discharging,
high in voltage and excellent in shelf life, so that they may
be used with high operational reliability for a prolonged
period of five to ten years. For this rèason, they are used
at present extensively in electronic `time pieces or as a
variety of memory backup power sources.
However, the presently used organic electrolyte cells
are primary cells, such that their service life is terminated
when used once so that they leave a lot to be desired
especially from economic considerations.
; For this reason, with the rapid progress in a variety of
electronic apparatuses, a strong demand has been raised for
rechargeable organic electrolyte cells that can be used
conveniently and economically for a prolonged time, and many
researches are being conducted for developing this type of
cells.
In general, me~al lithium, lithium alloys, such as Li-Al
alloys, electroconductive polymer materials, such as
~ 2~308~;
polyacetylene or polypyrrole, doped with lithium ions, or
intercalation compounds with lithium ions mixed into crystals
thereof, are used as the anodic material of the cell, while
an organic electrolytic solution is used as the electrolyte
thereof.
On the other hand, various materials have been proposed
as the cathodic active material. Examples of these materials
include TiS2, ~oS2, NbSe2 or V205, as disclosed in the
Japanese Laid-open Patent Publication No. 54836/1975.
The discharging reaction of the cell making use of these
materials proceeds as the lithium ions of the anode are
intercalated into the spacings between these materials,
whereas the charging reaction proceeds as the lithium ions
are deintercalated from these spacings towards the anode. In
other words, the charging and discharging proceeds by a
repetition of the reactions in which the lithium ions of the
anode make entrance into and exit from -the interlayer
spaeings of the cathode aetive material. For example, when
using TiS2 as the eathode active material, the charging and
discharging reaction may be represented by the formula
disehaxgingTiS2 ~ xLi ~ e C ~LiXTiS2 (I)
charging
With the conventional cathodic material, charging and
diseharging proeeeds by the above reaction. However, the
eonventional cathodic material has a defieiency that, with
;.:,,
~ ~oao~
the repetition of the charging and discharging reactions, the
discharge capacity thereof is decreased gradually. It is
because the lithium ions, once having made an entrance into
the cathode active material, tend to exit therefrom only with
i~ .
increased difficulties, such that only a limited fraction of
the lithium ions having made an entrance into the cathode
active material by discharging are returned towards the anode
by the charging reaction. In other words, the lithium ions
are caused-to rernain in the cathode in the form of LiXTiS2 so
that the number of the lithium ions taking part in the
ensuing charging reaction is decreased. The result is that
the discharge capacity of the cell af`ter the charging is
decreased and the cyclic service life characteristics of the
cell are correspondingly lowered.
OBJECT AND SUMMARY OF THE INVENTION
It is an object of the present invention to provide an
improved rechargeable organic electrolyte cell.
It is another object of the present invention to provide
a rechargeable organic electrolyte cell superior in charge-
discharge cycle characteristics.
It is another object of the present invention to provide
a rechaxgeable organic electrolyte cell which is charged and
discharge up to nearly full capacity of the active material
contained in the cell.
~ ccording to one aspect of the present inven-tion, there
~290~305i
is provided a rechargeable organic electrolyte cell which
comprises an anode containing Li, a cathode formed of LiMn204
and an organic electrolyte. The LiMn204 preferable has a
full width at half maximum between 1.1 and 2.1 of a peak at
2~ equal to 46.1 by X-ray diffraction analysis using FeK~
ray.
BRIEF DE~CRIPTION OF THE DRAWINGS
Fig. 1 is a chart showing charging and discharging cycle
characteristics of a rechargeable organic electrolyte
secondary cell making use of TiS2 and MoS2 as the cathode
material.
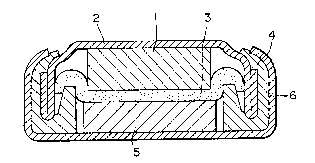
Fig. 2 is a diagrammatic sectional view showing an
exemplary rechargeable organic electrolyte secondary cell.
Fig. 3 is a char-t showing the result of an X-ray
diffraction analysis of LiMn204 syntheslzed from electrolytic
manganese dioxide and lithium carbonate.
Fig. 4 is a chart showing discharging characteristics of
an rechargeable organic electrolyte secondary cell according
to the present invention.
Fig. 5 is a chart showing charging characteristics of an
organic electrolyte secondary celi according to the present
invention.
Fig. 6 is a chart showing charge-discharge cycle
characteristics of an organic electrolyte secondary cell
according to the present invention.
~L290805
Fig. 7 is a chart showing the result of an X-ray
diffraction analysis of LiMn204 synthesized from electrolytic
manganese dioxide and lithium carbonate.
Fig. 8 is a chart showing the difference in the
discharging characteristics caused by the difference in the
full width at half maximum of the diffraction peak of LiMn204
employed in the cell.
Fig. 9 is a chart showing the difference in the charging
characteris-tics caused by the difference in the full width
half maximum of the diffraction peak of LiMnO4 employed in
the cell.
Fig. 10 is a chart showing the r~elation between the
discharging capacity of the non-aqueous electrolyte cell and
the sintering temperature of LiMn204.
Fig. 11 is a chart showing the result of an X-ray
diffraction analysis of LiMn204 synthesized from electrolytic
manganese dioxide and lithium carbonate.
Fig~ 12 is a chart showing an X-ray diffraction spectrum
of LiMn204 obtained by sinterinig manganese dioxide and
lithium iodide at 300 C.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
With a view towards achieving the above objects, the
present inventors conducted various and repeated
investigations into Einding a material less subject to
deterioration in the intercalation of the lithium ions so as
lX9~)8g)5
to be used as the cathode active material. As a result
thereof, we have obtained an information that a compound
LiMn2O4 having the splnel structure gives favorable results.
On the basis of such information, the present invention is
characterized in that it comprises an anode containing
lithium, a cathode formed essentially of LiMn2O4, and an
organic electrolyte~
The compound LiMn2O4 employed as the cathode active
material of the organic electrolyte cell according
to the present rechargeable invention may be easily obtained
by reacting lithium carbonate Li2CO3 and manganese dioxide
MnO2 by heating in 400 C or by reactin`g lithium iodide LiI
and manganese dioxide MnO2 by heati`ng in a nitrogen
atmosphere at 300 C. The compound LiMn2O4 itself is shown
for example in the United States Patent No. 4246253 as the
starting material for producing MnO2. However, when a cell
is prepared by using LiMn2O4 obtained in accordance with the
teaching of the United States Patent No. 4246253, that is, by
a method including sintering lithium carbonate and manganese
dioxide at a temperature of 800 to 900 C, as the cathode
active material of the cell, the capacity of the order of
only about 30 percent of the theoretical charging and
discharging capacity of the cell is obtained.
Above all, by using LiMn2O4 in which the full width at
half maximum of a diffraction peak at a diffraction angle of
s
46.1 in the case of in X-ray diffraction analysis using FeK~
rays is in the range between 1.1 and 2.1, as the cathode
active material of the rechargeable organic electrolyte cell,
the charging and discharging capacity nearly equal to the
theoretical capacity may be obtainèd. While LiMn204 may be
prepared by sintering lithium carbonate and manganese dioxide
in air, the full width at half maximum value of the
diffraction peak observed upon X-ray diffraction analysis is
changed by adjusting the sintering temperature. According to
the present invention, the compound LiMn204 in which the full
width at half maximum of the diffràction peak at the
diffraction angle of 46.1 in the` case of an X-ray
diffraction analysis using FeK~ rays is in the range of 1.1
and 2.1, is selectively employed. When the full width at
half maximum value is less than the above range, the desired
discharging are not achieved.
Lithium iodide may be used in place of lithium
carbonate, while the sintering may be performed in an inert
gas, such as nitrogen, instead of in air.
As the lithium containing material, employed as the
anode material, metal lithium, lithium alloys, such as LiAl,
LiPb, LiSn, LiBi or LiCd, electroconductive polymer
materials, such as polyacetylene or polypyrrole, doped with
lithium ions, or intercalation compounds with lithium ions
mixed into crystals thereof, such as TiS2 or MoS2 containing
0805
lithium in the intercalation spacings thereof, may be
employed.
As the electrolyte solution, non-aqueous organic
electrolytes may be employed in which a lithium salt is used
as an electrolyte and dissolved in an organic solvent.
Example of the organic solvent may include one or a
mixture of two or more of 1,2-dimethoxyethane, 1,2-
diethoxyethane, ~ -butyrolactone, tetrahydrofuran, 2-
methyltetrahydrofuran, 1,3-dioxolane or 4-methyl-1,3-
dioxolane.
Examples of the electrolyte may incIude one or a mixture
of two or more of LiCl04, LiAsF6, LiPF6,`LiBF4 or Li~(C6~5)4.
By using LiMnO4 as the cathode act~ve material of the
rechargeable organic electrolyte cell, the lithium ions that
have migrated towards the cathode by the discharging reaction
may be optimally deintercalated during the charging reaction.
In addition, when LiMn204 in which the full width at
half maximum of the diffraction peak at a diffraction angle
of 46.1 in case of an X-ray diffraction analysis using FeK~
rays is in the range between 1.1 and 2.1, is selectively
employed as the cathode active material of the non-aqueous
electrolyte cell, it hecomes possible to procure a charging
and discharging capacity of not less than 90 percent the
theoretical charging capacity of the material.
The description with reference to specific test examples
. ^ -:: '' '
.' ~ .
~2~0~(35
is given below. It is -to be understood that these examples
are for illustration only and are not intended to limit the
scope of the present invention.
OMPARATIVE EXAMPLE
The cycle characteristic of the Li/TiS2 or Li/MoS2
rechargeable organic electrolyte cell, making use of TiS2 or
MoS2 as the cathode active material, were investigated. The
results are shown in Fig. 1, from which it is seen that, wi-th
the rechargeable organic electrolyte cell making use of TiS2
or MoS2 as the cathode active material, the discharge
capacity of the cell is rapidly decreased after the
repetition of about ten charge-dischar`ge cycles, such that
the discharge current is only one half the`~original discharge
capacity of the cell. It is also seen that the discharge
capacity ~is continuously decreased with a further repetition
of the charge-discharge cycles.
EXAMPLE 1
; In accordance with the following production steps, a
button type cell shown in Fig. 2 was produced.
87 grams and 26 grams each of marketed manganese dioxide
and lithium carbonate were thoroughly mixed in a mortar and
the resulting mixture was heat-treated on an alumina boat in
a nitrogen gas at 400 C Eor 10 hours. The product obtained
after cooliny was subjected to an X-ray analysis, whereby a
chart of the X-ray analysis as shown in Fig. 3 was obtained.
~9~8~5
In comparison with the material represented by the formula
LiMn204 in the ASTM card, the chart was found to be
coincident completely with the X-ray diffraction chart for
LiMn204. Thus the material obtained by the above described
process could be identified to be LiMn204.
Then, 88.9 parts by weight of LiMn204 produced by the
above described process were admixed with 9.3 parts by weight
of graphite and then with 1.8 parts by weight of
polytetrafluoroethylene as the binder. The resulting mixture
was then press-formed into a pellet of 15.5 mm in diameter
and 0.3 mm in thickness under application of a pressure of 3
tons/cm2. The pellet thus produced was dried in vacuum at
300 C for five hours to a cathode pellet 5.
On the other hand, an aluminum foil of 0.3 mm in
thickness was punched to a disk shaped piece of 15.5 mm in
diameter, which was then spot welded to an anodic can 2. A
lithium foil of 0.3 mm in thickness was punched to a disk
shaped piece of 15 mm in diameter, which was then press
bonded onto the aluminum foil piece to form an anodic pellet
1 to be used as an anode.
On this anode was placed a non-woven propylene cloth as
a separator 3 and propylene carbonate with LiCl04 dissloved
therein at a rate of 1 mol/liter was added as an electrolyte
solution. A gasket 4 formed of a suitable synthetic material
was press fitted to the anode and the previously obtained
1 0
~;~90~305
cathode pellet 5 was placed on the separator 3. A cathodic
can 6 was placed over the cathode pellet and caulk for
hermetically sealing an openlng that may be present between
it and the gasket 4 to form an rechargeable organic
electrolyte cell having an outside diameter of 20 mm and a
thickness of 1.6 mm.
The sample cell obtained as above was subjected to a
discharge test through a resistor of 1 kiloohm. The
discharge curve shown in Fig. 4 was obtained.
The discharge reaction may be expressed by the following
reaction formula
Li+ + LiMn204 ~ e ~ 2LiM O
The completely discharged sample cel`l was then charged
with a current of 2 mA with the upper voltage setting of 3.1
V. The results are shown in Fig. 5. It is seen from this
figure that the charging voltage curve is extremely flat.
This possibly implies that deintercalation of lithium ions in
the charging reaction shown by the formula
2LiMnO2 ~ LiMn2O4 + Li+ ~ e
has proceeded smoothly.
The sample cell showing the charging and discharging
characteristics as described above was charged and discharged
repeatedly for investigating into cyclic charge-discharge
characteristics of the sample cell. It was seen that, as
shown in Fig. 6, deterioration in the discharge capacity due
129~80s
to the cyclic charging and discharging was not observed in
the least and the obtained rechargeable cell had truely
superior properties.
EXAMPLE 2 a
In the present Example 2, various LiMn2O4 samples were
prepared using various sintering temperatures and so-called
button-type cells were prepared with the use of -these samples
to investigate into the charge-discharge characteristics of
th~se cells.
First, in order -to produce LiMn2O4 having favorable
properties as the cathode active material of the organic
electrolyte cell, the sintering temperatures of LiMn2O4 were
changed variously to investigate into changes in the X-ray
diffraction peaks and in discharge capacities cause by these
changes in the sintering temperatures.
For producing the LiMn2O4 sample, 86.9 grams (1 mol)
and 18.5 grams (0.25 mol) each of marketed manganese dioxide
and lithium carbonate were mixed while being ground
thoroughly in a mortar. The resulting mixture was sintered
in air for one hour on an alumina boat at a sintering
temperature of 430 to 900 C.
The product was cooled and analyzed by the X-ray
di~fraction analysis using FeK~ rays and the measurement
conditions including the tube voltage of 30 kV, tube current
of 15 mA, measurement range of 2000 cps, scanning speed of
12
,:
.,
.
' ' ~' ., ,~
.. . . .
: . -: ,.. ~........... . . ;.
... ..
1/min., chart speed of 5 mmlmin., diffusion slit width of 1
and the light slit width of 0.6 mm. On collation with the
card index of the American Society for Testing Materials
(ASTM), the product could be identified to be LiMn2O4. Fig.
7 shows an X-ray diffraction spectrum of LiMn2O4 obtained at
a sintering temperature of 460 C, a~ an example. The full
width at half maximum oE the diffxaction peak at a
diffractlon angle of ~6.1 is 2.08, which is larger than
that of LiMn204 obtained by sintering at 800 to 900 C in
accordance with the conventional production process thereof.
The full width at half maximum data for LiMn2O4 obtained by
sintering at various other sintering temperatures are
summarized in Table 1.
Then, using the LiMn2O4 s~mples obtained at the
respective sintering temperatures as described above, organic
electrolyte cells such as shown in cross-section in Fig. 2
were prepared. 86.4 parts by weight of LiMn2O4 were thus
admixed with 8.6 parts by weight of graphite and 5 parts by
weight of polytetrafluoroethylene (Teflont m ) to a cathodlc
composition which was then formed a cathodic pellet 5 of 15.5
mm in diameter and 0.44 mm in thickness and having a weight
of 0.213 gram.
A marketed aluminum plate of 0.3 mm in thickness was
punched to a disk shaped piece of 15 mm in diameter and
bonded to an anodic can 2 by spot welding. A lithium foil of
1 3
~; .... . , ,1
: `:
0.18 mm in thickness was punehed to a disk shaped piece of15
mm in diameter and press bonded to the aluminum piece to an
anodic pellet 1 to form an anode.
Then a separator 3 was applied to the anode and a gasket
4 formed of a suitable synthetie material was fitted thereto.
Then, an eleetrolyte solution mixture of 1,2-dimethoxyethane
and propylene earbonate with LiCl04 dissolved therein at a
rate oE 1 mol/liter was introduced. The previously produced
cathodic pellet 5 was applied to the separator 3 and then
eovered by a cathodic ean 6, whieh was then eaulked for
hermetically sealing an opening or gap between it and the
anode to produce a so-called button type organic electrolyte
cell having a diameter of 20 mm and a thickness of 1.6 mm.
Using the LiMn204 samples prepared in this manner at the
various sintering temperatures, organic eleetrolyte cells A,
B, C, D, E, F, G, H, I, J and K were produeedO In Table 1,
these eell appellations are entered in assoeiation with the
sintering temperatures for the LiMn204 samples used in the
cells.
Investigations were made into charging and diseharging
eharaeteristies of the thus produeed organie eleetrolyte
eells A through K.
14
8~
TABLE 1
_ .
full width at
-ells sintering discharge half maximum at a
temperature capacity X-ray diffraction
angle of 46.1
_
A 430 C 24.0 mAH 1.80
B 450 25.9 2.10
C 460 25.6 2.08
D 480 24.9 1.91
E 500 23.5 1.55
F 520 20.3 1.10
G 550 17.4 0.93
ll 600 14.6 0.78
I 700 12.4 0.57
J 800 10.0 0.35
K 900 7.5 0.26
.
These organic electrolyte cells were respectively
connected to resistances of 1 kiloohm,and the discharge
characteristics were measured with the terminal voltage of
2.0 V. The results are shown in Table 8, wherein the cell
voltage V and the discharge time Hr are plotted on the
ordinate and the absissa, respectively. From this figure,
the mean discharge voltage may be read and converted into the
mean discharge current which may then be multiplied by the
duration of discharging until reaching the terminal voltage
to give the discharge capacity in terms of the ampere-hour
capacity, which is given herein by units of mAH since the
resistnace of 1 ]ciloohm is used in the present measurement
system. The discharge capacities obtained in this manner are
also shown in Table 1.
Then, with the terminal voltage being set to 3.1 V, the
~29~305
current of 4 mA was caused to flow through each of the thus
discharged cells, for measuring the charging characteristics.
The results are shown in Fig. 9, wherein the cell voltage V
and the charging time Hr are plotted on the ordinate and the
abscissa, respectively. The organic electrolyte cell
according to the present invention has extremely stable
charge discharge characteristics, as may be seen from Figs. 8
and 9 showing that the major portions of the curve for each
cell are flat, that is, do not show voltage changes with -the
charging time. This is an indication that intercalation and
deintercalation of lithium ions into and from the spacings
between the adjoining LiP~n2O4 layers occùr extremely promptly
and thus LiMn2O4 obtained in the above described manner has
superio properties as the cathode active material.
In Fig. 10, the relation between the discharge capacity
and the sintering temperature shown in Table 1 is shown. In
Flg. 10, the discharging capacity in mAH is shown on the
ordinate and the sintering temperature in C is shown on the
abscissa. It is seen from Table 1 and Figs. 8 and 10 that
the cells A, B, C, D, E and F have the excellent discharging
capacity of not less than 20 mAH and hence are may meet
practical demands and that the full width at half maximum
values of LiMn2O4 as the cathode active material of these
cells at an X-ray diEEraction angle of 46.1 are all within
the range of between 1.1 and 2.1. The full width half
16
maximum values may be controlled by changing the sintering
temperature of LiMn2O4, with the optimum sintering
temperature range being 430 to 520. It has been found that
the discharging capacity is gradually lowered when the
sintering temperature higher than the above range is
employed. The discharge capacity is similarly lowered when
the sintering temperature lower than the above range is
employed, such that, with the cell L making use of LiMn2O4
obtained by sintering at 400 C, the discharging capacity was
lowered to 19.1 mA~, as shown in Fig. 10. The X-ray
diffraction spectrum for this LiMn2O4 sample is as shown in
Fig. 11. It is found from this figure that, with the lower
sintering temperature of 400 C, parts of lithium carbonate
and manganese dioxide remain unreacted, so that desired
characteristics are not attained.
EXMAPLE 3
In the present Example, in preparing LiMn2O4, lithium
iodide was used in place of lithium carbonate shown in the
Example 1, while the sintering was performed in a nitrogen
atmosphere instead of in air.
50 grams (0.57 mol), 39 grams (0.29 mol) and 5.2 grams
each of marketed manganese dioxide, lithium iodide and
graphite were thoroughly mixed while being ground in a mortar
and the resulting mixture was press molded into a pellet
under a pressure of 3 tons/cm2. This pellet was placed on an
9V8~5
alumina boat and sintered in a nitrogen atmosphere at 300 C
for six hours. After sintered, the product was cooled and
washed with ethyleneglycol dimethylether. The product was
analyzed by X-ray diffraction analysis under the conditions
specified in the Example 2, and was identified to be LiMn2O4
on collation with the card index of ASTM. The X-ray
diffraction spectrum for this product is shown in Fig. 12.
The full width at half maximum value of a peak at a
diffraction angle of 46.1 was 1.57. In this figure, the
peak corresponding to graphite may also be seen in addition
to the peaks appearing in Fig. 7.
Then, to 95 parts by weight of L`iMn2O4 were added 5
parts by weight of polytetrafluoroethy`lene ~Teflon) as a
binder to give a cathodic composition. The ensuing
assemblying of the organic electrolyte cell was performed in
accordance with the method described in Example 2 to produce
the cell sample M. The discharge capacity of the cell sample
M was tested by a method according to the Example 2 and found
to be as high as 23.1 mAH.
EXAMPLE 4
In the present Example 4, LiMn2O4 was prepared by
sintering in a nitrogen atmosphere, as in Example 3, instead
of in air.
86.9 grams (1 mol) and 1~.5 grams (0.25 mol) each of
marketed manganese dioxide and lithium carbonate were
18
~.29~S
thoroughly mixed while being ground in a mortar. The
produced mixture was placed on an alumina boat and sintered
in a nitrogen atmosphere at 450 C for one hour. The product
was analyzed by the X-ray diffraction analysis under the
conditions stated in the Example 1 and thereby identified to
be LiMn2O4. The full width at half maximum value of the peak
at a diffraction angle of 46.1 was 1.60.
The ensuing assembling of the organic electrolyte cell
was performed by the method according to the Example 2 to
produce the cell sample N. The discharge capacity of the
cell sample was tested by a method according to the Example 2
and found to be as high as 22.9 mAH.
From the foregoing it is seen that,~ by using LiMn2O4 as
the cathode active material of the rechargeable organic
electrolyte cell, the lithium ions once migrated towards the
cathode during the discharging reaction may be optimally
deintercalated during the charging reaction, with the result
that the charge-discharge cycle life properties of the
rechargeable organic electrolyte cell may be lmproved
significantly.
In this manner there is provided an rechargeable organic
electrolyte cell suffering from only limited deterioration in
the cell capacity caused by repeated charge-discharge cycles
and hence superior in cyclic life characteristics.
When LiMn2O4 used as the cathode active material of the
1 9
rechargeable organic electrolyte cell has a specified full
width at half maximum of the diffraction peak at a
dlffraction angle of 46.1 in the range of 1.1 to 2.1, it
is possible to elevate the charge-discharge characteristics
of the cell obtained by using such cathode active material to
higher than 90 percent its theoretical capacity.
Since LiMn2O4 is a less costly material, it is not only
excellent from economic considerations as compared to
conven-tional cathode active materials, such as TiS2, MoS2,
NbSe~ or V2O5, but also contributes to energy saving in the
production process of the organic electrclyte cell.
. ' ' .