Note: Descriptions are shown in the official language in which they were submitted.
12~V~Z~::
~ETHOD OF PURIFYING EXHAUST GAS
The present invention relates to a dry method of
purifying exhaust gases wherein an absorbent of the Ca type
is used for absorbing and removing harmful acid substances
from the exhaust gas.
Hot exhaust gases discharged from boilers or
incinerators for waste materials usually contain 10 to 2000
ppm of harmful acid substances such as sulf~r oxides (SOx),
hdyrogen chloride (HCl) and hydrogen fluoride (HF). It is
required to remove such substances under pollution control
regulations. These harmful acid substances have~heretofore
been removed generally by the wet method wherein an absorbing
liquid or slurry containing an alkaline absorbent is brought
into direct contact with the exhaust gas as cooled to a
lower temperature to purify the gas. Although achieving a
high removal efficiency, this method has the problem of
involving difficulties in treating the resulting waste water,
necessitating reheating of the exhaust gas and being
expensive in equipment and running costs.
In view of the above problem, various methods have
been investigated as substitutes for the wet method. For
example, it has been proposed to adsorb harmful substances
by active carbon, followed by desorption, or to spray a
slurry of slaked lime into the exhaust gas as a semi~wet
'~
~9o9~
method. However, these methods still fail to achieve high
removal efficiencies. Although research was conducted on
a dry method wherein particles of a Ca-type absorbent
(quick lime, slaked lime, limestone, dolomite or the like)
are diffused through the interior of a hot furnace or flue,
this method has not been practiced except where the environ-
mental regulations are extremely slack as a special case,
because the absorbent is low in reactivity. For example,
the reactivity between the absorbent and Sx is up to about
20% if highest, while that of the absorbent with HCl is
not higher than 50%. It is generally expected that the
reactivity will improve with decreasing particle size,
whereas a reduction in the particle size does not lead to
a noticeable increase in the reactivity of lime.
An object of the present invention is to provide
a dry method of purifying exhaust gases which assures an
improved reactivity between a Ca-type absorbent, such as
slaked lime or quick lime, and harmful acid substances.
To fulfil the above object, the present invention
provides a method of purifying an exhaust gas having a
temperature in a range selected from the group consisting of
about 150 to 400C and about 900 to 1200C, characterized by
dispersing individually independent fine particles of a Ca-
type absorbent in a carrier gas to a concentration in the
range of about 50 to 500 g/Nm3 by a dividing-dispersing device
to which the carrier gas and the absorbent are supplied, the
--2
~ .
~9()~ZZ
absorbent being up to 10 microns in mean particle diameter,
introducing the resulting aerosol into the exhaust gas having
said temperature to uniformly mix the aerosol with the exhaust
gas and to produce a particle concentration in the exhaust gas
of about 1 to 20 g/Nm3 and thereafter introducing the exhaust
gas into a dust collector, whereby the fine particles of
absorbent absorbing harmful acid substances from the exhaust
gas are removed together with dust and soot.
The principle and embodiments of the present
invention will be described below with reference to the
accompanying drawings, in which:
Fig. 1 is a graph showing the relationship between
the mean diameter of particles and the apparent cohesiveness
thereof;
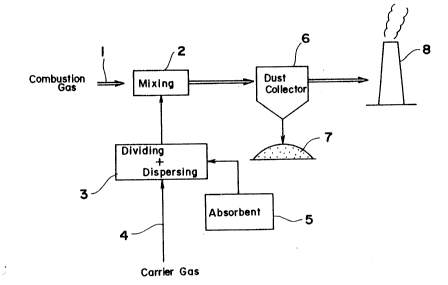
Fig. 2 is a diagram schematically showing an
embodiment of system for practicing the method of the
present invention;
; Fig. 3 is an enlarged fragmentary view in section
showing the system;
Fig. 4 is a view in longitudinal section showing
an injector which is an example of dividing-dispersing
device for use in the system;
Fig. 5 is a view in longitudinal section showing
an orifice tube which is another example of dividing-
dispersing device for use in the system;
Fig. 6 is a graph showing the relationship between
the mean particle diameter of a Ca-type absorbent (slaked
~J
lX909~2
lime) and the HCl removal ratio thereof; and
Fig. 7 is a graph showing the relationship between
the mean particle diameter of the absorbent (slaked lime)
and the Sx removal ratio thereof.
First, the principle of the present invention will
be described.
Generally, individually independent fine particles
(hereinafter referred to as "primary particles") of the order
of microns in size tend to agglomerate. This tendency
increases as the particle size decreases as shown in Fig. 1.
Accordingly, when particles of 1 to 2 microns in size are
sprayed into an exhaust gas through a mere pipe or nozzle as
in the conventional dry method, these primary particles form
secondary agglomerates before being dispersed into the
exhaust gas, so that the primary particles behave as coarse
particles in the exhaust gas. With the conventional method,
therefore, the absorbent failed to achieve noticeably improved
reactivity even when reduced in particle size.
On the other hand, it is known that secondary
agglomerates, when given a definite amount of dispersing
energy in a gas stream, are dispersed in the stream as divided
into individual primary particles. From this viewpoint, it
is useful to insert a Venturi (means for giving the dispersing
energy), for example, in an exhaust gas channel and to spray
an absorbent, which is already in the form of secondary
~9092Z
agglomerates, into the channel at a location upstream from
the Venturi. The absor~ent can then be divided into primary
particles before being uniformly mixed with the exhaust gas
to consequently remove harmful acid substances with an
improved efficiency. However, to divide secondary
agglomerates into the original primary particles, for example,
of up to 5 microns in size, it is required to use a greatly
constricted Venturi and to increase the velocity of the
exhaust gas to as high as several tens to 300 m/sec.
This entails a great pressure loss. Accordingly, to give
an increased velocity to the exhaust gas itself which originally
is in a large amount requires a great power consumption and
therefore is not practical. According to the present inven-
tion, an absorbent and a carrier gas are supplied to a
dividing-dispersing device which is used for dispersing
primary particles of the absorbent in the carrier gas flowing
in a small amount at a high speed to obtain an aerosol of
high particle concentration, which is then uniformly mixed
with an exhaust gas. Thus, a high removal efficiency or ratio
can be achieved with small power consumption.
Next, embodiments of the invention will be described.
With reference to Fig. 2, an exhaust gas 1
released from an unillustrated boiler or waste material
incinerator through an exhaust gas channel is introduced
into a mixing portion 2 provided in the channel. On the
--5--
~ ~9092~
other hand, a carrier gas 4 and a Ca-type absorbent S are
fed to a dividing-dispersing device 3, which divides the
absorbent S into primary particles and disperses them in
the carrier gas 4 to a high concentration. The aerosol of
high concentration from the device 3 is introduced into the
mixing portion 2, where the aerosol is uniformly mixed with
the exhaust gas 1. The Ca-type absorbent (e.g. quick lime
or slaked lime) reacts with the harmful acid substances
(e.g. SO2 and HC1) in the exhaust gas according to the
0 following equations.
CaO + SO2 + 12 2 CaSO4
Ca(OH) + SO + 12 2 - CaSO4 + H2O
CaO + 2HC1 CaC12 + H2O
Ca(OH)2 + 2HCl - CaC12 15 The absorber thus absorbing the harmful acid substances
is collected by a dust collector 6 along with soot, dust, etc.
and discharged from a lower portion of the dust collector 6
as indicated at 7. On the other hand, the purified exhaust
gas is released to the atmosphere through a chimney 8.
With the system described above, the concentration
of primary particles of the absorbent in the carrier gas is
50 to 500 g/Nm3, whereas the absorbent primary particle
concentration of the exhaust gas after the mixing has been
reduced to 1 to 20 g/Nm3. In other words, the amount of
carrier gas can be as small as 1/25 to 1/50 of the amount of
~X90922
exhaust gas. Thus, the amount of power consumption in this
case is much smaller than when the exhaust gas itself is
caused to flow at an increased velocity. Furthermore, the
decrease of the concentration due to the mixing reduces the
frequency of collision of the absorbent 2rimary particles,
consequently reducing the likelihood that the primary
particles will re-agglomerate.
Usable as the dividing-dispersing device 3 is an
injector, orifice tube, Venturi tube, jet mill, mechanical crusher
permitting the carrier gas to flow therethrough and having
a dispersing function, or the like. As shown in Fig. 4, the
injector comprises an innter tube 9 and an outer tube 10.
The carrier gas 4 is caused to jet out from the inner tube 9
and thereby given a higher velocity. The negative pressure
produced in the outlet portion of the outer tube 10 draws
out secondary agglomerates 5a of the absorbent 5 from a duct
lOa, divides them into primary particles 5b in the outlet
portion and disperses the particles in the carriergas. As
shown in Fig. 5, the orifice tube comprises a tube 11 to which
the carrier gas 4 and the absorbent 5 in the form of
secondary agglomerates 5a are supplied, and an orifice
member having an orifice 12. The carrier gas having the
secondary agglomerates entrained therein is given an increased
velocity when passing through the orifice 12, whereby the
agglomerates are divided into primary particles 5b, which
~ X909~2
are dispersed in the carrier gas. The Venturi tube has the
same construction as shown in Fig. 5 except that the orifice
member is replaced by a Venturi. The tube operates on the
same principle as the tube of Fig. 5. In the jet mill,
solids as entrained in a carrier gas are caused to collide
with one another and thereby reduced to smaller sizes.
Secondary agglomerates can be thereby divided into the
original primary particles of specified size, or primary
particles so sized as not to form secondary agglomerates
can be divided into smaller primary particles. The mechani-
cal crusher is used solely for dividing coarse particles into
primary particles of specified size and dispersing them in
a gas. In any case, the primary particles to be eventually
dispersed in the carrier gas need to be up to lO microns in
mean diameter. For the present method to remove harmful
acid substances with as high an efficiency as the wet method,
it is required that the primary particles be up to 5 microns
in mean diameter for removing HCl and l to 3 microns in mean
diameter when removing SOx.
In practicing the method of the present invention,
it is essential to assure that the primary particles of the
absorbent once dispersed will not re-agglomerate. For this
purpose, it is required to minimize the length of the high-
concentration aerosol channel from the dividing-dispersing -
device 3 to the mixing portion 2 and to eliminate a greatly -
--8--
1.~90!~2
bent portion from this channel to thereby preclude anyturbulence. vihen an injector, orifice tube or Venturi tube
is used as the dividing-dispersing device 3, the device is
preferably so disposed as shown in Fig. 3. In this arrange-
ment, the device 3 is attached directly to a bent portion 13aof the exhaust gas channel 13, and the outlet of the device 3
is oriented in the same direction as the flow of exhaust gas
flowing downward from the bent portion 13a. Accordingly,
the primary particles 5b from the dividing-dispersing device
3 is sprayed directly into the mixing portion 2 without
being passed through an intermediate conduit. This almost
completely eliminates the likelihood of re-agglomeration.
Preferably, the mixing portion 2 is provided with a diffuser
2a for promoting the dispersion of primary particles 5b in
the exhaust gas 1. The portion of the exhaust gas channel
13 from the bent portion 13a to the dust collector 6 has a
space of such a volume that the primary particles 5b will be
suspended in the exhaust gas 1 for about 1 to about 3 seconds
before reaching the dust collector 6. The diffuser 2a,
although basically the same as the Venturi, is not intended
to give an increased velocity to the exhaust gas 1 and thereby
divide secondary agglomerates into primary particles,
therefore is not greatly constricted and does not give rise
to the problem of pressure loss substantially. Primary
particles of the absorbent can be sprayed into the boiler
0~92~
or the waste material incinerator.
Examples of Ca-type absorbents usable in a high
temprature range of 900 to 1200 C are limestone [chiefly
CaCO3], quick lime [CaO], slaked lime [CaOH], dolomite
[CaMg(CO3)2], calcined dolomite [CaMgO2] and slaked dolomite
[CaMg(OH)4]. At high temperatures of 900 to 1200 C, lime-
stone, slaked lime, dolomite and slaked dolomite instantaneously
undergo thermal decomposition (with release of H2O, CO2)
to form porous, highly reactive quick lime or calcined
dolomite, so that these materials afford better results than
quick lime and calcined dolomite (commercial product is
usually crystallized). When used at temperatures above
1200 C, quick lime and calcined become progressively
crystallized and therefore lower in reactivity. On the other
hand, examples of Ca-type absorbers usable in a temperature
range of 150 to 400 C are quick lime, slaked lime, calcined
dolomite and slaked dolomite, among which slaked lime and
slaked dolomite are preferable to use.
Usually, a bag filter or electrical precipitator
is used as the dust collector 6.
The advantages of the present methQd will become
apparent from the following examples.
Example 1
Slaked lime was used as a Ca-type absorber for
the system of Fig. 2, in which the dividing-dispersing device
--10--
~ X9092~
was an injector (Model TB-1, product of Atsuji Tekko Co.,
Ltd.) for preparing an aerosol of high concentration. The
injector was inserted in an exhaust gas duct through which
a simulated exhaust gas having a temperature of 250 C and
containing about 1000 ppm of HCl was flowing, with the outlet
of the injector oriented in the direction of flow of the
exahust gas. To mix the aerosol with the exhaust gas
effectively, the exhaust gas duct was constricted at a
location immediately downstream from the injector to form a
diffuser. The injector was operated by passing compressed
air having a pressure of 5 kg/cm G at a flow rate of
6.0 Nm /hr, and slaked lime was thereby sprayed into the
exhaust gas duct at a rate of 5 kg/hr. It took about 2
seconds for the primary particles of slaked lime to reach
the dust collector.
The above procedure was repeated with use of
slaked limes of varying particle sizes (primary particles
of varying diameters). Fig. 6 shows the results.
In Fig. 6, indicated at A are HC1 removal ratios
achieved by slaked lime according to the method of the
invention, and at B HCl removal ratios achieved by slaked lime
according to the conventional dry method. It is seen that
the reactivity improves with decreasing particle size more
remarkably in the case of the invention than with the
conventional method.
--11--
~2~0922
Example 2
The same procedure as in Example l was repeated
with the exception of the following.
Simulated exhaust gas
Contained substance: about 1000 ppm of Sx
Temperature: about llOO C
Flow rate: about l900 Nm3/hr
Injector
Air flow rate: 6.3 Nm /hr
Rate of spray of particles: 7.4 kg/hr
Fig. 7 shows the results achieved by the above
experiment. Indicated at A' and B' in Fig. 7 are the results
achieved by the invention and the conventional method,
respectively. It is seen that the method of the invention
is effective also on SOx.
-12-