Note: Descriptions are shown in the official language in which they were submitted.
Title: ANTI-ATHEROSCLEROTIC TRISUBSTIUTED
UREAS AND THIOUREAS
BACKGROUND OF THE INVENTION
This invention relates to new ureas and thiourea
compounds useful- as pharmaceutical agents. The novel
compounds of the present invention are antiatherosclerotic
agents c~apabie of ameliorating atherosclerosis by counter-
acting the formation or development of atheromatous lesions
in the arterial wall of mammals. The invention also relates
to the chemical synthesis of the novel compounds disclosed
herein. In addition, the invention pertains to novel
pharmaceuticaI compositions for the utilization of
these compounds in the treatment of disease in mammals.
Further, the ~invention~contemplates methods for treating
atherosclerosis In a manner designed to prevent, arrest,
or~reverse the course of the disease.
~ A vari~ety of urea and thiourea compounds can be
found;in~the~l~iterature, for example, in~J. ~ed Che_
18, 102~4 ~(1975~ Chem. Absts. 95: 6758m (1981) and 91:
74.631g~(1979j~; U.S. Paten~t Nos. 2,688,039; 3,335,142;
3,~856~,952; 3,903,~130;~and 4~,252,957 and in West German ~
~Offenlegu~ngsschrlft 29~28 485. The compounds found in litera-
~tur~e;~and~disclosed~as~being useful herbicides, plant growth
regul~ators,~bac~cericides, pesticides, fungicides, algacides,~photogr~a~pb~i~c~sensitizers, antihelmintics, sympatholytics
and~anti~v;~irals.~ Those urea compounds found in Offen-
egungss~chr~ift~29~28~485~are disc~osed as use~ul in
~inh~ibi~t~ing~ id~absorptio~n. There are, however, no
l~iter~atu~re~references ~d~isclosing the trlsubstituted
urea~and~th~i~ourea~compoun~ds of the~present invention or
thelr~use ~in~the~trea~tment of a~therosclerosis or hyperlipidem~a.
- ~P3~h~
\~
- ~ - 61109-7181
Atherosclerosis is a form of arteriosclerosis character-
ized by lipid accumulation in and thickening of the arterial walls
of both medium and large-sized arteries. Arterial walls are
thereby weakened and the elasticity and effective internal size of
the artery is decreased. Atherosclerosis is the most common cause
of ischemic heart disease and is of great medical lmportance since
the occlusion of medium and large-sized arteries diminishes the
supply of blood to vital organs such as the heart muscles and the
brain. The sequelae to atherosclerosi include ischemic heart
disease, heart failure, life-threatening arrythmias, senility and
stroke.
The fact that cholesterol is a major component of
atherosclerotic lesions or plaques has been known for more than
lO0 years. Various researchers have studied the role of cholest-
erol in lesion formation and development and also, more important-
ly, whether lesion format:ion can be prevented or lesion develop-
ment arrested or reversed. Atheromatous lesions have now been
shown [Adams, et al., Atherosclerosis, 13, 429 (1974)] to contain
a greater quantity of:esterified as opposed to unesterified
cholesterol thsn th~e~surroundlng undlseased arterial wall. The
intracellular esterification of cholesterol with fatty acids:is
catalyzed by the enzyme~Fatty acyl CoA:cholesterol acyl transfer~
ase or ACAT and the accumulation and storage of cholesteryl esters
in~the~arterial wall;ls~associated with increased activity of this
sne~me~[Hashimoto~and Dayton, Athsrosolerosis, 28, 447 (1977)].
In add~ition, cholesteryI esters are removed from cells at a slower
: :: :
`
,
. ~ . .
\
- 2a - 61109-7181
rate than unesterified cholesterol [~ondjers and Bjorkerud,
Atherosclerosis, 15, 273 (1972) and 22, 379 (1975)]. Thus inhibi-
tion of the ACAT enzyme would diminish ~he rate of cholesterol
esterification, decrease the accumulation and storage of
cholesteryl esters in the arterial wall,
.
,
:
and prevent or inhibit the formation and development of
a~heromatous lesions. The compounds of the present
invention are very potent inhibitors of the ACAT enzyme.
Thus, these compounds are useful for controlling and
reducing the cholesteryl ester content of mammalian
arterial walls and decreasing the accumulation and
storage of cholesterol in the arterial walls of mammals.
Further, the compounds of this invention inhibit the
formation or development of atherosclerotic lesions in
`iO mammals.
The evidence that hyperlipidemia is one of the
factors involved in coronary heart disease is very im-
pressive. A most important study carried out in Framingham,
Mass. (Gordon and Verter, 1969) in over 5,000 persons
for more than 12 years established a correlation
between high concentrations of blood cholesterol and
increased risk of heart attack. Although the causes of
coronary artery disease are multipIe, one of the most
constant factors has been the elevated concentration of
20~ lipids in the blood plasma. A combined elevation of
cholesterol and triglycerides has been shown (Carlson
and Bottiger, 1972) to carry the highest risk of coronary
heart disease. The majority of patients with ischemic
heart disease or peripheral va~cular disease were found
to have hyperlipoproteinemia, involving very low-density
and/or low-density lipoproteins (Lewis, et al., 1974).
~ We have now found that certain members of this
class of compounds can safely and~effectively lower
serum~lipids in warm-blooded animals. Such action on
serum lipid~s is considered to be very useful in the
treatment of;atherosclerosis. For some time it has
~be~en~cons~idered desirable to lower serum-lipid levels
~and to~correct lipoprotein imbalance in mammals as a
~ preventive~measure against atherosclerosis. The compounds
35~ ~o~the ~present invention do not act by blocking late
: ~ :
.. :
stages of cholesterol biosynthesis and thus do not
produce accumulation oE intermediates such as desmosterol,
as equally undesirable as cholesterol itself. Compounds
with the combination of therapeutically fa~orable
characteristics possessed by those of the present in-
vention can be safely administered to warm-blooded
mammals for the treatment of hyperlipidemic and ather-
osclerotic states found in patients with or prone to
heart attacks, to peripheral or cerebral vascular disease,
and to stroke.
The compounds of this invention exhibit anti-
atherosclerotic activity and the invention should not
be construed as limited to any particular mechanism of
.
antiatherosclerotic action.
.
~ ~
- : :
: :
:: ~
$~
- 5 - 6110g-7181
UMM~RY OL~ Tll~ INV~N'~'lON
Thi~ invention relate8 to new trl~ub~ti~uted urea and
thiourea compounds, their preparatlon, pharmaceutlcal compo~itions
containing them, and their uge in the treatment of athe~o~clero~
More particularly, it i~ aoncerned with urea~ and thioureas which
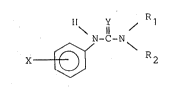
may be repr~sented by Formula I:
Il \ Y ~R
X ~ -C-N\
wherein X repre~ents at lea~t on~ ~ub~tituent ~eleeted from the
group con~isting of hydrogen, Cl-C4 alkyl, Cl-C4 alkenyl, Cl-C4
alkynyl, hydroxy, Cl-C4 alkoxy, phenoxy, mercapto, Cl-C4 alkylthio,
amino, Cl-C4 alkylamino, di-(Cl~C4 alkyl)amino, halo, trihalo-
methyl, Cl~Cq alkanoyl, benzoyl, Cl-C4 alkanamido, haloaceta~nido,
Cl-C4 alkanesulfinyl, benzenesulfonyl, toluene~ulfonyl, nitro,
cyano, carboxy, Cl-C4 carboalkoxy, carbamoyl, ~ul~amyl, methylene-
dioxy, phenyl, ortho-phenylene,tolyl, benzyl, halobenzyl, methyl-
benzyl, Y i8 Belect~d from the group con~i~ting o~ oxygen and
sulEur, with the proviso that when X i8 hydrogen Y mu~t be 6ulfur;
Rl and R2 are dl~ferent and are independently selected from the
~group consisting of C~-C12 alkyl, C4-C12 alkenyl, C4-C12 alkynyl,
C4-C12 cycIoalkyl, C~-C12 cycloalkylalkyl C7~Clg aralkyl, and
C7-C14 aralkyl in which an aromatic riny bears at least one
8ubstituent selected from the group con~i6tln~ o~ Cl-C10 ~lkyl,
Cl-C10 alkoxy, phenoxy, ben~yloxy, urethylene~ioxy, Cl~C4
alkylthio, phenyl, halo, trihalon~hyl, adaman~yl, Cl-C4 carbo-
alkoxy, and nltro wlth the pxoviso that when X compri6es one
D
~ ~:
~.D~
- 5a - 61109-7181
sub~tltuen~ ln the meta po~ltlon wlth th~ r~pect to the nl~ro~en
llnkage to the phenyl rlng and X i~ ~elected fro~ ~he group
consl~tinq of hydroxy, alkoxy, mercapto and alkythlo then at lea~t
one o~ Rl and R2 1~ othar than C4 alkyl.
.
'
: ~ , :
:
:
-6-
Preferred embodiments o~ the invention are those
in which Y is oxygen. More preferred are those in which X
represents at least one Cl-C4 alkyl or halo sùbstituent,
Rl is C7-Cl4 aralkyl or substituted C7~C14 aralkyl and ~2
is C4-Cl2 alkyl. The most preferred are those in which X
represents at least one methyl or chloro substituent.
Preferred specific embodiments involve l-ben-
zyl-l-(n-butyl)ureas, for example:
l-benzyl-l-(n-butyl)-3-(3-methylphenyl)urea
l-benzyi-l-(n-butyl)-3-(3-methylphenyl)urea
l-benzyl-l-(n-butyl)-3-(3-chlorophenyl)urea
l-benzyl-l-(n-butyl)-3-(3,4-dichlorophenyl)urea
l-benzyl-l-(n-butyl)-3-(3,5-dichlorophenyl)urea
l-benzyl-l-(n-butyl)-3-(2,4,6-trichlorophenyl)urea
lS l-benzyl-l-(n-butyl)-3-(3-chloro-4-methylphenyl)urea
l-benzyl-l-(n-butyl)-3-(2,4-dimethylphenyl)urea
l-benzyl-l-(n-butyl)-3-(2-methylphenyl)urea
l-benzyl-l-(n-butyl)-3-(4-methyIphenyl)urea
l-benzyl-l-(n-butyl)-3-(2,3-dimethylphenyl)urea
l-benzyl-l-(n-butyl)-3-(2,5-dimethylphenyl)urea
l-benzyl-l-(n-butyl)-3-(2,6-dimethylphenyl)urea
l-benzyl-I-(n-butyl)-3-(3,5-dimethylphenyl)urea
l-benzyl-l-(n-butyl)-3-(3,4-dimethylphenyl)urea
l-benzyl-l-(n-buty~ 3-(2,4,6-trimethylphenyl)urea
1-benzyl-1-(n-butyl)-3-(3-phenyl)thiourea
l-benzyl-1-(1,2-diphenylethylj-3-(2,4-dimethylphenyl)urea
l-benzyl-:1-[1-(3-methoxyphenyl)-2-phenylethyl]-3-(2,4-di-
methylphenyl)urea
~ l-bènzyl-1-[1-(:4-benzyloxyphenyl)-2-phenyl]-3-(2,4-di-
methylphenyl)urea
l-benzyl-1:-~1-(3-methoxyphenyl)-2-phenylethyl]-3-(3-tri-
flu~o:rome~thylphenyl)urea~
benzyl-l-(n-butyl)-3-(3-chloro-2-methoxyphenyl)urea
l~-benzyl-l-(n-butyl)-3-(5-chloro-2-methoxyphenyl)urea
: .
~ 3
--7--
This invention also relates to a method of re-
ducing the cholesterol content of the arterial walls of
mammals which comprises administering to said mammal an
effective amount of a compound as recited above.
This invention also'relates to a method of treat-
ing hyperlipidemia in mammals which comprises administer-
ing to said mammal an effective amount of a compound as
recited above.
This invention further relates to,a method of
inhibiting atherosclerotic lesion development in mammals
which comprises administering to said mammal an effective
amount of a compound as recited above.
This invention still further relates to a pharm-
aceutical composition which comprises an effective anti-
i5 -atherosclerotic amount of a compound as recited above in
association with a pharmaceutically acceptable carrier.
Finally, this invention relates to processes for
preparing,compounds as rècited above. One process especi-
ally useful for the preparation of trisubstituted ureas
and thioureas involves reacting an arylisocyanate
or arylisothiocyanate of formula II with a secondary amine
of formula III, wherein X, Y, Rl and R2 are as deined
above.
~ ` X ~ ~ HN
~R2
~ II III
A second~process for the preparation of ureas
and~th~ioureas involves reacting a compound ot formula IV;
~wherein~ A and B are leaving groups, which may be the same
or~ dif~ferent, selected from the group consisting of halo,
Cl-C4 alkoxy,~Cl-C4 alkylthio phenoxy, 4-chlorophenoxy and
: ~::
:
~5 ~ :
- ~ -
4-nitrophenoxy; with a secondary amine of formula III to
yield an intermediate of formula V and then reacting the
intermediate with an arylamine of formula V~ wherein X, Y,
Rl and R2 are as defined above.
A C B III > A ~ ~ 1 X ~ NH2
R2
IV V VI
A third process for the preparation of ureas
and thioureas involves reacting a compound of formula IV
with an arylamine of formula VI to yield an intermediate of
formulaVII, wherein X, Y and B are as defined above and
then reacting this intermediate with a secondary amine of
formula III
i5 A-C-B + X ~ ~H2 X ~ N-C-B - >
IV VI VII
:
~ DETAILED DESCRIPTION OF THE INVENTION
... . .
Many of the novel ureas and thioureas of this
invention are prepared by reacting arylisocyanates and
arylisothiocyanates with secondary amines. These re-
actions may be performed in aprotic~solvents such as
hexane, diethyl ether, toluene, tetrahydrofuran, and the
li~e~at temperatures from room temperature or below up to
the bo~iling point oE the solvent used. The ureas and
; thioureas are isolated by filtration or by evaporating
the~solvent~and they may be purified by recrystallization,
absorption chromatography, or distillation under reduced
pressure. An example of this process is the reaction of
~ ~ d ~ ~
_9_
2,4-dimethylphenylisocyanate with N-benzyl-N-(n-butyl)-
amine to yield l-benzyl-l-(n-butyl)-3-(2,4-dimethyl-
phenyl)urea.
Many of the secondary amines required for the
5 synthesis of the ureas and thioureas of this invention
are prepared by diborane reductions of the corresponding
amides. An example of this reaction is the synthesis
of N-(n-butyl)-2-chlorobenzylamine by diborane reduction
of N-(n-butyl)-2-chlorobenzamide. Certain of the amides
required by these reductions are prepared by acylation of
primary amines with carboxylic acids by methods well
known to those skilled in the art, for example, by con-
version of the carboxylic acid to the corresponding
carboxylic acid chloride using thionyl chloride and then
reacting the acid chloride wi~h the primary amine in the
presence of a base~ One method especially useful for this
transformation is the boron trifluoride etherate catalyzed
reaction of a carboxylic acid with a primary amine. An
example of this transformation is the boron trifluoride
etherate catalyzed acylation of 2-chlorobenzylamine with
3-methoxyphenylacetic acid to yield N-(2-chlorobenzyl)-
-3-methoxyphenylacetamide.
Certain of the novel ureas and thioureas of
this invention are prepared by reacting activated deriva-
tives of carbonic acid such as phos&ene, thiophosgene, or
phenyl chloroformate with secondary amines to yield an
intermediate, for instance, a disubstituted carbamyl
chloride. This intermediate is in turn reacted with an
arylamine to yield the urea or thiourea. The preparation
of the intermediate is conducted in an aprotic solvent
such as tetrahydrofuran, toluene, xylene, or the like at
temperatures from about room temperature up to the boil-
ing point of the solvent. The intermediate may be iso-
lated by evaporation and~purified by distillation if
necessary. The intermediate is then reacted with an
arylamine in an aprotic solvent such as dimethylacetamide
in the presence of a base such as sodium hydride at temp-
eratures from about room temperature up to the boiling
point of the solvent used An example of this process is
the reaction of phosgene with N-benzyl-n-butylamine in
~oluene to yield the intermediate N-benzyl-N-(n~butyl)-
carbamyl chloride, which is then reacted with 3-bromo-
aniline in N,N-dimethylacetamide in the presence of sodium
hydride to yield l-benzyl-l-(n-butyl)-3-(3-bromophenyl)-
urea.
Other of the novel ureas and thioureas of this
invention are prepared by reacting arylamines with acti-
vated derivatives of carbonic acid such as phosgene or
thiophosgene to yield an intermediate, for instance, an
arylcarbamyl chloride. This intermediate is then reacted
with a secondary amine to yield the urea or thiourea.
The preparation of this intermediate is conducted in an
aprotic solvent such as toluene or xylene at temperatures
from about room temperature up to the boiling point of
the solvent in the presence of a base, for example,
N,N-dimethylaniline.
The intermediate is then reaGted with a second-
ary amine in an aprotic solvent such as toluene at temp-
eratures from room temperature or below up to the boiling
point of the solvent. An example of this process is the
reaction of phosgene with 3-chloroaniline to yield the
intermediate N-(3-chlorophenyl)carbamyl chloride which is
~hen reacted with N-benzyl-n-butylamine to yield l-ben-
zyl-~l-(n-butyl)-3-(3-chlorophenyl)urea.
~:
9 ~ ~
The ureas and thioureas of this invention which
contain carboxy groups are prepared by alkaline hydroly
sis of the corresponding carboalkoxy ureas and thioureas,
prepared by the synthetic methods described above. Like-
wise, those which contain hydroxy, mercapto, or amino
groups are prepared by alkaline hydrolysis of the corres-
ponding O-acetyl, S-acetyl, and N-acetyl ureas and thio-
ureas, respectively, the latter also having been obtained
by the urea and thiourea syntheses described above.
Alternatively, the ureas and thioureas which contain
hydroxy groups are prepared by cleavageof the corresponding
methoxy compounds by Lewis acids such as borontribromide.
The ureas and thioureas of the present invention
are obtained as crystalline solids or distillable liquids.
They are characterized by distinct melting or boiling
points and unique spectra. They are appreciably soluble
in organic solvents but generally less soluble in water.
Those compounds which contain carboxylic acid groups may
be converted to their alkali metal and alkaline earth
salts by treatment with the appropriate metal hydroxides
and those which contain amino groups may be converted to
their ammonium salts by treatment with organic or mineral
acids. Both of these types of salts exhibit increased
water solubility.
The preparation and properties of the compounds
of this invention will be described in greater detail in
conjunction with the specific examples shown below.
The compounds of the present invention were
assayed for two types of biological activity related to
their potential use as antiatherosclerotic agents. Com-
pounds were tested in vitro for the ability to inhibit
the enzyme fatty acyl CoA:cholesterol acyl transferase
(ACAT) and in vivo for serum hypolipidemic activity as
measured by the ability to inhibit lipid absorption in
rats.
:
-~2-
The compounds were tested for their ability to
inhibit ACAT according to the following procedure:
Rat adrenals were homogenized in 0.2M monobasic
potassium phosphate buffer, pH 7.4, and centrifuged at
lO00 times gravity for 15 minutes at 5C. The super-
natant, containing the microsomal fraction, served as the
source of the cholesterol-esterifying enzyme, fatty acyl
CoA:choiesterol acyl transferase (ACAT). A mixture com-
prising 50 parts o adrenal supernatant, lO parts of
albumin tBSA) (50 mg./ml.), 3 parts of test compound
(final concentration 5.2 g./ml.) and 500 parts of buffer
was preincubated at 37C. for 10 minutes. After treat-
ment with 20 parts of oleoyl CoA (14C - 0.4 Ci) the
mixture was incubated at 37C. for 10 minutes. A control
mixture, omitting the test compound, was prepared and
treated in the same manner. The lipids from the incuba-
tion mixture were extracted into an organic solvent and
separated by thin-layer chromatography. The cholesteryl
ester fraction was counted in a scintillation counter.
This procedure is a mod~ification o that described by
Hashlmoto,~et.; al., Life Scie., 12 (Part II), 1-12 (1973).
~ The results of this test on representatlve
compounds of thi~s 1nvention appear in Table I.
:, ; ~ ~ : :
:~
:: :
: ~ ~
35~
,
:: : :~ :
-13-
Table I
Com ound % Inhibition
P
l-Benzyl-l-(n-butyl)-3-(3-methylphenyl)- 92.3
urea
.
l-Benzyl-l-(n-butyl)-3-(3-trifluoromethyl- 85.7
phenyl)urea
l-Benzyl-l-(n-butyl)-3-(3,5-dichlorophen- 90.7
yl)urea
l-Benzyl-l-(n-butyl)-3-(3,4-dichlorophen- 95.9
yl)urea
_
l-Benzyl-l-(n-butyl)-3-(3-chlorophenyl)- 88.6
urea
~ _ _
l-Benzyl-l-(n-butyl)-3-(2,4-dimethylphen- 91.3
yl)urea
_ _ _
l-Benzyl-l-(n-butyl)-3-(2-methylphenyl)- 78.8
urea
_
l-Benzyl-l-tn-butyl)-3-(4-methylphenyl)- 78.0
urea
_
l-Benzyl-l-(n-butyl)-3-(2,3-dimethylphen- ;85.8
yl)urea
_
l-Benzyl-l-(n-butyl)-3-(2,5-dimethylphen- 92.7
yl)urea
_ . _
l-Benzyl-l-(n-butyl~-3-t2,6-dimethylphen- 83.1
yl)urea
..
l-Benzyl-l-(n-butylj-3-(3,5-dimethylphen- 94.2
yl)urea
l-Benzyl-1-[1-(3-methoxyphenyl)-2-phenyl- 86.4
ethyl]-3-(2,4-dimethylphenyl)urea
_
- 14 -
Table I (continued)
Com o~nds % Inhibition
. _ P
l-Benzyl-l-[l-(4-benzyloxyphenyl)-2-phen- 93.0
ylethyl]-3-(2,4-dimethylphenyl)urea
_
l-Benzyl-l-(l,2-diphenylethyl)-3-(2,4-di- 95.0
methylphenyl)urea
l-Benzyl-l-(n-butyl)-3-(3,4-dimethylphen- 87.l
yl)urea
_ _ _ ._ _
l-Benzyl-l-[l-(3-methoxyphenyl)-2~phenyl- 88.l
ethyl]-3-(3-trifluoromethylphenyl)urea
_ _
l-Benzyl-l-(n-butyl)-3-(3-chlc,ro-2-methoxy 84.5
phenyl)urea
l-Benzyl-l-(n-butyl)-3-(5-chloro-2-methoxy 80.6
phenyl)urea
l-Benzyl-l-(n-butyl)-3-phenyl thiourea 82.4
_ _
l-(n-Butyl)-1-(2-fluorobenzyl)-3-(2,4-di-
methylphenyl)urea 83.6
l-(n-Butyl)-1-(4-fluorobenzyl)-3-(2,4-di-
methy1phenyl)urea 80.6
_ _ .
l-(n-Butyl)-1-~(2-chlorobenzyl)-3-(2,4-di-
methylphenyl)urea 95.5
, , .
l-(n-Butyl)-1-(2,6-dichlorobenzylj-3-(2,4-
-dimethylphenyl)urea 74.5
~ _ . _ _ .
.l-(n-Bu~tyl)-1-(4-bromobenzyl)-3-(2,4-di-
methylphenyl)urea 81.0
- 15 -
Table I (continued)
.
Compound % Inhibition
_
l-(n-Butyl)-l-~4-(n-butyl)benzyl) -3-(2,4 54 4
-dimethylphenyl)urea
_ ,
l-(n-Butyl~-1-(4-methylbenzyl)-3-(2,4-di- 96.7
methylphenyl)urea
l-(n-Butyl)-1-(4-tert-butylbenzyl)-3-(2,4 96.4
-dime~hylphenyl)urea
.. _ .
l-(n-Butyl)-1-(4-chlorobenzyl)-3-(~,4-di- 94.6
methylphenyl)urea
_ _ _ __ . .
l-(n-Butyl)-1-(4-methoxybenzyl)-3-(2,4-di 94 2
methylphenyl):urea .
: ~
l-(n-Butylj-l-(3~4-methylenedioxybenzyl)- 2
3-(2,4-dimethylphenyl)urea as
~ -- :
1-(n-Butyl)-1-(4-trifluoromethylbenzyl)-3 3 3
: -(;2,4-dimeth~yl~heny1)urea : 9 .
l-(n-Butyl)-1-(4-phenylbenzyl)-3-(2,:4-di- 97 1
~ethylphenyl:)~urea~ .
l-~(n-De~y~ benzyl-3-(2~4-dimeehyl- 96.1
pheny~1~)ur~ea~
1-(n-~But:y1):-1-(2-phenylethyl)-3-(2,4-di- 87.9
methy1~phenyl)urea ~
.
::
,t~r~
-16-
Table I (continued)
¦ ComDound % Inhibition
_ . r
l-(n-Butyl)-1-[2-(4-fluorophenyl)ethyl]
-3-(2,4-dimethylphenyl)urea 96.1
. _
l-(n-Butyl)-1-[2-(4-chlorophenyl)ethyl]
-3-(2,4-dimethylphenyl)urea 93.3
_ _ .
l-(n-Butyl)-1-[2-(3-methoxyphenyl)ethyl]
-3-(2,4-dimethylphenyl)urea 98.3
_ _
l-(n-Butyl)-1-(3-phenylpropyl)-3-(2,4-di- 97 4
~ethylphenyl)urea
l-(n-Butyl)-l-benzyl-3-(2,4,6-trimethyl- 75 8
phenyl)urea
_
l-(n-Butyl)-1-[4-(n-hexyl)benzyl]-3-(2,4-
dimethylphenyl)urea 93.8
.
l-(n-Tetradecyl)-l-benzyl-3-(2,4-dimethyl- 80 3
phenyl)urea
,,
l-(n-Octadecyl)-l-benzyl-3-(2,4-dimethyl- 19 7
phenyl)urea
,
l-(n-Butyl)-1-[2-(3-bromophenyl)ethyl]-3-
(2,4-dimethylphenyl)urea 97.0
~ : , .
~:~
. ~
-17-
Inhibition of choles~erol absorpcion was determined by feed-
ing male Sprague-Dawley ra~s, weighing 150-170 g., a 1%
cholesterol:0.5% cholic acid diet for 2 weeks. The
diet also contained compounds being tested at a dose o
0.3~ oE the diet. Con~rol rats were fed the same diet
without any compound. At the end of the test the rats
were sacrificed by decapitation. Blood is collected,
centrifuged at 1.5 kg times gravity for 10 minutes at
4C; and the serum is then analyzed for cholesterol and
1~ triglycerides enzymatically by the method of Trinder,
P., Analyst, 77,321 (1952) on a Centrifichem*400 Analyzer.
Livers are removed, a 0.4 g sample is taken from ~he center
of the large lobe, and the sample is subjected to saponi-
fication using 25% saturated potassium hydroxide in ethanol.
The resulting neutral sterols are extracted with petroleum
ether and extract analyzed for cholesterol. The effective-
ness of the compound in inhibiting cholesterol absorption
i~ measured by the lowering of either serum cholesterol or
liver choiesterol relative the values ~or control ra~s.
The resul~s o~ this test on typical compounds of
this invention appear in Table II.
Table II
_ _ _ _ _ _ .
_ Compound Result
l-Benzyl-l-(n-butyl)-3-~2,4-dimethylphenyl)urea Active
l-Benzyl-l-(n-butyi)-3-(2,6-dimethylphenyl)urea Active
I-Benzyl~ n-b~utyl)-3-(3,5~dimethylphenyl)urea Active
l-Benzyl-1-(1,2-diphenyle~hyl)-3-(2,4-dime~hyl- Active
phenylJurea
~
Trademark
.~j
, d
-18-
Inhibition o~ cholesteroi absorp~ion was a;so
de~ermined by feeding male Sprague-dawley rats wei~hing
150-170 g. a 1% cholesterol:0.5% cholic acid diet for 2
weeks. The diet also contained compounds being tes~ed a t
doses of between 0.01% and 0.1% of the diet. After the
rats had been on the test diet ior 9 days each rat is given
by gavage a sonicated mixture o~ [4-14C] choles~erol (6~ Ci),
G.2 ml. triolein, 10 mg. cholic acid, 20 mg. cholesterol
and 2 mg. of test compound in 0.8 ml. of 10% non-fat dry
miik. Feces were collected for each 24 hour period for the
remaining 5 days during which the rats were maintained on
the 1% cholesterol:0.5% cholic acid plus tes~ compound
diet. Fecal 14C-neutral sterois were extracted with petro-
ieum ether rrom saponified feces by ~he method of Grundy,
S. M. et. al., J. Lipid Res., 6, 397 (1565) and counted in
a scintillation counter. Acidic s~erols (Bile acids) were
extracted by acidifying the saponified feces and extracting
in chloroform:methanol (2:1) ana counting the chloroform
phase in a scintillation counter. Total extraction of
radioactivity (98-100%) from saponified feces is realized
by this procedure.
Radioactivity in liver and adrenals were deter-
mined by saponification and extraction into petroleum ether
and counting by scijntillation techniques. Total cholesterol
in liver and adrenals was determined by the cholorimetric
method of Zlatkis, A., et. al., J. Lab. Clin. Med., 41, 486
(1953j on saponified organic solvent extracted tissue pre-
pared by the method of Trinder, P., Analyst, 77, 321 (1952j.
Serum choles~erol and triglycerides were assayed enzymaticaly
by the method~of Allain, C. C., et. al., Clin. Chem. 20,
470 (1974) on a centrifichem 400 Analyzer. 14C-Cholesterol
in serum was determined by direct scintillation counting.
The effect of a test compound on cholesterol
absorption was determined by:
::
-19-
1. increase in excreted 14C-neutral sterol.
2. decrease in excreted 14C-acidic sterol.
3. decrease in 14C-cholesterol or 14C-cholesteryl
ester in the liver.
4. decrease in 14C-choles~erol or 14C-cholesteryl
ester in the serum.
A compound is considered active in inhibiting
cholesterol absorption if it meets at least the first ~wo
criteria.
The results of this test on a typical compound of
this invention appear in Table III.
~ Table III
_ . _ _ I
Compound Result
-- :-: _
lS l-Benzyl- } (n-butjl)-3-(2,4-dlmetbylphenyl)-
: : :
:
~" The;;tests~reported~ orshown in Tables l-III, inclusive
have~been actually carr~led out and the results therein
actually obtained or `concluded thererom.
:~
-20-
When the compounds are employed for the above
utility, they may be combined with one or more pharma-
ceutically acceptable carriers, e.g., solvents, diluents
and the like, and may be administered orally in such
forms as tablets, capsules, dispersible powders, granules,
or suspensions containing, for example, from about 0.5 to
5% of suspending agent, syrups containing, for example,
from about 10 to 50% of sugar, and elixirs containing,
for example, from about 20 to 50% ethanol, and the like,
or parenterally in the form of sterile injectable solu-
tions or suspensions containing from about 0.5 to 5%
suspending agent in an isotonic medium. These pharmaceu-
tical preparations may contain, for example, from about
0.5% up to about 90% of the active ingredient in combina-
tion with the carrier, more usually between 5% and 60% by
weight.
The antiatherosclerotic effective dosage of
active ingredient employed may vary depending on the par-
ticular compound employed, the mode of administration and
the severity of the condition being treated. However, in
general, satisfactory results are obtained when the com-
pounds of the invention are administered at a daily dos-
age of from about 2 milligrams to about 500 milligrams
per kilogram of animal body weight, preferably given in
divided doses two to four times a day, or in sustained
release form. For most large mammals, the total daily
dosage is from about 100 millisrams to about 5,000 milli-
grams preferably from about 10G millgrams to 2,000 milli-
grams. Dosage forms suitable for internal use comprise
from ~about 25 to 500 milligrams of the active compound in
intimate admixture with a solid or liquid pharmaceutic-
ally~ accept~able carrier. This GOSage regimen may be
adjusted to provide the optimal therapeutic response.
For example, seve~ral divided dcses may be administered
daily or the~dose may be proportionally reduced as in-
dlcated by~the exigenCies of the therapeutic situation.
'
., ~
~21-
A decided practical advantage is that these active com-
pounds may be administered orally as well as by intra-
venous, intramuscular, or subcutaneous routes if nec-
essary. Solid carriers include starch, lactose, di-
calcium phosphate, microcrystalline cellulose, sucrose,
and kaolin, while liquid carriers include sterile water,
polyethylene glycols, non-ionic surfactants and edible
olls such as corn, peanut and sesame oils, as are appro-
priate to the nature of the active ingredient and the
particular form of administration desired. Adjuvants
customarily employed in the preparation of pharmaceutical
compositions may be advantageously included, such as
flavoring agents, coloring agents, preserving agents, and
antioxidants eg. vitamin E, ascorbic acid, BHT and BHA.
The preferred pharmaceutical compositions from
the stand-point of ease of preEaration and administration
are solid compositions, particularly tablets and
hard-filled or liquid-filled capsules. Oral administra-
tion of the compounds is preferred.
These active compounds may also be administered
parenterally or intraperitoneally. Solutions or suspen-
sions of these active compounds as a free base or phar-
macologically acceptable salt can be prepared in water
suitably mixed with a surfactant such as hydroxypro-
pylcellulosè. Dispersions can also be prepared in gly-
cerol, liquid polyethylene glycols, and mixtures thereof
in oils. Under ordinary conditions of storage and use,
these preparations contain a preservative to prevent the
growth of microorganisms.
3Q The pharmaceutical forms suitable for inject-
able use include sterile aqueous solutions or dispersions
and sterile powders for the extemporaneous preparation of
sterile injectable solutions or dispersions~ In all
cases, the form must be steril~ and must be fluid to the
extent that easy syringability exists. It must be stable
under the conditions of manufacture and storage and-must
'
-22-
be preserved against the contaminating action of micro~
organisms such as bacteria and fungi. The carrier can
be a solvent or dispersion medium containing, for
example, water, ethanol, polyol (e.g. glycerol, propylene
S glycol and liquid polyethylene glycol), suitable mix
tures thereof, and vegetable oils.
Example 1
l-Benzyl-l-(n-butyl3-3-(2,4-dimethylphenyl)urea
_
A solution of 4.89 g. of 2,4-dimethylphenyl-
isocyanate in 100 ml. of hexane is added to a solution
of 4.41 g. of N-benzyl-n-butylamine in 150 ml. of hex-
ane and the solution is stirred at room temperature
for 2 hours and then evaporated. The residual solid
is recrystallized from pentane to yield l-benzyl-l-(n
-butyl)-3-(2,4-dimethylphenyl)urea, m.p. 70-71C.
With reference to Example 1 and Table IV,
only Example 1-4; 9-13; 15; 18; 29-30; 33; 39-40;43i 79-
81; 83; 86-89; 150; and 202-266, inclusive have been
actually carried out. Those compounds of the other
actual Examples in Table IV were prepared from the
appropriate arylisocyanates or arylisothiocyanates and
secondary amines by the method of Example 1. All
other Examples are simulated or predicted.
:
:
~ 2 3 ~ r s~
C.)
O ~ ~ O ~ o ~r
. In Cl~
. co ~ t~l t~ I~ ~n ~r co ~~ `J
a~ o ~ co ~ ~ o ~ ,~,,
_ ... . ... _
0
~ aJ
a) Ll ~ 4 4 1~ 4
) C ~t
. _ c c a) ~ o _ tl
^ C C C ~ ~ ~ C
H ~ ~ ~ C
~ ~ '1 .C S S .S ~ C O
au c ~ S C a~
_, :~ c c c :~ :~ Q. ~ ~ ~ ~ ~ ~ v v a) ~ o c c
Q O a~ ~ O C C
(~ Ql S .C ~ s s
E~ 6 ~ t~ ,C 5 ~ ~1 V ~ ~ V V ~ X ~
O ~ O ~ aJ a) ~ 4 ~ X O ~ 0 0
4 V 6 6 ~ 6 6 ~ ~ O V ~
S ~ 1 1 .C ~ I ~ O O
v S ~: Q ~ ~ ~ ~ ~ U~
a.~ V V u~ I I I I I I ` ` ~ I ^ ~ s
e ~ 6 ~J au ~l C~
I
l l l l l l l l l l l l l l l l l l l l
l l l l l l l l l l l l l l l l l l l l
, - . - ~ - ~ ~ ~ - - - - - ~ ~ - ~ ~ ~ ~ ~
~ ~ n ~ Q ~ ~ Q ~ ), Q Q ~ Q ~ Q Q n Q Q
1 1 ~ I 11111111111111111
Cl Cl 1 l ~ 1 I Cl Cl Cl ~::1 Cl Cl Cl ~I Cl Cl cl Cl Cl C
1: 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1, 1 1 1
N N N N N N N N N N N N N N N N N N N N
~ C C : ~: C C:: C C C C C C C C ~: C C C C
m m o: m m m m m m m m m m m m m m m m m
l l l l l l l l l l l l l l l l l l l l
_
: a) ::
Q~
E ~ ~ ~ ~ ~o t~ c~ ~ o ~ ~ r~ cn o
~x~ _ __
- 2 4 - ~ "~
_,
o ~o
a) S.. LJ ^
_ ~ c c Q~
a) _, c ~ o
3 ~ ~ c ~ o
x x
O ~ o a) s
~ ~ C C Ll ~ E
i ¦ U ¦ c c s o o o o c o o / ~) o C~ E
R~ : I I 1 ~ V U ~ c C ~
: ~ ~~ _ _ ~ ~ ~ _ _ ~i _
~ ~ _
: ~ : .a~: Q~ n ~ ~ R~ Q ~.~1 Q q ~
: ~; ~ ~C~ Cl;~ Cl CI ~CI Cl; ~I Cl Cl~ Cl ~ 1 1 I Cl Cl Cl Cl C~ 1
~ ` :~ : I
c~ o
O 3
:~: a
_
Q~
C ,C
Q, Q, ~ L~ C
S
~ ~ ~ ~ S
_ _ _. U
a) a~
e ~ s
~ ~ C o o o s ~ ~ V
c a~
,~ s 5~ o o o ~ ~ a) c ^ ~ e
X ~ c _~ ~ c ~
C ~ i O ~ :5 S ~ U ~ C C 'C5
O ~ C S aJ aJ I
t~ ~ r ~ ~Q. C a) Q. C S ~r
_ ~ ~ a~ ~ ~ u c~ S ~ C ~ a) s ~ ~ Q.
3 : ~ E v JJ v C c c s ~ ~ aJ c X s ~ ~ O ~
o ~ v ~I c ~: a) o ~ ,, s ~ ~ --
~` , ~ I er ~ ~ u ~ c s a~ ~ ~ ~ c s ~ S O I
e ~ I I 1 1 ~ Q~ ~ e s c ,, C4 ~ :~ c ~ v
O I o O O O s ~ ,~ ~ ~ ~ ~ ~ s ~ E ~ s
~ c~ ~ ~ ~ , O V
_1 . ~ O O 0 0 ~ C N I e ~ V ,c Q ~ e C~l I I
~ ~ I s ~ V ~ e I --
0 ,~ C S s I ,C ~ ~ ~rJJ E ~ 0 1 ~ I -- -- J~
E~ c o: t~ u c~ aJ I e ~
I I 1 1 1~ ~ I I -- II ~ I I -- I I ~1 ~ Q
: IIIIIIII~ V--
_ ~ _ _ _ ~ _ _ ~, ~_ _~ _ _ ~ t) ~ aJ ~J I
Q ~ I
' ~ ~ J~ ~ V J- ~: V 1~ 1 X ~ J-) C ~ ~ X ~J --
a) ~u u o c
: ~ Q ~ Q .Q 1~ :~ ~ ~ ~ t) ~ S ~; O C :~ ~ ~ ,C O ::~
: :~ I~ C~ C~ ~ ~ 1 Cl Cl Cl 1 1 1 I Cl Cl 1 I J-
IIIIIIIIIIIIII
~' I: 1 1 1 1: 1 1 1 1 1 1 1 1 1 1 1 1 1 1 C
~ ~ ~ N:~l N ~ N; ~ W N ~ N N N N N N N N N: N N N N N
: ~:C:CCCC~CCC~CCCCC~CC
: :~ ~ m ~ m m~ m ~ m m m m m m m m m m m ~ m m a~ I
~ : ~ :~1 ~
_
0~ : :
o ~ o
X
~: ~ :
_ _ ._ _ _
: :
~ : ~
- 2 5 ~
~ CO ~o
O I
~ r~
s U~ ,
. ' ,~, ,
~aJ ~
a) ~ ~a) --
c
c q) ,~
Ll ~
c ~ I
~ 5,1 C h ~ C Q~
s a~ c
c _ a) ~ ~ c ~ :~ ~
~1 ~ C :~ ~ ^ ~ :~ ~ ~ ~ ~ JJ C ~ ~ S C --
c ~ ~ ~ ~ aJ q) a~ _~ ~ a) I
~ a) c ~:: o ~ c ~
. ~. Q. S C `J S ~ S `J ~ I ~ ~ .C
C~ P~ ~ W S ~
v ~ X ~1 O c ~ ~ Ql w~ I
:~ I ,C O ~ ~ O ~ ,~ .C O O O E~ I C
C ~ 0~ S C 0 ~1 ~ S ~1 rl ~ O h ~ ~ C
~,, I o v ~ ~ :~ o ~ s ~a r ~ o ~: s ~ ~
~ O ) ~ ) S S ~--I w V I V ~ I U t~ w
c ~ o o e u ., c ~ ~ ~A ~1 I S
O O ~I Q rl --f ~J U I ~ er U ~r ~ ~
t~ ~ _~ S Ll ~ ~ I -- -- I ~ I c
_ C ~ U ~ I I I ~ -- ~ -- ~ ~ ~ I I _ I ~ ~I)
:: U I t~ .C
O I ~ I ~ ~ -- I ~ I ~ I I I I I ~ -I
~ ~ I ~ ~ ~ ~ ^ u :~ --
1 _ ~3 ~ I I I ~ ~I W U I
O ¦ ¦ ~ {5 w (~ ¦
A~ C.~ ~ ~~ U n~ ~ ¦
w : ~ U ~ ~ ~ ~1 ~ W AW L~
Q ~ ~ U o X ~ ~ ~: U ~ ~ v J~
~ U ~ ) UO~)C O ~ U ~ C
E l ~1 J--I >1>1~1a) ~ ~ ,c s o c ~ ~ ~ V o s w
x cl. V c u ~a '1 x I I I I I I I I I v s
w ~ u O ~ w c ~c ¦c ¦ s I c ¦ c ¦ c ¦ c I c I c ¦ `LS Ql
C: ~ 0 C ~ ~ ~
1111 ~1111111111111 ~ X
c 1: ~ ¦ c ¦ ~ ¦ c I ~ I c ¦ c ¦ ~ ~ ~ ~ c o
~ j I 1 , 1 I j I ~ ~ ~ ~ ~ ~ ~ _ _ ,~ V
I I I I I I I I ~
. ~ ~ ~ V ~ ~ V V V ~ V ~ ~ I
,~ 3 ~ I
Q Q Q Q .r~ Q Q
C s ~ c ~ s ~ s~ s .~s~ I I Cl Cl Cl Cl Cl cl Cl ~
~ W ` J W ~ ~ J ~J W ¦ ¦ I 1 ~
~ c c c c ~ ~ c ~ c c ~ c
~ AW ~ ~) W W ~)
~: I ~; C ~ S ~ S: ~ ~ S C C S C S S S S S ~ ~
~ ~ ~`J ~ ~ ~J N t~ ~r esl ~ ~r ~r ~r e ¦~ ~ ~) ~ ,A~5
_ ~ J
, ~ IIIIiIIIIIIIIIIIIII~
w ~ ~
A~ ~ ~ ~ CO
IL1 ~
`' ~ ;
-27~
`I
~ ~ ~D
o er
.
I ~ oooo
.
I o ~
,~ ~ s
C
a~
S O O L~
u~
o a) _
S ^
~ ~ -- ~ C L~ ~
s
e ~ s ~ a) ~ ~
,~ C
I I ~ ^ aJ_ LlL~ ~ ~ ~ ~,
I ~ ^ S 3
S~ ~ ~ ^
C ~ L~ ~ LJC ~ ~1 V C
a~
_ -- v I ~, s ~ ~ ~ ~ s _ s c a e ~1 aJ ,, c
I ~ ~ C Q. G~ ~ S ~ a)
QJ ~ ~ )J ~ a) ~ ~ s s ~ s c~ I s
:~ I ~1: ~ S ~ C :~ CL ~ I ~ ~1 er
C ~ ~ S ~ ~C .-- ~ ^ aJ ~ ~ S -1 ~ ~r a) ~ ~ ~1
,~ ~ c IJ ~ V ~ C ~ s ~a s ~ e s c~
s ~ ~ ,~ v -- s
c c s ~ s ~ c s C ~ e V V ~ ~ v
o a
O ~ ~- C ~ ~ s ~
_ ~ I ~ ~ I ~ ~ C~ JJ _ ~ I ~ rl I ~ ~ ~_ _1
~ I ~1 -~- r~ ~r ~ L: ~ aJ I ~ ~r ~ ~a ~ ~ I
o ~ I ~ ~
? ~ ~ ~ O ~ s ~I~ s ~ s ~r
H e ~ 4 ~
O r1 ~ I ` I ~J r~l I ~1 1 1 ~ ~ ~ I -- aJ ~
aJ C~ ~ ~ ~ ~ ~ ~ ~ ~ ~ X ~r ~ _ _ ~ _ I _ _
~1 C C: ~: -- I ~1 1 '~1 ~ JJ ~ I I I _ ~ ~ ~1 1
. ~ .~1 ~ I _ ~ ~ ~ ~ :~ ~ -- t~7 ~ --~ ~1 1 >~
~ s ~ c ~ ~ n -- _, I I ~ N -- C I
E~ Q. I ~ 1: ~ ~ ~ ~ I I I -~ ~ _ C C
C -- ~J ~ -- ' C ~ 1 ~ a) O ~ S --
X ~ J ~ S D N 12~
o ~ a~ :~ n -- ~ ~ ,, ~ ~ N N ~ O C ~ N
--II X: S I I I I I ~ ~1 ~ ~: C O ~1 a1 X C
~ _ O ~ Cl r~ ~ 1 ~ X ~ ~ O ~ O a
N ~I S 0. I ~ ~ n ~ o ,~ :~ s ~
C ~ s C 5~ o o ~, S X V ~
~c ~ x ~ Q. s O O c~ ~
~ , S ~ S S ~ C G~ U C ~SU S ~ ~ ~ _ E3
~ ~C-r1 ~1 ~ ~ Cl ~I Cl Cl ~ U ~1 ~ ~ ~ ~ ~ ~ ~
: ~ _
~ I
,~ e x _, ~ ,1 ,~ ,, ,, " ,, ,, ,, ,, ,_~ ,, ,_, ,, ,, ,,
1 -~1 0 1 ~
N ~ ~r ~ N---l: N N N N N N N N N N N N N N N N
:~: CtaUC:~CC,CCCCCCCCCCC~:: C
: m ~ --c ~ aJ ~ m m m m ~ m CD m m ~ m m m m m
s
~: : _ ~ -
a~
_I
e ,, ~ O r co ~ o
~ CO ~
.
,~
:
-28~ J
_ L~ . h
~ aJ C
O C4 ~1 ~ ~U O h ~ ~
S''~ ,c C 1-'
J ~ ~ L~ I C :~ ~ ~ ~ S S
~1 V S
S ~ ~
1: Ei r~l ~ ~ ~i ~ ~: lU ~ .C S O JJ
a~ a) c ~1 ~ a) s ~ ~ Q~ ,C aJ S ~ ~J
a~ . ~ .c ~ C S Q~
I ~ I s a
. ,~ r ~ S S ,~ S S ~1 ~ e e
,, ~ ~ e I ,~ s ~ ~ ~ s
V ~ ~ I O ~ ~ --~ S ~ ~ ~ aJ IJ O er er L~ ~ ~ C
c .,., ~ e ~
o au I I o v s .c ~ e ~ I I e o o o ~ ~ ~ s
c~ ~ ~ O ~ ~I JJ JJ E~ I I ~ ~r I ~ ~. ~ _~ ~ c ~4
_ c ~ e ~ r o I I ~ ~ o o ~
:~ ~5 - o ~ I e e ~ I ~ o o I o _, ~ c c s ~.
O I ~ ~ I O O L~ ~ O I C S a~ 4 s
I ~ ~ O ~ --~ O O L~ ~ U U C S ~ V
H e ~ s ~ ~- oI , Ll o s ~ ~ o -- I I ~ ~ ~ ~
O ~ J 1 I h O O O ~ t) S S ~ S e
aJ ~ -- ~ ~ ~ O ~ ~ ~1 C I U U ~ ~ -- -- ~ ~ JJ I
I ~ ~ I --I O O s ~ I I I s s a~ ~r
s ~ è --
I ~ c s
I I -- I I ~ ~ I S _I ~1 1 1 ~ I
:~ S ~~ 7 ~ I ~ ~ I I ~ v ~
N P~ ~ 8 I -- -- ~ I ~1 ~ _ I O N N -- -- t~ _I
c ,, s I ~ ~ e c ,~
~ ~ ~ C I I ~ r~ U ~ ~ ~
8 .~ U C) ~ :~ Q 1~ aJ
O X S C --I ^ --
~ ~ U ~
S 6 C C ~ ~ h It5 ~15 S S O S
. ~ I ~ ) X: V O C~ X JJ ~ ~J ~
aJ el' s s a~ v c L.l CJ tlJ ~ C aJ
E ~ ~ S O ") :~ IJ ~ S O U 1: t.) e c o ~ JJ
~ ~ C~ Cl Cl C~ Cl C) ~ ~r ~ Cl C~
:
.
: 1 `N N N N N N N N N N N N N N N N N N N N
CCC~ C~:CCCCCCCCC~:CCC
~ m m m m P: m m m m m m a: m m a~ m m :a m m
I I1~ 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
~ I ~ .
e I O ~ O ~
~ O 0 : 0 0 0 0 0 0 0 0 ~ 1 ~ r~l
: ~ : ~ :
_ ~
: :
--29--
~_ ~
fV
4 fO a ~ 0 fO ra
~ a) fV fV
_ 4 4 ra _ . 4 S_~ 4
_ fa fa ~ ra ~ a ~ fa ~ f5
fV ~ ra ~ ~ :>, 0 ~
~U ~I Ll C ~ 1-1 ~ flJ :5 4 C ~J 4 ~
:~ ~ , ra ra ~ -- ~ fV 4 3 ~1 ~1 ~ 1
c _ _ s c c a~ a~ fa ~a ~ c c ^
~ ~ a) ~ 4 ~ f~
4 ~ C :~1 0 ~ S S ~1
C C S :~ C t ~ 3 ~1 fV C L~ ~ C Ql Q~ ~ C
o a~ fV .C fV ~1 ~ C .S: fV O C ~IJ O O O fV
C.) ~ S S J~ S ~ ~1 aJ S U 4 4
_ C Q~ ~ fV: ~i S f-.
~ --I ~ ~ ~ ~ ~ ~ ~V C C ~ L~ O C~ ~ O ' ~ O
O ~ fV ~V: C fV rv' S) O Ll I 0 4 S S S 4
:~ ~ .C S ~ .~ Lj S ,C ~J ~ O ~ Ll O C) C~ ~,) O
H E~ .L)J-- -- ~ I I O O Q~ ~ O .C r-~ -- O ~ I I I --I
V rV I ~1) ~ ~ h h O O ~i C) S I --I .C ~ ~ e~ ,C
rv C~ E~ -- -- O O ~1 4 1 0 (~ S t.) -- -- -- U
~1 i I I I I I ~ O O t~ ~r I I O I I I I ~
Q ~ ~ ~ S S _~ ~1 1 -- ~r _ I ~ ~)
fa ~ S ~ I -- ~
E ~ I I ~ r
t` ~1 ~1 ~ ~r I I I I t~ ,C I IY~ ~ ~-1 ~ `
_ -- rv _ N N I I -- -- I ~ _ ~V I -- N ~ N --
~J --I E 1~l C - C ~ ~ II -- ~ C C C I
~ ~1 rv ~V I I ~ ~ ~ 1 fV rv rv ~)
U ~~ Q ~ 1 fV ~ Q ~ I
` ~V rv S ~ O ~ ,r)rv S X ~ O O _1 _
'O ~ V Ll ~ ~ ~ ~I r-~ ~V 0 ~ V rv ~ 4 ~ --I
~, fa fa c ~ o s ~ J f~ .C .J ~ O O S ~1
. X ~O --I ~ C ~ c o ~ U X C~ O O ~ X
aJ '~ f~V rv aJ O ~; 4 rv rv fa ~ ~ fV rv
.S~ O C C~C '~ J S . C) t~ S
I I I ~ I I 1~ 1 1 1 1 1 1 1 ~ ~ I I I I
1::1 C~ 1: Cl ~:;1 Cl Cl ~ r~ C
_ _, _ _ _ _ _ _ _ _ _ _ _ _ _ _ _, _ _
I 1 1~ 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
~ IIIIIIIII
N N N N N N N N N N N N N N N N N N N N
C C ~ ~ C C ~ C ~ ~ ~ C C ~ C ~ l C C
a~ rv v aJ rv ~v ~v fl~ rv ~J rv a) rv rv tv ~V a~ <V (V ~V
m ~ m ~ m a ~Q a a m m m m m ~ ~Q
~ l l l l ~l l l l l ~ l l l l l l l l l l
-- -
:
~l ~ ~
: E3: o ~1 ~ ~ ~ U~ ~ ~ o ~ ~ ~ e
fO N ~t N ~N N~ N N ~ ~ N ~ ~ ~ ~ ~'1 ~ ~) ~ ~ ~
: _ _
~30~ 3~
.__ _
U ~
~a ~ ,1 ~ ~ s c c c c ~ ~ ~~ a) 3 3
a~ o L~ ~ ~
a~ ~ ~ c ~ ~ o ~ s c 5 ~ S C
~ ~ C ~ .C O ~ J~
C ^ ~ s aJ ~ o o o o o o o o ~ ~ ~ ,i
.,1 ~ s~ ~ c a~ O O ~ S
o ~ ~ ~ o o o o
C ~ S ~ O ~ t) ~~ V JJ L S ~ ~ C C
o a) ~ o ~ o ~ ~ .c s s ~ ~ ~ ~ v ~ c ~ ~ a)
O ~ ,C O --1 0 ~ S S
_~: ~ ~ ~ ~ O I ~ O ~ ~ S C ~ C~.
~ o o u ~s ~ 1 c ~ c ~ ~ ~ o
O` L~ _1 ~ O .C ~ I I I I .C a1 a~ ~ ~ C _~ s
Ql 0~ S ~ ~1 U ~ ~ er ~ ~ U S S S aJ a) ~ ,C
` ` ` ` ~ ~ ~ ~ S ~: ~ ~ U V
O S ~ ~ O ~ ~ Q
)
~ rl I ~~ ~ I I I I I~ S ~; S ~
n ,~ J v 5: s o R ~3 ~1
E~ ~ ~ C~
` -- I ~ I .C ~ --~ ' ~ _~ I I I I I I I I
N N N N -- -- -- -- ~
1 1 ~ i C C ~: C I I I I I I I I I I
I,~ u :~ ~ Q n n ~2 1 1 1 1 1 1 1 1 1 1
X s o o ,~
~ C S~ O ~ J~ s ~ 1 ~ ~ ~ L)
U c a~ U ~ ~ C S a~ 3
o ~ ~ o u c u u ~ ~ ~ Q D ~ n n n ~
Cl ~CI Cl Cl t~ Cl Cl Cl CI Cl Cl Cl Cl Cl Cl
~III1111ilIIIIIIIIII
: ~ 1 1 1 1 1
: ~ 1
N N C~ ~ C C C C C C C C C C C C C C C C
m m ~ m m m a~ m ~ :a m ~ ~ m ~ m ~ a~
:
a1 ~ :~
:Q~ ~ o ,~ O
~ : :
:
-3~
_ _ .
a~
Ll Ll n~ ~
~u o aJ o o h ~IJ Q~ ~ a) aJ O
~ L~
3 :~ :3 S .C a O ~ S
_ O O O V J~ 1 0 ~0 0 0 0 JJ
,~ _ ~ s ~
a~ S ~ 5 V S h ~ S S ~C S _
~ V V ~ ~ ~ O ~ aJ ~ V JJ
.~ ~ ~ ~ c c ~ 3 o ~
V ~ ~ ~ ~ s V ~ o ~ ~ ~ ,1 s
C I v v ~ S aJ au a) ~) O
~ ~:1 S ,C S ~ ~ >1 ~ .S: ~I r-l ~1 ~ _ ~1 U S r S S 4
_ ~: ~ Q~ Qj S 5: C ~
~ ~ ~ ~ V L~ a~ x Q C C: C ~ O O O O ~
o ~ ~ a) a,) s o ~ c aJ . ~ 4 )~ S
:~ ~ S L: S ~ S S S .C S ~) O S O O O O t
o ~ 4 ~ O O O Q~ 0 5 L: S S S ~
C~ Ei E E~ ~ U O e ~ 4 4 4 O O 4 Qj ~ O t) C~ V
. ~ I 1 S ~r~ S O O O E~ E~ O O
r~ D V ~ ~ ~ 1 0 0 ~
~1~ 1 1 1 ~ ~ O I a) s s 5:: ~ i4 -~ O
E~ ~ 3 er e ~ r
~ ~~1: ~ ~ ~ ~ '1 N ~ ~ t~ I
____________________
I I I 1 1: 1 1 1 1 1 1 1 1 1 1 1 1 il I I
` :~ ~
. ~ ~ ~ ~ V~ U~ ~ ~ ~ ~ ~1 V~ ~ ~ ~ V~ V~
: ~8: Q: Si 8 8 Q 8 8 R 8 Q Q Ll 8 D 8 8 8 8 8
. ~ ~::1 C~ cl Cl Cl ~ Cl Cl s::l Cl Cl Cl ~:1 Cl Cl Cl Cl Cl C
:`
~1 ~
: : ~ N: N N N N N N N N N N N N N N N N N N N
; ~ C a) ~ C C ` ~ C C. C C ~: C C :: C C C
~m ~ m G: a~ m m m m m m P~ m :q m m m m m m m
aJ ~
:
o ~ ~ ~ ~ 1~ o ~1
X:~ ~ ~ ~1
w
--
:
~: :
-32~
_ _ _ .
a) ~ a
a :J o :~
O ,~ o
Ll '~ S
, ~ ~ V s
~ O r~ ~ h
a~
r ~ I O ~ ~ O ~J
~ 1 ~ 5 a) a) ~
o n~ _ OL~ ~ C .C
~ ~ O S ~
C: Ll O ~ ~J S O O S -- ~-1 ~ O O ~ -- C
-- ~ O ~ ~ ,C ~-I ~ V >1 S S ~1
~ 1 0 S O ~ ~ V ~1 C -- ,C ~ 1 ~ C Q~
a~ 1 ~ _ _ s aJ ~
~ ~ O V ,1:: ~I SC ~ .C ~ aJ ~1 ' ~ ~ S ~1
~ e " ~ L s
_ O ~: O S ~ Ei ~ V
~ ~ S ~ aJ aJ ~
-~- ~ s _ c ~ I s s ~ s c~ I s s ~ s
C E ~ ~1 ~ ~ I ~ ~ er ~ Q~ I V I
~: ~ s :~ c o ~1 aJ s ~
,~ ` ~I v ,-~ C Q, S ~ S JJ :~ ~ ` E s ~ ~ ~ ~ e
s s ~ ~, V -- s s ~ I o
~ c ~ C~ JJ Y ~ ~ aJ I v v -- ~ ~
o a.) ~ I I o
u ~: s~ ~ ~ ~ c ~a E ~ ~ O --~
_ C ~ ~ Y ~ ~ I ~ S
O er r1 .,C E a) I ~ O C)
O ~ ~ ~ v -~
:~ ~ o ~ ~: a~ ~ ~1: 1 ~1 ~ ~r er ~ I ~ s ~r ~r ~ S ~
E :~ ~ ~ E I ~ ^ ~ -- ~ ~ S ~ ~ ~ ~ ~ S O --
O ~1 1 ~ ~ I I N ~ V I -- ~ ~ ~ J~J 1: 1
~ C~ q~ ~ ~ r8: ~ er ~ er ~ ~ ~ ~ ~ I ~ ~ ~ a~
_I
~ , ,, , ~ ~, -- n -- ,, , , ~ N -- C I
E-~ ~ -- C C ~1 '~J -- C I
~ I ~ C~ : s ~ I) ~ s ~ I aJ _ v
: CN ~ I V ~ ~1 S 52 N ~ :~ :~ S ~1 --(
-- ~ I ^ a) N N ~ O C ~ N N Ql ~ Q
` 1` 1 1 1 ~ C C O ~ a) x c c _I s I
~: ~ ~ x ~ o n o ~ , v ~::1
1 ~ ~ o ~ :~ ~ ~ ~ s ~ I
_, _ ~ ~ a) ~ c ~ o o : ,t ~ x
a o a) V ~ o aJ ~ :~ a) ;~
C ~ ~ ~J: O t~ e. S ~ ~ C C
: u ~ ~1 X ~ ~ x t): o ~: r t ~, , ~ ~ , u v I O a~
~ J C O ~ c ~ I a) ~1 ~ o ~ s s
: .a~ ~ ,c ~ V S O C) c O c) -- ~r E -- E E ~ ~ Q-
1~ r c I C I c I C I q~ ~ ,1 ~ ~r ~ ~ ~ ~ ~ ~r ~ ~ ~ ~
_ _ _ ~
II11,1IIIIIIIIIII
: ~ ~ N N: N N N N N N ` N N N N N N N N N N N N
~` C ~: C ~: C:` C C C ': C ~: C C~ C C C: C C C C C C C
. : ~ ~ aJ ~
m m~ m cq ~ m a~ a m m m m m m m
l l l l l l l l l ~ l l l l l l l
: a~ ~ ::
~1
~ ~ o ~ ~ ~: ~ ~ ~ o ,~ D 1~ .0 a~
: X: : ~
:: ~ :
~ ~ --~
:
3 3 iL,- ~ ~ j,r
_ .
O
O ~ o ~ ~ ~ ,_
. In ~ t~ r`
. ~ ~ l O
'i u~ `D r` r` o ~ D O c~ O O O O ~ O O
.
C~ ~ ~
. ~, ~
~ C
L~ C C
s
,c
' ~ ~ C ~- -- ~ C ~ ~ c:~ ~ 1.
o ~ ~ ~ C
~1 o ~ a~ s -- ;~ ~ ~c ~
S ~ C ~ ~ ~ C E ~ ~
~J ~C ~ C C C ~ ~ al c ._ c ~ ~-~ E C ~ E
-- J~ aJ o ~ c s ~ s ~ ~ ~J 5
~1 S S S S a~ C~ ~ s C ~ I ~ ~
_ ~Q, ~, Q, V S --~ O- ~ C~ ~ ~ I ~ I
C ~ C C
E
~ S ~ S S S S S ~r1 ~ V ~ S Ll -- ~I S ~
C C~ S ~ ~ V V V ~ c ~ ~ ~ ~ 1 S --
~ ~ V E Q~ I ~ E
V ~ r-l X X F E~ e ~ ~ ~-~ E ~J E ~ I ~ E a~
C S :>. O O ~
O ~ ~` S S ~ ~ ~ ~ -' I ~ C~ ~ I ~ -- ' ''' --
~ ~ ~ V V V I I I ~
_ c ~ O ~J~1'r~r ~ I I ` ~ I ~ - N
~ I ~ E~E~ ' ` ` ~ ~ ~ ^ ~ - C~J c N - ~ S
O ~ ~C C~ -
a D~
a o I II I I I ~ . 9 1 --
O L~ O O O ~ ~ ~ ~ I X
al U O h 1.~ ~ I I I N ~ -- I N I ~ ~ I
_1 --I O O O -- -- -- C I--~ ~ C ^ ~ - ^ I C
s ~
1~ t.~ S S S ~ ~ ~ ~I N ~, 9 ~, N al ~ ~ I S
E-~ I ~ t) U N N N 0 ~1 C N _I N C C E N :~
~ I II c c c ~ v ~J C ,~. C ~ al o c s o
-- ~ ~ U a~ o ~ n a~ aJ D ~ .
, _ -- -- ~ ~ n ~ ~ --I D ~ D ~ ~ 0 9 ~
I I I O o o s I :~ _ 9 o x
I ~ ~ ~ Ll ~ C I V ~ O L~ _I
--IIIOOO-'--~c~1oc~C~
"~ I ~ I n ., ~ ~ ~ E ~
~ I S I ~ S ~;r
v ~, ~ ~ ~ ~ U ~o I c I E ~ E
a~-vvIII~IIIII III
s o ~ ~ -- -- -- -- ~ -- _ _ _ _ _ _ _ _ _
I I I I I I I I N
CI CI CI CI ~ I C
_ _ _ _ I I I I a~ I I I I I ~ I ' '
I I I I ~ ~ _ ~ ~ _
I ~ ~ V V V O V Ll Ll 1~ .U ~ ~I
N N N N m m m m m m ~a ~ ~ ~ ~ ~ P
c c c ~ l l l l l l l l l l l l l l l
G~ ClClClCl~Cl~CCCCCCCC
m m m -- -- -- -- -- -- -- -- -------- -- -- ~
IIIIIII~IIIIIIIIIII
a~
_I
o ~ ~ ~ ~ u~ o ~ ~ ~ ~ u~ ~o r~ oo
ooOOOOOooo--~--~--~
~ . ~ .
3~
--34--
__
o ,_ .
~ ~ ~ ~ I ~ ~ ~ ~ ~ ~ ~ ~ ,~
:~: ooo~oooooooo~ooooooo
_ _ ,
,~
>~
~ C aJ ~ ~ C
5 a.~ IJ ~ ~ ~ C C C ~ C C
C~ .. C r a. ,C r a~
CL ~ a.~ 5 ~ t~ a. a. CL ~ C) ~ C:)~ ~:;
~. ,~ r ~ r ~ ,~
a~ ~ C CL r C~
~ ~:: ~ ~ a) c ~ ~ ,c ,c ~ :J c ~ ,c
~ ~ J ~ ~ 5
6 ~ ~ c ~ a~ aJ aJ 6 a
~ F: ,~ c _ C~. C ~ C L~ ~ 6 E a ~ 6 6 6 a)
O ~~:) ~ I ~ ~ ~ ~ ~; E a~ 6 a~ 5 ~ ~ .
~ . C I ~ CL LJ r o~ 6 -~ c I I I ~J I I I
_ ~1 `5 ^ ~ ~V ~ ~ ~IJ ~ ~ ~ ~ 3 ~t ~ ^ ~ ~J ~5 1
o - ~ ~ E ~3 ~ :~ Ei I ~ I ~ ^ - C~l - - - ~J
~ ~ 6 .c r~
6 _ ~
I ~ I I I _
~- ~ 1 6 ~ I e ~r --~ -- aJ ~, ~ ~ ,
E I ~ I ~ i I I
I
;'~ S ~ I ~ r ~ ~
s ~ t s c I s c
~ _1 .U tU Ll IJ JJ .S
~ N :~ N ~1 0 0 a.l ~ a~ O (V
I ~ C N C ~
:~ C: I: N ~ I ~ P ~ C
C ~ ~ C N~-- C ~ ,~1 ~ I C C C O C C C :~
a~ c ~ s o ~ ~ c
.ç; Q. ~ ~ x :~ x ~ s s s o~ c .C s a
~ ~ ~ ~_ D N ~ O X O ~ Cl.
O X O,--1 ~ C :~ r~: O ~ N ~ ~ X O O O C~
~ 0 ~ Ll X ~ O ~ 0
o r o~ JJ ~ D O LJ ~ G) S S C S O O O E
~ ~ C x 0~ ~-) C x CL. ,~ ~ O
s ~ ~ O ~ ~ ~ ~ ~ c ~ ~J
o ~j: C 1~ o p Q. S .S 1 - E E Ei 6 U~
I1'~I1'r~IIII~IIII,IIII
~ ~ c ~ c s c c: ~ c ~ r ~ ~ ~ ~ ~
_ ~ _ C -- `' '' '' '' ~' -- --
,.,, j,,,,,, ,~ j, I I I I I ~
,, ,: I
I111,~,,,,,,,
: ~ ~ ~ ~ ~ ~ ~ ~ ~ _~
: ~ ~ ~ ~ ~ ~ ~ ~ 1 r1 ~1 ~~ 1
: J_l 1.. 1 ~I W Ll ~ Ll L~ 1 Ll ~ Ll U Ll ~ L~ .U Ll
~ C C C C G C C C C C C C C c C C C C C
~ _
: : 1: 1 ~ 1 : 11111111111
. ~ ~ r~ ~1 ~ ~ _ _ _ _ _ _~ _ ~ ~ ~ _
~' : ~ ~
E ~ o ~ o _ c~ o
x ~: ~ :~
-
`` : :: : : : :
:
~35-
., ._ ,.
(~ ~ o o
o ~ ,_ ~ ._ ~ ~ ~ ~ ~ ,_ ~o
.
o ~;r ~ ~ oo ~ ~o I
. ,~ ~ ~ ~ ~ ~ ~ C~l o o ~ ~ ~ ~
:~ o ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ o ~ o
I ta
~ U 0 t3
:,~ ~ ~ ~J ~ ~ ~ a~
c ~ C
C ~J I ~ a) ~ C ~ C 5
aJ ~ ~ 3) ~1 ~ 3 ~ ~ ~ a) Ll ~ ;~
fi "~ r ~ aJ O C :~
J ~ ~ C ~ C CL ;~ ~ ~ IJ ~I N
SJ ~ ~ ~ ~ a) :~ o ~4 c ~ ~ c
I ~ ~ r
~J ~ ~ r~ N Q. ~ tl. CC ,C I ~ D
- _ C 1C C ~ ~ ~ Llt~ 1 ~3 C ~ :~
c~ ~ ~ E ~ G) ~ o - o I x
_ C C: E; ~ ~ c .,~ r ~ O
~ ~t ~ o u ~ ~ ,~ c
1 c ~ ~ ~ ~ a) I a) ~ ~ ~ I ~ C`~
~ o
_ ~ ~ ~C ~ JI I C
a) r ~ C~l ~ ~ I _I ~ ~ ~ c~l ~1 a.l I
C ~u a~ r - I - I ~ I N
,~ ~ E3 ~ -- ~ I ~ ~ - ~ C ~ :~
., ~ ~-~ ' ' I I ~ ~ ~I ~ I a~ I N
C ~ ~~ C ~;
O t~
~ ~ . ~ ~ I N I ~ ~ x c~l D ~J
_ ~.C - ~ S ~~ ~ ~ C ~ N I N O ~ O
c~ ~ I ~ I ~ :~ a) ~ c ~_ c ,~
~ ~ ~ ~ N r D ~ t ~ ~ ~1 S
:~ O x -I 1: E I t 1 0 N ,~ ~ ~ a) I ~
1 ~ ~ c o N ~ C ---
Ei,~I ~ ~ ._ ,c~ E3 0
~ O ~~ ~ 1 ~ D
_I ~ ~~ N t N :~~ S
a
~a ~a~ s ~ O U ~ a~ s
E~ ~,~ ~ t~ ~ ~ S ~ ~ ,~
~: O O E t~
c ~ ~ o ~ ~
~J~ X O O ~ IJ C ~ r c I ,_
. c):~ ~ ~ ~ o I c~l I _ I ~ _ .1_~ ,_~ ;,~
E;c r c ~ ~ C
I S ~ S
~ ~ ~ _ I ~ I ~ i I I I I ~ N
-- ~ C X
I I 1 -~ ^ I Q ~1 ~ O
C~ C`J ~ 'V~ ~ ~ :~ N ~ ~ ~ ~ ~ S
`~ N C N N N N N-- O
I1 1 ~ 5 a~ C C t; C C I 1-
O ~ C
: I ~ :1 :~1 1 1 ,_, ~ ~ D D :~ D ~ I ~ -' O
o x o _~ o o o ,~ a
~ ~ ~_ ~ ~ ~ ~ C ~ ~ ~ ~ ~ I~ ~ ~ U ~ I t~.
's ~ ~ ~ C o~ o o ~ ~ ~
:~~ * ~ ~ c c ~ s ~ s
:: ;_ C~ C C C C C c ~ ~J ~ ~ ~ ~ ~ C C ~ t~
' ~ ~ ' ' ' ' ' ~ ' ' ' ' ' ' ' s ' ' c I ~
~ ~ ~: ~ t~ . ~1 1 ,
:
~ ~: :
: ~ ':~ ~ o ~ ~ ~ o
~ X ~ ~:~~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ _
:: ::: : ~: :
-36~
. _..... ~
. ~ o
t~ ~ ~
O ~ u~
. I ` a~
o ~ ~ I I o~ I
. ~ .,~ .~1 ~ ~ O ~
o o a~ o
_ _
.
~ I~
~ , ~
I I ~ I --
a~
E ~ ~ c ~ C
~
Ei 6 ~ E ~ ~--
C
I `J~ `J ^
- - ~ ^ - E3 a~
, ~ ~ _ ~ ,~ CL
~ I I ~ I I :~
_ :~ ~ ~ I ~ ~J r
~: N I I
~I~ C
.-~ ;-,~ --- E3 ~
E
O N N C N ~' ~ r~
:J s, C C a c I I -c
C O o~ a) D a.) ~ ;r I
o ~ ,~ J
t) ~ r
~_ C U 1~ 0 S~ N ~ ~1
~ ~ O O r-l C I ~
O ~ 5
.c ~I c
U tl EI ` '--
O _ I 1 1: C~ 1. _ _~
o~ ~ ~ ~ C~ O
, I I _ ~ O
E-~ ~
: ~ ~ ~ .* _ _
: ~ 3 I C x
S 1~ ~ I U) Q)
,1 J l 1~ J ~
Q~ o
._
: ~ ` ~ ~ ~:
I
C ~ C C ~ O ~ O
r r ,~; i r~ J
~1 ~ ~, O X ~ I I
~ X ~ O ~
: ~c : r ~ : o~ :: o ~ a~ I I
JJ ~ C
a,~
W
: I ~ : 3 I ~ ~: I ~ I ~ ~1
:
~ : ~ ~ ~ m ~
1 ~ I I
C~ ~"C: ~ C ~ C ~ C ~ C C
L) ~
~, I ! : ¦.C : I I .~ I
_ ~ ,
:
: ~ : ~
: ~ ~ ~ ~ ~ '~
x u~
: C~ .
:
-37-
Example 262
l_Benzyl_l_(n-butyl)-3-(3-chlorophenyl)urea
A solution of 1.56 g. of phenyl chloroformate in
50 ml. of ether is added dropwise to a stirred solution of
2.55 g. of 3-chloroaniline in 35 ml. of ether. The mix-
ture is stirred for one hour at room temperature and then
filtered. The filtrate is evaporated and the residue is
crystallized from hexane to yield phenyl N-(3-chloro-
phenyl)carbamate.
A solution of 1.46 g. of phenyl N-(3-chloro-
phenyl)carbamate in 15 ml. of tetrahydrofuran is added to
a solution of 1.92 g. of N-benzyl n-butylamine in 20 ml.
of tetrahydrofuran and the mixture is stirred under reflux
for 24 hours. The mixture is diluted with hexane and the
precipitate collected by filtration. Recrystallization
from pentane affords l-benzyl-l-(n-butyl)-3-(3-chloro-
phenyl)urea, m.p. 69-70C.
~ Exam~ e 263
l-Benzyl-l-(n-butyl)-3-(4-carboxyphenyl~urea
A solution of 5.30 g. of l-benzyl-l-(n-butyl)
-3-~4-carboethoxyphenyi)urea in 100 ml of ethanol is
treated with 25 ml of IN aqueous sodium hydroxide,
stirred under reflux for i6 hours, allowed to coo~,
acidif;ied with IN hydrochloric acid, and filtered. The
soiid is recrystallized from ethanol to yield l-benzyl-l-
-(n-butyl)-3-(4-carboxyphenyl)urea as a white solid.
Examnle 264
:
roxy-3-chlorophenyl)urea
; ~ ~ A so;lution of 1.73 g of l-benzyl-l-tn-butyl)
~-3-(2~-meth~oxy-3-~chlorophenyI)urea and 1.00 ml of boron
tribrom~ide in 40 ml of methylene chloride is stirred at
ambient temperature for 3 days and diluted with water.
~ ~The~ organic layer is separated, dried, and evaporated.
:
::
-38- ~ ~3~
The residue is crystallized from hexane to yield l-benzyl-
-l-(n-butyl)-3-(2-hydroxy-3-chlorophenyl)urea, mp 59-62.
Example 26$
N-(2-Chlorobenzyl)-3-methoxyphenylacetamide
A mixture of 12.5 g oE 3-methoxyphenylacetic
acid, 21.2 g of 2-chlorobenzylamine, 15.1 g of tri-
ethylamine, 19.3 ml of borontri~luoride etherate, and
500 ml of toluene is stirred under reflux for 18 hours
using a Dean-Stark moisture trap and allowed to cool. The
mixture is extracted with aqueous sodium hydroxide, dilute
hydrochloric acid, and water. The remaining organic
solution is then evaporated and the residue crystallized
from hexane to yield N-~2-chlrobenzyl)-3-methoxyphenyl-
acetamide as a yellow solid, mp ~9-91.
Example 266
N-(n-Butyl)-2-chlorobenzylamine
A solution of 21.2 g of N-(n-butyl)-2-chloro-
benzamide~in 100 ml of tetrahydrofuran is added with
cooling~to 200 ml of 1 M borane in tetrahydrofuran and
the mixture is stirred under reflux for 18 hours, allowed
to cool, and treated with 6 N hydrochloric acid. The
organic solvent ~is evaporated and the residue is partitioned
between ether andi aqueous sodium hydroxide solution. The
ethèr~ layer is separated~, dried, and evaporated. The
residue~is~distilled to yield N-(n-butyl)-2-chlorobenzyl-
amine~as a colorless liquid, bp 65-75 at 60~ .
:
3Q
:
~: