Note: Descriptions are shown in the official language in which they were submitted.
~e~ l c o ~,~Ft~
Field of application_of the invention
The invention relates to new diaryl compounds, pro-
cesses for their preparation, their use and medicaments
-5 containing them. The compounds according to the invention
are employed in the pharmaceutical industry for the prepara-
tion of medicaments.
Kno~n technical background
_
It is known that certain 1,4-dihydropyridine deriva-
tives subs~ituted in various ways have pharmacologically
useful properties. European Patent Applications 88,903,
94,159 and 106,276 may be mentioned as examples. Surpris-
ingly,`it has now been found that the new compounds des-
cribed in more detail below, which, in contrast to the com-
pounds of the prior art~ carry a piperidine ring which is
disubstituted in the 4-position, have particularly interest-
ing pharmacological properties by which they differ advan-
tageously from the compounds of the prior art.
Description of the invention
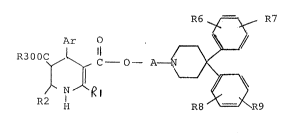
The invention relates to ne~ diaryl compounds of the
formula I
R6 ~ R7
2) ~ ~Rl ~ 9
wherein
Ar represents a ring of the formula
- z ~
R~
/~
Y ~R5
\//~
;n ~hich Y denotes oxygen (0)~ sulphur (S), vinylene
(-CH=CH-), azometh;ne (-CH=N-) or a group of ~he
formula
/ N ~ ~ / N
o I or S
N ~ N ~
R1, R2 and R3 are identical or different and denote hydrogen,
C1-C6-alkyl, C3-C7-alkoxyalkyl, aryl~ aryl-C1-C6-
alkyl or aryloxy-C1-c6-alkyl,
R4 and R5 are identical or different and denote hydrogen,
hydroxyl, halogen, n;tro, cyano, tr;fluoromethyl, ~1-
C4-alkyl~ C1-C4-alkoxy, C1-C4-alkoxy wh;ch is
completely or partlj substituted by fluor;ne, C1-C4-
alkoxycarbonyl, C2-C5-acyL, amino or mono- or di-
C1-C4-alkylamino,
R6, R7, R8 and R~ are identical or different and denote
hydrogen, hydroxyl, halogen, C1-C4-alkyl, C1-C4-
alkoxy, amino, mono- or di-C1-C4-alkylamino, or
C1-C4-alkoxy ~hich is completely or partly sub-
stituted by fluorine, and
A denotes straight-chain or branched C2-C5-alkylene,
which can be substituted by C1-c4-alkoxy or aryl,
and their salts.
C1-c~6-Alkyl is straight-chain or branched and
denotes, for example, a hexyl, neopentyl, isopentyl, butyl,
i-butyl, sec.-butyl, t-butyl, propyl, isopropyl or, in par-
ticular, ethyl or methyl radical.
C3-C7-Alkoxy represents, for example, a methoxyethyl,
sthoxyethyl, propoxyethyl, isopropoxyethyl, butoxyethyl, methoxy-
propyl,
- 3 -
2-methoxy-1-methylethyl or 2-ethoxy-1-methylethyl radical.
Aryl ~e~7W~ represents phenyl or substituted
phenyl with one or two substituents from the group compris-
;ng halogen, hydroxyl, nitro, cyano~ trifluoromethyl, C1-
C4-alkoxy, C1-C4-alkoxycarbonyl, C2-C5-acyl, amino and
mono- or di-C1-C4-alkylamino.
Aryl-C1~C6-alkyl is C1-C6-alkyl which ;s sub-
stituted by aryl. Aryl-C1-C6-alkYl is, for example, phen-
ethyl, 3-(4-chlorophenyl)-propyl or, in particular, benzyl.
Aryloxy-C1-C6-alkyl is C1-C6-alkyl which is
substituted by aryloxy. Aryloxy-c1-c6-alkyl is, for
example, phenoxyethyl.
Halogen denotes bromine and, in particular, fluorine
and chlorine.
C1-c4-Alkyl is straight-chain or branched and
denotes, for example, a butyl, i-butyl, sec.-butyl, t-butyl,
propyl, isopropyl, ethyl or, in particular, methyl radical.
In addition to the oxygen atom, C1-C4-alkoxy con-
tains one of the abovementioned C1-C4-alkyl radicals.
The methoxy radical is preferred.
C1-C4-Alkoxy which is completely or partly sub-
stituted by fluorine is, for example, 1,1,2,2-tetrafluoro-
ethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy or difluoro-
methoxy.
In addition to the carbonyl group, C1-C4-alkoxy-
carbonyl contains one of the abovement;oned C1-C4-alkoxy
radicals~ The methoxycarbonyl and ethoxycarbonyl radical
are preferred.
In addition to the carbonyl group, C2-Cs-acyl
4~
contains one of the abovement;oned C1-C4-alkyl radicals.
The acetyl rad;cal is preferred.
In add;tion to the nitrogen atom, mono- or d;-C1-
C4-alkylam;no contains one or two of the abovement;oned
C1-C4-alkyl rad;cals. D;-C1-C~-alkylam;no ;s preferred,
and especially dimethyl-, diethyl- or d;;sopropyl-am;no.
Straight-cha;n or branched C2-C5-alkylene is,
for example, tetramethylene, 1,2-dimethylethylene, 1,1-
dimethylethylene, 2~2-dime~hylethylene, isopropyl;dene, 1-
1û methylethylene, 2-ethylpropylene or, in particu~ar, ethylene
or propylene.
C2-C5-Alkylene which is subst;tuted by C1-C4-
alkoxy is, for example, 1-methoxy-propylene, 2-ethoxy-
propylene or 1,2-dimethoxyethylene.
C2-C5-Alkylene which is substituted by aryl is,
for example, 1-phenylethylene or 2-(4-chlorophenyl)-propyl-
ene.
Possible salts are all salts with acids. The
pharmacologically acceptable salts of the inorganic and
2û organic acids usually employed in the pharmaceutical indus-
try may be mentioned i~ particular. Pharmacologically un-
acceptable salts ~hich may be obtained, for example~ as pro-
cess products when the compounds according to the invention
are prepared on an ;ndustrial scale are converted into
pharmacologically acceptable salts by processes which are
known to the expert. Examples of suitable salts of this
type are water-soluble and water-insoluble acid addition
salts, such as the hydrochloride, hydrobromide, hydriodide,
phosphate, nitrate, sulphate, acetate, citrate, gluconate,
benzoate, hibenzate, fendizoate, butyrate, sulphosalicylate,
maleate, laurate, malate, fumarate, succinate, oxalate,
tartrate, amsonate, embonate, metembonate, stearate, tosyl-
ate, 2-hydroxy-3-naphthoate, 3-hydroxy-2-naphthoate or
mesylateO and also sal~s wi~h bume~anideO furos~mide, azo-
sem;de, galosemide, besun;de, piretan;de, etacrynic acid,
tienilic acid or 4-chloro-sulphamoyl-benzoic acid.
Radicals Ar which are to be singled out are the
phenyl, 3-fluorophenyl, 2-chlorophenyl, 3-chlorophenyl, 4-
chlorophenyl, 2,3-d;chlorophenyl, 2-cyanophenyl, 3-cyano-
phenyl, 2-methoxyphenyl, 3~methoxyphenyl, 2-trifluorome~hyl-
phenyl, 3-trifluoromethylphenyl, 3-(1,1,2,2-te~rafluoro-
ethoxy)-phenyl, 2-difluorome~hoxyphenyl, 3-di~luoromethoxy~
phenyl, 2-tolyL~ 3-tolyl, 4 tolyl, 2-pyridyl, 3-pyridyl,
2,1,3-benzoxdiazol-4-yl, S-methyl-2-thienyl and, ln part~cular,
2-nitrophenyl and 3-nitrophenyl radical.
Embodiments of the invention and preferred and par-
ticularly preferred embodiments are given in the claims~
Examples ~hich may be mentioned of compounds accord-
ing to the invention are:
3-ethyl S-C2 (4,4-diphenylpiperid-1-yl)-ethyl] 1,4-dihydro-
2,6-dimethyl-4-(3-nitrophenyl)-pyridine-3,5-dicarboxylate,
3-~2-methoxyethyl) 5-t3-~4,~-diphenylpiperid-1-yl)-propyl] 1,4-
dihydro-2,6-dimethyl-~-(3-nitrophenyl)-pyridine-3,5-dicarboxy-
late,
3-methyl S-C4~t4,4-diphenylpiperid-1-yl)-butyl~ 1,4-d;hydro-
2,6-dimethyl-4-(3-nitrophenyl)-pyridine-3,5-dicarboxylate,
3-methyl 5-~2-(4,4-diphenylpiperid-1-yl)-ethyl] 1,4-dihydro-
2,6-dimethyl-4-~2-nitrophenyl~-pyridine-3,5-dicarbo%ylate,
3-methyl 5-C2-~4,4-diphenylpiperid-1-yl)-ethyl] 1,4-dihydro-
2,6 diethyl-4-(3-nitrophenyl)-pyridine-3,5-dicarboxylate,
3-tprop-2-yl) S-C2-(4,4-diphenylpiperid-1-yl)-ethyl~ 1,4-di-
hydro-2,6-dimethyl-4-~3-nitrophenyl)-pyridine-3,5 dicarboxyl-
ate,3-hexyl 5-~2-(4,4-diphenylpiperid-1-yl)-ethyl] 1,4-dihydro-
2,6-dimethyl-4-(3-nitrophenyl)-pyridine-3,5-dicarboxylate,
3-(2-n-butoxye$hyl) 5-[2-~,4-diphenylpiperld-1-yl)-ethyl~
dihydro-2,6-dlmethyl~ 3-nitrophenyl)-pyridine-3,5-dicarb-
oxylate,
3~'
-- 6 --3-methyl 5-{2-C4,4-di(4-methoxyphenyl) piperid-1-yl]-ethyl~
1,4-d;hydro-2,6-dimethyl-4-(3-n;trophenyl)-pyridine-3,5-
dicarboxylate,
3-ethyl 5-~2-(4,4-diphenylpiperid-1-yl)-ethyl] 1,4-dihydro~
2,6-d;methyl-4-('-tr;~luoromethylphenyl)-pyr;dine-3,5-d;-
carboxylate,
3-methyl 5-l2-l~,4-d1phenylpip~r~d-1-yl)-~thyl~ -d~hydro-2,~-
dimethyl-~ (1,1,2t2-tetrafluoroethoxy)-ph~nyl~-pyridine-3,5-
d~carboxylate,
3-ethyl S-C2-(4,4-d;phenylp;per;d-1-yl)-ethyl~ 1,4-dihydro-
2,6-d;methyl-4-t2-d;fluoromethoxyphenyl)-pyr;d;ne-3,5-d;-
carboxylate,
3-ethyl 5-~4-(4,4-diphenylp;perid-1-yl~-butyl~ 1,4-d;hydro-
2,6-d;methyl-4-(2-d;fluoromethoxyphenyl)-pyr;d;ne-3,5-d;-
carboxylate,3-methyl 5-C2-(4,4-dihydroxyphenylp;per;d-1-yl)-ethyl] 1,4-
d;hydro-2,6-dimethyl-4-(3-n;trophenylj-pyr;d;ne~3,5-di-
carboxylate,
3-methyl 5-~2-(4~4-d;phenylp;perid-1-yl)-ethyl~ 4-(2,3-di-
chlorophenyl)-1,4-dihydro-2,6-dimethylpyridine-3,5 dicar-
boxylate,
3-methyl 5-C2-~4,4-d;phenylp;per;d-1-yl)-ethyl] 4-(2,1,3-
benzoxdia20l-~-yl)-1,~-dihYdro-2,6-dimethylpyridine-3,5-
d~carboxylate`,
3-methyl 5-C2-~4,4-d;phenylpiperid-1-yl)-ethyl] 4-(3-cyano-
phenyl) 1,4-dlhydro-2,6-dimethylpyr;d;ne-3,5-d;carboxylate,
3-methyl S-C2-(4,4-d;phenylp;perid-1-yl)-ethyl~ 1,4-d;hydro-
2,6-dimethyl-4-(2-methoxyphenyl)-pyridine-3,5-dicarboxylate,
3-methyl 5-C2-(4,4-diphenylpiperid-1-yl)-ethyl~ 1,4-dihydro-
2,6-dimethyl-4-(2-pyr;dyl)-pyr;d;ne-3,5-d;carboxylate,
3-methyl S-C2-(4,4-d;phenylp;perid-1-yl)-ethyl] 1,4 dihydro-
2,6~d;methyl-4-(5-methyl-2-th;enyl)-pyr;dine-3,5-dicarboxy-
late,
3-methyl 5-~2-C4-(4-chlorophenyl)-4-phenylpiperid-1-yl]-
ethyl3 1,4-d;hydro-2,6-dimethyl-4-(3-n;trophenyl) pyr;dine-
3,5-dicarboxylate,
3-methyl 5-C3-(4,4-diphenylpiperid-1-yl)-propyl] 1,4-d;-
hydro-2,6-d;methyl 4-(2-n;trophenyl)-pyrid;ne-3,5-dicar-
- 7
boxylate,
3-methyl 5-~3-~4,4-diphenylpiperid-1-yl)-propyl] 1,4-d;-
hydro 2,6-diethyl-4-(3-n;trophenyl)-pyr;d;ne-3,5-dicar~
boxylate,
3-~prop-~-yl) 5-C3-(4,4-diphenylp;perid-1-yl)-propyl~ 1,4-
dihydro-2,6-dimethyl 4-(3-nitrophenyl)-pyridine-3,5-di-
carboxylate,
3-hexyl 5-~3-(4,4-diphenylpiperid-1-yl)-propyl~ 1,4-dihydro-
2,6-dimethyl-4-(3-nitrophenyl)~pyridine-3,5-dicarboxylate,
3-(2-n-butoxysthyl) 5-~3-t~ diphenylpiperid-1-y1)-propyl~
1,~-dlhydro-2,6-dimethyl-~-(3-nitrophony1)-piperidino-3,5-
dicarboxylate,
3-methyl 5-{3-C4~4-di(4-methoxyphenyl)-piperid-1-yl]-propyl3
1,4-dihydro~2,6-dimethyl~4-(3-nitrophenyl)-pyridine-3,5-
dicarboxylate,3-ethyl S-C3-(4,4-diphenylpiperid-1-yl)-propyl~ 1,4 dihydro-
2,6-dimethyl-4-(2-trifluoromethylphenyl)-pyridine-3,5-di-
carboxylate,
3-methyl 5-l3-l~,~-diPhenylpiPerid-1-Yl)-propYl~ -dihydro-2,6-
dim~thyl-4-t3-(1,~,2,2-tetrafluoroethoxy)-phenyl]-pyridine-3,5-
dicarboxylate
3-ethyl S-C3-(4,4-diphenylpiperid-1-yl)-propyl~ 1,4-dihydro-
2,6-dimethyl-4-(2-difluoromethoxyphenyl?-pyridine-3,5-d;
carboxylate,
3-methyL S-C3-t4,4-dihydroxyphenylpiperid-1-yl)-propyl]
1,4-dihydro-2,6~dimethyl-4-(3-nitrophenyl)-pyridine-3,5-
dicarboxyla~e,
3-methyl 5-C3-~4,4-diphenylpiperid-1-yl)-propyl] 4-t2,3-di-
chlorophenyl)-1,4-dihydro-2,6 dimethylpyridine-3,5-dicar-
boxylate,3-methyL 5-C3-(4,4-diphenylpiperid-1-yl)-propyl] 4-~2,1,3-
benzoxdiazol-~-yl)-1,4-dihydro-2,6-dimothylpyridine-3,5-
dicarboxylate,
3-methyl 5-C3~(4,4-diphenylpiperid-1-yl)-propyl~ 4-(3-cyano-
phenyl)-1,4-dihydro-2,6-dimethylpyridine-3,5~dicarboxylate,
3-methyl 5-C3-(4,4-diphenylpiperid-1-yl)-propyl~ 1,4-di-
hydro-2,6-dimethyl-4-(2-methoxyphenyl)-pyridine-3,5-di-
carboxylate,
~9~
-- 8 --
3-methyl S-C3-t4,4-diphenylpiperid-1-yl?-propyl~ 1,4-di-
hydro 2,6-dimethyl-4-t2-pyridyl)-pyridine-3,5-dicarboxylate,
3-methyl S-C3-(4,4-diphenylp;perid-1-yl)-propyl~ 1,4-di-
hydro-2,6-dimethyl-4-tS-methyl-2-thienyl)-pyridine-3,5-
dicarboxylate, and
3-methyl 5-~3-C4-t4-chlorophenyl)-4-phenylpiper;d 1-yl~-
propyl3 1,4-d;hydro-2,6-dimethyl-4-t3-nitrophenyL)-pyr;dine-
3,5-dicarboxylate,
and their salts.
_ 9 _
The compounds of the formula I have a chirality
centre at the 4-pos;tion ;n the 1,4-dihydropyr;dine~ The
invention therefore includes both ~he enant;omers and, if
a further ch;rality centre ;s present, the diastereomers,
and mixtures and racemates thereof~
The invent;on furthermore relates to a process for
the preparation of the compounds according to the invention
and their salts. The process is characterised in that
a) cinnamic acid derivatives of the formula II
Ar
R300c~ H ~II)
R 2J~ o
are reacted ~ith enamine derivatives of the formula III
R6 ~ R7
)(C--~
or
b) cinnamic acid derivatives of the formula II are reacted
with ammonia and~ -ketocarboxylic acid derivatives of the
formula IV
._
o~ 7 ~IV~
_R8 R9
or
c) enamines of the formula V
R300C~ ~H
)l (V)
R2 ~NHz
S are reacted ~ith benzylidenecarboxylic acid derivatives of
the formula VI
~ b-- ~ (Vl)
or
d) keto compounds of the formula VII
R300C \
~ ~ ~VII)
: R2 0
are reacted w;ith ammonia and benzylidenecarboxylic ac;d
derivatives of the formula VI, or
,
- 11 -
e) aldehydes of the formula VIII
A~ ~VIII)
0~ H
are reacted ~ith enamines of the formula V and~ -ketocar-
boxyl;c acid derivatives of the formula IV, or
f) aldehydes of the formula VIII are reacted ~ith enamine
derivatives of the formula III and keto compounds of the
formula VII, or
g) 1,4-dihydropyrid;nes of the formula IX
Ar 8
R2)( ~ )(t (IX)
are reacted ~ith diaryl compounds of the formula X
R6 ~ R7
Ho - A__~ r ~ tX)
R8 - R9
as such or in the form of their salts, and, if desired,
resulting salts are then converted into the free bases or
resulting bases are converted into the salts, wherein Ar,
R1, R2, R3~ R4, R5, R6, R7, R8, R9, r and A have the above-
mentioned meanings and Z, together with the carbonyl group
to which it is bonded, represents a carboxyl group or a
reactive carboxylic acid derivative tfor example a carboxy-
lic acid halide).
3~
~ 12 -
The process accord;ng to var;ants a to f is carr;ed
out in suitableO preferably inert organ;c solvents.
Examples which may be mentioned are alcohols, such as
ethanol, methanol, isopropanol or tert.-butanol, ethers,
such as dioxane, diethyl ether, tetra~ydrofuran, glycol
monoethyl ether and ~lycol dimethyl ether, or other sol~
vents, for example polar solvents, such as dimethylform-
amide, dimethyl sulphoxide~ acetonitrile or hexamethylphos-
phoric acid triamide, or, in particular, chlorinated hydro
1û carbons, such as methylene chloride, chloroform or tetra-
chloroethylene.
The reaction temperatures can be varied ~ithin a
~ide range - depending on the reactivity of the educts. The
reaction is in general carried out at temperatures between
20C and 150C, preferably between 20C and 10~C and
in particular at the boiling point of the solYent used~
The process can be carried out under normal pressure
or under increased pressure, the reaction under normal pres-
sure being the rule and ;t being possible to apply increased
pressure, in particular, for reactions ~ith ammonia.
In carrying out the process according to the inven-
tion in variants a to f, the substances participating in the
reaction are as a rule in each case employed in molar
amounts, but, if desired, an excess (for example of ammonia
in variants b and d) can also be employed - depending on the
reaction conditions.
In carrying out the process according to variant 9,
similar reaction conditions to those for variants a to f are
used, but, if appropriate, additional measures are neces-
sary - depending on the nature of the substituent Z. For
example, if Z represents a hydroxyl group, the reaction is
preferably to be carried out in the presence of 3 condensing
agent which splits off or binds water (such as, for example,
dicyclohexylcarbodi;mide). If Z represents a halogen atom
~ 7
- 13 -
~for example a chlorine atom~, the reaction is to be carried
out, if desired, ;n the presence of a base Sfor example a
tertiary organic amine, such as triethylamine, or an ;n-
organic carb~nate, such as sodium carbonate).
The substances according to the invention are iso-
lated and pur;fied in a manner which is knoun per se, for
example by distilling off the solvent in vacuo and re-
crystallising the resulting residue from a suitable solvent
or subjecting it to one of the customary purification
methods, such as, for example, column chromatography on a
suitable carrier material.
Acid addit;on salts are obtained by dissolving the
free base in a suitable solvent, for example in a chlorina
ted hydrocarbon, such as methylene chloride or chloroform,
or a lo~ molecular weight aliphatic alcohol (ethanol or iso-
propanol), conta;ning the desired acid or to which the
desired ac;d is subsequently added.
The salts are obtained by filtration, reprecipita-
tion, precipitation ~ith a non-soLvent for the addition
salt or evaporation of the solvent.
the salts obtained can be converted into the free
bases by rendering them alkaline~ for example with aqueous
ammonia solution, and these bases can in turn be converted
into acid addition salts. Pharmacologically unacceptable
acid addition salts can in this manner be converted into
pharmacologically acceptable acid addition salts.
The starting compounds are known from the literature
or can be prepared by methods analogous to those known from
the literature. The cinnamic acid derivatives II and the
benzylidenecarboxylic acid derivatives VI can be prepared,
for example, by a method analogous to that of G~ Jones
~"The Knoevenagel Condensation" in Org. Reactions, Volume
~V, 204 et seq. (1967)3. The enamine derivatives III and
- 14 -
the enamines V are obtainable, for exampLe, by a method
analogous to that of A.C. Cope ~J. Amer. Chem. Soc. 67, 1017
(1945)~ etocarboxylic acid dèrivatives IV and keto com-
pounds VII can be prepared in accordance with the method of
D. ~orrmann ~"Umsetzung von Diketen mit Alkoholen, Phenolen
und Mercaptanen" ("The Reaction of diketene w;th alcohols,
phenols and mercaptans") in Houben-Weyl, Methoden der
Organischen Chemie ~Methods of Organic Chemistry), Volume
VII/4, 230 et seq. (1968)~ or Y. Oikawa et al. CJ. Org.
Chem. 43, 2087 t1978)]. The compounds IX are accessible
from corresponding starting compounds by variants analogous
to process variants a to f. Compounds X are obtainable by
reaction of corresponding piperidines (see, for example,
German Patent Specification 1,936,452) ~ith ~-halogenoaLkan-
ols.
The above preparation processes are mentioned onlyfor illustration, and the preparation of the compounds of
the formula I according to the invention is not restricted
to these processes. Rather, any modification of these pro-
2û cesses can be applied in the same manner to the preparationof the compounds according to the invention.
Preferred process variants are the variants a and c
The following preparatlon examples are ~ntended to
illustrate the inventlon ln more detall without lim~t1ng it m p
denotec melting point and b.p. represents boiling point
- 15 ~
Examples
1. 3-Meth l 5-~3-(4~4-diphenylpiperid-1-yl)-propyl] t,4-
_ . . Y_ ..
dihydro-2~6-dimethyL-4-(3~ni~ro~henyl)-pyridine-3,5-
dicarbo~ylate hydrochlor;de
Process variant e)
4.53 9 of 3-nitrobenzaldehyde, 3.45 9 of me~hyl
3-aminocrotonate and 11.38 9 of 3-(4,4-diphenylpiperid-1-yl)
propyl acetoacetate in 100 ml of 2-propanol are heated at
the boiling point under reflux overnight~ The cooled solu-
1û tion is concentrated to dryness and the residue whichremains is chroma~ographed over a silica gel column using
ethyl acetate as the eluant. The uniform product fractions
leave a solid foamed residue after concentration, the resi-
due is dissolved in methanol and ethereal hydrochloric acid
is added. The solution is concentrated, the solid residue
~hich remains is taken up in a little methanol and the title
substance is precipitated by addition of petroleum ether.
m.p.: from 135C (decomposition); yield: ~.3 g.
The s~arting compounds are obtained as follows:
a) 3 t4~4-Diphenylpiperid-1-yl)-propyl acetoacetate
23.6 9 of 3-(4,~-diphenylpip~rid-1-yl)-propanol
are dissolved in 100 ml of absolute toluene, and 16 ml of a
50X strength solution of diketene in acetone are added, with
stirring. After the mixture has been left to stand at room
temperature for several days (control by thin layer chroma-
tography), ;t is cGncentrated and the residue is dried under
a high vacuum. The pale yellow viscous o;l wh;ch rema;ns ;s
employed for the next stage without further puritication.
b) ~ .4-~i~h~nv1Pi~Qrid-1-yl)-oroDa~oL
bO 9 of ~,~-dlphenylplperidine, 2~.7 g of 3-bromo-
propanol, 116.4 9 of powdered potassium carbonate and
about 1 9 of potassium iodide are heated at the boiling
point under reflux and with vigorous stirring in SOO ml of
a 1:1 mixture of d;oxane and 1-butanol for about 48 hours.
After cooling, the mixture is filtered and the filtrate is
concentrated. The oily residue is taken up in ethyl acetate
and the solution is filtered again. After the filtrate has
~ 16 ~ 3~
been concentrated to dryness, the product is obta~ned as a
yellowish oily residue ~hich slo~Ly ~olidifies as a wax~
Yield: 44.8 9. ~i~h ~he~eal hydrochloric ac;d, ~he hydro-
chloride is obtained, and is recrystallised from 2-propanol.
m.p.: 226-7C.
Alternatively, tho startlng compound b~ i0 obtalned by
heating 352 9 of 4,4-diphenylpiperidine, 128 9 of sodium
hydroxide granules, 2.5 l of methylene chloride, 500 ml of
water, 218 y of 3-bromo-1-propanol and cataly~ic amounts of
a phase transfer catalyst (for example benzyltri~ethylammon-
ium chloride) at the boiling point under reflux for 10 hours~
~he organic phase is separate~ off and washed ~ith water and
the collected aqueous phases are extracted ~ith ~ethylene
chloride. After the combined or~anic phases have been dried
~ith sodium sulphate, the clear brownish solution is
evaporated to dryness. The resinous brown residue is taken
up in 4.5 l of boiling petroleum ether (boiling range 100-
140C), the insoluble residue is filtered off hot from the
solution and the fi~trate is cooled. After being left to
stand overnight~ the title compound is obtained as the free
base in the form of colourless coarse crystals. m.p.: 97C;
yield: 303 g.
The followlng starting compounds are obtained analo-
gously:
4~ diphenylpiperid-1-yl~-butanol, m p of the hydrochloride:
209-212C,
2-~4,~-diphenylpiperid-1-yl)-2-methylpropanol, m.p.: 115-116C,
3-t4,4-di-(4-methoxiphenyl)-plperld-1-yl]-propanol, m.p of the
hydrochloride: 130-t34~C ~contains 1 equivalent of mathanol in
the orystal),
3-(4,4-diphenylplperid-1-yl)-2-methyl-2-propanol, m.p. of the
hydrochloride: 1~ 3C,
2~(4,~-diphenylpiperid-1-yl)-ethanol, ~.p of the hydrochloride:
197-199C.
3y reactlon of the above alcohol~ with a solution of di-
ketene analogoulsy to Example 1a, the correspondin~ acetoacetates
are obtained which are further reacted without purification.
- 17 -
Process var;ant c)
15~38 9 of 3-~4,4-d;phenylpiper;d-1-yl)-propyl
2-acetyl-3-(3-nitrophenyl)-acryla~e and 3.~5 9 of methyl
3-aminocrotonate ;n 100 ml of 2-propanol are heated at the
boiling point under reflux overn;ght. The cooled solut;on
;s evaporated to dryness, the foamed solid residue ;s taken
up in a little methylene chloride and ethereal hydrochloric
acid is added. After rene~ed concentra~ion to dryness and
taking up of the solid residue in a little methylene chlor-
ide~ ethyl acetate is added until a slight cloudiness per-
sists. After the mixture has been left to stand in a
refrigerator, the title compound crystallises out overnight
in the form of finè, slightly yellowish-coloured flakes
(microscope)D m~p.: 230 231C (decomposition); yield:
13.6 9.
Other solvents which are ~employed for the condensa-
tion reaction are: tert.-butanol, dioxane, tetrahydrofuran
and chlorinated hydrocarbons~ The yields of product are
60-80X of theory.
Other salts of the title compound 1 which are pre-
pared are:
hydrobromide: m.p.: 229-230C (decomposition), fine plate-
lets (from ethyl acetate and diisopropyl ether);
fumarate: m.p.: 144-145C (decomposition), fine flakes
(from ethyl acetate);
maleate: m.p.: 1S1-152C (decomposition), clusters of
coarse needles (from ethyl acetate).
The free base of the title compound 1 is obtained
when the condensation batch is concentrated to dryness and
the foamed solid residue is taken up in a little methylene
chloride; after addition of diisopropyl ether until a fine
cloudiness remains~ the base crystallises out in the form
of fine platelets, after being left to stand in a refrigera-
tor. m.p.: 145 147C.
The startin~ compound 3-(4,4-diphenylpiperid-1-yl)-
propyl Z-acetyl-3-(3-nitrophenyl)-acrylate (cisltrans isomer
mixture) is obtained as follows:
- 18 ~
40.14 9 of 3-(4,4-d;phenylp;per;d-1-yl)-propyl
acetoacetate, 15~97 9 of 3-nitrobenzaldehyde, 8.0 ml of
acetic acid and 0.5 ml of piper;d;ne are heated at the
boiling point in 300 ml of benzene using a water separator.
After 1.9 ml of ~ater has been separated off, the cooled
solution is washed uith saturated sod;um bicarbonate solu-
tion and then ~ith waterY After the organic phase has been
dried uith sodium sulphate, the clear red-brown solution is
concentrated to constant weight under a high vacuum. The
red-brown viscous residue obtained is employed directly for
the condensation, ~ithout further purif;cation. Yield: 52 9
of crude product. Other entraining agents which are suit-
able are: toluene and chlorinated hydrocarbons. The yield
of crude product is 90-10~% of theory.
~ y reacting the free base ~ith an equimolar amount
of fumar;c acid, the fumarate of the starting compound is
obtained; m.p.: from 128C (decomposition), clusters of
fine needles, from ethyl acetate.
3y reaction ~ith ethereal hydrochloric acid, the
hydrochloride is obtained: m.p.: 152-155~ (fine platelets,
from ethyl acetate and d;ethyl ether).
Process_variant a
134.6 g of methyl 2-acetyl-3-(3-nitrophenyl)-
acrylate, 204.5 9 of 3-t4,4-diphenylpiperid-1-yl)-propyl
3-aminocrotonate and 4.5 ml of acetic acid in 2.7 l of
anhydrous dioxane are heated at the boiling point under
reflux and under a n;trogen atmosphere for 20 hours. After
cooling, 45 ml of concentrated hydrochloric acid ~37X) are
added to the mixture~ seed crystals of the title compound 1
are added and the mixture is left to stand at roam tempera-
ture for 24 hours. The precipitate is filtered off with
suction, washed with dioxane and diisopropyl ether and then
dried in vacuo at 75C. The product ~270 g) is parti-
tioned between 1.5 l of methylene chloride and aqueous
ammonia solut;on (pH 11), the organic phase is dried over
sodium sulphate and the solvent is then distilled off in
i vacuo. The residue is dissolved ;n 2.5 l of d;o%aneO 35 ml
of concentrated hydrochlor~c acid (37X s~rength) are added
and the mixture is seeded and left to stand for 40 hours.
The product which has crystallised out ;s fil~ered off w;th
suction, washed ~ith dioxane and then diisopropyl ether and
dried at ~00C ;n vacuo. The t;tle substance ;s obtained
as a pale yellow powder (m;croscope: small needles).
m.p~: 198-200C; yield: 246 9.
The starting compound 30(4~4-diphenylp;perid 1-yl)-
propyl 3-aminocrotonate is prepared as follo~s:
260.5 9 of 3-(4,4~diphenylpiperid-1-yl)-propyl
acetoacetate in 1.6 l of 2-propanol are st;rred overnight
~;th 260 ml of concentrated ammonia solution. The fine,
slightly ochre coloured prec;p;tate ~h;ch has separated out
is f;Ltered off ~ith suction and ~ashed with coLd 2-propanol,
diethyl ether and finally petroleum ether. m.p.: 144-
150C; y;eld: 225 9. After addition of a further 100 ml
of concentrated ammon;a solut;on to the filtrate and after
the mixture has been left to stand in a refrigerator for
several days, a further 12 9 of product of identical melting
point are obta;ned.
Alternatively, the starting compound is obtained if
gaseous ammon;a is passed ;nto the solution of 3-(4,4-d;phenyl-
p;perid-1-yl)-propyl acetoacetate in 2-propanol, with
stirring, until no further precipitate separates out.
Yield: about 90% of theory.
2. 3-Methyl 5-C2-S4,4-diphenylpiperid-1-yl)-ethyl~
1,4-d;hy~dro-2,6-dimethyl-4-(3-nitrophenyl)-pyridine--3~5
dirarboxYlate hydrochloride
. _
The title compound of m.p.: from 137C (decomposi-
tion); y;eld: 8.5 9, is obtained analogously to Example 1
from 4.53 9 of ~-nitrobenzaldehyde, 3.45 9 of methyl
- 20 -
aminocrotonate and 11 9 of 2-(4,4-d;phenylpiperid-1 yl~-
ethyl acetoaceta~e in 100 ml of ~-propanol.
/
/
/
/
/
/
3. 3-Methyl ~ iphenylp;per;d-1-yl?-propyl~
4-(3-cyanophenyl)-1 4-dihydro-2 6-dimethylpyridine-3 5-
d;carbol~ d hlor;de
The title compound of m.pr 136-146C (slow
del;quescence, amorphous, precipitated ;n petroleum ether);
y;eld: 5.9 9, is obta;ned analogously to Example 1 from
3.93 9 of 3-cyanobenzaldehyde, 3.45 9 of methyl 3-am;no-
crotonate and 11~38 9 of ~-(4,4~d;phenylpiperid-1-yl)-propyl
acetoacetate in 80 ml of tert.-butanol.
- 21 -
4.
~'~
pyr ~ e h ~
The title compound of m.p. from 155C (slo~ deli-
quescence, amorphous, precipitated from petroleum ether);yield: 13.6 9, ;s obtained analogously to Example 1 from
9 o~ 2-l~,4-diph~nylp1perid-l-yl)-2-m4thylpropyl
2-acetyl-3-(3-nitrophenyl)-acrylate and 3.45 9 of methyl
3-aminocrotonate in 1ûO ml of tetrahydrofuran after a reaction
time of 12 hour~.
5. 3-Ethyl 5-C3-(4,4-diphenylpiperid-1-yl)-p-opyl]
1~4-dihydro-2~6-dimethyl-4-(3-nitrophenyl)~ eyridine-
3,5 dicarboxylate hydrochlor;de
The title compound of m.p. 230-232C (fine angu-
lar crystals, from acetonitrile and diethyl ether); yield:29.7 9, is obtained analogously to Example 1 fron 13.16 9
of ethyl 2-acetyl-3-(3-nitrophenyl)-acrylate and 18.91 9 of
3-(4,4-diphenylpiperid-1-yl)-propyl 3-aminocrotonate in
100 ml of tetrahydrofuran after a reaction time of 6 hours.
6. 3-Methyl 5-C3-(4,4-diphenylpiperid-1-yl)-propyl~
1,4-dihydr ~ 6-dimethyl-4-C3-(1,1~2 ~ tetrafluoroethoxy3-
phenyl~-pyridine-3,5-dicarboxylate hydrochloride
The title compound of m.p. 189-192C; yield: 57 9,
is obtained analogously to Example 1 from 40.33 9 of methyl
2-acetyl-3-C3-(1,1,2,2-tetrafluoroethoxy)-phenyl]-acrylate
and 37.85 9 of 3-(4,4-diphenylpiperid-1-yl)-propyl 3-amino-
crotonate in 400 ml of tetrahydrofuran and 0.5 ml of glacial
acetic acid after a reaction time of 14 hours.
~ 22 - ~
7. 3~M ~ 5-C2~(4~4-diphenyle~perid 1-yl)-ethYl~
1~d~o-2,c~-dimethyl-4~13-~ ~roethoxy)-
~henyll-pyridin~o3L5-dicarboxylate hydrochloride
The ~it~e co~pound o~ m.p. 130-140C (slow del;-
quescence, amorphous, precipitated from petroleum ether anddiethyl ether 1:1); yield: 6.2 g, is obtained analogously
to Example 1 from 4.03 9 of methyl 2-ace~yL-3-l3-(1,1,2,2-
tetrafluoroethoxy)-phenyll-acrylate and 3.4 9 of 2-(4,4-
diphenylpiperid-1-yl)-ethyl 3-aminocrotonate in 80 ml of
2-propanol after a reaction time of 8 hours.
8. 3-l2-MethoxY~thvl) 7-~2- U.~ henvl~i~erid-1-vl)-
~hvlL 1~4-~ihvd~Q-2 6-di~th~ -(3-n~rop~nv~L-
-Dy~ldin~ r~ox~la~ ru~p~
The title compound of m~p. from 130C (slow deli-
quescence, fine platelets, from ethyl acetate and diethylether); yield: 3.7 9 is obtained analogously to Example 1
from 4.99 9 of 2~(4,4-diphenylpiperid-1-yl)-ethyl 2-acetyl~
3-(3^nitrophenyl)~acrylate and 1.6 9 of 2-(2-methoxyethYl1
3-aminocrotonate ;n 6û ml of tetrahydrofuran and 0.5 0l of
glacial acetic acid after a reaction time of 4 hours.
9. 3-~2-Methoxveth~l~ 5-~3-(~l4-diphenvl~i~eri~-1 -Yl ) -
pro_yl~ 1,4-dihydr ~ 6-dimethyl-4-(3-nitrophenyl)-
pyridine-3,5-dicarbo~ylate fumarate
The title compound of m.p. 184~185C (angular
flakes, from ethyl acetate and diethyl ether); yield: 5.3 9,
is obtained analogously to Example 1 from 5.12 9 of 3-(4,4-
diphenylpiperid-1-yl)-propyl 2-acetyl-3-(3-nitrophenyl)-
acrylate and 1.6 g of 2-(2-methoxyethyl) 3-aminocrotonate in
80 ml of tert.-butanol after a reaction time of 5 hours.
~ ~33~
-- 23 --
10. 3-MethYl 5-~3- ~4,4 d;-(4~me~hoxyphenyl)-piperid--1-yl~
~ __~_. ... _ __ ___ . ___
~r__e 3,5-dicarboxYlate hvdrochlor;de
_ _ _ . ~ _ . _ . . ~ _ _ _
The title compound of m.p. from 138C (slo~ deli-
S quescence, amorphous, precipitated in petroleum ether);
yield: 4.9 9, is obtained analogously to xample 1 from 4.4 9
o-f 3-C4,4-di-t4-methoxyphenyl)-piperid-1-yl]-propyl aceto-
acetate, 1.15 9 of methyl 3-aminocrotonate and 1.51 9 of 3-
nitrobenzaldehyde in 60 ml of tert.-butanol after a reaction
10 time of 12 hours.
11. 3-Methyl 5-C4-(4,4-diphenylpiperid-1-yl)-butyl~
1~4-dihydro-2~6-dimethYl-4-(3-nitrophenyl)-pyrid7ne-3~5-
_ _ _ _ _ _ _ . . _ _ _ _ _
d;carboxylate fumarate
The title compound of m.p. 123-126 (fine needles,
15 from ethyl acetate and methylene chloride); yield: 3.9 9, is
obtained analogously to Example 1 from 3.94 9 of 4-(4,4-
diphenylpiperid-1-yl)-butyl acetoacetate, 1.15 9 of methyl
3-aminocrotonate and 1.51 9 of 3-n;trobenzaldehyde in 80 ml
of tert.-butanol after a reaction time of 6 hours.
20 12. 3-Methyl 5-C1,1-dimethyl-2-~4,4-diphenylpiperid-1-yl)-
ethyl~ 1~4-dihydro-?,6-dimethyl-4-(3-nitrophenyl)-
pyridine-3 5-dicarboxylate hydrochloride
The title compound of m.p. from 148C (slow deli-
quescence, amorphous, precipitated from petroleum ether);
25 yield: 11.3 9, is obtained analogously to Example 1 from
15.8 9 of 1,1-dimethyl-2-(4,4-diphenylpiperid-1-yl)-ethyl
2-acetyl-3-(3-nitrophenyl)-acrylate and 3.45 9 of methyl
3-aminocrotonate in 120 ml of 2-propanol after a reaction
time of 15 hours.
- 2
13. ~ 4 ~ -di enylpiperld-1-y ~ PX~
pyr-d e-3 ~ oxylate hydrochloride
The title compound of m.p. from 126C (slow deli-
S quescence, 3morphous, precipitated from petroleum ether anddiethyl ether 1:1); yield: 2.8 9, is obtained analogously
to Example 1 from 3.8 g of 3-t4,4-diphenylp;perid-1-yl)-
propyl 2-acetyl-3-t2-difluoromethoxyphenyl)-acrylate and
1.3 9 of ethyL 3-aminocrotonate in 60 ml of tert.-butanol
after a reaction time of 20 hours.
14. 1~4-Dihydro-2,6-dimethyl-4-t3-nitrop ~ dine-
3 ~ icarb_xy_lic acid 3-C3-(4c4-diphenylpiperid-1-yl~-
propyl~ ester
6.8 9 of 3-(2-cyanoethyl) 5-C3-t404-diphenylpiperid-
1-yl)-propyl~ 1~4~dihydro-2,6-dimethyl-4-(3-nitrophenyl)-
pyridine-3,5-dicarboxylate hydrochloride are stirred with
40 ml of 0.5 N sodium hydroxide solution and 100 ml of
dioxane at room temperature for 3 hours. After most of the
dioxane has been distilled off, 15 ml of 2 N hydrochloric
acid are added to the residue. The milky/cloudy solution
is extracted 4 times with 100 ml of chloroform/n-butanol
t3:1) each time and the combined organic phase is washed
~ith 50 ml of saturated sodium chloride solution. The
organic phase is concentrated to dryness and the solid
residue is recrystallised from chloroform/methanol t1:1).
m.p. 192-195C (decomposition); yield: 504 9.
The starting compound 3-t2-cyanoethyl) 5-~3-(4~4-
diphenylpiperid-_-yl)-propy ~ -dihydro-?,6-dimethyl 4-(3-
nitrophenyl)-pyr din ~ -dicarboxylate is obtained as
follo~s:
8.1 g of 2-cyanomethyl 2-acetyl-3-(3-nitrophenyl)-
acrylate and 10.6 9 of 3-(4,4-diphenylpiperid-1-yl)-propyl
- 25 -
3~aminocrotonate are heated at the bo1Ling point under
reflux in 120 ml of 2-propanol and 0.5 ml of glacial acetic
acid for 4 hours. After most of the solvent has been dis-
tilled off and 2 portions of 50 ml of toluene e~ch have been
added, the residue is concentrated to dryness. The residue,
~hich has foamed as a sol;d, ;s taken up ;n ;sopropanol and
d;ethyl ether is added to the clear solution until the
cloud;ness f;rst persists. After the mixture has been left
to stand in a refrigerator, the starting compound crys~al-
lises out in the form of fine plateLets. mOp.: 158-160C;
y;eld: 14.3 9.
With ethereal hydrochlor;c acid, the hydrochloride
of the starting compound ;s obta;ned, and is recrystallised
from methylene chloride/methanol. m.p.: 184-194C (slo~
deliquescence).
15. 3-Ethyl 5-C3-(4,4-diphenylpiper;d-1-yl)-propyl~
1,4-dihydro-2,6-dimethyl-4-C3-(1,1,2,2-tetrafluoroethoxy~-
phenyl~-pyr;d;ne-3~5-dicarboxylate hydrochlor;de
_ _ _ . _ _ _ _ _ _
The title compound of m.p. 196-197C (decomposition,
from methylene chloride and diisopropyl ether); yield: 48.9 9,
is obta;ned analogously to Example 1 from 22.2 9 of 3-
~1,1,2,2-tetrafluoroethoxy)-benzaldehyde, 12.9 9 of ethyl
3-aminocrotonate and 37.9 9 of 3-(4,4-diphenylpiperid-1-yl)-
propyl acetoacetate in 28û ml of tert.-butanol after a
reaction t;me of 14 hours.
16. 3-Ethyl 5-~3-(4,4-diphenylpiperid-1-yl)-propyl~
1~4-dihydro-2,6-dimethyl-4-(3-difluorometho~yphenyl)-
pyridine-3,5-dicarboxylate hydrochloride
The tltle compound of m.p. 197-198C ~fine plate-
lets from methylene chloride and ethyl acetate); yield:
5.1 9, is obtained analogously to Example 1 from 1.6 9 of
- 26 -
3-difluoromethoxyb~nzaldehyde, 1~3 9 of ethyl 3 aminocro~on-
ate and 3.8 9 of 3-(4,4-d;phenylp;per;d-1-yl)-propyl aceto-
acetate in 80 ml of tetrahydrofuran af~er a reaction t;me
of 4 hours.
17. 3 Methyl 5-C3-~4~4-d;phenylp;per;d-!-yl)-propyl~
4-(2 3-dichloroDhenyl)-1~4-dihydro-2,6-dimethylpyr;d;ne-
3~5-dicarboxylate h ~rochloride
The ~itle compound of m.p. 228~230C (fine needLes
from ~ethanol and ethyl acetate); yield: 4.2 9, is obtained
analogously to Example 1 from 2~72 9 of methyl 2-acetyl-3-
(2,3-dichlorophenyl)-acrylate and 3.7~ 9 of 3-~4,4-diphenyl-
piperid-1-yl)-propyl 3-am;nocrotonate ;n 100 ml of
2-propanol after a reaction time of 7 hours.
18 3-M~thvl 5-r~ b~ n~erid-1-vl~-DroDv~
(2~ -benzQx~!~azol-4-v~ dihvdro-2.6-~Lim~thvl-
Dvr~ e-3.5-~farboxvla~ hydrochloride
The tltle compound of m p. 208-210C ~fine lumpy crystals
from ethyl acetate and methylene chloride); yield: 4 1 ~, is ob-
tained analogously to Example 1 from 2.5 9 o~ methyl 2-acetyl-3-
t2,1,3-benzoxdiazol-~-yl)-acrylate and 3.8 g of 3-(4,4-diphenyl-
piperid-l-yl)-propyl 3-aminocrotonate in 60 ml of tetrahydrofuran
and 0 5 ml of acetic acid after a reaction Sime of 4 hours
19. 3-Methyl 5-~3-(4,4-d;ph~nylpiperid-1-yl)-propyl~
1,4-dihydro-2,6-dimethyl-4-(3-fluorophenyl)-pyridine 3,5-
d;c~rboxylate hydrochlor;de
The title compound of m p. 213-216C Ifine, angular
crystals from methylene chloride and diisopropyl ether); yield:
4.8 g, iQ obtained analogously to Example 1 from 2.44 9 of methyl
2-acetyl-3-~3-fluorophenyl)-acrylate and 4.39 9 of 3-(4,~-diphe-
nylpiperid-1-yl)-propyl 3-aminocrotonate in 50 ml of tetrahydro-
furan after a reaction tlme of 5 hour~.
3~7
~ 27 -
.- 20.3-Me~L 5~C3~(4,4-diphenylpiper~d~ l)~roPy~
10_dihydro-2,~5-dimethyl-4-(2-tri~luorom~thylphenyl)-pyr;-
d i n e -3 5-g~L~
.
Thc title compound of m.p, 158-162C [flna, cublcal
crystal~ from methylene chloride ~n~ dl~sopropyl ~ther); yield:
.2 g, ls obtalned ~nalogously to Example 1 from 2.7 9 of methyl
2-acetyl-3-(2-trifluorom~thylphenyl)-~crylate and ~ of 3-
~ diphenylplp~rid-l-yl)-propyl 3-aminocrotonate in ~0 ml of
tert.-butanol after a reaction time of ~ hour~.
21. 3-Eth l 5-C3-~4 4-di henYl i erid-1-yl)-propyl]
. Y~ . P -... P P
104-t-2--cyanoehe~L~ro-2~6-dimethylpyridine-3~5
dicarboxyLate_hydrochloride
The title compound of m.p. 178-181C (flne necdles from
ethyl acetate and m~thylene chlorlde); yisld: 8.~ 9, ls obtalned
analogously to Example 1 from 3.93 9 of 2-cyanobenzaldehyde,
3.~5 9 of methyl 3-amlnocrotonate and 11.38 ~ of 3~ -dlphenyl-
piperid-1-yl~-propyl acetoacetate in 80 ml of Z-propanol after a
reaction time of 10 hours.
22. 3-Methvl 5- U -l4 ~4-dl~hsnvl~iDeri~- ? -~1~ -pro~vl ~-l2-
~hloro~henvl~ -dihvdrQ-~6-dimethvlDvridine-3.5-
dicarboxvlate hvdrochlori~
The title compound of m.p. 150C (decomposition) (fine,
needles from methylene chloride and diisopropyl ether); yield:
3.3 9, is obtained analogously to Example t from 3 6 9 of methyl
2-acetyl-3-(2-chlorophenyl)-acrylate and 5.7 9 of 3-~,4-di-
phenylpiperid-1-yl)-propyl 3-aminocrotonate in 60 ml of tetra-
hydrofuran and 0.5 ml o~ acetic acid after a reaction time of 8
hours.
~ 3
- 28 -
Commercial Usefulness
_~ _.A
The compounds of the formula I according to the
;nvention and their salts have useful properties which
render them commerciaLly useful. In particular, they are
active vasodilators with properties as coronary therapeutics.
The pharmacological activity of the compounds accordin~ to
the invention, ~hich is coupled with a low toxicity, mani-
fests itself ;n particu(ar in a reduction in blood pressure
wh;ch starts slowly, is po~erful and lasts for a long time.
Moreover, the compounds according to the invention have
peripheral, coronary, cerebral and renal vasodilating pro-
perties and salidiuretic properties.
In their excellent activity, ~hich is coupled with
a low toxicity and the absence of substantial s;de effects,
the compounds according to the invention differ in a sur-
prising and advantageous manner from the compounds of the
prior art. Examples of advantageous properties ~hich may
be mentioned are: the slow onset of the reduction in blood
pressure, the degree of reduction in blood pressure, the
long period ~hich the reduction in blood pressure lasts, the
good ease of control of the reduction in blood pressure, the
only slight increase in heart rate, which disappears on
repeated administration, the excellent bioavailability, the
wide therapeutic range, the absence of side effects on the
central nervous system, the absence of kinetic interactions
with other substances, the absence of-tolerance development,
the balanced physical properties and the high stability.
The e~cellent activity of the compounds of the
formula I according to the invention and their salts enables
them to be used in human medicine, possible ind;cations
being, in particular, primary (essential) and secondary
hypertension of all degrees of severity, coronary heart
diseases (coronary insufficiency, angina pectoris, myo-
cardial ;nfarction etc.), disturbances in.peripheral and
cerebral circulation (cerebral apoplexy, temporary
- 29 -
disturbances in cerebral circulation, narrowing of the renal
artery otc. ), cardi~c in~ufficl~ncy 3nd d1~eases ~asQ~ on an ln
creased ret~ntlon o~ water and sodium.
The invention thus furthermore relat~s to a method
for the treatment of mammals, in particular humansO suffer-
ing from one of the abovement;oned d;seases. The method is
character;sed in that a therapeutically effective and
pharmacologically acceptable amount of one or more compounds
of the formula I is administered-to the s;ck ;ndiv;dual.
The invention also relates to the compounds of the
formula I for use ;n the treatment of the d;seases ment;oned.
The invention likeuise relates to the use of com-
pounds of the formula ~ ;n the preparat;on of medicaments
which are employed for combat;ng the d;seases ment;oned.
The invention furthermore relates to medicaments
containing one or more compounds of the general formula I.
The medicaments are prepared by processes uhich are
known per se and are familiar to the expert. As med;caments,
~he pharmacologically active compounds (= active compounds)
according to the invention are employed either as such or,
preferably, in combination with suitable pharmaceutical
auxiliaries in the form of tablets, coated tablets, cap-
sules, suppos;tories~ plasters (for example as TTS), emul-
sions, suspensions or solutions, the content of active com-
pound advantageously being between 0.1 and 95%.
The expert is famil;ar w;th what auxil;aries aresuitable for the desired medicament formulations on the
basis of his expert knowledge. In addition to solvents,
gelling agents, suppository bases, tabletting auxiliaries
and other active compound excipients, it is possible to
use, for example, antiox;dants, d;spers;ng agents, emulsi-
f;ers, antifoaming agents, flavour correctants, preserva-
- 30 -
tives, solubilising agents, dyestuffs or, in particular,
permeation promoters and c~mplexing agents (for example
cyclodextrins).
The active compounds can be adm;nistered orally or
parenterally ~in par~icular perlingually, intravenously or
percutaneously).
In general, it has proved advantageous in human
medicine to administer the active compound or compounds,
when these are administered orally, in a daily dose of about
0.01 to about 10, preferably û.ûS to 5 mg/kg of body weight,
if desired in the form of several, preferably 1 to 4, ;ndi-
vidual doses, to achieve the desired results. In the case
of parenteral treatment, similar or (especially when the
active compounds are administered intravenously) as a rule
lo~er doses can be used. ~ith a dosage increasing from
initially small amounts, a lower dose is administered at the
start of the treatment and this is then slowly changed to a
higher dose. When the desired therapeutic result has been
achieved, the dose is reduced again to a lower dose.
The particular optimum dosage required and the mode
of administration of the active compounds can easiLy be
determined by any expert on the basis of his expert know-
ledge.
If the compounds according to the invention and/or
their salts are to be employed for the treatment of the
diseases ment;oned, the pharmaceutical formulations can also
contain one or more other pharmacologically active con-
st;tuents from other groups of medicaments, such as other
vasodilatorc, antihypertenslves, ~-receptor blockers, B-receptor
blockers, ACE-inhibitors, nitro-compounds, cardiotonics, diure-
tics, saluretics, alkaloids etc., such as nifedipine, dihydrala-
zine, prazosine, propranolol, labetalol, captopril, isosorbide
dinLtrate, digoxin, mefruside, clopamide, spironolactone, chlor-
thalidone, furosemide, polythiazide, hydrochlorothiazide, reser-
pine, dihydroergocristine, rescinnamine, Rauwolfia total alka-
loids etc.
- 31 -
Pharmacology
The ant;hypertensive act;vity of the compounds
according to the invention can be demonstrated on the model
of spontaneously hypertensive rats.
S To determ;ne the antihypertensive action, the com-
pounds l;sted below are adm;nistered once daily, by means of
a stomach tube, on four successive days in the doses men-
tioned to in each case 6 rats (s~rain SHR/N/I~m/Bm o , 250-
350 9) with hypertension of ge~et;c orig;n (RR > 180 mmHg).
The blood pressure is in each case measured 6 and, if
appropriate, 2 or 24 hours after administration of the sub
stance.
The blood pressure measurement is carr;ed out in a
heated chamber at 36C in order to achieve better circula-
t;on in the tail artery. For this, the animals are trans-
ferred to perforated sheet metal cages and the measurement
is made 20 - 40 minutes after warming up has been started.
To measure the systolic arterial pressure, an annular cuff
with an inflatable rubber membrane, for suppressing circula-
tion, and an annular piezocrystal transducer, to recordpulse waves, are pushed onto the tail. When the blood
stream has been suppressed in the tail artery, the cuff
pressure is reduced continuously. Return of the pulse waves
as the pressure is reduced is recognised and expressed
automatically as the systolic blood pressure (Buhler, R. et
al.: Microprocessor-based automation of blood pressure
measurement in the conscious rat. Proceedings of the 4th
international symposium on rats with spontaneous hypertension
and related studies, Rascher, R. et al. (eds.), Schattauer
Verlag, Stuttgart, New York, 1982, pages 410-413). The
pulse signals and pressure course are recorded graphicaLLy
for evaluation.
For acclimatisation to the measurement process, the
animals ar~ trained for 14 days before the substance is
- 32 ~X~ f
tested. In the second week of training, blood pressure prevalues
are recorded. The groups of animals receiving the substance are
tested against a control group.
In the table which follows, the compounds investigated are
labelled by serial numbers, which are allocated as follows:
Serial No. Name of the compound
1 3-Methyl 5-~3-(4,4-diphenylpiperid-1-yl~-propyl]
1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-
pyridine-3,5-dicarboxylate hydrochloride
2 3-Methyl 5-[2-(4,4-diphenylpiperid-1-yl)-ethyl]
1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-
pyridine-3,5-dicarboxylate hydrochloride
3 3-Methyl 5-[3-(4,4-diphenylpiperid-1-yl)-propyl]
4-(3-cyanophenyl)-1,4-dihydro-2,6-dimethylpyridine-
3,5-dicarboxylate hydrochloride
4 3-Methyl 5-[2-(4,4-diphenylpiperid-1-yl)-2-methyl-
propyl] 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-
pyridine-3,5-dicarboxylate hydrochloride
3-Ethyl 5-[3-(4,4-diphenylpiperid-1-yl)-propyl]
1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-
pyridine-3,5-dicarboxylate hydrochloride
6 3-Methyl 5-[3-(4,4-diphenylpiperid-1-yl)-propyl~
1,4-dihydro-2,6-dimethyl-4-t3-(1,1,2,2-tetrafluoro-
ethoxy)-phenyl]-pyridine-3,5-dicarboxylate hydro-
chloride
7 ~-(2-Methoxyethyl) 5-~2-(4,4-diphenylpiperid-1-yl)-
ethyl] 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-
pyridine-3,5-dicarboxylate fumarate
8 3-(2-Methoxyethyl) 5-[3-(4,4-diphenylpiperid-1-yl)-
propyl] 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-
pyridine-3,5-dicarboxylate fumarate
33 12~
Serial No. Name of the compound
. _____.
9 3-Methyl 5-{3-t4,4~di-~4-methoxyphenyl)-piperid-
1-yl]-propyl} 1,4-dihydro-Z,6-dimethyl-4-(3-nitro-
phenyl)-pyridine-3,5-dicarboxylate hydrochloride
1D 3-Methyl 5-[h-(4,4-diphenylpiperid-1-yl)-butyl]
1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-
pyridine-3,5-dicarboxylate fumarate
11 3-Methyl 5-t1,1-dimethyl-Z-(4,4-diphenylpiPerid-
1-yl)-ethyl] 1,4-dihydro-2,6-dimethyl-4-~3-nitro-
phenyl)-pyridine-3,5-dicarboxylate hydrochloride
12 3-Ethyl 5-~3-(4,4-diphenylpiperid-1-yl)-propyl]
1,4-dihydro-2,6-dimethyl-4-(2-difluoromethoxy-
phenyl)-pyridine-3,5-dicarboxylate hydrochloride
13 3-Ethyl 5-~3-(4,4-diphenylpiperid-1-yl)-propyl]
1,4-dihydro-2,6-dimethyl-4-(3-difluoromethoxy-
phenyl)-pyridine-3,5-dicarboxylate hydrochloride
14 3-Methyl 5-[3-(4,4-diphenylpiperid-1-yl)-propyl]
4-(2~3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-
pyridine-3,5-dicarboxylate hydrochloride
3-Methyl 5-t3-~4,4-diphenylpiperid-1-yl)-propyl]
4-(2,1,3-benzoxdiazol-4-yl)-1,4-dihydro-2,6-di-
methylpyridine-3,5-dicarboxylate hydrochloride
- 34 - ~29~
Serial No. Name o~ the compound
16 3-Methyl 5-[3-t4,4-diphenylpiperid-t-yl)-proPYl~
1,4-dihydro-2,6-dimethyl-4-l3-~luorophenyl)-pyrl-
dine-3,5-dicarboxylate hydrochloride
17 3-Methyl 5-~3-t4,4-diphenylpiperid-1-yl)-propyl~
1,~-dihydro-2,6-dimethyl-4-(2-tri~luoromethyl-
phenyl)-pyridine-3,5-dicarboxylate hydrochloride
18 3-Ethyl 5-[3-t4,~-diphenylpiperid-1-yl)-propyl]
4-t2-cyanophenyl)-1,4-dihydro-2,6-dimethylpyridine-
3,5-dicarboxylate hydrochloride
19 3-Methyl 5-~3-(4,4-diphenylpiperid-1-yl)-propyl]
4-t2-chlorophenyl)-1,4-dihydro-2,6-dimethyl-
pyridine-3,5-dicarboxylate hydrochloride
Table I shows the precentage reduction in blaod pressure (~P)
following oral administration to rats for the representatives of
the compounds according to the invention.
- 35 -
Table I
X change (6P) in genetically hypertensive rats following a single
daily oral administraiton on four successive days ~N=6/dose); the
dose in mg/kg is calculated on the free base.
6P ~X changes vs. control)
Serial Dose mean values of the given points
No. of time of measurement
~mol/kg) (mg/kg) 2h 6h 24h
(1st~4th (1st to 4th (1st+3rd
day) day) day)
1 5 3,05 -47 -30 -7
10 6,0g -50 -37 _9
2515,24 -48 -45 -25
2 2514,89 -51 -36 -2
3 2514,74 -48 -41 -19
4 2515,60 -51 -44 -25
10 6,24 -51 -41 -20
6 2517,02 -21 -21 -10
7 2515,99 -44 -33 0
8 2516,34 -48 -2 a - 5
9 10 6,70 -53 -45 -14
10 6,24 -40 _~ -2
11 5 3,12 -54 -33 -15
12 2516,12 -31 -11 +4
13 2516,12 -41 -29 -6
14 2515,84 -49 -43 -30
2515,17 -49 -41 -16
16 2514,57 -46 -38 -7
17 2515,82 -44 -30 -17
18 2515,09 -37 -25 -1
19 2514,98 -43 -28 -2