Note: Descriptions are shown in the official language in which they were submitted.
lZ9ZE~87
Method ln the bleachlnq of cellulose Pulp
Technlcal Fleld
The present lnvention relates to a method for measuring
the chemical content of llquors used ln the bleaching of
cellulose pulp. The method can be applled ln con~unctlon
wlth the bleaching of all cellulose pulps, lrrespectlve of
thelr llgnin contents, l.e. lncludlng, for example, cheml-
cal pulp, chemlmechanical pulp, semlchemlcal pulp and mecha-
nical pulp.
Back~round Prlor Art
Cellulose pulp is bleached for a number of reasons. One
reason ls to decrease the lignln content of the pulp,
whereas in the case, for example, of chemlcal pulps the ob-
~ectlve is to remove the lignln totally. Another reason for
bleachlng pulp is to lncrease its brightness. It ls often
endeavoured to both remove llgnin from the pulp and to ln-
crease the brightness thereof.
Different bleachlng agents have different effects on
cellulose pulp, and can be divided into bleaching agents
whlch remove lignln and bleachlng agents whlch preserve the
lignin. Examples of lignln removing bleaching agents lnclude
chlorlne, chlorine dioxlde, hypochlorite, chlorite, oxygen
gas and ozone. Various kinds of peroxide, dithlonlte and
boron hydride constitute examples of lignln preservlng
bleachlng agents. Some of these bleachlng agents possess
both of the aforesaid properties. The pulp ls normally
treated wlth alkali between the various bleachlng stages and
washed thereafter to remove dissolved materlal.
2 129Z887
A number of parameters must be taken lnto account when
uslng a glven bleachlng agent, ln order to achleve the
result deslred. For example, lt ls normally necessary to
check or monitor pulp consistency, temperature, tlme and pH,
and also preferably to control these parameters. Another
lmportant parameter ls the bleachlng agent content, partly
of the actual bleachlng llquor as such and partly subsequent
to mixlng the bleaching llquor wlth another llquld phase ln
the absence or presence of the pulp. There are many theorles
as to how a bleachlng stage should be controlled. Dependlng
upon the method of control applled, the bleachlng agent
content ls determlned lmmedlately after mlxlng the agent
wlth the pulp, at some tlme durlng the bleachlng stage, or
upon completlon of the bleachlng process. The bleachlng
agent ls often charged to the system in excesslve quantl-
tles, ln whlch case the amount of bleachlng agent that
remalns ls normally of lnterest.
A relatlvely common method of controlling the supply of
bleaching agent to the system, and therewith the amount of
bleachlng agent contalned by the pulp suspenslon ls one of
measuring the redox potentlal of the suspension and of
regulating the charge of bleachlng agent golng out from a
predetermined set-polnt value of the redox potentlal, so
that the set-polnt value ls reached. In the case of bleach-
lng chemlcals, thls method can only be used wlthln a very
narrow content range. In addltlon thls partlcular method of
measurlng the redox potentlal ln the pulp suspenslon is
dependent on temperature, to which is added the problem of
coatings on the measuring electrodes.
From the chlldhood of bleachlng technology up to the
present day, samples of bleachlng agent solutlons have been
taken manually (and are stlll taken manually) at relatlvely
wlde tlme lntervals, these samples being titrated manually,
e.g. iodometrlcally, to arrlve at a given bleaching agent
content.
3 lZ9~887
The samples are taken at one or more locatlons wlthln
the bleachlng department, e.g. from the actual bleachlng
llquor before lt ls mlxed lnto the pulp, or from the sus-
pension llquor subsequent to mixlng the bleachlng llquor
into the pulp, l.e. durlng an ongolng bleachlng process, or
from the suspenslon llquld subsequent to completlon of a
bleaching process, l.e. ln order to determlne the resldual
content of bleachlng chemlcals of the suspenslon llquor.
Thls partlcular methodology results ln poor coverage of what
actually takes place durlng bleachlng of the pulp, partly
because the samples are taken at random, and partly because
of the relatlvely long perlod of tlme that lapses from the
tlme of taklng the sample to the tlme at whlch the operator
ln the bleachlng department recelves the lnformatlon con-
cernlng the content of the bleachlng agent concerned.
With regard to strongly oxldatlve bleachlng agents, suchas chlorlne and chlorlne dloxlde, lt has been suggested ln
Swedlsh Patent Speclflcatlon No. 731412g-3 (399 966) that
samples of liquor contalnlng these bleachlng agents shall be
reacted wlth a chemlluminescent reactant, leadlng to the
emlsslon of light. The total amount of llght emltted ls
measured, and the value obtalned provldes data relatlng to
the content of the bleachlng agent concerned. The method ln
questlon ls lntended prlmarlly for determlnlng both the
chlorlne content and the chlorlne dloxlde content of one and
the same bleachlng llquor sample, which has prevlously been
lmposslble to achleve to any satlsfactory degree of success.
Thls method can be àpplled to partlcular advantage ln
bleachlng stages ln whlch a mlxture of chlorlne and chlorlne
dloxlde ls used.
4 ~Z9Z8~37
summarY of the Inventlon
Technlcal Problem
There has been found wlthln the cellulose pulp lndustry,
and partlcularly when uslng strongly oxldatlve bleachlng
agents, the need for an automated method of analysls whlch
s whlle belng rellable and requlrlng but llttle malntenance,
spans a wlde range of bleachlng chemlcal concentratlons, and
whlch can be applled at relatlvely low costs.
Solutlon
The present lnventlon satlsfles thls need and relates to
a method for measurlng the chemlcal content of bleachlng
llquor wlthln the cellulose pulp lndustry, ln whlch method a
bleachlng llquor sample ls admlxed wlth one or more reagents
of whlch at least one 16 chemllumlnescent resulting ln llght
emlsslon, and the lntenslty of the llght emltted ls deter-
mlned as a measure of the chemlcal content, characterlzed bycontlnuously advanclng a flow of reagent through a condult
system; lntroduclng a small quantlty of sample llquld spora-
dlcally or lntermlttently lnto the flow of reagent so as to
produce a chemllumlnescent reactlon; contlnulng the reac-
tlon, and therewlth the emlsslon of llght, over a glvenperlod of tlme, so that the llght lntenslty ls such as to
fall wlthln the measurlng range of a llght sensltlve or re-
sponslve devlce; constantly measurlng the llght lntenslty
after sald glven tlme lnterval at repeated measurlng opera-
tlons; and convertlng the measured value of llght lntensltyto the content of bleachlng chemlcal.
~ 5 129Z887
The condults used when applylng the method accordlng to
the lnventlon are preferably transparent, at least wlthln
the reglon thereof ln which the light sensitive measurlng
devlce ls located, although lt is fully posslble to use a
condult system ln whlch all parts thereof are opaque. In
thls latter case, one end of a flbre optlc cable can be con-
nected to a non-transparent condult system at a glven
location therein, whlle the other end of the cable can be
connected to a llght responsive measurlng devlce.
Accordlng to one preferred embodlment of the lnventlon,
the reagent ls supplled from a contalner through a condult
system whlch can be closed, so that substantlally all unused
reagent ls returned to the contalner.
It ls also preferred that the sample llquor contalnlng
the bleachlng chemlcal concerned ls dellvered through a con-
dult system to a mlxlng locatlon at whlch, vla at least one
multl-path valve, elther a small amount of the sample llquor
ls lntroduced lnto the flow of reagent or the sample llquor
ls conducted beyond the flow of reagent tc an outlet.
Instead of lntroduclng the sample llquor lnto the rea-
gent flow ln small quantltles, vla a valve and a condult
loop connected thereto, the sample llquor can be introduced
lnto the reagent flow by means of so-called hydrodynamlc
ln~ectlon, as deflned herelnafter, while uslng a short sec-
tlon of the reagent flow located ln the condult system.
That part of the reagent flow whlch comes lnto contact
wlth the sample llquor and reacts therewlth to cause llght
to be emltted ls caused to leave the condult system, through
a sample plpe fltted wlth a valve, subsequent to measurlng
the lntenslty of the llght emltted.
In accordance wlth the lnventlon, lt ls also posslble to
take separate samples of llquor contalnlng one or more
bleachlng chemlcals at selected locations ln the pulp mlll
6 lZ9Z8~7
and to lntroduce sald samples lnto the reagent flow with the
ald of a volumetrically graduated devlce. One example of
such a devlce is a syrlnge provided wlth a cannula whlch can
be lnserted through the wall of the condult carrylng the
reagent. The dlstance ln length and/or tlme calculated
between the locatlon a~ whlch the sample ls lntroduced and
the locatlon at whlch the lntenslty of the llght emltted ls
measured must be exactly the same at each tlme of measurlng,
even ln the case of the samples ~ust mentioned.
The llght responslve devlce wlth whlch the intenslty of
the llght ls measured, may be of any sultable known klnd. In
accordance wlth the lnventlon, however, the devlce is pre-
ferably ln the form of a dlode capable of convertlng llght
energy lnto electrlc voltage resultlng ln a reglsterable
slgnal. It has surprlslngly been found that the output slg-
nal obtained from the dlode has the form of a curve of sub-
stantlally normal dlstrlbutlon, l.e. a so-called Gauss
curve. Both the area of the curve and lts peak helght, l.e.
the dlstance from the top of the curve down to lts base, can
be related dlrectly to the amount ln whlch a glven bleachlng
chemlcal ls present ln the sample. Thls has been establlshed
by lntroduclng lnto the aforedescrlbed condult system a
sample of llquor whlch contalned a bleachlng chemlcal of
known concentratlon and by measurlng the llght lntenslty at
a glven polnt of tlme, whlch resulted ln the prlntlng of a
curve of the aforesald klnd.
The analysls method accordlng to the lnventlon can be
used wlth all bleachlng agents that are capable of reactlng
ln a chemllumlnescent fashlon. The lnventlon ls partlcularly
useful for analyslng bleachlng llquors that contaln chlo-
rlne, chlorlne dloxlde, hypochlorlte, chlorlte, or peroxlde,
ln some form or another.
The analysls method accordlng to the lnventlon can be
used to advantage both when manlpulatlng actual bleachlng
llquors as such, for example ln bleachlng llquor preparatlon
processes, and for bleachlng department monltorlng and/or
controlllng purposes.
7 12~8~7
The invention also relates to apparatus for measuring
the chemical content of bleaching llquor in the cellulose
pulp industry, thls apparatus lncludlng means for storlng a
chemilumlnescent reagent solutlon, a closed conduit system
whlch lncorporates a branch llne capable of being connected
to an outlet and through which the reagent solutlon ls ad-
vanced by means of a pump means, a further condult system
for advanclng a bleaching llquor sample solutlon with the
aid of said pump means or some other pump means, and means
for lntroduclng a glven quantity of sample solutlon lnto the
reagent solution conduit system, by displacing the reagent
solution with the sample solution, characterized in that the
reagent solutlon condult system has a speclflc length cal-
culated from the locatlon at whlch the sample solution is
introduced lnto sald system to a location ln a sald system
at whlch a llght responslve device measures the lntenslty of
the light emltted as a result of a reactlon between the che-
mllumlnescent reagent solutlon and the sample solutlon con-
talnlng bleachlng agent.
Advantaqes
The method accordlng to the lnvention affords a number
of advantages. For example, wlth the aid of the lnvention,
it is possible to determlne the content of a glven bleachlng
chemlcal, e.g. hydrogen peroxlde, ln a bleaGhlng llquor,
from small to large chemlcal contents, wlthout needlng to
prepare the sample ln any partlcular way, e.g. by hlghly
dllutlng the sample. Another advantage ls one of low che-
mical consumption, whlch enables the cost of procurlng the
chemlcials to be kept at a low level. Even though all chemi-
luminescent reagents are highly expensive, calculated inkilograms for example, the fact that the conduit system
according to the invention is closed means that only very
small volumes of reagent wlll pass to the outlet. These
small volumes are also ensured by the fact that in accor-
dance wlth the inventlon it is possible to use liquor deli-
very pipes or hoses of very small cross-sectional area.
-~ 12~Z887
The actual analysls method as such has also been found
to be particularly rellable. It ls especlally surprislng
that the reglstered curve form of the slgnal obtalned when
repeatedly taklng measurements of one and the same bleachlng
llquor sample has a practlcally congruent appearance.
In addltlon the method according to the lnventlon ls
hlghly flexlble and has a hlgh avallablllty. The perlodlclty
at whlch the varlous analyses are made can be declded upon
gulte readlly by the operator responslble. Slnce the reagent
solutlon, ln accordance wlth a preferred embodiment of the
lnventlon, ls pumped constantly around a condult system ln
whlch no reagent solutlon ls lost, lt ls posslble for the
temporal dlstance between the sampllng occaslons to be
selected ln perlods whlch are ln excess of hours and down to
some tenths of a second.
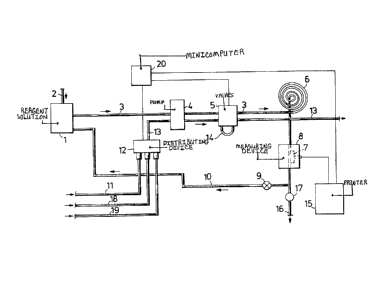
Brlef Descrlptlon of the Drawlnqs
Flgure 1 lllustrates an array of apparatus for carrylng
out a preferred embodlment of the method accordlng to the
lnventlon. Flgure 2 lllustrates an alternatlve method for
lntroduclng sample llquor lnto the reagent llquor condult
system, and Flgure 3 lllustrates an example of the measurlng
slgnal recorded ln curveform when practlslng a preferred
embodlment of the lnventlon.
DescrlPtlon of the Best Embodlment
lnltlally, preferred embodlments of the lnventlon wlll
be descrlbed wlth reference to Flgures 1, 2 and 3, followed
by a number of worklng examples.
The manner ln whlch the method accordlng to the lnven-
tlon can be applled concretely ls made evident in Flgure 1.
The reference 1 deslgnates a contalner ln whlch the reagent
solutlon ls fitored. When necessary, further reagent solutlon
ls supplled through the condult 2. The composltlon of the
reagent solution may vary, although at least one of the com-
ponents must always be a chemlcal whlch ls chemllumlnescent,
9 lZ9Z887
l.e. a ~ubstance whlch reacts wlth certaln other substances
whlle emlttlng llght at the same tlme. There are several
chemllumlnescent reagents which can be used ln the method
accordlng to the lnventlon. One well known substance of thls
klnd ls deslgnated luminol. In additlon to lumlnol, the
reagent solutlon normally also contalns copper chlorlde,
60dlum bicarbonate and sodium carbonate dlssolved ln water.
The reagent solutlon ls passed, vla the conduit 3 and the
pump 4, to two four-path valves whlch form a unit 5. Any
known pump whatsoever may be lnstalled at the posltlon 4. A
perstaltlc pump ls preferred however. When thls type of pump
ls used, the condult 3 may comprlse a Teflon* hose. It has
been found ln practlce that a Teflon*hose havlng a dlameter
of 0.7 mm functlons admlrably. Teflon*hoses are transparent.
The method accordlng to the lnventlon ls not llmlted to the
use of such hoses or condults however. It ls fully posslble
to use hoses or conduits whlch are not transparent. However,
ln thls case, ln accordance wlth a preferred embodlment of
the lnventlon, that part of the condult or hose sltuated in
the reglon where the llght responslve measurlng devlce 7 is
located shall be transparent. Thls sectlon of the condult
may comprlse a Teflon~ hose or a condult sectlon of some
other llght-permeable materlal, such as a glass pipe. In
those lnstances when no analysls ls carrled out, the
pumpable reagent 601utlon ls pumped stralght through the
unlt 5 and through the loop 6, for further transportatlon
through ehe measurlng devlce 8 lncorporatlng a light re-
sponslve device 7 and back to the contalner 1, vla the
condult 10 fitted wlth the valve 9. It ls essentlal that the
reagent solutlon ls advanced at a constant rate of flow. It
has been found, for example, that a flow rate of 2.5 ml
reagent solutlon per mlnute can be used.
The sample llquor, l.e. the llquld whlch contalns the
bleaching chemlcal whose concentratlon ls to be determlned
by analysls, ls advanced by means of the pump 4 through the
condult 11, the distributing device 12 and the condult 13 to
* trade mark
~2 ~Z ~
the valve unlt 5. ~hen no analysis is carrled out, the samp-
le liquor ls caused to enter the valve unit 5 on one slde
thereof, whereupon the llquor passes through the loop or
by~pass 14 and out of the unlt 5 on the opposite side there-
of~ for continued passage through the condult 13 to an out-
let. ~hen the sample llquor ls to be analysed, the two val-
ves in the unit 5 are set ln a manner to interrupt the flow
of reagent 601ution through the conduit 3 by a volume (or
length) whlch corresponds to the volume of llguid (or the
length) present 1n the loop 14. Subse~uent to introduclng
the sample into the condu1t 3, the ~alves are re-set to the
~tarting po~ition. As a result hereof there is o~talned in
the condult 3 to the right of the unlt 5 a sample liquor
cylinder of given length which ls embraced by reaction solu-
tion on both 6ide~ thereof. As thls takes place, the reac-
tlon liquor begin6 to penetrate and mlx with the sample
llquld from both 6~de thereof, resulting in a chemilumine-
scent reactlon, whlch cau~es light to be emitted. It has
been found difflcult wlth the use of known llght lntensity
meter~ to mea~ure the intensity of the l~ght in the imme-
diate vicinlty of the locatlon at whlch the reagent and the
bleachlng chemlcal mlx together and obtain at the same tlme
a dlstinct or characteri~tlc signal, i.e. a measurement
value whlch correlates to a given concentratlon of the
bleaching chemical. Consequently, test6 were carried out in
which the aforesald cyllnder of 6ample liquor was permltted
to pa66 through a conduit loop 6 of given length to a posl-
tion ~ which lncorporated a measurlng apparatus (e.g. in the
form of a light impervious box) provided ~ith a llght re-
sponsive measuring device 7. The afore6aid length, or dimen-
sion, of the loop, or coll, ls directly convertible to a
given di6placement in t~me. It h~s ~urprislngly been found
t~at with the aid, e.g., of a photodlode placed in the imme-
diate vicinlty of the condult wlthin the box B and at a
given time di~tance from the moment at which the 6ample
liquor was introduced, it is po66ible to mea~ure the inten-
sity Df the light emitted ~o as to obtaln a 6ignal ln
1 ~ 1292887
the form of a curve on the prlnter 15 whlch stands ln dlrect
relatlonshlp wlth the concentratlon of the bleachlng cheml-
cal concerned ln the sample llquor. The photodlode converts
llght to electrlc voltage. In order to obtaln a useable
curve, the measured slgnal ls preferably ampllfled before
belng prlnted by the prlnter 15.
~lth regard to the aforesald dlsplacement ln tlme, l.e. the
tlme perlod between lntroduclng the sample lnto the condult
carrylng the reagent and measurlng the lntenslty of the
light emltted, thls tlme displacement ls dependent on a
plurallty of factors, such, as lnter alla, the dlameter of
the condult, the rate of flow, the volume of sample llquor.
Convenlently, the chemllumlnescent reactlon ls allowed to
contlnued for 2-60 seconds, preferably 15-30 seconds. The
most essential factor ln this connectlon, however, ls that
all measurements are taken wlth exactly the same tlme exten-
slons. The aforementloned preferred tlme lnterval ls prlma-
rlly appllcable when analyslng bleachlng llquor that con-
talns hydrogen peroxlde. Consequently, ln order to enable
each lndlvlual bleachlng agent used lndustrlally to be ana-
lysed lt ls necessary to determlne approprlate reactlon
tlmes, prlor to measuring the llght lntenslty, wlth the ald
of sultable tests. For example, the reaction tlme required
for chlorlne-contalnlng bleachlng agents, such as chlorlne,
chlorlne dloxlde and hypochlorlte, ls much shorter than that
requlred for hydrogen peroxlde.
Any suitable llght responslve measurlng devlce may be
used, as an alternatlve to the aforesald photodlode. Exam-
ples of such devlces lnclude phototranslstors, photoresls-
tors and photomultlpllers.
The sample llquld may be advanced through the system atany deslred rate of flow. The flow rate of the sample liquor
ls of secondary lmportance~ slnce lt ls the volume, and then
prlmarlly the length of the loop 14, whlch determlnes the
amount of sample on whlch the analysls ls carrled out. The
12 i ~Z ~7
loop 14, or shunt, ls completely fllled wlth llquor as the
sample llquor ls advanced, lrrespectlve of the speed at
whlc~ the sample liquor ls transported. By uslng the same
pump 4 as that used to transpor~ the reagent solutlon, and
the same type of hose as that used for said reagent solu-
tlon, for example, a Teflon hose havlng a dlameter of 0.7
mm, the same flow rate ls also obtalned, for example 2.5 ml
of sample llquor per mlnute.
When uslng the aforedescrlbed analysls system for con-
trolllng bleachlng processes, the condult 11 ls preferablyconnected to an arrangement of apparatus whlch enables a
flow of ~ample llquor to be taken whlch ls substantlally
totally free of pulp flbres. Any sultable known klnd of
sampllng apparatus can be used. For example, there are found
on the market apparatus whlch can be partlally lnserted
lnto, for example, a condult through whlch a pulp suspenslon
contalnlng a glven bleachlng agent ls transported. That part
of the apparatus, or devlce, lnserted lnto the pulp suspen-
sion conslsts of a slotted tube, and part of the suspenslon
llquor ls caused to pass through the slots and lnto the tube
for further transportatlon to, e.g., a buffer vessel, from
whlch the flow of sample llquor, or lndlvldual samples of
the bleachlng llquor, can be taken for analysls purposes. In
order to ensure that the sample llquor ls totally free of
flbres and also of other lmpurltles, such as resln partlcles
etc., lt may be necessary to pass the sample llquor through
a wlre fllter of some 6ultable klnd.
Subsequent to taklng the aforesald measurement, the re-
acted chemlcals are permltted to pass through the condult 16
to an outlet. Thls 16 effected by closlng the valve 9 ln the
condult 10 and openlng the valve 17 ln the condult 16. The
valve 17 need only be held open for a very short perlod of
tlme. As a result hereof the reagent solutlon taken from the
contalner 1 ls passed back thereto durlng, e.g. at least
13 1292887
95% of the tlme, resultlng ln extremely low consumptlon and
therewlth low reagent chemlcal costs.
In order to comprehend the slgnlflcance of the measured
slgnal wlth regard to the content of a glven bleachlng
chemlcal ln the sample llquor, lt ls necessary to callbrate
the system by lntroduclng bleachlng agent solutlons of known
content of a glven bleachlng agent. It has been found ad-
vantageous to callbrate the system whlle uslng one and the
same chemllumlnescent bleachlng agent, but ln dlfferent con-
centrations, l.e. a solution contalnlng the bleachlng agentin a relatlvely low concentratlon and a further solutlon
contalnlng sald bleachlng agent ln a relatlvely high concen-
tratlon. These solutlons are lntroduced lnto the system
through the conduit 18 and the condult 19 respectively. By
measurlng the peak height on the curve obtalned wlth re-
6pectlve solutlons and placlng sald peak helght in relation
to the known concentratlon ln, for example, grams of bleach-
lng agent per llter of 601ution, lt 16 possible when analys-
lng a 6ample contalnlng an unknown quantity of bleachlng
agent, 6ub6equent to 6tudylng the recorded curve and its
peak helght, to convert the 61gnal in question to informa-
tlon concerning the bleachlng agent content of the sample
llquor in gram6 per liter.
The 6y6tem 6hould be callbrated at unlform time inter-
vals, 61nce certaln dl6crepancle6 can occur in the system.Such dl6crepancle6 can be cau~ed by aglng of the hoses used
ln the 6y6tem, whlch ln turn affect6 the flow rate, the flow
rate belng an lmportant parameter whlch must be held con-
6tant ln order to achleve a 6atlsfactory analysls result.
The pump or pump6 lncorporated ln the 6ystem must al~o be
nonltored and checked. If 60 de61red, the analysl6 system
lllu6trated ln Flgure 1 can be manlpulated manually, lm-
plylng that the operator manually 6tart6 the pump 4, closes
the valve 17 and open6 the valve 9 and permits the reagent
601utlon to clrculate around the 6ystem for a short period
prior to carrying out an analy6i6. During this period the
14 12~Z887
sample is also pumped ln lts respectlve condults through the
system, vla the condult 13 to She aforesald outlet. The
valves in the valve unit S are re-set at a selected polnt ln
tlme, so as to temporarily lnterrupt transportatlon of the
reagent solution and to introduce the content of the loop or
~hunt 14 into the condult 3 so that the chemilumlnescent re-
action commences. Subsequent to transportlng the aforesaid
determined volume of sample liquor through the loop or coll
6 and past the photodlode 7, during slmultaneous reactlon
wlth the reagent and the resultant emlsslon of llght, the
valve 17 ls opened and the valve 9 ls closed for a brlef
period of time, in order to transport consumed chemicals to
the outlet through the condult 16.
It ls assumed that the arrangement of apparatus lllus-
trated in Flgure 1 wlll enable the sample llquor to be tran-
sported contlnuously to the outlet over longer or shorter
perlods of tlme. It 15 qulte posslble, however, ln accor-
dance wlth the present lnventlon to supply lndlvidual samp-
les of the bleaching liquor, optionally over relatlvely long
lntervals of time. When the lnvention ls applied in this
manner, it may be sultable to use two pumps at the locatlon
4, a first pump for continuously transportlng a constant
flow of reagent solution, as described above, and a second
pump, together with an associated condult system, for trans-
portlng the optlonally llmlted volume of sample llquor that
ls avallable. The amount of sample liquor on whlch the ana-
lysis ls carried out i5 also determined in this case by the
length of the loop 14 (and a condult area whlch possibly
deviates from that of the conduit 3).
In accordance with one preferred embodiment of the in-
vention, the analysls system ls controlled by a mlnlcomputer
20, lnstead of being controlled manually. The minlcomputer
can be programmed specifically to control, for example,
whlch of the liquors carrled ln the conduits 11, 18
15 lZ~ Z ~7
or 19 shall be transported through the system. The mlnl-
computer can also be programmed to control the posltlons of
the valves ln the valve unlt 5, and it is also posslble to
program the computer to read the peak helghts of the llght
lntenslty curve prlnted ln the prlnter 15. The computer ls
readlly capable of convertlng the measured slgnal to the
correspondlng correct concentratlon ln, for example, grams
per llter of the bleachlng agent concerned. Thus, the minl-
computer can be programmed to ensure that samples are ana-
lysed at given time intervals, for example every third or
fifth minute, and to ensure that a calibratlon is made at
glven tlme lntervals, for example each 60th minute.
Figure 2 lllustrates and alternatlve method of lntro-
duclng the sample to the advanclng reagent solutlon when
carrylng out the method accordlng to the lnventlon.
The reagent solutlon ls transported through the condult
22 for example wlth the ald of perlstaltic pump 21, to the
llght measurlng device 23, for further transportatlon
through the condult 24 back to the reagent solutlon
contalner (not shown). The sample llquor can be transported,
for example by means of a perlstaltlc pump 25, through the
condult 26 to a part 27 o the condult 22, whlch ls common
to both condult systems, and from there back through the
condult 28.
When no analysls ls to be carrled out, the valve 29 ln
the sampllng condult 30 ls closed and the valve 31 open,
whlch means that the reagent solutlon ls caused, by means of
the pump 21, to clrculate ln a constant flow ln the closed
system lncorporatlng, lnter alla, the condults 22, 27 and
24. When the sample llquor ls to be analysed, the pump 21 ls
stopped and the pump 25 started up. The sample llquor ls
transported to the condults 26, 27 and 28 for a perlod of
time of such long duratlon as to posltlvely ensure that all
16 lZ9Z~
the reagent solutlon wlthln the conduit sectlon 27 has been
replaced wlth sample llquor. A suitable tlme ln thls respect
ls from 10 to 20 seconds. The pump 25 ls then stopped and
the pump 21 started up.
As thls takes place, the sample ls pressed ln the condul~
sectlon 27 to the rlght of the reagent solutlon whlle slmu-
lataneously lntroduclng the chemllumlnescent reactlon caused
by penetratlon of reagent solutlon from both sldes of the
cyllndrlcal body of sample llquor, the length of thls
cyllnder correspondlng to the length of the condult sectlon
27. There ls no rlsk of the sample llquor escaplng vla the
condult 28, slnce thls condult ls already filled wlth sta-
tlonary llquor. Thus, the sample llquor ls forced to the
rlght by means of the pump 21, through the condult 22 and to
the llght intenslty measurlng devlce 23. In reallty, that
part of the condult 22 located between the condult sectlon
27 and the measurlng devlce 23 ls conslderably longer than
lllustrated ln Flgure 2 and preferably lncorporates a loop
or coll, slmllar to that referenced 6 ln Flgure 1.
Subsequent to measurlng the lntenslty of the llght emltted,
the consumed chemlcals are caused to leave the system
through the outlet, vla the sampllng conduit 30 and the
valve 29. The valve 31 ls kept clo6ed whlle thls takes place.
The deslred quantlty of 6ample liquor to be analysed ls
determlned by the selected length of the condult sectlon 27.
The method of supplylng the sample lllustrated ln Flgure 2
is deslgnated hydrodynamlc ln~ectlon.
Figure 3 lllustrates an example of the slgnal whlch can
be detected when carrylng out the analysls method accordlng
to the lnventlon. The lllustrated curves were obtalned when
analyslng a chemllumlnescent bleachlng agent whlle uslng the
analysls system lllustrated ln Flgure 1. The lntenslty of
the llght emltted was measured by means of photodlode. As a
result of the chemllumlnescent reactlons llght ls emltted
wlthln the vlslble wavelength range. Photodiodes responslve
to llght wlthln the wavelength of 420-675 nanometers are
17 1292~7
sultable for use when practlslng the lnventlon. ~y posi-
tioning the photodiode in the lmmedlate vlcinlty of the con-
duit and allowlng the photodiode to convert the transmltted
light to a voltage lt ls posslble, wlth the ald of an ampli-
fylng unlt, to record the measurement slgnal ln the form ofthe curves lllustrated ln Flgure 3. The tests were carried
out on three mutally different bleaching llquors, l.e.
bleaching liquors contalnlng one and the same bleaching
agent, but ln three dlfferent concentrations.
Wlth regard to the bleaching agent havlng the lowest
concentration, three tests were carried wlth the analysls
system at three mlnute lntervals. These tests correspond to
the three curves shown on the left of the Figure. It wlll be
seen that the three curves are practlcally fully congruent.
Both the surface of the curve and the peak height thereof
can be used as a measurement of the amount of bleaching
chemical present in the sample. The peak helght has been
found the most sultable parameter for use in practice. Dup-
llcated measurements have been made on bleachlng llquors
contalnlng hlgher quantltles of bleaching chemlcal, and
surprlslng conformlty was also obtalned ln thls case ln the
appearance of both the measured curves ln respectlve tests.
The curves lllustrated ln Flgure 3 have a markedly narrow
conflguratlon, l.e. the curves have a small base ln relatlon
to thelr helght. The appearance of the curve ls dependent to
a large extent on the speed to whlch the prlnter 15 ls set.
Slnce the helght has been found to be the parame~er of prl-
mary interest, a low prlnter speed was selected ln order to
economlse on prlntlng paper. It has been found that when the
prlnter speed ls lncreased, the curves present a substan-
tlally normally dlstrlbuted conflguratlon. The conflguratlon
of the curve devlates from a strlctly normal dlstrlbutlon
prlmarlly due to a delay of the slgnal wlth respect to the
rlght-hand curve half, l.e. the right-hand curve half ls not
a complete mlrror image of the left-hand curve half.
18 1;~ 8~7
The fact that when practlsing the method accordlng to
the lnventlon such readily readable slgnals are obtalned dS
evidenced by the curves ln Flgure 3 ls surprising. The
reason why thls is so ls not known. One posslble explanatlon
may be that when the mlxture of the sample llquor and the
reagent (i.e. the part of the sample liquor which remains in
a non-reacted state subsequent to moving through the long
transport path up to the measurlng location) reaches the
measuring apparatus 8 llght of a certaln lntenslty ls stlll
emltted and that when this light enters the measurlng fleld
of the photodiode recording of the signal begins, whlle the
slgnal increases the more centrally the llght emltted ls
located ln the centre of the measurlng fleld of the photo-
diode, and conversely decreases ln pace wlth the llght emls-
sion as lt approaches the other end of the measurlng field,
and finally terminates completely as it leaves the field.
Thls explanation is purely theoretical and has not been con-
flrmed scientifically.
Example 1
The present invention can be used particularly for ana-
lysing various types of peroxides, such as hydrogen peroxide
for example.
The following tests were carried out in order to ascer-
tain the rellabllity of the method according to the inven-
tlon. A reagent solutlon having the following composition
was prepared.
1 part lumlnol 0.015 M dissolved in
0.1 M NaOH
0.1 part copper chloride 0.02 M
1 part carbonate buffer 33.2 g Na2C03 +
53.2 g NaHC03 per llter
4 parts water
1 9 ~2~Z887
Thls reagent solutlon was used when carrying out analysls
wlth the use of the arrangement of apparatus lllustrated ln
Figure 1. The analysls system was calibrated partly wlth the
ald of a hydrogen peroxlde solution, which contained 4 grams
per liter, and partly wlth the ald of a hydrogen peroxlde
solutlon whlch contalned 18 grams per llter. Samples of
bleachlng llquor were then pumped from a full scale cellu-
lose pulp bleachlng department. The bleachlng llquors con-
talned varying quantltles of hydrogen peroxlde and were
transported through the condult 11, the dlstributlng unit
12, the condult 13 and the loop or shunt 14, up to the unlt
5, where they were lntroduced lnto the condult 3 through
whlch the reagent solutlon was transported. 20 mlcrollters
of sample llquor were used ln the analysls, thls quantlty
correspondlng to the volume of the loop 14. When the sample
arrlved at the photodlode 7, the lntenslty of the llght
emltted was measured and the recorded ~lgnal converted lnto
grams of hydrogen peroxlde per llter, in the afroedescrlbed
manner.
The condults 3, 11, 13 and 14 used ln the tests com-
prlsed Teflon hose havlng an lnternal dlameter of 0.'7 mm. A
perlstaltlc pump was used ln location 4, whlch gave a flow
rate of 2.5 ml/mln, both ln re6pect of the reagent 601utlon
and of the sample solutlon. The reactlon tlme was 27
seconds, l.e. the tlme from lntroducing the 6ample solutlon
lnto the condult 3 at locatlon 5 ln the form of an elongated
llquid cylinder and embraced on both short sides or end-
walls by the reagent solution, to the time of measurlng the
llght lntensity by means of the photodlode 7.
In addition to analyslng the various bleachlng llquors
in accordance wlth the inventlon, the llquors were also sub-
~ected to lodometrlc tltration.
The results obtained ln respectlve analyses are set
forth ln Table 1 below.
~Z~ 7
Table 1
Sample Iodometrlc tltratlon Analysis accordlng to
Number the lnventlon
g H202/1 g H202/1
1 15.5 15.5
2 10.6 10.2
3 14.0 14.1
4 12.4 12.5
It wlll be seen that good agreement was obtalned between the
measurlng results achleved ln accordance wlth the lnventlon
and the results obtalned wlth the conventlonal, manual lodo-
metrlc tltratlon processes long used ln practlce.
s It 15 important to note that when practlslng the method
accordlng to the lnventlon lt ls possible to obtaln data
concernlng the hydrogen peroxide contents of a bleachlng
llquor both rapldly and directly, even when the content ls
comparatlvely hlgh. Thls has prevlously presented a problem.
Peroxldes, such as hydrogen peroxlde, are a common
bleachlng agent used when bleachlny mechanlcal and cheml-
mechanlcal pulps ln partlcular. Varlous suggestlons have
been put forward as to how such bleachlng processes should
be carrled out. One 6uggestlon proposes that a bleaching
agent solutlon contalnlng a large quantity of hydrogen per-
oxlde ls mlxed rapldly lnto the pulp at a relatlvely low
pulp conslstency, whereafter the pulp ls pressed to a rela-
tlvely hlgh pulp conslstency.
The llguor pressed from the pulp, whlch llquor stlll
~0 contalns a relatlvely large amount of hydrogen peroxlde, ls
recycled and mlxed wlth freshly supplled pulp subsequent to
addlng further hydrogen peroxlde, l.e. fresh hydrogen per-
oxlde. Thls bleachlng method lnvolves the constant
21 lZ~'Z~7
manlpulation of llquors that contaln large quantltles of
hydrogen peroxlde, and slnce thls chemical ls expenslve lt
ls hlghly deslrable that the hydrogen peroxlde content of
the liquors can be determined correctly and qulckly. The
analysis method according to the invention satisfies pre-
clsely thls desideratum.
The method accordlng to the invention can be applied
equally as well wlth other types of peroxldes, includlng
organlc peroxldes. Excellent results are also achleved when
the analysis method accordlng to the lnventlon ls applled ln
con~unctlon with bleachlng processes whlch use other strong-
ly oxldatlve bleachlng agents, such as bleaching agent solu-
tions tha~ contaln chlorlne, chlorlne dloxide, hypochlorlte
etc.