Note: Descriptions are shown in the official language in which they were submitted.
~ ~2~34 ~ ~
OBTAINING ENHANCED BONDING BETWEEN
SURFACES BY LASER BEAM AND ADHESIVE
The present invention relates to a method
for making an impro~ed adhesive bond.
A need has long existed for a method of
enhancing the adhesive bonding characteristics o~
various materials. This need ha~ extended to enhancing
the bonding characteristics of materials which are
essentially clean surfaces, e.g., solvent cleaned
surfaces, as well as enhancing the bonding
characteristics of contaminated surfaces, e.g., surfaces
covered with a protective coating such as a wax or oil
coated surface. These needs have developed for examp~e
out of the automotive and aircraft industries'
dissatisfaction with current surface treatment
techniques and bonding results.
~; 20
~'
32,850A-F -1-
~ -~ 2 ~2~3~
An elegant explanation of material treatment
using energy beams comprising laser beams is contained
in U. S. Patent 4,122,240 granted to Banas et al.
Figure 1 therein shows a plot of absorbed power density
versus interaction time. At relati-~ely low absorbed
power density applied for relatively long times, mater-
ials can be heated to appreciable depths without melt~
ing the surface and thus can be subjected to "trans-
formation hardening." At very high absorbed power
density applied for extremely short periods of time to
a surface coated with a thin layer of preferably black
paint (which enhances absorption of the laser beam)
surface vaporization of the paint is so violent that a
shock wave of su~ficient amplitude moves through the
material that can result in "shock hardening" (see U.
S. Patent 4,401,477 to Clauer et al.). ~t mid-absorbed
power density applied for intermediate time periods,
the irradiated material can be vapoxized to a signifi-
cant depth for "hole drilling" applications. At lower
power density applied for longer time periods, the
material can be melted to significant depths for "deep
penetration welding." Using approximately the same
absorbed power density as in "deep penetration welding,l'
but for shorter periods of time, Banas et al. achieved
"skin melting." In skin melting a thin layer of the
material irradiated is melted but not vaporized and
then rapidly self-cooled.
Langen et al. in U. S. Patent 4,368,080 apply
0.5 to 16 joules/cm2 per pulse and a pulse time of from
1 to 100 microseconds to clean rust from metallic
objects prior to painting. The power density used by
Langen et al. is relatively low to prevent melting or
vaporization of the parent metal, but high enough to
~z~
3 6~693-3892
vaporize the rust or to conver~ the rust to a form not detrimental
to subsequent paint performance.
In Japanese published Patent Application No. 55~119181,
published September 12, 1980, by Iuchi et al.~ a laser is used to
remove oil from steel plates prior to painting. The oil ls
compounded wi~h ligh~ absorbing chemicals to improve the
efficiency of conversion of the light energy into heating the oil
fllm and only the oil is vaporized, not the underlying steel. In
Japanese published Patent Application ~o. 56-116867, published
1~ September 12, 1981, by Maeda et al., zinc galvanized steel sheets
are pollshed to remove the zinc coating, irradiated with a laser
beam to remove residual ~inc, phosphate treated and then painted.
Patent No. 211,801 from the German Democra~lc Republic
claims a process for the modification of surface propertie~ of
se~i-finished products and molded materials made of olefin
copolymers. Examples of the process use ionizing radiation, such
as corona discharge and energy beams from an electron accelerator,
to heat the surface to a temperatuxe between 340 and 410K. Other
forms of ionizing radiation are suggested without examples, such
2Q as ultraviolet, X-ray, gamma or laser radlation and particle
radiation such as alpha or beta radiation. Improvement of the
adhesiveness, printability, metallizability and varnishability is
taught in this patent.
The present invention is a new utili~y for surfaces
modified by an energy beam, e.g., a laser beam. This new utility
is enhanced bonding characteristics using an adhesive to bond
together at leaqt two surfaces, enhanced relative to a comparable
9~
4-- .
untreated surface or surfaces. In many cases, enhanced
bonding characteristics are also shown to be achieved
relative to a comparahle solvent washed surface or
surfaces. The term "enhanced bonding characteristic~"
refers to a bond having at least one of the following
- enhanced properties: at least a 20 percent stxonger
bond as demonstrated by-a standard lap shear test; a
more durable bond upon exposure to a sodium chloride
solution/h`igh humidity environment as demonstrated
using the durability/lap shear test of Example 4; a
more durable bond upon exposure to moisture as demon-
strated by at least a 25 percent shorter crack exten-
sion in a modified standard crack extension wedge test;
a stronger bond as demonstrated by at least a 25 per-
cent shorter initial crack in a modified standard wedgetest; at least a 25 percent longer time of immersion in
a boiling water bath before debonding in the test of
: Example 29; at least a 20 percent higher pull strength
as demonstrated by the ASTM 1876-72 peet test; at least
a 20 percent higher impact strength as demonstrated by
a modified ASTM D-256-81 impact test; at least a 20
percent higher torque strength using the torque test of
Example 30; or at least a 25 percent reduction in the
area of bond failure at the interface between the
adhesive and the bonded surface in any of the tests
above with a co~mensurate increase in failure within
the adhesive itself or in the bonded material.
Prior techniques for enhancing the bonding
characteristics of materials include sandblasting, shot
peening, brushing, pickling with acid, anodizing and
washing with solvents, see for example, "Adhesives
Technology Handbook," A. H. Landrock, 1985, Noyes
.
,r~3,~
-5
Publications, ISBN 0-8155-1040-3. All of these tech-
niques have undesirable features such as waste disposal
of spent chemica~s. Nevertheless, surface treatment
usually results in enhanced bondability. For example,
galvanized steel sheets formed into automotive door
panels are generally coated wi~h a lubricant prior to
forming to extend forming die life and ~o preserve the
surface finish of the panel. ~owever, before the panel
can be fastened to other parts with an adhesive, the
lubricant usually needs to be removed from the panel,
e.g., by washing with a solvent, for yet improved
bondability. Often, even better bondability can be
obtained by additionally roughening or etching the
panel surface to be bonded by sandblas-ting and/or
chemical treatment, such as a phosphate treatment. The
present invention cleans the surface of a material and
can also be used to vaporize or melt the material
itself or otherwise modify ~he surface, said modified
surface especially suitable for adhesion purposes.
Terms
The term "magnesium-containing material"
means (1) magnesium or (2) alloys having ma~nesium as
the predominant component.
The term "aluminum-containing material" means
(1) aluminum or (2) alloys having aluminum as the
predominant component.
The term 'Icopper-containing material" means
(l) copper or (2) alloys having copper as the predomi-
nant component.
~34~
--6--
The term "titanium-containing material" means
(1) titani~m or (2) alloys having titanium as the
predomina~t componen-t.
.
The term "coact" means the adhesive inti-
- 5 mately associates with the treated surface chemistry
and/or topGgraphy. The-chemical association can include
various chemical bonding phenomena and surface wetting
phenomena. The topographical association can include
load transfer between the adhesive ana the surface.
The term "stainless steel" refers to steel
alloys having greater than lO percent chromium.
- The term "galvanized steel" refers to an
alloy of iron which has been coated with a layer of
zinc, cadmium or other similarly effective metal or
metals in rontrolling the rate of corrosion of the
steel itself.
The term "energy beam" refers to one or more
electomagnetic radiation beams and/or one or more
particle beams effective to modify the surface of a
material. An example of an effective electromag~etic
beam source in the invention is a laser. An example of
a particle beam believed effective in the invention is
an electron be~m.
.
The term "material" relates to a solid sub-
stance such as a metal, an alloy of metal, a metal
composite, a metal laminate, a polymer, a polymer
composite such as fiber reinforced polymer and com-
~; posites comprising metals and p-olymers, as well as
inorganic solids such as ceramics and semiconductors.
'- .
-7- 1293~ ~0
Modi~ied surfaces having enhanced bonding
characteristics have been observed to have one or more
of the following surface modification effects: (a) a
surface which has had any oil, wax, mold, release agent
or other quch organic compound vaporized to produce a
surface equivalent to a solvent washed surface; ~b) an
altered surface of a metal or an inorganic material with
the energy beam to produce a diminishme~t of surface
carbon in excess of effect (a) above of at least
20 percent after said altering step as measured by x-ray
photoelectron spectroscopy; ~c) an altered surface of a
metal with the energy beam to vaporize at least
20 percent of the native metal oxide on the surface as
measured by x-ray photoelectron spectroscopy; (d) a
heated surface with an energy beam to melt the surface;
(e) a heated surface with an energy beam to not only
melt the surface but also to vaporize it; (f) an altered
surface with an energy beam to generate a roughened
surface thereby generating load-bearing structures; (g)
a heated surface to vaporize inorganic chemicals from
the surface as measured by x-ray photoelectron
spectroscopy; (h) an altered alloy composition of the
surface as measured by x-ray photoelectron spectroscopy;
or (i) an increased oxide thickness by 20 percent or
more after altering the surface as measured by x-ray
photoelectron spectroscopy.
The invention is a method for making an
article comprising an adhesive bond between at least two
surfaces of said article, said method comprising the
steps of: (a) placing at least one surface of said
article in the path of an energy beam for a duration
32,850A-F -7-
,,
~9;~ O
effective to modify said at least one surface; and ~b)
contacting said at least one modi~ied surface with an
adhesive comprising said adhesive bond, said adhesive
being of a type effective to coact with said at least
one modified surface to produce enhancement of the
bonding characteristics of said adhesive bond.
A pre~erred energy beam source is a pulse
laser and the specific energy density per pulse at the
surface to be treated preferably is coordinated with the
pulse time of the laser. For example, when the pulse
time is between 1 and 100 nanoseconds, the energy
density is preferably between 0.005 and 100 Joules/cm2
per pulse and more preferably between 0.05 and
10 Joules/om2 per pulse. Generally a 100 fo~d change
in pulse time requires a corresponding 10 fold change
in pre~erred energy density.
Another preferred energy beam source is a
continuous wave laser and the specific energy density
per time of treatment duration at the sur~ace to be
treated preferably is coordinated with the treatment
duration time for any one point treated on the surface.
For example, when the duration time is between 0.1 and
10 milliseconds, the energy density is preferably
between 5 and 10,000 Joules/cm2 per duration time and
more preferably between 50 and 1,000 Joules/cm2 per
duration time. Generally, a 100 fold change in duration
time requires a corresponding 10 ~old change in
3 preferred energy density.
Materials beneficially bonded by the method
of the invention include polymers, metals and inorganic
materials such as glass.
32,850A-F -8-
.... .
` ` 9 ~334`~a3
The invention also includes an article made
using the method of the invention.
The invention is also a method for preparing
a surface formed of a material of a type capable of
being treated to produce an enhanced bondable surface
by using the method below, said method comprising the
steps of: placing said surface in the path of a beam of
electromagnetic radiation having an energy density
selected to produce beneficial enhancement of the
bonding characteristics of the surface; applying said
beam for a duration effecive to modify the surface; and
applying an adhesive of a type effective to coact with
the treated surface to produce a stronger bond than
with respect to the nontreated surface.
Brief Description of The Drawinqs
Figure 1 shows an apparatus useful for the
method of the invention.
Figure 2 shows photomicrographs of laser
treated aluminum of Example 9.
Figure 3 shows photomicrographs of laser
treated titanium of Example 12.
Figure 4 shows photomicrographs of laser
treated silicone rubber of Example 19.
Figure 5 shows photomicrographs of laser
treated tin of Example 20.
Figure 6 shows photomicrographs of laser
treated graphite fiber reinforced epoxy composite of
Example 21.
~' ,
.~ "
-10~ 3~
Figure 7 shows photomicrographs of laser
treated cold rolled steel of Example 22.
Fiyure 8 shows photomicrographs of laser
treated copper of Example 23.
Figure-9 shows photomicrographs of laser
treated molybdenum of Example 24.
'' '
Figure 10 shows photomicrographs of laser
treated tungsten of Example 25.
Figure 11 shows photomicrographs of laser
treated glass of Example 30.
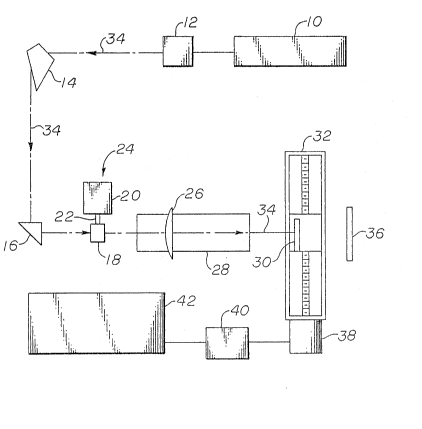
An apparatus useful for modifying surfaces to
be bonded accor~ing to the method of the invention is
typically illustrated in Figure 1 and involves a laser
10 and means 14, 16 and 26 for guiding the beam rom
the laser to the surface of the object to be treated.
:~ . A preferred laser 10 is a Q-switched Nd:YAG laser, but
other lasers which are preferred include gas lasers,
C2 lasers, and excimer lasers. The Kirk-Othmer
"Encyclopedia of Chemical Technology," Third Edition,
Volume 154, pages 42~81, John Wiley & Sons, New York
: (1979), describes various types of lasers and their
uses. The apparatus of thP invention may also comprise
;. one or more lasers or a laser with beam splitting means
adapted for the purposes of the invention.
',
The beam 34 issuing from laser 10 ca~ be
altered by a harmonic generator 12 capable of reducing
the wavelength of the beam proportional to a selected
~ .
.: ~
.
3~-J~
integral factor, and optical components, such as a
prism 14 to spacially separate differing frequencies of
the laser beam, a right-angle prism 16, and a preferred
cylindrical focusing lens 26 which focuses the laser
beam onto a surface to be'treated 30 and results in a
- generally elliptical shaped area of surface treatment
at any one time.- The length of said ellipse is deter-
mined by the diameter of the laser beam and can be made
longer by placin~ a dive~rging lens, not show~, in th~
laser beam 34 as is well known in the a'rt. The dotted
line 34 between laser 10 and lens 26 can further repre-
sent an optical fiber for directing the beam 34 at the
surface to be treated.
- A power meter 24 can be disposed between the
rlght-angle prism 16 and the ~ocusing lens 26. The
power meter essentially has two parts, a detector
head 18 integrally connected by means 22 with a con-
ventional analog readout meter 20. The detector
head 18 can be placed in the path of the laser beam to
` 20, detect the average power of the laser beam.
The cylindrical focusing lens 26 is disposed
on a translation stage 28. The translation stage 28
includes a track for moving the l'èns 26 parallel to the
path of the beam to focus and defocus the beam at the
sample surface 30 to be treated. The translation
stage 28 can be manually operated or operated by
xobotic means or by a motor.
The sample to be treated 30 is disposed on a
sample translation stage 32. The sample surface 30 is
moved on the translation stage 32 relative to the
~,
3~L~JC~
-12-
beam 34. Preferably, the sample surface 30 is moved
perpendicular to the beam 34. The translation stage 32
can be operated by a stepping motor 38 or, alterna-
tively, by a robotic means or by manual means (not
shown). Preferably the translation stage 32 is a
- controlled X-Y translational stage or a combination
-g translation stage-rotating wheel. SUCh items are
easily available commercially, for example, from Velme~
Company in Bloo~field, New York. Other means of moving
the energy beam relative to the surface to be treated
can include optical fibers attached to robot arms that
have 2-6 degrees axis of freedom or industrial grade
X-Y-Z gantry style platforms. It is convenient to
control translation stage 32 with a driver 40 which in
turn is operated by a computer ~2. The computer 42 can
easily control the number of pulses per area or the
treatment duration when using a continuous wave laser
on the surface 30 and the amount of overlap between
successive areas treated may be easily regulated. As
long as the energy density for treating the surface is
maintained, the relative movement between the laser
beam path and the surface to be treated can be as fast
as possible. Preferably, each area treated overlaps at
least somewhat with the area treated previously. In
many embodiments of the invention where there is a
visible change in the surface resulting from the laser
treatment, it is preferable that there is an overlap of
the visibly affected areas from those areas previously
treated. In ~he case of polymer materials, it is
generally believed that the depth of apparent vapor-
ization of the polymer is preferably deep enough to
(a) remove any mold release agents, (b) roughen the
surface, or (c) expose the fibers which can then bene-
ficially interlock with the applied adhesive. Once the
13 3L ~3~'~
sample 30 has passed through the beam 3a~ a beam block
36 can be used to trap the beam 34. The invention can
also incorporate more than one energy beam, e.g., two
or more lasers.
- 5 The energy beam or beams can be site-specific,
that is, focused on a se-lected area of a surface, such as
a metal surface with a high degree of accuracy to insure
that the beams do not affect the appearance and/or other
characteristics of neighboring surface~areas, namely, of
areas which do not require any treatment or whose
treatment is already completed. The amount of energy
transferred to an area of surface may be controlled by
focusing or defocusing the energy beam, controlling the
- exposure time of the beam and adjusting the output power
of the energy beam source. Any vaporized material may
be easily exhausted from the treatment area using
conventional exhaust, so as not to affect the health
and/or comfort of the attendants and not to contribute
to the pollution of the surrounding atmosphere.
.~ .
The energy beam source must satisfy certain
criteria. The first criterion is that the enersy beam
source must be capable of producing an extremely high
energy density at the surface to be treated. For this
invention, the critical parameter is absorbed energy
rather than incident energy. However, absorbed energy
is difficult to quantify and for the case where a laser
is used as the beam source, the proportion of energy
absorbed varies widely, for example, with differences
in the material to be treated and the condition of the
surface to be treated. The second criterion is that the
absorbed energy must be converted into sufficient thermal
.
.
-14- ~g3~ ~ ~
energy to vaporize and/or melt a surface layer of said
material or to otherwise modify the topography and/or
chemistry of the surface. The third criterion is that
the energy beam applied to any specific area of the
material must be applied for a relatively short time to
control the depth of any melting or charring of the
material. This ~riteria must be observ~d to preven~
melt through or burn through of the material to be
treated.
Using either a pulsed laser or a continuous
wave laser, or a plurality of lasers, the exposure time
o~ the laser onto the object may be used to control the
amount of energy directed to the surface of the object.
- The optimal exposure time and energy content of the
energy beam depends upon the chemical composition of
the surface of the object, the shape of the surace of
the object, the surface roughness of the object, the
movement of the surface of the object relative to the
beam, the angle the beam strikes the surface of the
object, the type of laser being used, and on the ulti-
mate application desired for the object being treated.
The preferred type of laser used in the
invention depends on the specific application. Among
the preferred lasers for metal and polymer surfaces is
a Q-switched Nd:YAG laser. Among the preferred lasers
for ceramic and polymer surfaces is a pulsed or con-
tinuous wave Co2 laser. It is believed that an excimer
laser is also preferred for metal, polymer and ceramic
surfaces.
J
Generally when a longer pulse time laser is
used, the amount of laser energy focused onto a given
-15~ 3~
area per pulse needs ~o be increased. When using a
laser having a pulse time between 1 and 100 nanoseconds,
preferably the enersy density of each la~er pulse is in
the range of from 0.005 to 100 joules/cm2, and more
preferably in the range of from 0.05 to 10 joules/cm2
per pulse. When using a pulsed laser having a pulse
time between 100 and 10,000 nanoseconds, prefer-ably the
energy density of each laser pulse is in the range of
from 0.05 to 1,000 joules/ cm2 per pulse and more
preferably in the range of from 0.5 to :L00 joules/cm2
per pulse. When using a laser having a pulse time
between lO and 1,000 microseconds, preferably the
energy density of each laser pulse is in the r~nge of
from 5 and 10,000 joules/cm2 per pulse and more pre~er-
- 15 ably from 50 to 1,000 joules/cm2 per pulse. When us:Lng
a laser having a pulse time between 1 and 100 micxo-
seconds, preferably the energy density of each laser
pulse is in the range of from 5 to 10,000 joules/cm2
per pulse and more preferably from 50 to 1,000
joules/cm2 per pulse. Similarly, when using a pulsed
laser having a pulse time of 0.1 nanoseconds, it is
believed that the preferred energy density of each
laser pulse is in the range of from about 0.001 to
about 5 joules/cm2, and more preferably from 0.01 to 1
joules/cm2 per pulse. When using a continuous wave
laser, the relative rate of movement between the laser
beam and the surface to be modified and the intensity
of the beam need to be controlled to similarly achieve
beneficial modification. When using a continuous wave
laser and a duration of treatment between 0.1 and
10 milliseconds, preferably the energy density is
between 5 and 10,000 joules/cm2 per duration time, and
more preferably between 50 and 1,000 joules/cm2 per
duxation time. When using a continuous wave laser and
-16- ~Z9347~
a duration of treatment between 10 and 1,000 milli-
seconds, preferably the energy density is between 10
and 20,000 joules/cm2 per duration time and more pre-
ferably between 100 and 2,000 joules/cm2 per duration
time. When using a continuous wa~e laser and a dura-
- tion of treatment between 0.001 and 0.1 millisecond,
preferably the energy density is between 0.5 and
1,000 joules/cm2 per duration time and more preferably
be~ween 5 and 100 joules/cm2 per duration timè. When
using a continuous wave laser and a duration of treat-
ment between 0.01 and 1 microsecond, preferably the
energy density is between 0.05 and 1,000 joules/cm2 per
duration time and more preferably between 0.5 and
100 joules/cm2 per duration time.
.
The determination of joules/cm2 per pulse is
made by interrelating two measurements. The first
measurement is a determination of the specific laser
beam energy per laser beam pulse. This measurement
includes the use of a laser optical power meter as is
well known in the art. Generally, laser power meters
; show the average power of the laser beam in average
watts. The number of average watts reported by the
; laser power meter is divided by the number of laser
beam pulses per second to obtain the number of joules
per pulse. The pulse time is that time over which
about 66 percent of the beam energy is emitted by the
laser. The second measurement is a determination of
the area on the surface to be modified that is impacted
i~ by the laser beam. This measurement is made by placing
ii^ 30 Zap-It brand laser thermal sensitive paper (Kentek
Inc., ~anchester, New Hampshire), or an equivalent
paper, on the surface to be treated followed by
* -r~ ~ ~ J~ ~f~ ~ ~
. .
-17
examination of the resulting visible effect of a singl~
laser pulse. An envelope is dxawn around the visibly
affected area of the pulse and the area of the envelope
is calculated as is well known in the art of geometry.
Finally, the number of joules ~er pulse is divided by
- the affected area in cm2 to obtain the joules/cm2 per
pulse. - -
The determina~ion of joules/cm2 per unit of
treatment time for any one point a continuous wave
laser is made by interrelating two measurements. The
first measurement is a determination of the laser beam
power per cm2. Using a laser optical power meter, the
number of watts reported is divided by the area of the
- surface treated by the laser at any one time as deter-
mined above to obtain watts per cm2. The second
measurement is a calculation of the duration o~
exposure o any one point. The width of the treated
area in cm at any one time in the direction of surface
movement relative to the laser beam is divided by the
relative movement velocity in cm per second. Then the
watts per cmZ is multiplied by the treatment duration
to obtain the joules/cm2 per unit of treatment dura-
tion.
The method of the invention can be used for
enhancing the adhesion performance of any surface
~ormed of a material of a type capable of being treated
with an energy beam to produce an enhanced bondable
surface, such as a metallic sheet-forming surface, a
polymer or a ceramic. Examples of such a surface
include galvanized steel, an aluminum-containing
material, or a magnesium-containing material. The
.
. , ~.. , . ~
. . .
3~L f'(~
-18-
method has been generally observed to enhance metallic
surfaces in the tests conducted by the inventors without
being limited to a particular metal.
In some applications it is desirable to
- 5 modify only one surface for enhanced bonding. For
example, when bonding dissimilar surfaces, one~surface
may reguire modification to enhance its performance to
a level approximately equal to the other surface.
Additionally, it is sometimes desirable to modify only
one surface to predispose a bond to fail in a pre-
dictable and beneficial manner not unlike the desire
~ for rolled cellophane~adhesive tape to come off its
- roll with the adheslve layer bonded to only one side of
- the cellophane.
The invention can be used to bond different
materials where both surfaces are treated. As an
example, it is not generally possible to spot weld
aluminum to steel while the present invention can be
used to enhance the adhesive bonding of aluminum to
steel, galvanized steel to glass fiber reinforced
plastic (SMC), aluminum to SMC, and other dissimilar
materials generally without limitation.
The specific àdhesive used is not critical in
the invention as long as said adhesive coacts with the
modified surface to produce an enhanced bond. Pre-
ferred a~hesives which work within the scope of the
in~ention include urethanes, acrylics and epoxies.
Gther adhesives which work within the scope of the
invention include silicone adhesives, cyanoacrylates
and thermoplastic hot melts like polyimides. Alter-
natively, other hot melts, may work within the scope
and teaching of the invention.
Q ~ ,k
~ `\
-19~ 3~ ~
The specific curing conditlons for the a &e-
sive used in the invention are not critical. Generally,
the curing conditions are recommended by the manufa--
turer of the adhesive for a given application. Prefer-
ably, the adhesive is applied to the mo,~ified surfacewith a minimum of delay in time. However, tests indi-
cate that when a modified surface was k~pt covered (in
a drawer), enhanced bonding performance was observed
even when the adhesive ~as applied one week and later
after surface modification.
With respect to the temperature at which
enhanced bonding is proved by lap shear or wedge test-
ing, said testing is generally done at room temperature
- but can also be done at temperatures higher or lower
than room temperature, e.g., at 180F or at 0F.
Wikh respect to the examples, the treated
surface generally has appeared amenable to a wide
variety of adhesives rather than appearing adhesive
specific to particular adhesives.
Urethane based adhesives are well known and
widely used to adhere plastic and/or metal adherends
together. The choice o~ urethane adhesives over other
adhesives is based in part upon their outstanding
characteristics with respect to bond strength, chemical
inertness, tensile strength and handling character-
istics.
One component of a urethane based adhesive
generally is an isocyanate-terminated prepolymer com-
pound. Such a compound is normally prepared by react-
- 30 ing a polyisocyanate with a polyhydroxy compound or
.
~ 20~ 3~;~
other compound containing labile hydrogen atoms that
will give a positive Zerewitinoff test. The isocyanate
group reacts with the labile hydrogen atom to form a
urethane group. A molar excess of the isocyanate is
added so that the resulting compound contains fre~
isocyanate groups.
The other component of the urethane based
adhesive is generally a cross-linking mixture compris-
ing an admixture of polyhydroxy compound free of iso-
cyanate groups and selected urethane catalysts. Whenthe two components are admixed, for example, in a high
shear mixing head and then applied to a surface, a
reactive hydrogen can interact with a free isocyanate
- group chain extended and cross-linked with an isocyanate~
terminated prepolymer to form a cured adhesive.
Other adhes.ives which work within ~he scope
and teachings of the present invention include epoxy
adhesives. A variety of epoxy adhesives work within the
scope of the present invention including those which
are rapidly curable at elevated temperatures and espe-
cially adapted ~or use on automotive assembly lines to
adhesively bond metal and/or polymeric parts. Numerous
t~pes of epoxy adhesives exist on the market, such as
the epoxy described in U. S. Pate~ts 4,459,398;
4,467,071; and 4,485,229. Epoxies and epoxy resins
which have increased adhesive strength contemplated for
use within the present invention include epoxy resin
formulations which are either pure or contain additives
which enhance the properties of the epoxy resin.
Exemplary enhanced epoxy resin compositions include
those described in U. S. Patents 4,002,598i 4,011,281;
. .
. ~ . ~
-21~ 3~
4,139,524; 4,1g6,701; 4,147,857; 4,178,426; and
4,219,638. Curable epoxy resins which are polymerized
by ionic addition mechanisms and ofter. reguire high
curing temperatures and long setting times can be used
within the scope and teachings of the invention.
Essentially, any epoxy adhesive capable of fQrming a
tight polymer network, ~haracterized-bv durability,
good adhesion, good water, chemical and/or
heat-resistant qualities can be used within the scope
of the invention.
Additionally, combinations of epoxies and -
acrylic based adhesives can be used. For example, the
adhesive described in U. S. Patent 3,684,617 dealing
with an adhesive mixture of acrylic based monomer and
epoxy resin can be used within the scope o~ the inven-
tion. Also, a nonreactive composite adhesive described
in U. S. Patent 3,994,764 may be used within the scope
of the invention.
Acrylic adhesives can work within the scope
and teachinss of the present invention. Acrylic adhe-
sives which include polymers and copolymers formed from
acryli~ and methacrylic acids and their derivatives can
be applied to the laser treated surface and provide the
enhanced bonding properties. It is anticipated that a
variety o~ other adhesives will also have utility
within the defined invention. These adhesives include
carboxylic polymeric adhesives, polysulfide adhesives,
phenolic resin adhesives, amino resin adhesives, ethyl-
ene copolymer based hot melt adhesives, polyvinyl
acetal adhesives, anaerobic adhesives, polyamide
adhesives and polyethylenimine based adhesives.
lZ~3'~
-22-
These adhesives can optionally contain other
materials. Other optional ingredients which can be
added to either component include thickeners~
stabili~ers, flame retardants, metal particles, fibers,
fillers, thixotropes and the like. The adhesives which
are usable within the scope and teachings of the present
invention can be prepared by a variety of methods, e.g.,
one and/or two-part components using a variety of curing
processes.
Adhesive materials can in ~ome cases be used
in conjunction with a primer as is well known in the
art. Here and in the claims such primers are considered
to be adhesive materials.
Thc adhesive coating disposed on the surface
of the material and treaSed in accordance with the
inventive method, can Porm a more durable bond, as well
as a stronger bond.
The invention can be carried out in alter-
native environments, such as a protective atmosphere
composed of nitrogen, or alternatively, an environment
containing an inert gas, such as argon or alternatively
; 25 in an environment containing a reactive gas such as
hydrogen. It is contemplated that the inventive method
could be practiced in an atmosphere containing a mixture
of gases which would enhance the treatment of the
surface for enhanced bonding.
The novel features which are considered as
characteristic of the invention are set forth in
particular in appended claims. The apparatus for the
32,850A-F -22-
-23- ~93~ '~
practice of the method, both as to its construction and
its mode of operation, together with additional features
and advantages of the metnod will be best understood
upon perusal of the follo~ing detailed description of
certain specific embodiments with reference to the
accompanying drawings as shown in the following
examples and comparative- examples.
Example 1
The apparatus for treating an object for
' 10 enhanced adhesion includes a Q-switched Quanta-Ray~
Nd:YAG model no. DCR-2 laser which produces 30 pulses
per second. The laser produces radiation at a wave-
length of 1.06 microns with a maximum average power of
- about ~8 watts.
The laser beam is directed at the object to
be treated by a system of optical compo~ents. The
components route and focus the beam. The components
are constructed of high ~uality quartz, Sl- W grade.
The beam is directed by 90 degree prisms ESCO model
no. 1125250 and focused to a line image about 1/2 inch
long by a cylindrical 50 cm focal length lens, ESC0
model no. Bl-20100.
The objects to be treated, 1 x 4 inch solvent
cleaned (the term "solvent cleaned" in this and all
examples herein means rinsing three times with methyl-
ene chloride unless otherwise indicated) 2024-T3 alu-
minum alloy panels 1/16 o~ an inch thick, are placed in
a holder on a translation stage synchronized with the
laser pulse repetition rate and translated through the
- 30 beam. The translation apparatus includes a Velmex
~ 'T~ ~. J~
-24- 1293~7~
, ~
Unislide AgO00 series translation stage. The slide of
the translation apparatus is driven by a stepping motor
from Superior Electric, model M061-FD08. The stepping
motor is controlle~ by a Superior Electric~model STM 103
controller and the controller is operated by a Commodore
64 computer for co~plex maneuvers or manually operated.
The panels are translated through ~le laser
beam path at 18 mm/sec and the final 1/2 inch portion
of each panel is treated. The lens to panel distance
is 35 cm.
Four pairs of panels are treated at each of
several energy density levels between 0.14 and 2.16
- joules/cm2 per pulse. Each panel pair is then bonded
together applying a high strength two-part urethane
15 adhesive cured at 135C for 45 minutes (Dow~XPR no.
0544-00923-05-1 urethane adhesive systems) containing
; 0.5 percent, 0.004 inch diameter glass beads to insure
an evenly spaced "glue line" as is wel- known in the
art, only to the treated portion of each panel with a
1/2 x 1 inch lap joint of the panel ends. Table I
lists the lap shear strength (measured using ASTM test
D-1002) of each pan~l pair so bonded in relation to
laser energy density. The lap shear str~ngth of the
panels treated with 0.8 to 2.3 joules/cm2 per pulse is
about 50 percent higher than those with no treatment.
The lap shear strength shown in Table I is an average
of four tests and generally such tests show a statis-
tical variation ~relative standard deviation) of about
ilO percent. Unless otherwise indicated, all examples
herein involved testing in quadruplicate at any one
laser energy density treatment.
~c/~ .,k
.
-25- ~93~ ~
TABLE I
,
hAP SHEAR TESTING OF LASER TREATED ALUMINUM
PANELS ~ONDED TOGETHER WITH A URE~NE ADHESIVE
~aser Energy Density,
Joules/cm2 per pulse La~Shear Stren~h, psi
O 2,300
0 4 2,200
0~6 2,500
0.8 3,300
1.0 3,800
- 1.2 3,~00
1.7 3,500
2.3 3,600
Exam~le 2
The apparatus of Example 1 is used in
conjunction with 1 x 4 inch solvent cleaned magnesium
panels 1/16 inch thick prepared as cold chamber die-cast
magnesium alloys of type AZ 91B produced by The Dow
Chemical Company. This alloy contains 9 percent alu-
minum and 1 percent æinc. The sample to lens distance
is 25 cm.
The samples are moved through the beam at a
rate of 15 mm/sec. These magnesium alloy panels are
then bonded and tested as in Example 1. The results
listed in Table II show that lap shear strength
increases with increased laser energy density. The
bond stxength increases approximately 60 percent over
that obtained from the untreated panels.
~. .
-~6- 12~
TABLE II
LAP SHEAR TESTING OF LASER TREATED MAGNESIUM
PANELS BONDED TOGETHER WITH A URE~NE ADHESIVE
Laser Energy Density,
- 5 Joules/cm2_per ~ se La~ Shear Streng~h,
:.
O - 1,200
0.1 1,600
0.3 1,700
0.4 2,000
0.8 1,800
1.2 2,200
Exam~le 3
The apparatus of Example 1 is used in connec-
tion with 1 ~ 4 inch hot dip zinc galvanized steel
panels (Deep DQSK, G-60 automotive grade) 1/33 inch
thick. The panels are immersed in a water based oil
emulsion, H. A. Montgomery no. DF-4285 and allowed to
drain for a period of 15 to 45 minutes. The panels
are translated through the laser beam at 7.4 mm/sec.
The sample to lens distance was SO cm.
These galvanized steel panels are then bonded
and tested as in Example 1. The lap shear strength of
the bonded panels versus the laser energy density is
listed in Table III. Laser treatment in the energy
density range of from 1.2 to 5.2 joules/cm2 per pulse
produced an increase in bond strength of almost an
order of magnitude over that of the untreated material.
.
.
,
27- 1~934'~
As the laser power increases to 1.2 joules/cm2 per
pulse, the lap shear strength increases to about
2,000 psi and remains at about that level for higher
laser energy densities. The lap shear strength of non-
oiled solvent cleaned panels of galvanized steel isabout 1,300 psi.
.
TABLE III
.
LAP SHEAR TESTING OF LASER
TREATED OILED GALVANIZED STEEL PANELS
BONDED TOGETHER WITH A URETHANE ADHESIVE
Laser Energy Density,
Joules/cm2 per pulse_ Lap Shear Strength, psi
0 300
1.2 1,000
1.4 2,100
. 1.9 1,700
.4 2,000
2.8 . 1,900
3.3 ~,100
5.2 2,000
Example 4
The apparatus of Example 1 also can be used
to improve ~he durability of bonding. Thirty-two sets
of 4 hot dip zinc galvanized steel panels axe first pre-
:~ 25 treated with an oil emulsion as in Example 3. Two of
the panels of galvanized steel in each set are then
translated through the laser beam at 7.4 mm/sec using
a sample to lens distance of 50 cm and a laser ener~y
:;
-28- 1Z~3~
density of approximately 1.60 joules/cm2 per pulse.
The other two panels of each set are not treated with
the laser and instead are solvent cleaned to remove the
oil. Table IV shows the bond strength of laser treated
and solvent cleaned panels bonded together as in Ex~nple 1
versus the length of time the panels are exposed to a
- moist salty environment in a test known as the Generai
Motors Scab Corrosion Test. This test is performed
over a period of 32 days and involves testin-g the lap
shear strength of the panels bonded as in Example 1.
This experiment involves 32 cycles, where for each
cycle, the bonded panels are placed for 22.5 hours in a
cabinet with a relative humidity of 85 percent and a
temperature of 145F, followed by 0.25 hour immersion
- 15 in a 5 weight percent NaCl solution in water and then
1~25 hours in a dry, room temperature area follcwed by
a test of lap shear strength. After 7 cycles, the test
results reveal that the untreated panels lost all lap
shear strength and fell apart. In contrast, the laser
treated panels maintain a bond for 32 days at a strength
. of at least 500-600 psi.
-29~ 34~;~
TABLE IV
BOND DURABILITY UPON EXPOSURE TO
MOISTURE AND SALT FOR ~ASER TREATED AND
SOLVENT CLEANED GALVANIZED STEEL PANEL~
Lap Shear Strength Lap Shear Strength
Days ofin psi for the in psi for the a
Exposure Solvent Cleaned Panelsa Laser Treated Panels -
0 1,30~ ~,000
1 600 1,400
4 600 1,400
7 0 1,300
14 0 1,300
18 0 900
0 600
1~ 28 0 500
32 0 600
a- Note, these data are the average of 2-3 determina-
- tions.
~,
Example 5
The apparatus of Example 1 is used in conjunc-
tion with hot dip zinc galvanized steel panels (Deep
DQSK, G-60 automotive grade) and an acrylic adhesive.
; (Hardman~red/white two-part acrylic adhesive, ~ured at
- 325F for 30 minutes). The galvanized steel panels are
treated with an oil emulsion as in Example 3 prior to
laser treatment. The sample to lens distance is 35 cm,
the sample translation is 15 mm/sec through the laser
~ T,'~Je
_30_ ~29~
beam and the laser energy density is 0.6 joules/cm2 per
pulse. The oily galvanized panels with no laser treat-
ment before bonding have a bond strength of about
900 psi. The laser treated panels have a bond strength
of approximately 1,600 psi. This result represents
over a 75 percent increase in bond strength for the
laser treated panels bonded with acrylic: adhesive in
comparison with untreated panels bonded with acrylic
adhesive.
Example 6
The apparatus of Example 1 is used in connec-
tion with treating 1 x 4 inch panels of sheet molding
compound (calcium carbonate/glass fiber filled poly-
- ester resin containing zinc stearate as a mold release
agent) 1/16 inch thick bonded with Hardman red/white
two-part acrylic adhesive, containing 0.5 percent,
0.020 inch diameter glass beads, cured according to
label directions. The panels are laser treated without
any application of oil or other material to the surface
2Q of the panels. The lens to sample distance is 35 cm,
the sample translation speed is 16 mm/sec and the laser
; energy density is 0.6 joules/cm2 per pulse. Polymer
panels bonded without laser treatment have bond
strengths of about 100 psi. The laser treated panels
show a lap shear strength of about 500 psi. The
increase in lap shear strength is greater than S00
percent for laser treated glass fiber filled polymer
panels as compared to the untreated panels. Examina-
tion of the laser treated surface by x-ray photoelec-
tron spectroscopy indicated that substantially all ofthe mold release agent was removed by the laser treat-
ment as measured by the diminishment of zinc from the
surface after laser treatment.
-31- ~293470
In addi~ion, 1 x 4 inch panels of acrylonitri~e-
butadiene-styrene (ABS) plastic sheet, 1/16 inch thick,
are laser treated as above and bonded with either a Dow
two-part urethane adhesive as in Example 1 cured at
150F for 30 minutes or bonded with Hardman red/white
two-part acrylic adhesive cured at room temperature,
containing 0.5 percent, 0.020 inch diameter glass
beads, cured according to label directions. The lap
shear strength of the laser treated panels is so high
that the panels themselves break in testing at about a
lap shear strength of 900 psi and for comparison the
lap shear strength of the panels without laser treatment
is about 400 psi.
- Example 7
In Examples 1-6 the test method is ASTM
D-1002 for lap shear strength. In this example and in
many of the following examples the test method is wedge
test ASTM D-3762, modified as describ~d below. In the
modified ASTM D-3762 test, 1 x 4 inch metal panels 1/16
inch thick are bonded together with an adhesive and
then a wedge is forced between the panels as sho-~n in
Figure 3. The initial crack length is a function of
several factors including the tensile strength of the
\ bond. The bond strength can be so poor that the test
panels fall apart, i.e., an initial crack length in
excess of 3 inches. After measuring the initial crack
length, the wedged panels, with wedge in place, are
placed in a 49C, 100 percent relative'humidity envi-
ronment for 75 minutes and then the crack growth after
exposure (herein termed "crack extension") is measured.
If the bond has poor resistance to moisture, then the
crack extension can be large and again if large enough
the test panels can fall apart. The percent relative
:: `
-32~ 3 ~t~'~
standard deviation precision of the wedse test is
generally believed to be about ~ 12 percent.
The system of Example 1 is used to laser
treat solvent clean~d 2024-T3 aluminum alloy p~nels.
The cylindrical lens is changed to one having a 25 cm
focal length and the panels are placed-21 cm fEom this
lens. Between the laser and the cylindrical lçns is
placed a polarizing filter. By~adjusting the polar
izing filter, different laser power can be directed
upon the panels while operating the laser itself at
full power (18 watts).
The panels are translated back and forth
- through the laser beam at 12 mm/sec to completely treat
one side of each panel with about a lSO percent overlap
of each pulse treated area. The treated surace of
each panel is then coated with American Cyanamid~epoxy
primer no. BR-127, cured at 250F for 1 hour and bonded
to an identically treated panel with 3M~epoxy adhesive
no. AF-163-2, OST, Grade 5, cured at 250F for 1.5
hours under vacuum at 100 psi and wedge tested.
:
As listed in Table V, with no laser treatment
the initial crack is about 37 mm and this crack grew
about 30 mm upon exposure to moisture. At 0.7 to 1.6
joules/cm2 of laser energy per pulse, the initial crack
is significantly reduced and the crack grew only about
2 mm to about 5 mm upon exposure to moisture; a signif-
icant improvement in both bond strength and bond dur-
ability upon exposure to moisture according to the
wedge test.
7rcr Je Oarrk
.
.
.
-33~ ~93
TABLE V
WED'~E TESTING OF LASER TREATED ALUMINUM
PANELS BONDED TOGETHER WITH AN EPOXY ADHESIVE
~ Laser Energy Density, Initial Crack Crack
Joules/cm2 per pulse Length~ mm Extension, mm
0.0 37 30
0.7 12 ~ 5
0.8 12 2
1.1 13 2
1.4 12 2
1.6 12 2
Example 8
This is a comparative example of several prior
axt surface preparation techniques. Panels of solvent
cleaned 2024-T3 aluminum alloy are sanded with 80, 320 or
600 grit sandpaper (190, 29 and 17 microns, respectively),
polished with 5 ~ alumina, sandblasted with 54 grit
aluminum oxide, or wire brushed with 13 mil wire. The
soxhlet cleaned panels are placed in a Whatman*LTD cel-
lulose soxhlet thimble and sox~let extracted with tol-
uene for 4 hours (~37 rinse cycles). The panels are
then coated with American Cyanamid epoxy primer no.
BR-127 and bonded together with 3M epoxy a &esive no.
: AF-163-2 as in Example 7 and then wedge tested, see
Table VI.
~ ~T,Qd~ ~nc"k
_34- ~2~3~
TABLE VI
WEDGE TESTING OF SEVERAL PRIOR ART
SURFACE PREPARATION TECHNIQUES FOR ALUMINUM
PANELS BONDED TOGETHER WIT~ AN EPOXY ADHESIVE
Surface Treatn.ent Initial Crack Length ra k Extension
80 Grit Sandpaper 24 mm 21 mm
320 Grit Sandpaper 22 mm 22 mm
600 Grit Sandpaper 17 mm 15 mm
Polished Surface 49 mm >27 mma-
10 Sandblasted 15 mm 14 mm
Wire Brushed 20 mm 15 mm
- Solvent Cleaned 36 mm >41 mma-
Soxhlet Cleaned 30 mm >~7 mma'
a- Panels ~ell apart in the humidity cabinet.
The data in Table VI, when compared to the
results obtained using the present invention, see
Example 7, demonstrate the superiority of the present
invention over the surface treatments listed in Table VI
; as indicated by the wedge test.
Exam~le 9
The system of Example 7 is used to again treat
; solvent cleaned 2024-T3 aluminum alloy panels except
that the polarizing filter is removed and the laser
power is adjusted as in Example 1. The panels are
placed about 25 cm from the 25 cm focal length cylin-
; drical lens.
.
_35_ ~ 2~ 3 ~7~
,
The degree of overlap of the area covered by
each laser pulse is about 150 percent. The pulse time
of the laser depends on the power setting, being about
10 nanoseconds above 6 watts and from 10 to 40 nano~
: 5 seconds between 1/2 watt and 6 watts, see Table VII.
: TABLE VII
PULSE TIME OF THE LASER USED AS A
: FUNCTION OF ENERGY DENSITY AND o~rpuT POWER
,
: Laser Power Laser Energy Density,Pulse Time in
10 in Watts/Sec Joules/cm2 per pulseNanoseconds
0.5 0.7 40
l 0.8 25
2 l.1 20
3 1.4 16
4 1.6 12
1.9 11
. 6 2.2 10
` 10 3.3 10
~: 15 4.7 10
18 5.5 10
:~
The data in Table VII is pro~ided to allow
~ conversion from laser power to energy density and pulse
:~ time for the conditions described above. The following
Table VIII shows example data of enhanced bonding char-
acteristics.
`""' ' .
-36~
The treated aluminum panels are then coated
with American Cyanamid epoxy primer no. BR-127 and
bonded together with 3M epoxy adhesive no. AF-163-2 as
in Example 7 and -then wedge tested, see Table VIII.
TABLE VIII
WEDGE TESTIN~ OF LASER TREATED-.ALUMINUM
PANELS BONDED TOGEl~R WITH AN EP~.XY ADHESIVE
Laser Energy Density,Initial Crack Crack
Joul~s/cm2 ~er pulseLen~th, mm Extenslon, mm
10 0.0 40 30
0.7 19 20
0.8 12
1.4 11
15 1.9 12
~ 207 11 3
The data in Table VIII indicate that even at a
laser energy density of 0.7 joules/cm2 per pulse, the
bond strength and durability is improved relative to the
nonlaser treated panels.
Figure 2 shows electron photomicrographs of
aluminum treated with: (a) a single 0.8 joules/cm2
pulse, shown at 3,000X magnification; and (b) an alu-
minum surface showing overlapping 2.7 joules/cmZ pulses,
~ 25 shown at 1500X magnification. These photomicrographs
:~ show evi.dence o~ surface vaporization and/or surface
~`
-37~ 3~
melting along with roughening of the surface. X-ray
photoelectron spectroscopy of the treated surfaces
listed in Table VIII indicates that even at 0.7
joules/cmZ per pulse of laser energy density, the
percent carbon on the surface of the aluminum is sig-
nificantly diminished versus the untreated surface.
Optimization of economic factors using the
invention can result from using full laser powex with a
defocused laser beam to treat a larger~area of surface
with each pulse. In this event, the linear velocity of
relative movement between the laser beam and the surface
to be treated can be higher and still effectively treat
the surface for enhanced bonding characteristics.
Example 10
The system of Example 9 is used to treat
panels o~ solvent cleaned 304 grade stainless stee~.
The treated panels are then coated with American
Cyanamid epoxy primer no. BR-127 and bonded together
with 3M epoxy adhesive no. AF-163-2 as in Example 7
and then wedge tested, see Table IX.
~,~
~ TABLE IX
.
WEDGE TESTING OF LASER TREATED STAINLESS STEEL
PANELS BONDED TOGETHER WITH AN EPOXY ADHESIVE
.
Laser Energy Density, Initial Crack Crack
~ 25 Joules/cm2 per Pulse Length, mm Extenslon, mm
,~ .
0 15 22
1.4 17 7
2.7 8
5.5 17 3
~38-
~3~
The data in Table IX show that optimum bond
strength as indicated by initial crack length requires a
specific treatment power range while bond durability as
indicated by crack extension is improved at all laser
power levels tes~ed.
.
ExamPle 1-~
The system of Example 9 is used to treat
panels of solvent cleaned hot dip zinc galvanized
steel (Deep DQSK, G-60 automotive grade~. The treated
panels are then coated with American Cyanamid epoxy
primer no. BR-127 and bonded together with 3M epoxy
adhesive no. AF-163-2 as in Example 7 and then wedge
tested, see Table X.
TABLE X
WEDGE TESTING OF LASER TREATED GALVANIZED STEEL
PANELS BONDED TOGETEER WITH AN EPOXY ADHESIVE
Laser Ener~y Densit~, Initial Crack Crack
Joules/cm2 per pulse Len~th, mm Extension, mm
0.0 11 2
. 1.~ 2 0.3
.2.7 2 0.2
5.5 4 0.3
The data in Table X show laser treatment
improves both initial crack length and crack e~tension
for galvanized steel under the condikions studiedO How-
ever, it should be noted that the laser treated panels
,:
-39~
curled back upon wedge entry and thus the only conclu-
sion believed to be shown by the data in Table X is
t~at laser treatment improved bond strength.
. X-ray photoelectron spectrosc:opy of the 0 and
1.4 joules/cm2 per pulse treated panels indicates that
b~fore laser treatment the surface contains about
70 percent carbon, and ~hat after treat~ent the surface
is essentially 100 percent zi~c oxide.
Example 12
The system of Example 9 is used to treat
panels of wire brushed and nonbrushed solvent cleaned
Ti6Al4 titanium alloy. The treated panels are then
- coated with American Cyanamid epoxy primer no. BR-127
and bonded together with 3M epoxy adhesive no. AF-163-2
and then wedge ~ested, see Table XI.
T~BLE XI
: WEDGE TESTING OF LASER TREATED TITANIUM
PANELS BONDED TOGETHER WITH AN EPOXY ADHESIVE
Laser Energy Density, Initial CrackCrack
~;20 Joules/cm2 per pulse Lenqth, mmExtension, mm
O O O 24a 36a-
0.0 2ob- 8b.
104 20 4
2.7 22 4
5.5 24 11
:
Not wire brushed.
b- Wire brushed.
'
~' .
-40- ~3~
The data in Table XI show no improvement in
bond strength at the laser powers tested as indicated
by initial crack length. However, an improvement in
bond durability is seen at 1.4 and 2.7 joules/cm2 per
pulse of laser energy as indicated by the crack exten-
sion data in Table XI.
Figure 3 shows electron photomicrographs of:
(a) the titanium alloy before laser tre~tment at 3,000X
magnification; and (b~ aftex laser treatment with a
single 1.4 joules/cm2 pulse at 2,900X magnification
that also shows evidence of apparent surface melting.
.
X-ray photoelectron spectroscopy of the 0 and
- 3 watt treated panels indicates that before laser
treatment the surface contains about 50 percent carbon
and only about 2 percent titanium. After treatment the
surface contains about 15 percent carbon and 19 percent
titanium (present as titanium dioxide).
- Example 13
The system of Example 9 is used to treat
panels of solvent cleaned 15 microinch surface ground
cold rolled steel. The treated panels are then coated
with American Cyanamid epoxy primer no. BR-127 and
bonded to an identically treated panel with 3M epoxy
adhesive no. AF-163-2 as in Example 7 and then wedge
tested, see Table XII.
:~ :
,. ...
-41~
TABLE XII
WEDGE TESTING OF LASER TREATED COLD ROLLED STEEL
PANELS BONDED TOGETHER WITH AN EPOXY ADHESIVE
Laser Energy Density,Initial Crack Crack
5 Joules/~mZ per pulse Length, mm Exte~siGn, mm
0 20 7
2.2 22
2.7 2~ 6
3.8 23 7
5.5 22 7
The data in r~able XII show no significant
improvement in either initial crack lenyth or in crack
extension under the conditions studied. When the sol-
vent cleaned ground cold rolled steel panels are treated
as listed in Table XII, bonded using a urethane adhesive
as in Example 1 and subjected to the lap shear testr no
significant improvement in lap shear strength results.
Example 22 describes wedge testing of laser treated
cold rolled steel panels and Example 28 describes lap
shear testing of laser treated oily cold rolled steel
panels.
Photo~icrographs o~ the 5.5 joules/cm2 per
pulse laser treated ground steel surface showed a
general smoothing of the surface, relative to the
untreated ground surface, with evidence of overall
apparent surface melting.
.
-42- ~g3~
E~ample 14
The system of Example 9 is used to laser
treat panels of solvent cleaned mirror smooth chrome
plated steel. The treated panels are then coated with
American Cyanamid epoxy primer no. BR-127 and bonded
together with 3M epoxy adhesive no. AF-163-2 as in
- Example 7 and then wedge tested, see Table XIII.
.
TABLE XIII
WEDGE TESTING OF LASER TREATED CHROME PLATED
STEEL PANELS BONDED TOGETHER WITH AN EPOXY ADHESIVE
Laser Energy Density, Initial Crack Crack
Joules/cm2 per pulse_ Lenqth, mm Extension, mm
0.0 45 13
0~8 6 0
1.4 6 0
2.7 14 3
5.5 16 6
The data in Table XIII show that under the
conditions studied both bond strength and bond dur-
ability are improved by laser treatment as indicatedby the wedge test.
Example 15
The laser of Example 9 is replaced with an
excimer laser having a waveleng~h of 0.249 micron, a
pulse width of about 10 nanoseconds, a pulse frequency
of 30 Hertz and a maximum power of 6 wat-ts. The excimer
''
~ 3 ~7~
laser is used to treat panels of aluminum, stainless
steel and copper at a laser energy density of approxi-
mately 10 and 0 joules/cm2 per pulse. The treated
surface of each panel is then coated with ~merican
Cyanamid epoxy primer no. BR-127 and bonded to an
identically treated panel of the same metal with 3M
epoxy a &esive no. AF-163-2 and then wedge tested.
In each case, the laser treatment significantly enhances
the bonding characteristics of the panels as indicated
by the wedge test.
Example 16
The system of Example 9 is used to treat
panels of solvent cleaned 2024-T3 aluminum alloy panels.
- In this example the Q-switch of the laser is turned off
and as a result the laser pulse length is about 100
microseconds. The cylindrical lens is replaced with
a conventional converging lens and the laser pulse is
focused to a spot 0.6 mm in diameter on the object to
be treated in order to compensate for the longer laser
pulse length. The translation stage is controlled so
that there is about 150 percent coverage of the panel
surface with the laser pulses. The treated panels are
then coated with American Cyanamid epoxy primer no.
, BR-127 and bonded to an identically treated panel wi-th
3M epoxy adhesive no. AF-163-2 as in Example 7 and then
wedge tested, see Table XIV.
.. .- - . , .,, ~.,
_4g- ~293~70~
TABLE XIV
WEDGE TESTING OF LASER TREATED ALUMINUM
PANELS BONDED TOGEl~IER WITH AN EPO~ ADHES IVE
Laser Energy Density,Initial Crack Crack
Joules/cm2 per pulse Len~th, mm Extension; mm
S
0 37 39
140 3~ L8
210 15 10
.
The data in Table XIV indicate that signif-
icant enhancement of bond characteristics was observed
at the highest laser power studied as indicated by the
wedge test. This example demonstrates the success~ul
use of a relatively long pulse length laser in the
invention.
15. Comparing the data in Table XIV (Q-switch off3
with the data in Table VIII and Table VIII (Q-switch on~
indicate that delivering a given laser energy density in
a xelatively short pulse (Q-switch on) is more efficient
than a relatively long pulse (Q-switch off).
Example 17
The s.ystem of Example 9 is used to treat ~ .
panels of solvent cleaned 2024-T3 aluminum alloy panels.
;~ The treated panels are then bonded together with Locktite
Super Bonder~495 cyanoacrylate-type adhesive containing
. .
0.5 percent, 0.004 inch diameter glass beads to insure
an evenly spaced "glue line" as is well known in the art.
s~Trc~d~ ~,k
.
. .
,: ,, .
., ..... , , .... .-`
-45-
TABLE XV
WEDGE TESTING OF LASER TREATED ALUMINUM PANELS
BONDED TOGETHER WITH A CYANOACRYLATE ADHESIVE
Laser Energy Density, Initial Crack Crack
-Joules/cm2 per pulse_ Length, mm Extension, mm
0.0 54 ~ >23a
1.5 36 3
a- Panels fell apart in the humidity cabinet.
- The data in Table XV show that both the
initial crack length and crack extension are improved
with the laser treatment.
i
Exa~le 18
The system of Example 9 is used to treat
panels of solvent cleaned, Bonderized, ~phosphate treated)
electrogalvanized steel. The treated panels are then
coated with American Cyanamid epoxy primer no. BR-127
; and bonded to an identically treated panel with 3M
epo~y adhesi~e no. AF-163-2 as in Example 7 and then
wedge tested, see Table XVI.
-46~ 34~
TABLE_XVI
WEDGE TESTING OE LASER TREATED
BONDERIZED ELECTROGAr.VANIZED STEEL PANELS
BONDED TGGETHER WITH AN EPOXY ADHESIVE
- - S Laser Energy ~ensity,Initial Crack - Crack
Joules/cm2 per Pulse Length, mm Extension, mm
.
0.0 8.0 - 2.2
0.8 7.6 0.7
1.4 8.1 0.0
2.7 8.~ 0.0
5.5 6.7 0.4
The data in Table XVI indicate tha~ although
bond strength was not significan~ly improved by laser
treatment as indicated by the initial crack length, bond
durability was significantly improved as indicated by
the crack extension under the conditions studied.
.
Example 19
The system of Example 9 is used to treat
detergent washed panels of Duro Inc. 50A red silicone
rubber. The test panels are not the usual 1 x 4 x
1/16 inch, but rather are 1 x 4 x 1/8 inch. The
. treated panels are then bonded together with 3M
icotch-Weld no. 2216 B/A flexible a &esive (3 parts
"A" to 2 parts "B" by volume) cured 1 hour at room
temperature and 1 hour at 180F and then subjected to
the ASTM D-1876-72 peel test, see Table XVII.
~Tr~c/e ~ rk.
_47- ~2~3~ ~
TABLE XVII
PEEL TESTING OF LASER TREATED SILICONE RUBBER
PANELS BONDED TOGETHER WITH SCOTCH-WELD ADHESIVE
Laser Energy Density,Peel Strength Pounds
Joules/cm2 per pul-seper Linear Inch
- o . o 0 . 1
1.35 ~ a.3
5.5 13.6
The data in Table XVII indicate that the bond
strength as indicated by the peel test was significantly
improved after laser treatment.
Figure 4 shows 400X magnification electron
photomicrographs of silicone rubber: (a) before laser
treatment; (b) after overa~l treatment with 1.4 joules/-
cm2 per pulse of laser energy; and (c) after overalltreatment with 5.5 joules/cm2 per pulse of laser energy.
Figure 4 shows evidence o~ apparent surface vaporiza-
tion. Figure 4(c) shows evidence of general overall
apparent surface vaporization and roughening.
Example 20
The system of Example 9 is used to laser
treat panels of solvent cleaned pure tin. The
treated panels are then coated with American Cyanamid
epoxy primer no. BR-127 and bonded together with 3M
epoxy adhesive no. AF-163-2 as in Example 7 and then
peel tested using the ASTM 1876-72 peel test, see
Table XVIII.
~ '.
-48- ~2~34~
TABLE XVI I I
PEEL TESTING OF LASER TREATED TIN PANELS
BONDED TOGETHER WIl~ AN EPOXY ADHESIVE
Laser Energy Density, Peel Strength, Pounds
`oules/cmZ per pulse per Linear Inch
` 3 . 6 `
1.4 8.9
:~ 2-7 7.0
5.5 3.5
- 10 The data in Table.XVIII show that at the
intermediate laser energy densities per pulse, the peel
strength is significantly improved relative to no laser
treatment.
~ Figure 5 shows 3, 000X magnification electron
:~ 15, photomicrographs of tin: (a) before laser treatment;
:~ (b) after overlapping treatment with 1.~ joules/cm2
~` laser pulses; and (c) after overlapping txeatment with
2 . 7 joules/cm2 laser pulses. Although there is no
signifi,cant apparent difference between Figure 5(a) and
5(b), the data in Table XVIII indicates that the surface
shown in Figure 5~b~ was apparently beneficially modi-
fied even though,there is no apparent surface melting
;~ and roughening o~ the type shown in Figure 3(b) or
Figure 2~a). Figure 5(c) does show evidence of apparent
~ ~ 25 surface melting and/or vaporization.
: .
~ '' ' .
~ .
~Z~3~
Exam~le 21
- The system of Example 9 is used to laser
treat panels of solvent cleanec. aircraft grade graphite
fiber reinforced epoxy composite panels, 4 x 1 x 0.040
~- 5 inches, ~60 volume percent Hercules IM-6 graphite
fibers, 40 volu~e percent epoxy resin~. The treated
panels are bonded together with Hardman blue~beige
urethane adhesive, containing 0.5 percent, 0.020 inch
-glass spheres, and tested for lap shear strength using
the ASTM D-1002 lap shear test. Another set of treated
panels are ~onded together with Hardman yellow epoxy
adhesive containing 0.5 percent, 0.020 inch diameter
glass spheres, and tested for lap shear strength using
the same test. The test results are shown in Table XIX.
~ Tr~ c/e /q'~a~iÇ
~50- 12~
TABLE XIX
LAP SHEAR TESTING OF IASER TREATED GRAPHITE FIBER
REINFORCED EPOXY COMPOSITE PANELS BONDED TOGETH~R
WITH A URETHANE ADHESIVE OR AN EPOXY ADHESI~E
-~ Lap Shear Lap Shear
Laser Energy Density, Strength, psi Strength, psi
Joules/cm2 per pulse_ Urethane Adhesive Epoxy Adhesive
0.0 2,800 1,845
1.4 3,020 --
5.5 3,010 ~,350
The data in Table ~IX indicate that the laser
treatment resulted in stronger bonds as indicated by the
lap shear test or both adhesives at all laser energy
densities per pulse studied but with a signficant
improvement in lap shear strength shown only for the
epoxy adhesive. Using the urethane adhesive without
- laser treatment shows most of the bond failure at the
interface between the adhesive and the panel, whereas
with laser treatment the bond failure occurred mostly
in the adhesive. Therefore, it is believed that the
use of a stronger urethane adhesive would have resulted
ln even higher lap shear test results than shown in
Table XIX.
.
~` Figure 6 shows electron photomicrographs of
the graphite fiber reinforced epoxy composite panels:
(a) before laser treatment at 400X ma~nification; (b)
after overlapping treatment with 1.4 joules/cm2 pulses
.
.
-51~
at 400X magnification; (c) after overlapping treatment
with 5.5 joules/cm2 pulses at 400X magniXication; and
(d) the same treatment as (b) above but at 3,000X
magnification.
Figure 6(a) sho~s the graphite fibers just under the
surface-of the -epoxy resin. Figure 6~b) shows the expo-
sure of the fibers, apparently due to vaporization of
the epoxy resin around the fibers. Figure 6~c) shows
in addition to the exposure of the fibers some apparent
fiber damage that nevertheless does not appear to detract
from bonding performance.
Example 22
- The system of Example 9 is used to laser -treat
panels of solvent cleaned mill finish cold rolled steel
~surface not ground). The treated panels are then
coated with American Cyanamid epoxy primer no. BR-127
and bonded togethex with 3M epoxy adhesive no.
AF-163-2 as in Example 7 and then wedge tested, see
Table XX.
20TABLE XX
,.
WEDGE TESTING OF LASER TREATED COLD ROLLED STEEL
PANELS BONDED TOGETHER WITH AN EPOXY ADHESIVE
Laser Energy Density, Initial Crack Crack
Joules/cm2 per PuIseLen~th, mm _Extension, mm
.
250.~ 26 24
0.8 13 2
1.4 12
2.7 12 2
5.5 ll 4
-52- lZ~3~7~
The data in Table XX indicate that the laser
treatments studied resulted in stronger and more dur-
able bonds as indicated by the wedge test relative to
no laser treatment.
- 5 Figure 7 shows electron photomicrographs of
the steel panels: (a) before laser treatmen~ at 400X;
(b) after treatment with overlapping 2.7 joules/cm2
pulses at 400X; (c) before laser trea~ment at 3,000X-
magnification; and (d) after treatment with overlapping
2.7 joules/cm2 pulses at 3,000X magnification. Figure
7(b) and (d) show apparent surface melting and roughen-
iny unlike the surface discussed in Example 13 and it
is believed that the roughening shown in Figure 7(b)
- and (d) may be partly responsible ~or the improved
performance shown in Table XXI with laser treatment.
Example 23
The system of Example 9 is used to treat
panels of solvent cleaned copper. In this example the
; panels are 1/8 inch thick instead of the more usual
1/16 inch thickness. The treated 1 x 4 inch panels are
then coated with American Cyanamid epoxy primer
no. BR-127 and bonded to an identically treated panel
with 3M epoxy adhesive no. AF-163-2 as in Example 7 and
then wedge tested, see Table XXI.
.
~53~ ~29~4~
TABLE XXI
WEDGE TESTING OF LASER TREATED COPPER
PANELS BONDED TOGETHER WIT~ AN EPOXY ADHESIVE
Laser Energy Density,Initial Crack Crack
5 -Joules/cm2 per pulseLen~th, mmExtension, mm
43 20
1.~ 47 3
2.7 30 8
5.5 47 12
- 10 The data in Table XXI show that optimum bond
strength as indicated by initial crack length rec~uires
a specific power range while bond durability as indi-
cated by crack extension is improved at all laser power
levels tested. In the wedge test, low yield strength
materials such as copper require thicker panels (than
the usual 1~16 inch thick panels) to prevent the panels
from simply curling back as the wedge is inserted between
~ the bonded panels.
:
Figure 8 shows 3,000X magnification electron
- 20 photomicrographs of the copper panels: (a) after laser
treatment with a single 1.4 joules/cm2 pulse; and (b)
after laser treatment with overlapping 1.4 joules/cmZ
pulses. Figure 8(a) and (b) show apparent surface
melting and roughening after laser treatment.
_54- ~Z~3~ ~
Exam le 24
The system of Example 9 is used to laser
treat panels of solvent cleaned pure molybdenum. The
treated panels are then coated with American Cyanamid
epoxy primer no. BR-127 and bonded together with 3M
epoxy adhesive no. AF-163-2 as in Examp:Le 7 and then
; wedge tested, see Table XXII.
TABLE XXII
WEDGF TESTING OF LASER TREATED MOLYBDENUM
PANELS BONDED TOGETHER WIT~ AN EPOXY ADHESIVE
Laser Energy Density,Initial Crack Crack
Joules/cm2 per pulseLenqth, mm Extension, mm
.
48 a.
1.4 23 a-
152.7 35 a-
a- Panels fell apart in the humidity cabinet.
The data in Table XXII indicate an optimum
in bond strength enhancement at an energy density of
1.4 ioules/cm2 per pulse under the conditions studied
as indicated by the initial crack length of the wedge
test.
Figure 9 shows 400X ma~nification electron
photomicrographs of the molybdenum panels: (a) before
laser treatment; and (b) after laser treatment with
1.4 joules/cm2 pulses. Figure 9(b) shows apparent
surface melting and roughening after laser trea~ment.
-55- ~ 29 3~ 3
Exam~le 25
The system of Example 9 is used to laser
treat panels of solvent cleaned pure tungsten. The
treated panels are then coated with American Cyanamid
S epoxy primer no. BR-127 and bonded together with 3M
epoxy adhesive no. AF-163-2 as in Example 7 and then
wedge tested, see Table XXIII. ' ~~
TABLE XXIII
WEDGE TESTING OF LASER TREATED TUNGSTEN
PANE1S BONDED TOGETHER WITH AN EPOXY ADHESIVE
Laser Energy Density,Initial Crack Crack
Joules/cm2 per PulseLength, mm Extension, mm
-
62 a.
2.7 47 a-
a- Panels fell apart in the h~midity cabinet.
The data in Table XX~II indicate almost
exactly a 25 percent reduction in initial crack length
after laser treatment.
Figure 10 shows 1,500X magnification electron
photomicrographs of the tungsten panels: (a) before
laser treatment; and ~b) after a single 2.7 joules/cm2
pulse. Figure 10(b) shows apparent surface melting and
roughening after laser treatment.
-56~ 3~7
Example 26
The system of Example g is used to treat
0O006 inch thick solvent cleaned aluminum foil panels.
The treated panels are bonded together with Dow Corning
Silastic~732 RTV adhesive cured at room temperature for
- 3 days at 70 percent relative humidity or with 3M
-Scotch Weld 2216 B/A epo~y adhesive cured for 1 hour at
room temperature and l.hour at 180F both containing
0.5 percent, 0.004 inch diameter glass spheres, and
then peel tested, see Table XXIV.
TABLE XXIV
- PEEL TESTING OF LASER TREATED
AL~MINUM FOIL BONDED TOGETHER WITH A
SILICONE ADHESIVE OR AN EPOXY ADHESIVE
Peel Strength Peel Strength
Pounds per Pounds per
Laser Energy Density, Linear Inch, Linear Inch,
Joules/cm2 per pulse Silicone Adhesive Epox~ A &esive
0.0 0.2 8.0
1.4 7.2 13.8
The data in Table XXIV show that laser treat-
ment significantly improves bond strength as indicated
by the peel test for both adhesives tested.
Example 27
The system of Example 9 i5 used to treat
0.055 inch thick panels of solvent cleaned Ti6A14
titanium alloy with overlapping 1.4 joules/cm2 pulses.
~-rf c~ C~ ~ ~Y1" ~ k
~2~3~
The panels are bonded together with Langley Research
Center Thermoplastic Polyimide primer and adhesive
resin and tested for lap shear strength~ The primer
coated panels are cured for 1 hour at 3~5F prior to
applying the adhesive to the panels which is heated for
105 hours at 625F under vacuum at 200 psi to bond the
panels together. For comparison, addi-t.ional panels are
chromic acid anodized according to airc:raft industry
standards and bonded together and tested as above. ~f
all the prior art treatments for titanium alloy, the
chromic acid anodizing process is o~ten preferred
despite waste disposal considerations for the spent
chromic acid bath liquors. The comparative data is
shown in Table XXV.
TABLE XXV
LAP SHEAR TESTING OF hASER TREATED TITANIUM
PANELS AND CHROMIC ACID ANODIZED TITANIUM PANELS
BONDED TOGETHER WITH A THERMOPLASTIC POLYIMIDE ADHESIVE
Treatment Lap Shear Strenqth, psi
Laser 4,640
Anodized 4,430
The data in Table XXV show that the laser
treatment provides a stronger bond than the anodizing
treatment but not a significantly stronger bond.
Importantly, the data show that the present invention
approximately equals the best of the prior treatments
for titanium alloy with regard to lap shear testing
- without a problem of waste disposal of spent bath liquors.
~ . .
-58~ 3 ~70
The system of Example 1 is used to laser
treat panels of oiled cold rolled steel. The panels
are bonded together with a urethane adhesive as in
Example 1 and then tested for la~ shear strength, see
. Table XXVI.
- TABLE XXVI
.
LAP SHEAR TESTING OF LASER TREATED
OILY COLD ROLLED STEEL PANELS
BONDED TOGETHER WITH A URETHANE ADHESIVE
Laser Energy Density, Lap Shear
- Joules/cm2 per Pulse Strength, psi
2.,200
0.8 2,500
1.1 3,600
1.4 4,200
1.6 5,500
1.9 4,200
2.2 5,200
2.7 5,000
3-3 4,200
4.0 3,200
.
The data in Table XXVI indicate that above a
laser power density of about 1 joule/cmZ, a significant
improvement in bond strength is observed with an apparent
optimum in improvement around 2 joules/cm2. The lap
- shear strength of solvent cleaned and nonlaser treated
' :,
.~
-59-
~lZ~39,t7~
panels is about 4,500 psi. Therefore, tne improvements
in bond strength shown in Table XXVI are believed to be
at least partly attributed to a vaporization of the oil
from the surface of t~e steel with the laser treatment.
Example 2g
- - The laser of Exam~le 28 is replaced with a-
100 watt (maximum) CO2 continuous wave :Laser. The
cylindri~al lens is removed from the system and the -
laser beam is instead focu~ed to a spot approximately
OoO1 inches in diameter. The translation stage is
adjusted to move the sample to be treated at a velocity
of about 5 inches per second. The laser is adjusted
for 50 watt output and thus the energy density of the
- laser beam directed to any one spot on the sample to be
treated is about 160 joules/cm2. The duration of
exposure for any one spot to be treated is calculated
to be about 2 milliseconds.
The above system-is used to laser treat
1 x 4 x 1/8 inch panels of plate glass with overlapping
coverage of the treated areas (about 150 percent cover-
age). The laser treated panels are bonded together with
Hardman Kalex "Blue Urethane" two-part urethane adhesive
with 0.5 percent, 0.020 inch diameter glass beads added
to insure an evenly spaced "glue line." This adhesive
is recommended by the manufacturer for use with glass
and is rated as having a very good resistance to water.
The bonded panels are immersed into boiling
water and examined periodically. After 24 hours of
this exposure, none of the laser treated and bonded
panels fell apart and could not be pulled apart by
hand. For comparison, nonlaser treated panels bonded
~* If aJe ~1~k
~60- 1Z~3~ ~
as a~ove fell apart in the boiling water after 0.6 to 2
hours.
Example 30
The system of Example 29 is used to laser
treat 1 x 4 x 1/4 inch panels of plate glass. A
stainless steel, General Motors approved automotive
interior windshield mirror mount is bonded to the laser
~ treated glass surfac~ with ~ardman "Orange" two-part
epoxy adhesive (which is recommended by the manufac-
turer for bonding stainless steel to glass and is rated
as having good water resistance) premixed with glass
beads as in Example 29. A 1 x 4 x 1/16 inch carbon
steel panel is bonded to the other side of the glass
- panel with the same adhesive as above.
The bonded assembly above is immersed into
boiling water for 4.5 hours and then subjected to the
industry specified torque test, i.e., a torque wrench
is attached to the mirror mount and the torque required
to peel the mirror mount away from the glass is measured.
The mount could not be peeled away from the glass
without actually breaking the glass (at about 150 inch
pounds of torque) with failure occurring in the glass
.itself with no failure at ~he a & esive/glass interface.
For comparison, a nonlaser treated glass panel was
bonded, boiled and tested as above. The mirror mount
cleanly peeled away from the glass at about 70 inch
pounds of torque.
Figure 11 shows electron photomicrographs of
the lasex treated glass panels: (a) before laser
treatment at 400X magnification; ~b) after laser treat-
ment at lOOX magnification; (c) before laser treatment
~ rrc~ Je YY~a rk
, .
-61- 1293~ ~
at 3,000X magnific2tion; and (d) after laser treatme~t
at 4,000X magnification. Figure ll(b) and (d) show
apparent surface roughening.
Example 31
The system of Example 29 is used to laser
treat 1 ~ 4 x ;/8 inch panels of Ashland Chemical Co.- -
"phase alpha" sheet molding compound and the panels are
bonded together with ~ardman ~'blue" acrylic adhesive
which is recommended by the manufacturer for bonding
fiber reinforced plastics. The adhesive is premixed
with 0.5 percent, 0.02 inch (O.51 mm) diameter glass
beads and the adhesive is cured for 1 hour at room
temperature and then at 250F ~121C) for 1 hour. The
bonded panels are tested for lap shear strength which
averages 450 pounds per square inch (3.1 MPa) ancl the
panel itself breaks without bond failure. Panels not
laser treated but bonded as above have lap shear
strengths averaging 160 pounds per square inch ~1.1
MPa) and the bond fails at the interface between the
adhesive and the panel.
Exam~le 32
The system of Example 9 is used to laser
; txeat panels of 2024-T3 aluminum at a laser energy
density of 1.4 joules/cm2 per pulse. The panels are
bonded together (1/2 x 1 inch [12 x 25 mm] overlap of
the ends of the panels as in a lap shear test) with -
Hardman "blue/beige" two-part urethane adhesive, con-
taining 0.5 percent, 0.004 inch (0.1 mm) diameter glass
~- beads cured as in Example 31. The bonded panels are
then impact tested by General Motors side impact test
~modified from the ASTM D-256-81 impact test) wherein
the pendulum impact head strikes at right angles to the
~ ,
.
-62~ lZ~347~
bond. The bonded panels show an average impact strength
of more than 5 foot pounds. Nonlaser treated panels
bonded as above show an average impact strength of
0.4 foot pounds.
Example 33
The system of Example 9 is used to laser
treat 1/2 x 4 x 0.022 inch panels of gold clad Kovar~M
alloy at a la~er energy density of 1.4 Joules/cm2 per
pulse. The gold thickness is 0.0025 inches (24 carat
gold). The laser treated gold surface of the panels are
bonded together with Dow Corning Sila~tic RTV-732
silicone adhesive containing 0.5 percent, 0.020 inch
diameter glass spheres and allowed to cure for 2 days at
room temperature and peel tested at a crosshead speed o~
2 inche~ per minute. The peel strength is 5.5 pounds
per linear inch.
Without laser treatment but bonding as
2~ above, the peel strength is 2.0 pounds per linear inch.
Example 34 ~
A Gentec Model DD-250 TEA C02 laser is
focused with a spherical ZnSe lens of focal length
100 mm to achieve the energy density indicated in
Table XXVII. The pulse length of this laser has a peak
including approximately 50 percent of the energy of
0.1 microsecond in length. The remainder of the energy
is included in a tail approximately 1.0 microsecond in
length. The results in Table XXVII indicate no
improvement in initial crack, durability, or mode of
failure with laser treatment approximately 5 Joules/cm2
per pulse.
32,850A-F -62-
,~
, ~
~.~.,
-
-63- ~293~
TABLE XXVII
Laser Energy Density, Initial Crack Crack
Material Joules/cm2 p~r pulse L~ mm Extension, mm
Aluminum 0 36.1 3405
5.2 3~.8 42
_____________________ _______ __ _______________________
Cold 0 40 8.5
Rolled 5.2 40 7.6
Steel
lO The following will reveal the gist of the
present invention that others can, by applying current
knowledge, be readily adapted for various applications
without admitting features that, from the standpoint of
prior art, fairly constitute essential characteristics
of the generic and specific aspects of this contribution
to the art and, therefore, such adaptions should and
are intended to be comprehended within the meaning and
range or equivalence of the appended claims.