Note: Descriptions are shown in the official language in which they were submitted.
~ llL.ll~lION O~ YLAS~IA
DACKGROlJN~ OF TIIE INVbN~IuN
The present invelltion relates to the filtration
of macromolecules from fluids, and more particularly to
~he removal of undesirable macronnolecules from plasr~a
solutions, by ehe process termed thermoEiltration.
The separation of undesirable solutes from
blood plasma through plasma filtration is a known method
of treating diseases, wherein such diseases have in
common undesirable elevated leYels of plasma solutes,
such as toxins, excessive antibodies, and other
metabolic factors. Successful treatment o such
diseases involves the renloval of the undesirable plasnla
solutes from the blood plasma by membrane filtration.
Various methods of plasma filtration, including
cascade filtration, double filtration, arld
cryofiltration have been developed. However, these
methods contain a number of ulldesirable characteristics
which lioli~ their usage.
Applicants have Iloted a nuolber of parameters
associated with performance, including module design,
membrane properties, plaslna composition, and plasma and
filtration t~mperature. Characteristics o~ the module
which affect flow dynamics and in turn performance
include, area, fluid and film dimensions, as well as
properties of the separatin~ menlbrane, including polynlcr
type and microstructural features such as pore size,
pore tortuosity, pore len~th, and pore number.
Variations in the plasma's composition also
affects its filtration. Plasllla frool patients with
;9a~
--2-
diferent disease stutes or with differin~ macromolecule
contents have di~ferent filtrati~n characteristics.
Manipula~ion uf the plasloa to effect changes in pH or
elee~rl)lyte composition and the addition of
anticoagulants such as hep~rin or other
macromolecule-aggre~ating additives such as polyethylene
glycol will effec~ filtraLion performance. Cenerally
such manipulations are carried out for the purpose of
augmenting the separation, by macromolecule a~gregation
or precipitation, of one or a ~roup of solutes from the
plasma .
Because of the number of paral~eters afecting
filtration performance, temperature selection and its
control has been demonstrated to be a key parameter in
fluid separation. In order to au~ment the selective
removal in a particular macromolecular range, it is
extrelnely important to operate within the proper
temperature range. In this respect, significant
differences have been noted for comparable conditions of
filtration (similar oper~ting flows, modular types a~d
plasma types) between cascade and double filtration,
which operate at near ambient temperatures, and
cryofiltration, which operates at temperatures below a
set physiologic temperature.
Temperature control offers many advantages over
the other parameters in that temperature control is the
easiest controlled physical parameter, and that
temperature control Inay be co~lbined with the use of
various complexing a~ents to increase the sensitivity of
macromolecule removal. A specific exalnple of this can
be shown in the case of cryofiltration, where the
addltion o~ heparin aids irl the formation of cryogel by
forming complexes wi~h fibronectirl and fibrino~en at
temperatures below 25C.
935~
-3-
Filtratior1 at sub physiologic telnperaturt- is
effective for the removal of plasma constitue~nts silllilar
in size but diÇfering in temperature sensitivity. A
number of autoimmune diseases can be treated in this
~ashion, as has been described in the literatureO The
effectiYeness of the treatmen~ is attributed to the
formation and removal of cryogel, which is composed of
high concentrations of the macromolecules associated
with autoimmune disease sta~es. Thus, the separation in
cryofiltraLion is not based on molecular size a~
physiologic temperature but on molecular size at reduced
temperatures.
Ilowever, opera~ion at a reduced tempf~ra~ure
can, in fact, reduce the selectivity of molecule
separation when size differences are great since
aggregation or complex formation of small molecules may
also occur at reduced temperatures. Therefore, for
separation based on size diferences at physiologic
temperatures, i~ may be more advantageo-ls to avoid
cryogel formation.
Accor~ingly, it is arl object of the present
invention to provide an improved means of removing
undesirable macromolecules from fluids in an effective
and efficient manner.
SU~1hlARY OP THE INVENTION
~ . ._
In one aspect, the present invention relates to
a method of selectively removing macronlolecules from a
plasma solution including the steps of providing a
plasma solution containing the macromolecules to be
removed, heating and/or maintaining the plasma at a
temperature near or above normal body temperature but
Z8
-4-
below the boilin~ point oL the plasrllu solution, and
filtering the warmed plasma solution while at a
temperature near or above normal body tenlperature but
below the boiling point with a membrane filter to remove
more selectively rnacromoleeules from the plasma
solution. In addition, by heating the plasma solution,
various macromolecules present within the plasma
solution may ~ecome inactivate~ or denatured, aiding in
their selective removal throu~h plasma filtration.
In another aspect, the present invention
concerns a method of selectively removing macromolecules
from a plasrna solution including the steps o~ securing a
blood flow from a specimen, separating the blood flow
into a concentrated cellular element strealn and a plasma
stream containing the macromolecules to be removed,
heating or maintaining the plasma stream containing
macromolecules to be rernoved to a temperature near or
above normal body temperature but below its boiling
point, filtering the warmed plasma stream while at a
temperature near or above normal body temperature but
below its boiling point with a Inembrane filter to remove
more selectively macromolecules from the plasma solution
to form a filtered plasma stream, combining the filtered
plasma stream and said cellular element stream to form a
processed plasma stream, and cooling and/or returning
the processed stream plasma to the specimen.
In still another aspect, the pIesent invention
concerns a l~ethod of controlling conditions o~
lipoproteirl abnormalities in a living organism by
selectively removing nlacrolrlolecules from a plasma
solution including the step of securing a blood flow
from a living organism, separating the blood flow into a
concentrated sellular element stream and a plasma stream
1;~93g~
- s-
containing ~he macrolllolLcules to be relnoved, heating or
maintaining the plasma strealn containing macromolecules
to be removed to a ~emperature near or above normal
body temperature but below its boiling point, filtering
the heated plasma streanl while at a temperature near or
above normal body temperature but below its boiling
point with a membrane filter to remove more selectively
macromolecules from the plasma solution to form a
filtered plasma stream, sombinin~ the filtered plasma
stream and said cellular element strea~ to for~ a
processed plasma stream, and cooling and/or returning
the processed plas~a stream to the living organism.
In still another aspect, the present invention
concerns an apparatus for relnoving nlore selectively
macromolecules from a plasma solution co~prising a means
of receiving and dividing a plasma containing solution
containing macromolecules procured from a speciQen into
a concentrated cellular element stream and a plasma
stream, a means of receiving, heatin~ and/or maintaining
the plasma strea~ to a temperature near or above normal
body temperature but below its boiling point, a means of
receiving and filtering the heated plasma stream to
remove selective macromolecules, a means for receiving
the filtered plasma strea~ from the filter ~eans and for
receiving the concentrated cellular element stream and
combining the streams to form a processed strea~
substantially free of the macromolecules intended to be
remove and a means of receiving and/or cooling the
combination stream to normal body temperature for
returning said fluid to the specimen~
3~2~3
-6--
BRIF~ DESCRIPTION OF THE DRAWlNGS
The following is a brief description of the
drawings which are pr~sented for the purpose of
illustrating the invention and not for the purpose of
limiting same.
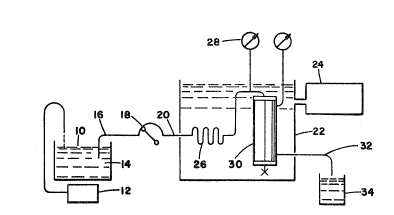
Figure 1 shows the circuit used in in vitro
filtration;
Figure 2 shows the extracorporeal circuit used
in ex vivo or clinical filtration;
Figure 3 shows the amount of cholesterol
removed at varying temperatures for in vitro filtration;
Figure 4 shows the mean sieving coefficients
with various kinds of plasma. ~Kuraray Eval 4A module;
In vitro at 37C);
Figure 5 shows the particle size distribution
of the lipoprotein for the FHC plasma and the dog plasma
with various cholesterol levels; and
Figure 6 shows the pos~ treatment recovery in a
dog of LDL-VLDL and HDL cholesterol.
DETAILED DESCRIPTION OF THE INVENTION
Applicant has discovered that thermofiltration
offers many advantages over the conventionally known
methods of plasma filtration. Thermofiltration is the
removal of macromolecules from plasma by warming the
plasma to a selective temperature near or above the
normal pllysiologic temperature but not above its boiling
point and filtering the warmed plasma with a membrane
filter having a porosity that will remove the
~~3~
-7-
desired macromolecules. The critical a~lvantages
demonstrate~ by thermoEiltration include the abili~y to
filter a mucll greater volume of plasma at higher
tempera~ures because plasma exposed to the higher
temperature has less of a tendency to form deposits o
undesirable solutes on the membrane media and the more
selective removal of macromolecules based upon
differences in sieving coefficients at higher
temperatures.
Evaluations of multiple membrane filters or
plasma filtration based on filter material, pore size,
~` and structure indica~e that the Kuraray Eva ~4A module
(ethylene and vinyl alcohol copolymer, Kuraray Co.,
Japan) and other modules of similar properties are
particularly well suited for plasma solute fractionation
by thermofiltration. Other suitable filters include
those which utilize filter media consisting of
polysulfone, polypropylene, nylon, polyester, cellulose
acetate~ collagen and the like~
Applicants have demonstrated that sieving
coefficients of some macromolecules are significantly
higher at 37C and 42C than at 25C Eor the Kuraray
Eval 4A module. (Table I below). Particularly
noteworthy are the higher sieving of HDL cholesterol,
IgG, ~ibringen, total protein, and albumin at 37 to
42C. In addition, as a result of the reduction of
cryogel formation at these higher temperatures, a much
greater volume of plasma can be filtered. This is
because at near or above normal physiologic temperatures
solute aggregatiorl is kept to a minimulll, and separation
is due to the molecular si~e differences of the solutes
and not the aggregate compositions.
rra~e mar~
-8--
Table I: Sieving coefficents for various macramolecules.
_ . , . . _ .
Volume
TP Alb Glb ~ib I~G IgA IgM PIocessed (ml)
25C 0.61 0.71 0,48 <0.06 0.36 0.21 0.15 1,000
37C 0.74 ~.82 0.59 0.13 0.55 0.51 0.17 1,000~3,000
42C 0.~ 0.86 0.59 0.26 0.57 0.46 0.18 1,000~3,000
Volume
T Chol LDL HDL TG Processed (ml)
25C 0.07 0.03 0.71 0.13 <11000
37UC 0.06 0.02 0.84 ~.11 1,000~3,000
42C 0.04 0.03 0.97 0.13 1,000~3,000
All macromolecules are from the same plasma source. ~eparin
dosage: 1,000 U/L.
TP: total protein; Alb: albumin; Glb: globulin; Fib:
fibrinogen; T Chol: total cholesteroli LDL: low density
lipoprotein; HDL: high density lipoprotein; TG: triglycerides.
Moreover, thermofiltration is an effective
method of removing pyroglobulins from plasma solutions.
Pyroglobulins are serum globulins that precipitate or
gel upon heating. Normally, pyroglobulins are not found
in serum of norlnal individuals. Rather they are readily
associated with macroglobulinemia and other
lymphoproliferative or multiple myeloma disorders.
93'~
g
HeatinB a serum col~taining pyroglobulins to 55-56C
results in gel forma~ioll, wllicll can be cf~ectively
removed froln the serum through plasma ~iltration.
Similarly, proteins and other immunogloblJlins which
ef~ectively denature or coagula~e upon heating may also
be selectively removed throu~h ther~ofiltration.
As a consequence of the above advan~ages of
thermofiltration over conventionally known methods of
plasma separation, ~hermofiltration can be used to
selectively remove pathologic macromolecules from blood
in on-line and off-line plasma treatments, while at the
same time allowing for the passage or return of
beneficial plasma pro~eins. The advantage of this type
of treatment can be clearly shown in the therapeutic
control of cholesterol.
Cholesterol has been determined to be an
impor~ant component of arterial plague formation in
artherosclerosis as well as in hypercholosterole~ia.
Cholesterol circulates in the blood liJIked to large
protein molecules. One form of cholesterol carrying
protein, called low-density lipoprotein (LDL~ 9 is known
to promote atherosclerosis. About two-thirds or more of
total blood cholesterol is transported in LDL. Another
orm, called high-density lipoproteiil (HDL), is known to
be protective against the disease process. Therefore,
the selective removal of LDI. and maintenance of HDL is
important in the treatment of atherosclerosis and the
therapeutic control of hypercholesterolemia.
Recently, plasma exchange has been utillzed for
the removal of plasma and its replacement with
electrolyte solutions and/or plasma products in familial
hypercholesterolemia patients. However, these methods
~LZ93~
-10-
are non-selective and remov~ proportionately low density
lipoproteins (I.DI.) with high-density lipoproteins (IIDL)
and other plasma proteins which are beneficial to the
patient. In addition, several methods have been studied
for the selective remo~al oE LDL, including anti-
LDL-antibody Sepharose columns, and combinations of
heparin precipitation and bicarbonate dialysis, however,
meIDbrane filtration offers many advantages over these
methods in terms of biocompatibility and treat~ent cost
effectiveness.
The selective removal of LDL cholesterol from
plasma by thermofiltration can be demonstrated under
both in vitro and ex vivo conditions. In vitro pertains
to conditions of experim~ntation i~ a laboratory
setting, whereas ex vivo pertains to conditions of
extracorporeal circulation with living organisms.
I. In Vitro ~:iltration
. .
Various types of plasma are used in vitro to
evaluate the temperature effect on selective cholesterol
removal. The in vitro filtration tes~s are carried out
with the various types of plasma at varying temperatures
according to the extracorporeal circuit demonstrated in
Figure 1.
In Figure 1, oIle unit per ml. heparin (heparin
sodium injection, Invenex Lab., OH) is added to plasma
pool 10 wherein the plasma is kept at approximately 37C
by h~at controller 12 and uIa~netic stirrer 14. Plasma
is drawn from plasma pool 10 into line 16 and fed into
plasma pump 18 of a plasma flow rate of l5ml/min. The
plasma is pumped from plasma punIp 18 into line 20 and
then into water bath 22 which is controlled by
thermo-re~ulater 24. Within water bath 22, the plasma
TrAde ~a~
~33~
- 1 1
passes throlJgh h~at exc~langer 26 an(l passes by pressure
gauge 28 into ~ilter 30, where the l.PI. ctlolesterol is
retained and the ~II)L cholesterol and albumin
substantially pass through. From filter 30, the
filtered plasma minus LDL cholesterol flows through line
32 into filtrate collection pan 34.
The following specific examples further
illustra~es the practice of the present invention.
EXAMPLE I
Familial type II hypercholes~erolemic plasma
(FHC) was procured by repeated centrifugal plasma
exchange. The in vitro filtration tests were carried
out with the PHC plasma and the Kuraray EVAL 4A membrane
filter ae temperatures of 4, 25~ 37, 42 and 47C,
respectively, according to the extracorporeal circuit
demonstrated in Figure 1 and as described above. The
mean sieving coefficients (sc~, the plasma volume
processed, and the ~otal amount of LDL and llDL
cholesterol were determined at the above temperatures by
the following calculations.
Concentration in ~iltrate
sieving coefficient (sc) - (mg/dl)
Concentration in filter
inlet ~mg/dl~
wherein a sieving coefficient oE
0.9 to 1 indicates little or no
separation or removal of the
macromolecule from plasma, and a
sieving coefficient of O to 0.1
indicstes substantially total
renloval of macromolecules from
plasma.
~3~3~3
-12-
Removal Alnount (g) ~ Concentration in plasma pool (g/~l)
X(l-sc) x Processe~ Volume (~1)
RL.SUL'!'S
Table Il outlines the volume processe~ and the
mean sieving coefficients for total cholesterol, 5IDL
cholesterol, LDL cholesterol, and albumin at varyin~
temperatures. l`he results ~ndicated that greater than
85~ of the total cholesterol and 90~ of LDL cholesterol
were removed while oYer 70~ of albuolin and 6a~ of HDL
cholesterol were passed throu~h ~he filter. The sieving
coefficients of tlDL cholesterol and albumin increased
with increasing temperature while LDL choles~erol was
independent of temperature.
ABL~ ~I. Mean sieving coefficients and plasma volumes
processed at varying temperatures; in vitro
filtration test of ~uraray ~val 4A (1.0 M
surface area) using fanlilial hypercholestero-
l~mic plasma.
Processed M n Sieving Coefficients
Temp Volume l'otal H~L LDL
(C) (ml) Chol Chol Chol Alb
_ . . .
4 365 0.10 0.5~ O.U7 0.72
1135 0.11 0.76 0.10 0.84
37 1780 0.12 0.~7 0.~6 0.81
42 2150 0.16 0.72 0.08 0.91
~7 2350 0.14 0.79 0.0~ 0.9q
~.;2g3~
Figure 3 denlonstrates that cholesterol removal
differs at varying temperatures. A~ 37 to 42C, the
removed LnL cholesterol amollnt is largest (4.5~ module),
while IIDL cholesterol is below O.lg. Removal amount per
module was limited by the maxinluln Pt~ 0~ mln tlg)
permitted.
Conclusion: Th~rnlofiltration is highly
effective in selectively removing large quantities of
LDL cholesterol from plasma while retaining lar~e
quantities of useful HDL cholesterol and albumin undér
in vitro conditions. The in vitro membrane filtration
of FtlC plasma with the EVAL 4A filter permits near
complete rejection of LDL cholesterol with high sieving
or retainment of HDL cholesterol and albumin. The
sieving coefficients of HDL cholesterol ~nd albumin
increased with increasing temperature, while the sieving
coefficient of LDL cholesterol was near comple~e
rejection at all temperatures. Thus, membrane
filtration near or above physiologic temperatures, i.e.,
thermofiltration, improves the selectivity of LDL
cholesterol removal over that of HDL cholesterol and
albumin.
Moreover, thermo~iltration also permits higher
plaslna volumes to be processed. This is a result of the
reduction of cryogel formation and less removal of
solutes not intended to be removed at the elevated
temperatures. Also, higher volumes of plasma are
processed and lar~er quantitics of cholesterol are
removed per unit module.
-14-
IX~MPI~
In vitro module ~iltration ~ests were carricd
out wi~h normal human plasma (NHP) and sclerosin~
cholangitis plasma (SCP) usin~ the Eval 4A (copolymer of
ethylene and vinyl alcohol; surface area 2~0 ~2) at
4, 25, 37, 42, 47 and 52~C, respectively, according
to the extracorporeal circuit demonstrated in Figure 1
and as described above. The NHP was procured by
filtration at 37C of outdated citrated plasma using the
Toray PS-05 plasma separator (polymethylmethacrylate;
surface area O.5m2; Toray Industries, Japan). The SCY
was procured by membrane plasma exchange. The SCP
differed from the NH~ in that the SCP had 1.5 fold
higher fibrinogen and four fold higher LDL cilolesterol
concentrations with similar levels of albumin and
sntithrombin III when compared to NHP.
All filtration tests were carried out with a
plasola flow of 30 ml/min. Changes in inlet pressure
were monitored as a function of the transmembrane
pressure and reflect membrane plugging.
Samples obtained pre and post filtra~ion were
analyzed for various biochemical solutes including
albu~in (Alb), fibrinogen (Fib), total cholesterol (T
Chol), LDL cholesterol (LDL Chol), HD~ Cholesterol (HDL
Chol), antithrombin III (AT III) and heparin. Alb was
measured with an autoanalyzer (SMA-II, Tech~icon
Instrument Co., Tarrytown, NY) by the bromocresol green
method. Fib was measured by the Fibrosystem (B~L,
Ockeysville, MD). T chol and triglycerides were
measured with an autoanalyzer (AA II, Technicon Inst.
Co.) using the cholesterol oxidase-peroxidase enzymatic
- l s-
method. L~L chol was culculated as: 1' chol - ~IDL chol -
1/5 triglycerides. II~L chol was oleasLIIe(l by th~
dextran-sll1fato-Mg2~ precipitation metilo~l.
Antithrombin III and heparin were measured by the
Protopath antithrombin III and heparin synthetic
substrate assay (American Dade, Miami, FL).
~SULTS
_
Pigures 4A and 4B show the volumes processed
and the sieving coefficisllts for the filtration of NHP
and SCP over the temperature range of 4 to 52C, In
both plasmas hi8her volumes were processed as the
temperature was increased from 4 to 52C. ~owever, the
volume processed did not increase at temperatures over
42C and dropped significantly at 52C. Sievings for
Alb ~ncreased with increasing temperature from 4 to
42C and thereafter also dropped. A similar tendency
can be seen in ~ibrinogen. Significant increases of IIDL
chol sieving were observed in the temperature range of
4 to 42C where no major changes in LDL chol were
noted. HDL chol sieving also dropped at 52C. Total
removal of LDL chol and removal raeios of HDL chol and
Alb to LDL chol were liste(l in Table III. Maximum
removal amounts of LDI. chol and minimal removal of HDL
chol an~ Alb versus LDL chol removal were obtained at
42C,
3~;~8
-16-
TABLE III: Total removal an~ ratios of ~IDI./LDL and
albumin/LDL choles~erol removal using NHP an(l SCP
patients plasma at varying temperatures.
Plasma Temp ~ 4 25 37 42 47 52
NHP LDL Chol (mg) 130 347 932 1854 1549 198
.. _
HDL chol 0.95 0.76 0,45 0.190.23 0.48
LDL chol
Alb 31.1 18.2 10 5 5.66.5 14~1
_ _ ~ __ .
LDL chol
. . _ . . . _
SCP LDL chol (mg) 887 1458 2368 3130 2790 1216
_ _ _ _ _ _ _ _ _
HDL chol 0.0160.021 0.008 0.001 0.007O.OS
LDL ohol
Alb 6.2 5.6 ~,~ 2.2 3.24.8
LDL chol
. . .HP - normal llulllan plasma SCP ~ sclerosing chol~ngitis
plasma
Conclusion: These results suggest that operation at
near physiologic tempeIature is promising for prevention
~35~
-17-
of heparin induced aggregation which occurs below
physiologic temperature and filter p~ugging caused by
these deposits when smaller pore size membranes are used
to separate molecules. As shown in Figure ~A and 4B,
filtration above physiologic temperature ~up to 47C)
produces higher volumes processed and a higher passage
of albumin and }IDL chol. These results indicate tha~
plasma filtration near or above 37C, thermofiltration,
is promising for clinical use in the separation of
plasma solutes based on size differences (i.e., LDL
selective separation vs. HDL sieving).
II. Ex Vivo Filtration
Ex vivo filtration is the continuous on-line
filtering of plasma with living organisms. The ex vivo
filtration tests were carried out at 37C according to
the extracorporeal circuit demonstrated in Figure 2.
Referring to Figure 2, blood is drawn from the
artery of a living organism illtO line 36 and fed into a
pump 38 from which it is pumped into a line 40, passing
by pressure gauge 42 and into plasma separator 44, where
the plasma and blood cellular elements are separated.
The concentrated blood cellular elements are fed into
line 46, while the plasma is fed into line 48. From
line 48, the plasma flows through pump S0 to line 52
where it enters water bath 54 controlled by
thermo-regulator 56. Within water bath 54, the plasma
passes through heat exchanger 58 and by pressure gauge
60 into filter 62, where the LDL cholesterol is
subs~antially retained and the HDL cholesterol, albumill,
and other low molecular weight macromolecules
subs~antially pass through.
9~
Prom ~ilteI 62, the ~iltere~! plaslna which is
essentially deficient in LDL cholesterol flows through
line 64 an~ is in~ermixe(l witll the blood cellular
elements of line 46. The mixture tllen is either cooled
to body temperature in heat exchanger 66, or passed into
line 68 where it passes pressure gauge 70 and is fed
into line 72 and returned to the vein of a living
organism in a continuous process.
Ihe following specific examples further
illustrate the practice of the present invention.
Example 3
As a result of the si~ilarity of lipoproteins
and its suitable body size for on-line filtration, a
hypercholestrol dog was the model chosen for ex vivo
filtration. The thyroidectomized/diet canine model is
well established and has been studied extensively.
Using this model, three different choles~erol level
ran~es up to 600mg/dl (normal, 120 mg/dl) were evaluated.
Arterio-venous (AV) fistulae were constructed
in two healthy male mongrel dogs weighing from 24 to 30
kg. As a control, dogs were maintained with a normal
diet (lab Canine Diet 5006; Lab Chow, St. Louis, M0).
As a middle cholesterol concentration model, the same
dog was maintàined with the same diet a~ter surgical
thyroidectomy. As a high cholesterol concentration
model, thè dog was maintained with a special diet that
consisted of the normàl meal with 4% hydrogenated
coconut oil and 0.75% cholic acid added (TD 75337,
Taklad, Madison, WI) after thyroidectomy.
Ex vivo filtration tests were carried out in
the dogs at different cholesterol levels under general
12~392B
_19_
anesthesia with nitrous oxide gas and nelnbutal injection
~Nembutal sodium sollltion, Abbott Lab, I~.) An AV
fistula was used for blood access and Z00 unit/kg
heparin was injected as an anticoagulant. Plasma was
separate~ in the on^line extracorporeal system using a
membrane plasma separa~or ~Mitsubishi 60TW,
polyethelene, Mitsubishi Rayon Co , Japan). The
separated plasma was filtered using the same method as
in vitro filtration (at 37C) and the filtered plasma
was then recombined and returned to the dog (Fig. 3).
Blood and plasma flows employed were 60 and 15 ml/min,
respectively. One calculated plasma volume was
filtered. ~ight hundred to 1000 ml of lactated Ringer's
solution was infused intr~venously during the
extracorporeal circulation.
Samples were drawn simultaneously from the
arterial and plasma lines of the filter inlet and outlet
when one half and one volumc were processed, and pre-
and post-treatment. At one hour and 1, 3, 7, 14 and 21
days post treat~ent, bloo~ samples were taken following
14 eo 16 hours fasting. Dogs were fed the same diet as
before filtration ad libitum. Biochemical measurements
included cholesterol and tri~lyceride (automatic
analysis AA II, Technicon Instrument Co., NY), HDL
cholesterol (Dextran Sulfate MgCl~ precipit~tion
procedure), in addition to routine serum multiple
analysis (SMA-II system, Technicon Instrument Co.) and
hematological analysis (autolnated cell counter, Coulter
Electronics Inc., FL).
The LDL cholesterol concentration of huulan
plasma was calculated using the equation: total
cholesterol - IIDL cholesterol - l/S triglyceride. The
LDL-VLDL choles~erol concentration in dogs was
calculated as follows: total cholesterol HDL
~ 39~
~20-
cholesterol. 'rhe lipoprotei~ fractioIls wcre prepared
~or analysis by preparutive ultracentlifuge (human LDI.;
1.006'd ~1.063, human HI)L; 1.063 'd ~1.21, canine
LDL-VLDL; d ~1.06~, canine }IDL; 1.087' d <1.21~, where d
~ density. Lipoprotein particle sizes were measured
using negative straining electron ~icrographs. These
fractions oE canine lipoproteins are no~ homogeneous but
are comparable ~o the fractions obtained by
precipitation me~hods.
R}.SULT
Table IV outlines choles~erol concen~rations at
the various stages of the dog model. Total cholesterol,
particularly LDL-VLD~ cholesterol, was increased and the
ratio of L~L-VLDL cholesterol to ~IDL cholesterol was
increased over 10 times. Albumin showed no significant
change. On-line plasma filtration was carried out at
each cholesterol level. Transmembrane pressure (Ptm)
of the plasma separator was stable throughout the
procedure. Sieving coefficients of albumin and total
cholesterol and other macromolecules were over 95~. ~he
Ptm of the macromolecule ~ilter increased gradually
during the perfusion. The Pt~ values at one plasma
volume processed ranged from 10 to 256 mmHg.
Significant differences in Ptm changes were not
dependent on cholesterol concentrations.
3~12~
-21-
ABLE IV. Cholesterol an~l albumin levels on canine
hypercholesterolemic model;
CH0LESTEROL_LEVEL (~ /dl) Albumin level
TOTAL l~l)L LDL-VLDL
1; 137 ~ 2~115 ~ 15 21 ~ 9 3.00 ~ 6 (~3~
II; 395 ~ 30181 ~ 13 214 ~ 17 3.25 ~ 0.07 (n=2)
III; 600 + 14 219 ~ 30 382 16 3.25 ~ 0.21 (n~2)
. . .
I; Normal dog with normal diet
II; Thyroidectomized dog with normal diet
III; Thyroidectomized dog with 4~ hydrogenated
coconut oil and 0.75~ cholic acid addition
on norJnal diet
Table V outlines mean sieving coefficients at
the varying cholesterol level. LDL-VLDL cholesterol was
highly rejected by the plas~a filter, whereas albumin
and HDL cholesterol showed high sieving. The sc of the
LDL-VLDL cholesterol ~ecreased with increasing
cholesterol.
3~ ~
ABLE V. Mean sievin~ coefficients of macrolllolecule
filter; ex vivo on~ e fiitration test (37C)
of Kuraray Eval 4A on differer1t chol~st~rol
levels.
CHOLESTE~OL
TO~AL tlDL LDL-YLDL AL~UMIN
I; 0.60~0.lO 0.63~0.09 0,3~0.07 0.~8~0.07 ~n~3)
II; 0.42~0,08 0.61*0.ll 0,32~0.0l 0.95~0.06 (n=2)
III; 0.34~0.05 0.59~0.09 0.l9~0.04 0.93~0.04 (n=2)
Mean + Standard Deviation
-
Pigure 5 shows the particle size distribution
of the lipoprotein for the FHC plas~a and the dog
plasmas with various cholesterol levels. The size
difference between the HDL and LD~ of FllC plasma was
grea~er than that of ~he dog plasmas. The particle
diameters and ~eviation of LDL-VLDL in do~ plasmas also
increased, but not in as great de8ree as with the hu~an
plasma. The HDL size was not significantly different
among the groups.
Figure 5 shows the post treatment recovery of
HDL-VLDL and HDL cholesterols. Recovery of LDL-VLDL
cholesterol was prolonued in th~ hi~her cholesterol
level groups. For Groups ~1 and III, it took about 2
weeks to return to pre-treatment values. IIDL
cholesterol recovery was constant and returned to
pre-v~lues within 7 days for all groups. Pi~ure 6 shows
~9~3~2~
-23-
the changes in the LDL-Vl,DI. cholesterol/l-lDL cholesterol
ratios. The ratio decre~sed during the post filtration
periods and was maintained a~ a lower level for 14
days. The tendency for a hi~her reduced ratio for
longer periods in comparison to the pre or post
treatment values was greater in Croup III which had the
highest cholesterol.
Conclusion: The data indicates that lipoprotein
particle size and sieving coeficients are highly
cholesterol concentration dependent. As the cholesterol
concentration increases, lipoprotein particle size
(particularly LDL-VLDL) increases, sieYing decreases and
the total cholesterol (LDL-VLDL cholesterol) removal
increases.
A comparison of the sieving coef~icients
indica~es that canine LDL-VLDL sieving is greater than
that of human LDL. This correla~ion is explàined by the
particle size study which indicates larger deviations
and overlaps between canine LDL-VLDL and HDL, ~aking it
more difficult to separate the lipoproteins in canines
than in humans. These results indicate that
thermofiltration would be quite effective in humans in
regard to the selective re00val of lipoproteins.
Moreover, the data indicates that there is a
more prolonged recovery of the LDL-VLDL cholesterol in
the more hypercholesterolenlic stages, while HDL
cholesterol recovery remains relatively normal. The
reduction of LDL choles~erol with preservation of HDL
cholesterol by thermofiltra~ion and the prolonged
recovery of LDL-VLDL cholesterol with the maintenance of
lower LDL/~IDL ratio is higllly sugKestive oE an effective
method of treating lipoprotein abnormalities.
~3~8
~,,
Exall!ple IV
Initial clinical thermofiltration procedures
were perormed on a secon(lary hypercholesterolemia
patient. The patient selected for the trial was a 39
year old man who ha~ a high concentration (210-450
mg/dl) of cholesterol and a very high LDL/HDL
cholesterol ratio (8-30) due to the cholestasis of
sclerosing cholangitis.
The thermofiltr~tion tests were carried out
according to the on-line system exhibited in Figure 2.
The blood flow was set at 100 ml/min and the plasma flow
at 30 ml/min. The Toray PS-05 (Toray Industries, Tokyo,
Japan), and Asahi Plasmaflo~(AP 0511: Asahi Medical Co..
Tokyo, Japan) modules were used as the plasma separator,
and the ~VAL 4A 2.0 m2 (~uraray Co.. Osaka~ Japan) was
used as the plasma ~ilter. The filter and a warmer
plate were wrapped with an electric heatin~ pad to
maintain the temperatule at 37C in the cryochamber of
the Cryomax ~Cryomax 360; Parker Biomedical, Irvine, CA,
U.S.A.). For anticoagulation, 5000 U of heparin was
injected as a bolus prior to initiation of the
extracorporeal circulation. The processed plaslna volume
and transmembrane pressure were monitored continuously
throughout the procedure. The Eiltration was carried
out until the transmenlbrane pressure (Ptm) o~ the
plasma filter reaches 500 mm Hg. Samples were drawn
simultaneously from ~he plasma inlet and outlet lines of
the ~ilter when the Ptm reached 150 and 300 ~m tl8 to
calculate the sieving coefficients (sc). Solute sieving
was calculated as concentration in the ~iltrate divided
by the concentration in the plasma inlet to the plasma
~ilter. Biochemical measurements included cholesterol
and triglyceride (automatic analysis AAIII Technicon
~a~le m~r~
633
2 5 -
Instrument Co., Tarrytowl-l, NY, U.S.A.), all~ HU~
cholesterol (dextran sulEclte MUcl2 precipitation
procedure), in addition to routine serum lnul~iple
analysis (SMA-II, Technicon Instrument Co.). The filter
was remove~ f 1`01!1 the circuit following plugKing, and the
plasmapheresis procedure was changed to plasma exchange,
using 53 albumin solution as a substitution fluid.
Plasma exchange was continued until one calculated
plasma volume (2893 ml) was processed by plasma exchange
alone.
In vitro filtration studies were done using the
same filter, the same temperature, and plasma from the
same patient as described in Example I.
~ESULTS
The procedure was well tolerated by the patient
and no adverse reac~ions were noted. No substitution
fluid was used during the thermofiltration phase. To a
Ptm Of 500 mul ~Ig. 1355 + 275 ml (1160 and 1540) ml of
plasma were filtered and 1117 ~ 183 ml (980 and 1253 ml)
were filtered to a YtD, of 300 mm Hg. The course o
Ptm versus the filtered volume was comparable to in
vitro studies with this patient's plas~a, as was the
filtered volume (1060 ml processed to 300 m~ Hg of Pt~
in vitro).
There was near complete rejection for LDL +
VLDL cholesterol (sc ~ 0.02) and high passage of albumin
(sc ~ 0.75) and HDL cholesterol sc~0.7B). Fibrirlogell
sievin~ was low (0.07). These results were comparable
to the in vitro filtration results. (Table Vl below).
~2~3~3Z~3
-2~-
_ABLF. VI
Concentration nnd SieVill~ coefficients (sc) of solute
(mean ~ SD)
Clinlcal (Ex Yivo) n
Concentration sc
.
Total protein (g/dl) 6.8 ~ 1.00.64 ~ 0.09
Albumin (g/dl) 3.2 ~ 0.20.75 + 0.0~
Total cholesterol (mg/dl) 181 ~ 47 0.09 ~ 0,00
HDL cholesterol (mg/dl) 17 ~ 7 0.78 ~ 0.09
LDL cholesterol (m~/dl) 140 ~ 40 0.01
LDL-YLDL cholesterol (mg/dl) 1~3 ~ 41 0.02 _ 0.01
Fibrinogen (mg/dl) 286 ~ 84a. 07 ~ 0.03
In vitro *~
Concentration sc
Total protein (g/dl) 6.2 0.3 0.72 0.02
Albumin (g/dl) 3.6 ~ 0.10,82 ~ 0.03
Total cholesterol (mg/dl) 274 ~ 2 0.06 ~ 0.01
HDL cholesterol (m~/dl) 11 ~ 3 0.84 1 0.08
LDL cholesterol (mg/dl) 228 ~ 120.02 ~ 0.02
LDL-VLDL cholestercl tm~/dl) 263 ~ 50.03 ~ 0,02
Fibrinogen (mg/dl) 298 ~ 28 0.13 ~ 0.03
HDL: hi8h density lipoproteins; LDL: low denslty
lipoproteins; VLDL: very low density lipoproteins;
Ptm: transmelnbrane pressure.
* Mean value of four samples, taken at Pt~n of 150 m
Hg and 300 ~Inlltg froln each of two treatments.
~ Mean value o~ three filtration tests at the same
conditions.
~3~
- 27-
(`_NCI.US ION
Sieving coefficients of LDL cholegterol (0.~2),
HDL choles~erol (~ 78) and albumin (0.75) demonstrate
che selectivity of thermoÇiltration. These results are
comparable to in vitro filtrations tests using the
plasma of the same patient. The advanta~e of this
system compared to plasnla exchange is the maintenance o
~IDL (antiathero~enic lipoprotein) and other essential
plasma solutes that would be discarded in plasma
exchange. Thermofiltration is more selective than
membrane schelnes without temperature control and simpler
to apply, as it does not require other plasma treatment
steps or the addition of potentially harmful chemical
additives. Moreover, abnormal concentra~ions of various
proteins (such as immulloglobulins) can also be
effectively removed by thermofiltration.
While there haYe been described herein what are
at present considered to be the preferred embodiments of
this invention, it will be apparent to those skilled in
the art that various changes and modifications may be
made therein without departing from the invention, and
it is, therefore, intended in the appended clai~s to
cover all such changes and modifica~ions as fall within
the true spirit and scope of the invention.