Note: Descriptions are shown in the official language in which they were submitted.
~2g~213
METHOD FOR CONTROLLING ROCK DISSOLUTION AND PIPE
CORROSION DURING OIL ~ELL STEAM INJECTION
The present invention relates to a method for
~5 inhibiting roc~ dissolution during steam injection, such
as gravel pack dissolution and carbonate and silicate for-
mation dissolution. More particularly, the invention
; relates to a method for preserving the reservoir rock or
gravel packing in oil wells ~eing subjected to steam
enhanced oil recovery techniques.
BACKGROUND OF THE I~VENTION
In the production of crude oil from wells, steam
injection has been used to recover immobile heavy oils and
to enhance the oil recovery from older wells where the
natural field pressures are too low for unassisted produc-
tion.
Steam stimulation and steam Elooding are tech-
niques generally used in reservoirs of high-viscosity
oil. The techniques involve injection into the well of a
high temperature steam (approximately 250~ or greater) in
cycles of thousands of cubic meters at a time. Steam used
in enhanced oil recovery operations is a fluid which is a
mixture of a vapor phase and a liquid phase. The quality
of this steam generally ranges from 60-~0~. Thus, large
quantities of liquid water are concurrently injected into
the well bore with the vapor phase.
A typical oil well consists of a casing which
lines the inside surface of the weLl bore and a length of
tubing which extends downwardly through the casing. The
casing serves to protect the tubing in the event of damage
to the latter. Sucker rods extend through the tubing and
terminate in a pump which reciprocates in the tubing and
forces the oil upwardly therethrough. The lower end of
~LZ9~13
01 -2-
the tubing extends into the oil zone and has perforations
therein through which the oil flows thereinto.
Many wells which are subjected to steam stimula-
tion have the lower end of the tubing filled with a gravel
pack. A liner is positioned on top of the gravel pack and
serves as a seat for the oil pump. The function of the
gravel pack is to filter and prevent sand from being pro-
duced with the well fluid. The sand is erosive and if notfiltered, would damage the pump. The gravel used to pacX
the well consists of granular sand grains. This material
is principally quartz or silica.
Silica has a very low solubility in water at
neutral pH and low temperatures, but this solubility rises
sharply as temperature and pH are increased. For pH
values above 11.0 and ~emperatures above 177C., the dis-
solution rates are orders of magnitude higher than at
neutral/ambient conditions.
When groundwater or river or lake water is used
in a steam generator to generate steam, the gaseous vapor
phase of the steam, and the residual liquid phase of the
steam have undesirable reaction characteristics with the
reservoir rocks. The residual liquid water phase produced
in a steam generator generally has a pH in excess of 11Ø
The gaseous or vapor phase of the steam, when condensed,
has an acidic pH of a~out 4.0-4.5. This partitioning is
due to the C02 contained in the so~rce water which is
volatile and enters the vapor phase leaving the residual
liquid phase deficient in anionic components and thus
produces a pH rise proportional to the lost anionic carbo-
nate species. The presence of CO~ in the vapor phase
produces a correspondingly low pH in the liquids condensed
from the vapor phase.
Coupled with the high fluid temperatures, both
the residual liquid phase and the liquids Erom the
condensed vapor phase are capable of rapidly dissolvin~
the gravel packs, such as quartz-rich sands or bauxite
with its associated impurities, or reservoir rocks, such
4Q as sandstone, car~onate, diatomite, porcellanite and the
;213
01 -3-
like. In the event of failure o the gravel pack, the
well begins to produce sand with the eventual shut-down of
O the well. Alternatively, the formation collapses and the
permeability is reduced.
Not only is the rate o~ silica dissolution quite
rapid, but the water in the well becomes saturated within
a short distance from the point at which the fluid con-
tacts the surface of the silica. This is significant inthat the dissolution o silica tends to be localized
rather than diffused over a wide area of the zone, result-
ing in the face of the zone receding significantly.
In addition to the dissolution of the gravel
pack due to the large quantities of water injected, there
is a danger of the face of the formation also being dis-
solved. If this occurs to a sizable extent, the formation
caves in and even the tubing or the casing could collapse
and result in the loss of the well.
Still further, these large silica or carbonate
losses at the well ~ore may precipitate out as the fluid
reaches s~persaturated conditions as it passes through the
zone. The precipitation of the silica or carbonate in the
zone may result in loss o zone permeability and a resul-
tant shut-in.
The costs resulting from such well failures are
imposing. Recently, one large oil producer estimated a
well failure rate of 34~ due to failure of gravel packing
or zone related problems due to steaming. The approximate
cost o reworking a well presPntly runs over $35,000.
Increasing the time between the workovers would realize
significant savings.
Dissolution of the gravel pack has been shown to
be primarily a function of the pH and temperature of the
injected liquid-phase water. Prior attempts at solution
of the problem have focused on these aspects. For exam-
ple, by Xeeping the pH of the injected hot water below 10,
gravel pacX dissolution can be decreased sharply. This
may be accomplished by (1) selection of feed wa~ers having
low total carbonate concentrations (less than 10 mg to~al
Z:13
01
carbonate/L), (2) treating the feed water with HCl to
yield the desired effluent pH, (3) using a total deionizer
to remove both cations and anions from the feed water, or
(4) protectively coating the gravel and/or reservoir
rocks.
With regard to the first proposed solution,
selection of feed waters is often impractical as the large
quantities of water used are not availa~le from a choice
of sources. With regard to the second proposed solution,
the use of HCl to neutralize the bicarbonate alkalinity
suffers from considerations of cost as well as feasibility
of the ,nethod. That is, addltion of too much acid will
cause severe corrosion oE the steam generator and too
little will result in insufficient depression of the pH to
alleviate silica loss. With regard to the third proposed
solution, the cost oE a total deionizer is prohihitive,
both in terms of capi.tal costs as well as daily mainte-
nance costs. With regard to the fourth proposed solution,
complete coating oE the gravel with a material, such as
soybean lecithin described in U.S. Patent No. 4,323,124,
is not assured and driving the material out into the
reservoir toward the production well is impractical.
Furthe.rmore, this patent does not address the problems of
formation dissolution out in the formation away from the
well bore.
U.S. Patent No. 3,43a,443 discloses another
approach for a solution to the problem through the use of
alkali metal silicates to saturate the water phase with
silica and thus, hopefully, preventing the dissolution of
siliceous formation material. However, alkali metal sili-
cates are costly and the process also requires careful pH
control.
Still other oil recovery processes as described
in U.S. Patents Nos. 3,500,931, 4,222,439, and 4,223,731
utilize chemicals, such as ammonium hydroxide, ammonium
bisulfite, ammonium sulfite in separate injection steps to
enhance oil recovery. However, these processes utili~e
4~ the chemicals in a separate step, generally not includiny
-
12~Z~ 3
01
steam, and do not recognize the problems associated with
the pH partitioning between the vapor phase of the steam
and the residual water phase of the steam during the steps
S that include steam injection. Still another oil recovery
process described in U.S. Patent 4,441,555 utilizes a
carbonated water flooding step before a steam drive to
enhance the recovery of viscous oil. This process also
does not recognize the problems of pH partitioning during
steps that require a steam drive.
Thus, it would be highly desirable to have a
process of reducing the pH of the residual liquid p'nase of
the steam. It would also be desirable to have a process
of solving the previously not addressed problem of the
gravel pack and formation dissolutions caused by the
decrease in the pH of the vapor phase of the steam (upon
condensation) in a steam enhanced oil recovery process. A
Eurther optional and beneficial advantage would be to have
the process compatible with stearn-surfactant enhanced oil
recovery processes. A process meeting these criteria
would have additional desirable benefi-ts obvious to the
ordinary skillea artisan, such as uses for in situ solu-
tion mining, and the like.
SUMMARY OF THE INVENTION
The dissolution of silica frorn the gravel pack
in the well bore area and the dissolution o-f silica and
carbonate from the formation is caused by a chemical reac-
tion between water and silica, silicate minerals, and
carbonate minerals which is catalyzed by heat and either
alkalinity or acidity or both. According to -the present
invention, these dissolutions are inhibited by adding a
salt, comprising a vola~ile cationic component and a non-
; volatile anionic component, to the steam generator feed
water to reduce the pH of the residual liquid phase of the
steam to within the range of from 8 to lO, preferably in
the range of from 8.5 to 9.5; monitoring the pH of the
residual liquid pha~e; and continually adjusting the addi-
tion of the salt to maintain the pH within the range of
from 8.0 to 10Ø A nonvolatile anionic component is an
;Z 13
anion which remains in the residual liquid phase of the
steam. A volatile cationic compon nt is a cation
capable o~ moving into the vapor pha~e of the steam.
The concentration of the salts is dictated by the
concentration of the total carbonate species and the pH
of the feed water. Total carbonate species includes
H~CO3, HCO3-, and CO3=. The ~toichiometric amount of
the appropriate salt~ with re~pect to the total
carbonate species in the ~team ~e~d water i added to
optimize the pH decrease in the residual liquid phase of
the steam while minimizing the cost of the required
chemicals. The amount of salts added to the feed water
is controlled to cause the pH of the residual liquid
phase to be in the range of from 8 to 10, preferably in
the range of rom 8.5 to 9.5. When the pH of the
residual liquid phase is above 10, the dissolution rate
of siliaa becomes unacceptably high. When the pH of the
residual liquid phase is below 8 the corrosion rate of
the well pipe becomes unacceptably high. In addition to
~O steam drive enhanced oil recovery processes, the
invention can be used with steam dump/heap leaching
operation3 in mining and ~or steam in situ mineral
mining.
Other aspects o~ this invention are as follows:
In a process for the recovery of hydrocarbons from
a silicate or carbonate containing hydrocarbon-bearing
formation pene~rated by at least one steam injection
well and at least one production well spaced therefrom,
the process which inhibits foxmation dissolution while
prevQnting pipe corrosion which comprises:
(a) injecting steam at said steam in~ection well
to displace hydrocarbons toward said production well/
said steam produced from a water having added thereto a
sufficient amount of a salt to reduce the pH of the
residual liquid phase of said steam to within the range
r
~Z9~213
6a
of from 8.0 to 10.0, said salt comprises a volatile
cationic component and a nonvolatile anionic component;
(b) monitoring the pH of said residual liquid
phase and continually adjusting the addition of said
sal~ to maintain the pH within the range of from 8.0 to
10.0; and
(c) producing said hydrocarbon~ at said production
well.
ln a process ~or the recovery oP hydrocarbons from
lo a silicate or carbonate containing hydrocarbon-bearing
formation penetrated by at least one production well
spaced therefrom, the process which inhibits formation
dissolution while preventing pipe corrosion which
comprises:
(a) injecting ~team at said production well, said
steam produced from a water having added thereto a
sufficient amount of a salt to reduce the pH of the
residual li~uid phase of said steam to within the range
of from 8.0 to lO.0, sai~ salt comprises a volatile
cationic component and a nonvolatile anionic component;
(b) monitoring the pH of said residual liquid
phase and continually ad~usting the addition of said
salt to maintain the pH within the range of from 8.0 to
10.0; and then
(c) producing said hydrocarbons at said produc~ion
well.
In a process for the recovery of hydrocarbons from
a silicate or carbonate containing hydrocar~on-bearing
formation penetratad by at lQas~ one steam injection
well and at least one production well spaced therefrom,
the process which inhibits formation dissolution while
preventing pipe corrosion which comprises:
(a) injecting steam at said steam injection well
to displace hydrocarbons toward said production well,
said steam produced from a water having added thereto a
6b 1;~9~13
sufficient amount of an ammonium salt selected from the
group consisting of ammonium sulfate and ammonium
chloride to reduce the pH of the residual liquid phase
of said steam to within the range of from 8.5 to 9.5;
(b) monitoring the pH of said residual liquid
phase and the temperature oP the steam as it is injected
in the steam injection well;
(c) continually calculating the effective pH o~
said injected st~am;
(d) continually adju~ting th~ addition of said
ammonium salt to maintain the effective pH of said
injected steam within the range of ~rom 8.5 to 9.5: and
(e) producing said hydrocarbons at said production
well.
A method for inhibiting silica dissolution while
preventing pipe corrosion for use in the vicinity of a
well penetrating a hydrocarbon-containing formation,
comprising:
(a) injecting steam into said well, said steam
having a liquid phase and a vapor phase, and including
an effectlve amount of a salt to reduce the pH of said
liquid phase of said steam to a range of about 8.0 to
about 10.0 said ~alt comprising a volatile cationic
component and a nonvolatile anionic component; and
(b) monitoring the pH of said liquid phase of said
steam and continually adjusting the addition of said
salt to maintain the p~ of said liquid phase of said
steam within the range of from about 8.0 to about 10Ø
A method for recovering hydrocarbons from a
silicate or carbonate con~aining hydrocarbon-bearing
formation penetrated by at least one well, wherein said
method inhibits formation dissolution while preventing
pipe corrosion, said method comprising the steps of:
(a) injecting steam at said well, said steam
produced ~rom a water having added thereto an ef~ective
~f ~
~`..
6c ~. 29~3
amount of a salt to reduce the pH of the residual
liquid phase o~ said steam to within the range o~ from
about 8.0 to about 10.0, said salt compri~ing a volatile
cationic component and a nonvolatile anionic component;
and
(b) ~onitoring the pH of said residual liquid
phase of said st~am and continually adjusting the
addition of said s21t to main~ain ~he pH of said liquid
phase within the range of ~rom about 8.0 to about 10Ø
~RI~F ~S~RI~TION OF TH~ ~RAWING~
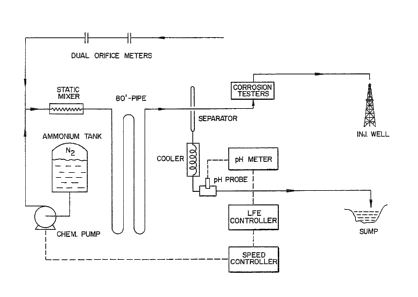
FIG. 1 is a schematic diagram o~ the field test
facility used to test this invention.
FI 2 is a plot of the ef ~ect of pH on corrosion
rate and silicate dissolution rate.
DETAI~D ~Ç~T~Q~ ~F '~ INVENTION
The problems of gravel pack and silicate formation
dissolution are a function of the pH of the steam
generator feed water and its carbonate concentration.
For example, the dissolution of the bicarbonate ion upon
heating according to the formula:
Feed Water Vapor Phase Liquid Phase
HCO3- c~ CO2 ~ OH- (1)
causes the pH of the vapor phase o~ the steam (upon
condensation) to drop and the pH of t~e residual liquid
phase
3LZ~ 213
01 ~7~
to increase. During steam injection, the vapor phase of
the steam typically enters the upper part of the produc-
tion interval while the alkaline liquid residual fluid
phase enters the lower part of the production inter~aL. ~ 3
However, regardless of where the vapor or liquid phases of/~Z
the steam enter the production intsrval, the p~ partition-
ing causes ~ravel pack and formation dissolution and
increases overall maintenance costs and time.
This problem is simply and effectively addressed
~y adding to the steam generator water a sufficient amount
of a salt to reduce the pH of the residual liquid phase o
the steam to within the range of from 8.0 to 10.0, moni-
toring the pH of the residual liquid phase and continuallyadjusting the addition of the salt to maintain the pH
within the range of from 8.0 to 10Ø
The pH of the residual liquid phase of the steam
i9 measured at about 120F. 'rhe addition of the ammonium
salt can be controLled based on either the pH measured at
120F or, preferably, the effective pH at the temperature
of ~he injec~ed steam. The e~fective p~ at the tempera-
ture of ~he injected steam can be caLculated by solving
~ the system of equations descri~ing the equilibrium rela-
tions among the controlling aqueous components in the
fluid phase and using the appropriate thermodynamic data
and mass balance constraints.
The salt must have a volatile cationic component
and a nonvolatile anionic component. Useful salts are
ammonium salts that include ammonium sulfate, ammonium
chloride, ammonium, ammonium hydroxide, ammonium acetate,
ammonium nitrate and mi~tures thereof, having a volatile
cationic, i.e., NH4~, and a nonvolatile anionic component,
i.e., SO4=, Cl , OH , CH3CO0 , NO3-. In view o~ its cost
advantage, ammonium sulfa-te is preferred, although ammo-
nium chloride is preferred if a more soluble salt is
réquired. The ammonium cation decomposes and ammonia
moves into the vapor phase, according to the formula:
2~3
--8--
01
Feed WaterVapor Phase Liquid Phase
NH + <~~~ NH3 ~ H+ (2)
05
The ~H3 increases the pH of t.he vapor phase of the steam
upon condensation and the H+ neutralizes the OH in the
liquid phase and reduces its alkaline pH. The nonvolatile
anionic component also drives the equilibrium of the
residual liquid phase of the steam toward a reduced pH.
: The amount of salt required initially depends on
the to~al carbonate ion concentration in the steam
generator feed water~ This concentration can be deter-
mined by standard carbonate titration methods, such as CO2
coulometric methods. The desired initial amount of the
salt is added to the feed water entering the steam
generator to reduce the pH of the residual liquid phase of
the steam to within the range of Erom 8.0 to 10.0, prefer-
~ ably within the range of frorn 8.5 to 9.5.
:: :
. 30
: :: : : :
~ 35
:::
~ 40
~9~213
01 _9_
Once the pH has been reduced to the desired
range, the pH must be maintained within -that range. This
05 is accomplished by monitoring the pH of the residual
liquid phase and continually adjusting the addition of the
salt to maintain the desired pH.
Oxygen in the feedwater to the steam generator
cannot be tolerated since severe corrosion will occur. A
1~ chemical oxygen scavenger, such as sodium sulfite, should
be used in t~e feedwater to keep the oxygen concentration
in the feedwater below 30 ppb.
EXAMPLE 1
FIG. 1 is a schematic diagram of a field test
IS facility to test this invention. The test facility can be
divided into three parts: the injection system, the auto-
matic pH control system, and the corrosion testers.
The Injection Sys _
The feedwater used in this experiment was well
water which had been softened and pipelined to the steam
generators. The major ion species present in the feed-
water were sodium, chloride, sulfate, and bicarbonate.
Their concentrations were nearly constant during the
experimental period. The average concentrations of ~icar-
bonate and sulfate were 272 mg/l and 1263 mg/l, respec-
tively. The steam flow rate and quality were continuously
monitored using a duel orifice meter system.
Ammonium sulfate was used ~in this field trial
because of the high concentration of sulfate in the feed-
water. The amount of sulfate that was added to the water
as ammonium sulfate represented only a 15% increase in the
overall sulfate concentration. 240 pounds of ammonium
sulfate was mixed with 570 gallons of water to obtain a
solution of approximately 0.8M in ammonium ion. The dis-
solved oxygen which saturates the chemical solution duringmixing was removed using sodium sulfite as an oxygen
scavenger. In addition to che oxygen scavenger, a nitro-
gen blanket was kept on top of the chemical solution.
; Nitrogen was injected into the tank at approximately twice
the rate of chemical solution output.
~L2~213 .
--10--
01
Ammonium solution was pumped to the steam line
with a pump havin~ a d-c variable speed motor. An in-line
05 2-inch static mixer was used to mix the ammonium solution
with the steam. In order to provide an adequate residence
time for ammonium salt to decompose, an 80-foot long
extension pipe was added to the line. With the extension,
the steam residence time increased from 0.3 seconds to 3-4
seconds.
Automatic pH Control_System
The pH of the li~uid raction of the steam was
used to determine the rate of chemical injection. Thé
system consisted of a separator, a pH meter, a micropro-
cessor, and a pump motor speed controller.
The separator wa~ made of two one~inch diameter
pipes welded to a sec*on of three--inch steam injection
line. The liquid collection pipe was welded to the bottom~
oE the steam line and the vapor collection pipe was welded
to the top. The vapor-liquid separation took place within
the one-inch pipes. Good separation was achieved as long
as the flow rates were kept sufficiently low. The fluids
then flowed from the separator through cooling condensers
after which the fluid was sampled as warm water.
After the fluid was cooled, the liquid fraction
of the steam was allowed to flow continuously from the
vapor-liquid separator into a bronze vessel containing p~
electrodes. T~e pH of the solution was monitored with an
industrial pH meter system.
lZ~13
The output from the pH meter was sent to a strip
chart recorder and a microprocessor proportional con-
05 troller which was programmed to maintain a specific pH.
The controller output then went to a speed con-
troller which amplified the signal and supplied the appro-
priate power to alter the speed of the chemical pump motor
that pumped the ammonium solution into the steam line.
Corroslon Testers
The corrosion test system was placed directly in
the steam line just downstream o the 80 foot pipe. Three
methods were used to determine the corrosion rate: corro-
sion coupons, corrosion nipples, and a corrosimeter.
Although the dissolution of silica decreased as
the pH of the residual liquid phase decreased, the corro-
sion of the well pipe increased dramatically when the pH
decreased below 8Ø (See Figure 2) Therefore, the pH of
that phase should be kept above 8Ø
Since the titration method is difficult to per-
Eorm in the field, once the carbonate concentration is
determined, the amount of ammonium salt to be added is
adjusted by monitoring the pH of the residual liquid phase
of the steam. If the pH is too high, then more salt is
added. If the pH is too low, then less ammonium salt is
added. The pH should be adjusted through the addition of
the compound to the range of from 8.0 to 10 for the
residual liquid phase.
The following table illustrates suitable stoi-
chiometric concentrations of ammonium sulfate and ammoniumchloride for steam generator feed waters having total
carbonate concentrations from 50 ppm to 1000 ppm.
;213
O 1 --1 2--
TABLE
Steam Feed WaterAmmonium Sulfate Ammonium Chloride
Carbonate Conc. (ppm) gt~ /1 g/bbl g/1
0550 ppm 8.6 0.054 6.97 0.044
250 ppm 43.0 0.27 34.8 0.22
1000 ppm 172. 1.08 139 0.88
For a typical 350-barrel per day (BPD) steam
injection project with a carbonate concentration of
250 ppm, the daily ammonium sulfate salt consumption is
about 15 kilograms. The chemical cost is only about
1.5 cents per barrel of injected steam. However, this
causes about a 20-fold decrease in the silica dissolution
lS rate at an adjusted pH of about 9.1.
The process is suitable for use within cyclic
steam injection or patterned steam injection involving the
use of injection wells and production wells, i.e.
seven-spot, five-spot, nine-spot, their inverted forms,
and the like. In addition, it can be used with surfac-
tants and steam-foam drives. Suitable surfactants are
known anionic, cationic or nonionic surfactants. Pre-
ferred surfactants for steam-foam enhanced oil recovery
drives are known as alpha olefin sulfonates and their
25 dimers ~s described in U.S. Patent 3,721,707
Furthermore, the steam drive does
not have to be continuous so lony as any displacing medium
does not substantially alter th~ preferred pH of the
residual liquid phase of the steam in the formation.
Example 2
Experiments with a made-up feed water containing
0.004M NaHCO3, .004M NaCl, and O.OOlM Mg504 indicate that
the residual liquid phases pH is reduced from a pH of
about 11.1-11.4 to about 9.6 with the addition of 50~ of
the stoichiometric amount of (NH4)2 5O4 added and further
reduced to a pH of about ~.8-9.1 with an addition of 100
of the stoichiometric amount of ammonium sulfate.
The pH of the condensed vapor phase increased
from about pH 4.5-4.8 without the ammonium sulfate ~o
' ;. ~,,~'q'` `
~Z9~2~3
-13-
01
about pH 8.5.
The addition of the stoichiometric amount of
05 NH4C1 to a similar made-up feed wa-ter caused the pH of the
residual water to drop from about pH 11.1-11.4 to about pH
9.2 and increased the condensed vapor phase pH from a~out
pH 4.5-4.8 to about pH 8.4.
Actual experimental ~ield work produced better
results. Water used to feed steam generators in a steam
10 flood had a carkonate con~tra~tion roughly from about 50 ~ J~
ppm to about 100 ppm and a residual ~luid pH of about ll.S ~ ~3
and a condensed vapor phase pH o~ a~out 4.5. The addition
of about 0.6 ~/1 of (NH~)2 S04 increased the pH of the
lS condensed vapor to about pH 6 and reduced the pH o~ the
residual liquid to about pH 7.5. Furthermore, the pH of
both phases could be adjusted by adjusting the addition of
the ammonium salt.
The process has been described with respect to
particularly pre~erred embodiments. Modifications which
would be obvious or apparent to the ordinary skilled
artisan are contemplated to be within the scope o~ the
invention. For exarnple, the invention is suitable to
reduce the di~solution of not only sand or gravel packs
but also carbonate Eormations and siliceous formations,
such as sandstone, diatomite, and porcellanite.
~0