Note: Descriptions are shown in the official language in which they were submitted.
33~
-- 1 --
This patent appllcation is a division of
application No. 514,378 filed on July 22, 1986.
Technical Field
This invention relates to synthetic intermediates
for preparing substituted di-t-butylphenols which are anti-
allergic agents. Pharmaceutical compositions containing
such compounds, pharmacological methods for using such
intermediates.
Background of the Inventlon
The leukotrienes are a group of biologically
active mediators derived from arachidonic acid through the
action of lipoxygenase enzyme systems. There are two groups
of leukotrienes derived from the common unstable precursor
Leukotriene A4. The first of these are the peptido-lipid
leukotrienes, the most important being Leulcotrienes C4 and
D4. These compounds collec-tively account for the
biologically active material known as the slow reacting
substance of anaphylaxis.
The leukotrienes are potent smooth rnuscle
contracting agents, particularly on resp:iratory smooth
muscle, but also on other tissues as well. In addition,
they promote mucous production, modulate vascular
permeability changes and are potent inflamma-tory mediators
in human skin. The mos-t important compound in -the second
group of leukotrienes, namely dihydroxy fatty acids, is
Leukotriene B4. This compound is a potent chemo-tactic agent
for neutrophils and eosinophils and, in addition, may
modulate a number of other functions of these cells. It
also affects other cell types such as lymphocytes and, for
example, may modulate the action of suppressor cells and
natural killer cells. When injected ln vivo, in addition
to promoting the accumula-tion of leukocytes, Leukotriene
B4 is also a potent hyperalgesic agent and can modulate
129~3~7
vascular permeability cllanges througll a nelJtrophil
dependent mecl~anism. soth groups of ]eukotrienes are
formed following oxygenation of arachidonic acid tllrough
the action of a lipoxygenase en7Jyme. See, for example,
D. M. Bailey et al., ~nn. ~_s. Med. Chem., 17, 203 (19~2).
RESPIRATOI'~Y CONDITIONS
~ sthma. q'he leukotrienes are potent spasmogens
of human trachea, broncllus, and lung parenchyma, and when
administered to normal volunteers as aerosols are 3,800
times more potent than histamine at inducing a 50~ decrease
in air flow at 30% of vital capacity. They mediate
increases in vascular permeability in animals and promote
mucous production in human bronchial explants. In
addition, Leukotriene B4 may also mediate mucous production
and could be an important mediator of neutrophil and
eosinophil accumulation in asthmatic lungs. Lipoxygenase
products are also thought to be regulators of mast cell
degranulation, and recent studies with human lung mast
cells have suggested that lipoxygenase inhibitors ~but not
corticosteroids), may suppress antigen-induced mast cell
degranulation. In vitro studies have sllown that antigen
challenge of human lung results in the release of
leukotrienes and that, in addition, purified human mast
cells can produce substantial amounts of leukotrienes.
There is, therefore good evidence that the leukotrienes are
important mediators of human asthma. Lipoxygenase
inhibitors would, therefore be a new cla6s of drug6 for the
treatment of astllma. See, for example, 13. Samuelsson,
Science, 220, 568-575 (19~3).
SKIN DISEASES
Psoriasls~ Psoriasis is a human skin disease
which affects between two and six percent of tlle
lZ~;33~
population. There is no adequate tllerapy for psoriasis and
related skin conditions. The evidence for leukotriene
involvement in these diseases is as follows. One of tlle
earliest events in the development of prepapillary lesions
is the recruitment of leukocytes to the skin site.
Injection of Leukotriene B~ into lluman skin results in a
pronounced neutrophil accumulation. There are gross
abnormalities in arachidonic acid metabolism in human
psoriatic skin. In particular, highly elevated levels of
free arachidonic acid can be measured as well as large
amoun`1ts of lipoxygenase products. Leukotriene s4 has been
detected in psoriatic lesions, but not in non-involved
skin, in biologically significant amounts.
lS ALLERGIC CONDITIONS
Leukotrienes can be measured in nasal washings
from patients with allergic rhinitis and are greatly
elevated following antigen challenge. Leukotrienes may
mediate this disease through their ability to regulate mast
cell degranulation, to modulate mucous production and
mucocillary clearance, and to mediate the accumulation of
inflammatory leukocytes.
Leukotrienes may also mediate other diseases.
These include atopic dermatitis, gouty arthritis, gall
bladder spasms and ulcerative colitis. In addition, they
may have a role in cardiovascular disease becallse
Leukotrienes C~ and D~ act a.s coronary and cerebral
arterial vasoconstrictors and tllese compounds may also have
negative inotr(ipic effects on the myocarclium. In addition,
the leukotrienes are important mediators of inflammatory
disease through their ahility to modulate leukocyte and
lymphocyte function.
Many substituted di-t-hutylphenols are known.
Generally these compounds may be useful as antioxidants.
~2~337
Some of these compounds are also known to be active
antiinflammatory agents.
Compounds wherein 2,6-di-t-butylphenol is
substituted in the 4 posltion by an unsubstitu-ted phenyl or
certain simply-substituted phenyls are known
antiinflammatory agents. See, for example, U.S. Patent
4,172,151 and references cited therein. The compound 2,6-
di(tertiary-butyl)-4-(4'-carboxyphenylimino)-2,5-
cyclohexandiene-1-one is disclosed in Chemical Abstracts
67:81701n.
No compounds wherein a 2,6-di-t-butylphenol is
substituted in the 4 position by an anilino group wherein
such anilino group is substituted by a moiety including
carboxy, tetrazolyl, N-methyl-tetrazolyl, or N-
trifluoromethylsulfonyl are known.
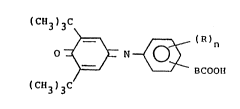
Summary of the Invention
This invention relates to compounds of formula:
( CEE ) C
3 3 \
O _ ~ = N~ )n
~==~ BCOOII
( CEI 3 ) ~C
-
where R is hydrogen, lower alkyl, lower alkoxy, lower
alkylthio, hydroxy, halogen, amino or lower acylamino; n is
0, 1 or 2, provided that when n is 2, the R substituents
combined contain no more than 6 carbon atoms:
and B is a carbon-carbon bond, lower alkylene, lower
alkenylene, lower alkylene containing one ether or thioether
o
link in the alkylenechain, or -CNHCH2-; with the proviso
that -BCOOH is bonded to thephenyl ring in ortho or meta when
~%~
- 5 --
B is a carbon-carbon bond.
The compounds of the invention are useful as
synthetic intermediates for preparing certain of the
antiallergic compounds which are disclosed and claimed in
canadian co-pending applications Nos. 514,386 and 514,378.
These compounds are also useful as inhibitors of mammalian
leukotriene biosynthesis. As such, these compounds are
useful therapeutic agents for treating allergic conditions,
particularly asthma. Pharmaceutical compositions comprising
such compounds, pharmacological methods of using such
compounds are also described. Moreover, it is believed that
certain of the antiallergic compounds disclosed in said
copending application No. 514,386 are prodrugs of certain
antiallergic compounds disclosed herein. For example, N-( 3-
carboxyphenyl)-N- ( 3,5-di-ti-butyl-4-hydroxyphenyl)
succinamic acid disclosed in said copending application No.
514,386 is believed to possibly be a prodrug of 3-(3,5-di-t-
butyl-4-hydroxyanilino)benzoic acid which is disclosed
herein.
Detailed Description of the Invention
The invention of the p ar ent application N~
514,378 relates to compounds of formula (I):
)3C R'
HO - ~ N ~ R)n
(C~13)3C/
wherein R is hydrogen, lower alkyl, lower alkoxy, lower
l~g5~
-- 6 --
alkylthio, halogen (preferably chloro or fluoro), amino,
lower alkylamino, di(lower)alkylamino, lower acylamido or
hydroxy, and n is 0, 1 or 2 with the proviso that if n is 2,
then all R substituen-ts combined contain no more than 6
carbon atoms; R' is hydrogen, lower alkyl, acetyl or
trifluoroacetyl; A is carboxyl, tetrazolyl, N-methyltetra-
zolyl or -CNHSO2CF3: and when A is carboxyl, B is a carbon-
carbon bond, lower alkylene, lower alkenylene, lower
alkylene containing one ether or thioether link in the
o
alkylene chain, or -CNHC~I2-; when A is tetrazolyl or N-
o
methyltetra~olyl, B is a carbon-carbon bond, -CH2- or -CNH-;
l
and when A is -CNHSO2CF3, B is a carbon-carbon bond; and
derivatives of compounds wherein A is carboxyl selected
from the group consisting of the lower alkyl esters,
(lower)alkylamino(lower)alkyl esters, pharmaceutically
acceptable (lower)alkylamino(lower)alkyl ester acid-addition
salts and pharmaceutically acceptable carboxylate salts, and
derivatives of compounds wherein B is te-trazolyl selected
from pharmaceutically acceptable alka]i metal and alkaline
earth salts of the tetrazolyl moiety.
Presently preferred are compounds wherein the
group -B-COOH, -B--tetrazolyl or -B-N-methylte-trazolyl is
I -
oriented para or me-ta to the -N- linking group.
Presently preferred compounds are those wherein A
is carboxyl.
Presently preferred as B is a carbon-carbon bon.
When B is alkylene it is preferably methylene. When B is
alkenylene it is preferably ethenylene.
When R is lower alkyl, lower alkoxy or lower
~2~3~
alkylthio, it is presently preferred to be methyl, methoxy,
or methylthio respectively. The presently preferred R group
is hydrogen.
By "lower" as used in connection with "alkyl" and
"alkylene" is meant that such groups contain one to about
four carbon atoms. Most preferred alkyl groups contain one
or two carbon atoms. By "lower" as used in connection with
"alkenylene" is meant that such groups contain two to about
four carbon atoms.
In the compounds of Formula (I) wherein A is
tetrazolyl, two tautomeric forms of tetrazolyl exist as is
known to those skilled in the art. Tautomerism does not
exist in tetrazolyl moieties where the tetrazolyl ring is
substituted on a nitrogen atom by methyl. Instead, two N-
methyl isomers are obtained, one in which the methyl group
is in the 1-position, the other in which it is in the 2-
position. All such tautomers and isomers are within the
scope of the invention of the parent application.
It is well known to the art that pharmaceu-
tically acceptable salts such as alkali metal, alkalineearth, aluminum and other metal and amine salts of
pharmaceutically active acids are the equivalents of the
acids in terms of activity, and in some cases may even offer
advantages in absorp-tion, formulation and the like.
Pharmaceutically-acceptable carboxylate salts oE the
compounds of formula (I) which contain carboxyl as A are
prepared in an inert atmosphere by reaction of the acid with
a base and subsequent evaporation to dryness, preferably
under mild conditions. The base may be organic, e.g.,
sodium methoxide or an amine, or inorganic, e.g., sodium
hydroxide. Alternatively, the cation of a carboxylate salt,
e.g., sodium, may be displaced by a second cation such as
calcium or magnesium when the salt of the second cation is
more insoluble in a selected solvent.
~2~;.3~37
-- 8
other useful derivatives of the compounds of
formula (I) which contain carboxyl as A include alkyl
esters, alkylaminoalkyl esters, and salts of the latter. In
the ester derivatives, the hydrogen portion of the
carboxylic acid group is replaced with an alkyl or
substituted alkyl, preferably an alkylaminoalkyl group.
According to the present invention, esters of the
compounds of formula (I) may be obtained as interrnediates
during the preparation of the adidic compound. In some
cases, the esters may be prepared directly using standard
synthetic methods. These esters of the present invention
may exhibit antiallergic activity, but they are primarily of
interest as synthetic intermediates, although in some
instances hydrolyzable or salt-forming esters may be of
interest as therapeutic agents. Preferred esters are alkyl
esters and alkylaminoalkyl esters having one to four carbon
atoms in the alkyl group. Especially preferred are
alkylaminoalkyl esters such as the dimethylaminoe-thyl esters
which will form salts, e.g., hydrochlorides.
Ester derivatives may be obtained by alkylation of
an alkali metal salt of the compound in dimethylformamide
with an alkyl iodide or dialkylaminoalkylchloride, or by
starting with esters instead of acids in Scheme I, Step (1)
below.
Pharmaceutically acceptable a]kali metal and
alkaline earth salts may also be prepared of compounds of
formula (I) wherein A is tetrazolyl by methods known to
those skilled in the art.
The preferred compounds of formula (I) are 4-(3,5-
di tertiary-butyl-4-hydroxyanilino)benzoic acid, 3-(3,5-di-
tertiary-butyl-4-hydroxyanilino)benzoic acid 5-C3-(3,5-di-
tertiary-butyl-4-hydroxyanilino)phenyl~-tetrazole, and 5-C4-
(3,5-di-tertiary-butyl-4-hydroxyanilino)phenyl~tetrazole.
Compounds of formula (I) may be prepared by the
3~
- 8a -
method of Scheme I, wherein A, R, and B are as defined
above, and R' is hydrogen.
~o~ME I
(CH3)3C
(CH3)3C
II III
(1) \~
(C 3)3C
~ )n
(CH3)3c
IV
~2) /
(CH3)3c
B-/~
(C113)3C
V
3-~7
The reaction of step (1) is a Lewis acid catalyzed
condensation of the known compound 2,6-di(tertiary-butyl)-p-
benzoquinone (II) and a substituted aromatic amine (III).
Suitable substituted aromatic amines for preparing compounds
of formula (I) wherein A is carboxyl are known compounds
such as the aminobenzoic acids, for example, 3- and 4-
aminobenzoic acid, the aminophenylacetic acids,
aminophenylbutyric acids, aminophenyl-thioacetic acids,
aminophenyloxyacetic acids, alkyl aminophenylaceta-tes,
aminophenylcinnamic acids, and the like. Similarly,
suitable tetrazolyl-substituted aromatic amines for
providing cornpounds of formula (I) wherein A is tetrazolyl
are known such as 5-(3- or 4-aminopheny])tetrazoles.
Suitable I,ewis acid catalysts include boron
trifluoride, tin tetrachloride, titanium tetrachloride and
the like.
The reaction of step (1) is carried out by com-
bining the reactants in an inert solvent such as an ether,
for example, tetrahydrofuran, and hea-ting gently, if neces-
sary. The products of formula IV, which forms the subjectof the present invention, are novel solids which are readily
isolated and may be recrystallized from polar solvents.
The reaction of step (2) is a reduc-tion of the
imino quinone system of the intermediate of formu:La IV to an
amino phenol. According to the present invention, it is
readily accomplished using catalytic reduction wi-th hydrogen
gas in an inert solvent when A is carboxyl. It may be
carried out under neutral conditions or in the presence of
basem, for example, an equimolar amount of base. Suitable
catalysts include platinum or palladium on charcoal.
Chemical reduction can also be carried out, for example,
with sodium thiosulfite, or zinc and acetic acid to provide
compounds wherein A is carboxyl according to the invention,
or tetrazolyl. Chemical reduction is preferred when B
~2~;33~
-- 10 --
contains a double bond.
Compounds of formula (I) wherein R' is alkyl and A
is carboxy are prepared from a compound of forrrlula V
(obtained above) by reacting the compound with an alkyl
halide, particularly an alkyl bromide or an alkyl iodide.
This reaction may be carried out in a solvent such as N,N-
dimethylformamide, optionally in the presence of base. When
base is present, the carboxyl will generally become
esterified, and therefore subsequent hydrolysis by
conventional methods may be desired.
Compounds of formula (I) wherein R' is acetyl or
trifluoroacetyl and A is carboxy are prepared from a
compound of formula (V) by reacting the compound with the
appropriate anhydride.
Compounds of formula (I) wherein A is N-
methyltetrazolyl are preferably prepared by alkylating an
alkali metal salt of the corresponding compound of formula
(I) wherein A is tetrazolyl with methyl iodide.
Compounds of formula (IV) wherein A is -C-NHSO2CF3
may be prepared from the corresponding compound of formula
(IV) of the invention, wherein A is carboxy, via reaction of
that compound with thionyl chloride and subsequent reaction
of the resulting acid chloride with sodium trifluoromethane-
sulfonamide. Catalytic reduction provides compounds of
Q
formula (I) wherein A is -CNHSO2CF3.
Compounds of formula (I) wherein A is tetrazolyl
may also be prepared by the method of Scheme II wherein R, n
and B are as defined above and R' is hydrogen.
Z~3~
SCII EME I I
3 ) 3
O--~ O -~ N112~ ~C~n
( CH3 ) 3C
II VI
\ (1)
\~
( Cll 3 ) 3C
EEC11
(C113)3C
V I I
(~
(C 3)3 (4) (CIJ3)3C
HO ~}NIJ ~ > E10 <~NH (~
3 ) 3 ( 3 ) 3
VIII \ X
\ (3)
(C113)3C
3 0 IIO ~ NII~B ( R ) n
( C 3 ) 3 >= N
HN
N
I X N /
~L2~.53~7
-l2-
The reaction of step (1) of Sclleme II is a ~ewis
acid catalyzed condensation similar to step ~1) of Scheme I
except that here an aminoni~rile of Formula VI is used in
place of the substituted aromatic amine used in step (1) of
Scheme I. Compounds of Formu]a VI are known or may be
prepared by conventional methods. I'he reaction is
conducted as described in connection with ~tep (l) o~
Scheme I. The product of step (1) of Scheme II is a novel
intermediate of Formula VII.
Tlle reaction of step (2) of Scheme II is a
reduction of the type (and performed using the method of)
step ~2) of Scheme I to provide a novel intermediate of
Formula VIII.
In step (3), the intermediate of Formula VIII is
reacted with sodium azide in the presence of ammonium
chloride and lithium chloride. The reaction is preferably
conducted in N,N-dimethylformamide and is conducted under a
nitrogen atmosphere and accompanied by heating.
In step (4), the intermediate of formula VIII is
hydrolyzed, in an inert atmosphere, by known means such as
with sodium hydroxide in aqueous ethanol, to provide
compounds of Formula x.
Compounds of formula (I) wherein R' is alkyl
and ~ is carboxy, tetrazolyl or N-methyltetrazolyl may be
prepared by alkylating tlle intermediate of Formula VIII by
conventional methods prior to conducting step (3) or (4).
Again, as in Scheme 1, compoun~ls of Eorrnu:la (I)
wherein R' is acetyl or trifllloroacetyl may he prepared
from the compourlds of Formula IX arlcl X l)y reacting the
compound with an appropriate anhydride as discussed
previously.
Similarly, N-methyltetraæolyl derivatives may be
prepared as described in connection Wittl Scheme I above.
The anti-allergic activity of the compounds o
Formula I may be demonstrated via a variety of biological
assays including in vltro assays for measuring inhibition
of lipoxygenase activity and leukotriene synthesis, and in
_ivo assays for inhibiting bronchoconstriction.
;3~
-l3-
More specifically, a suitable assay for
demonstrating inhibition of lipoxygenase activity by the
compounds of Formula I utilizes lipoxygenase isolated from
mammalian lung tissue, for example, the lung tissue of
S guinea pigs. An example o~ such an assay is that describe~
by sen ~7.i7, et a]., ~nal. siocllem~ 34, ~ (1970). 'l'he
inhibition of lipoxygenase activity is measured by a rapid
and sensitive spectrophotometr;c technique. rhe compounds
of Formula (I) exhibit an IC50 (the
concentration at which 50~ of the enzymatic activity is
inhibited) of less than about 100 micromoles per liter.
Preferred compounds exhihit an tC50 oE less than about 50
micromoles per liter. Most preferred compounds exhibit an
IC50 of less than about 10 micromoles per liter.
The activity of the compounds of Formula I may
also be demonstrated in a more specific test for
leukotriene biosynthesis inhibition. This test utilizes
the cell free leukotriene biosynthesis system of M.
Steinhoff et al., Uiochim. Biophys. ~cta. 6~, 2~ (1980),
which consists of homogenized rat basophil leukemia cells.
Leukotriene synthesis is initiated by the addition of
arachidonate. Solutions are centrifuged and supernatants
assayed using a radioimmunoassay developed as described by
Aeringhaus et al., FEBS Letter 146, 111-114. Drugs are
dissolved in ethanol or dimethyl sulfoxide and preincubated
for flve minutes. Phenidone is used as a positive control.
The compounds of Formula I exhibit an IC50 of 100
micromoles per liter or less. Preferred compounds exhibit
an IC50 less than 25 micromoles per liter, and most
preferred compounds exhibit an lC50 of ]ess than 10
micromoles per liter.
The compounds of Formula I are relatively
inactive as inhibitors of cyclooxygenase. This is
important in order for there to be good _n _ iVO
antiallergic activity. ~ conven;ent in v_tro method for
.
,: , .
-14- ~295~
measuring cycloox~genase activity is an assay wherein the
amount of thromboxane s2 production is measured in a whole
blood clotting assay. The thromboxane s2 production is
measured by a radioimmunoassay as described by Patrono, et
al, Thromb. Res. 17, 317 (1980). Tlle compounds of Formula
I do not show appreciable activity at concentrations of 100
micromoles per liter.
The i_ vlvo test used to demonstrate
anti-allergic activity may be any of those known to those
skilled in the art. Preferably, bronchoconstriction in
sensitized guinea pigs is measured upon antigen challenge.
This te~st is described in broad terms by Piechuta, et al.,
Immunology, 38, 3B5 (1979), and more speci~ically by
Hammerbeck and Swingle, Int. Archs. Allergy Appl. Immun.
74, 84-90 11984). It is used in a modified form as
follows: Male l~artley guinea pigs (250-600 g) are dosed
with a eompound of Formula I in an amount generally about 1
to ~0 mg/kg. Fifteen minutes later the animals are aerosol
challenged with either water or ovalbumin at a
concentration of 10 mg per ml. The animals are then placed
under an inverted dessicator jar ~18 x 14 cm) with a
eonstant flow o~ air coming into the chamber from a
eompressed-air source to prevent hypoxia. ~ir flow leaving
the ehamber and fluctuations due to respiration are
monitored through a separate outlet with a Fleisch No. 0000
pneumotachograph (available from Beckman Instruments, Inc.,
Schiller Park, Illinois) coupled to a Beckman 'l'ype 1
dynograph (available ~rom ~eekmall Instrumellts).
Aerosoliæation through a thi~] outlet is made via a No. 40
DeVilbiss nebulizer (available from l'he DeVilbiss Company,
Somerset, PA) for 90 seconds at 150 mm llg. The
charaeteristic respiratory patterns observed are summations
o two air exehange processes occurring simultaneously in
the chamber. One exchange process is due to inspiration
and expiration of air into and out -~ the animal, while the
other exchange process is due to the air flow into and out
o~ the chamber due to respiratory movements. The tracing
s~
- 15 -
obtained is the mechanical representation of the summation
of those flows. Superimposed on the tracings was a
characteristic spiking ('notching'), which appears to be due
to an exaggerated expiratory movement, the frequency of
which correlates with the severity of the broncho-
constrictive reaction. The frequency of notching for 15
minute periods beginning 4 minutes after the beginning of
the aerosol challenge is used for comparing various
treatments. Effects are considered significant if the t
value achieves p <0.05. Compounds of formula (I) exhibit an
intraperitoneal ED40 of lOO mg per kg or less when tested in
the above model. Preferred compounds exhibit an ED40 of 20
mg per kg or less. Most preferred compounds exhibit an
ED40 of 10 mg per kg or less and are effective orally.
The imine intermediates of formula (IV) according
to the present invention are also active as an antiallergic
agent and are believed to be reduce in vivo to the corres-
ponding compounds of formula (I). Specifically, according to
the present invention, 4-amino-3-(2,6-di-t-butycyclohexa-
dienon-4-ylideneamino)benzoic acid, 4-(2,6-ditertiary-butyl-
cyclohexadienon-4-ylideneamino)benzoic acid, 4-acetamido-3-
(2,6-di-tertiary-butylcyclohexadienon-4-yli.denearrllno)benzoic
acid, 3-(2,6-di-tertiary-buty:Lcyclohexad:ienon-4-yliderle-
amino)benzoic acid and other intermediates such as: ~-(2,6-
di-tertiarybutycyclohexadienon-4-ylideneamino)hippuric acid
and, 4-(2,6-ditertiary-butylcyclohexadienon-4-ylideneamino)
cinnamic acid, have been found to exhibit useful activity in
the above described in vivo assay involving bronchoconstric-
tion. The 3-(2,6-di-tertiary-butylcyclohexadieno-4-ylidene-
amino benzoic acid, when administered in vivo to a dog, hasbeen found to be converted to the compound 3-(3,5-di-ter-
tiary-butyl-4-hydroxyanilino) benzoic acid.
One of the preferred compounds of formula (I)
namely 3-(3,5-di-tertiary-butyl-4-hydroxyanilino)benzoic
acid has been found to be active as a bronchodilator in the
33~
small airways of the guinea pig as de-termined using the
method described in L. Diamond et al., J. Appl. Physiol.:
Respirat. Environ. Exercise Physiol., 43 (6), 942-948
(1977)-
Thus, compounds of formula (I) are antiallergic
agents exhibiting in vivo activity in mammals. The
pharmaceutical compositions contain sufficient compound of
formula (I) in a dosage form suitable for inhibiting the
mammalian biosynthesis of leukotrienes, or for the treatment
desired. The effective concentration of the formula (I)
compound in the composition will vary as required by the
mode of administration, dosage form, and pharmacological
effect and level desired.
For treating pulmonary conditions such as asthma,
the mode of adminstration may be oral, parenteral, by
inhalation, by suppository and the like. Suitable oral
dosage forms are tablets, elixirs, emulsions, solutions,
capsules, including delayed or sustained release dosage
forms. Dosage forms for administration by inhalation
include aerosols and sprays which may be administered in
metered doses if desired.
For treating other allergies or allergic
reactions, the compound of formula (I) may be administered
by any conventional mode, for example, orally, parenterally,
topically, subcutaneously, by inhalation and the like. The
oral and parenteral dosage forms are as described for
pulmonary treatment. The topical application dosage forms
include ointments, sprays, controlled release patches,
powders, solutions and the like.
For treating inflammation, the mode of
administration may be oral, parenteral, by suppository and
the like. The various dosage forms are as described above.
For treating skin diseases such as psoriasis,
atopic dermatitis and the like, oral, topical or parenteral
administration is useful. For topical application to the
~ Z~33~7
-1 '7 -
diseased area, salves, patches, controlled release p~tches,
emulsions, etc. are convenient dosage forms.
For treating cardiovascular contlitiorls any
suitable mode of administration may be usecl.
In addition to the common dosage forms listed
above, the compounds of Formu]a I may also be administered
for various utilities and indications or for inhibiting
leulcotriene synthesis by conventional controlled release
means and/or delivery devices.
In preparing suitable dosage forms, conventional
compounding procedures and ingredients, for example,
diluents, carriers, etc. may be used. Examples of suitable
solid carriers are lactose, terra alba, sucrose, talc,
gelatin, agar, pectin, acacia, magnesium stearate, stearic
acid, and the like. Examples of suitable liquid carriers
are syrup, peanut oil, olive oil, water, and the like.
Similarly, the carrier or diluent can include any time
delay material well known to the art, sucl- as glyceryl
monostearate or glyceryl distearate, these being useful
alone or, for example, in combination with wax.
The f~llowing Examples are provided to ill~lstrate
the invention Of the parent application and the pr~sent in-
vention, but are not intended to limit the invention.
~XAMPLE 1 - Preparation of 4-(3,5-Di-tertiary-butyl-4-
hydroxyanilino)henzoic ~cid
Step A
~ mixture of 22 g (0.10 mole) o
2,6-dl(tertiary~butyl)-p-ben%oquillorle, 13.7g (0.10 rnole) of
4-aminobenzoic acid, 175 ml of tetrahydrofuran and 1 ml of
boron trifluoride: diethyl ether complex was lleated on a
steam bath for 1.25 hours. l'he mixture was allowed to cool
to about 20C over 16 hours under a nitrogen atmosphere.
Evaporation provided a solid whicll was washed with hexarle
and recrystallized from ethanol to provide orange solid
2,6-di(tertiary-butyl)-4-(4~-carhoxypllenylimino)-2,5-
cyclohexadien-1-one, m.p. 305-309~C. AnalysiS: Calculated
for C21H25NO3: %C, 74.3; ~ll, 7.4; ~N, 4.1; Found: ~C, 74.2;
~EI, 7.4; %N, 4.1.
To a solution of 5.0 g (0.0147 mole) of
2,6-di(tertiary-butyl)-4-(4~-carboxyphenylimino)-2,5-
cyclohexadien-l-one in 300 ml of ethanol was a(~ded 0.25g of
5 percent palladium on cllal-coal. It was suhjected to
hydrogenation in a Paar apparatus ancl filtered. This
solvent was removed by evaporation under vacuum and the
residue was recrystallized from a 5:2 (v/v) ethanol-water
mixture to provide light-orange crystals of
4-(3,5-di-tertiary-butyl-4-hydroxyanilino)henzoic acid,
m.p. 241-243C. Analysis: Calculated for C21l~27NO3: ~C,
73.9; %I~, 8.0; ~N, 4.1; Found: ~C, 73.9; ~I, 7.9; fiN, 3.8.
EX~MPLE 2
Using the method of Example 1, 2,6-di(tertiary-
butyl)-p-benzoquinone was reacted with 3-aminobenzoic acid
to provide red-orange crystals of 2,6-di(tertiary-butyl)-
4-~3'-carboxyphenylimino)-2,5-cyclohexadien-l-one, m.p.
230-231C. Analysis: Calculated for C21l~25NO3: %C, 74.3;
~H, 7.4; %N, 4.1; Found: %C, 74.1; ~H, 7.6; ~N, 3.7.
EX~MPLE 3-6
Using the general method of Example 1, the
aminobenzene starting materials of Formula III, shown in
Table I below were reacted with 2,6-di(tertiary-butyl)-p-
benzoquinone to provide the imine products indicated in
Table I.
-19-12~ 37
Tal)l e
Ex~m~le Starting Matel-ia1 I'ro~ t ~t F~rn1uJ~ lV
_ No. ~r rormula III~ - ('!' P~ `C)
3 4-~m;l~opllenylacet;c ((113)3 ~ ~-N <(~ ~ -CII~COOII
acid (~ 3(
( ] ~ n
4 NH2 ~(~NIIC1~7(,0011 (`< ~- N~-CNHC112(,()011
(239.~ 240)
~COOII ~ 3 ~ ~N~ COOII
1~2N ( 3 ) 3(;
( 225 . 5-226 )
( Cll 3 ) 3C ~C00~1
6 ~12N 1~) lcO()~l O~N--/~
CH30~ ( C113 ) 3(~ CH3
( 226 . 5-228 )
EXI~MPLE 7
To a mixture of 200 ml of ethanol and 23. 8g
(0.0701 mole) of 2,6-di(tertiary-butyl)-4-(4'carboxyl-
phenylimino)-2,5-cyclohexadien-1-one was added 2.9 g (0.072
mole) of sodium hydroxide in 20 ml of water. To this
mixture was added l.Og of 10~ pal]adium on charcoal,
followed by the addition of 50 ml of water. The mixture
was reduced by agitating on a Paar apparatus for about 16
hours. Celite*was added to the mixture, and the mixture
was Eiltered through a bed of Celite* The mixture was
acidified with 6N hydrochloric acid, and the resulting
yellow solid precipitate was collectecl by filtration to
provide 4-l3,5-di(tertiary-butyl)-4-hydroxyanilino]benzoic
acid, m.p. 241-242C.
* Trade-mark
~29,5~ t~
--20-
EX~MPLE 8
-
To a mixture of 200 ml of ethallol ancl 25.0g
(0.0736 mole) of 2,6-di(tertiary-butyl)-~-
(4'-carboxyphenylimino)-2,5-cyclohexadien-l-one and 12 g
l0.087 mole) of potassium carbonate warmed on a steam batl
was added l.Og of palladium on charcoal. rhe mixture was
reduced using a Paar apparatus for 2 hours. The mixture
was diluted with 300 ml of water, filtered throtlgh celite,
and the filtrate acidified with 6N hydrocllloric acid. ~'he
yellow solid precipitate was collected by filtration to
provide 4-l3,5-di(tertiary-butyl)-4-hydroxyanilinolbenzoic
acid, m.p. 241-242C.
EX~MPLES 9--13
Using the general method of Example 7 or 8 the
imine intermediates obtained in Example 2-6 were reduced to
provide compounds of Formula I shown in Table II below:
.53~7
O
V U~
d ~ a
rtl~o ~ ~ r~ J
O
V X X X X ~
~) ~ W W rll rll r,r
~n
C r~ o
O o
~o
C~ I I I I
~ ~ m u~ m n
C r~ ~ o
c~ o r-- ~ m
a)
m o= ~
O ~
u ~ m
~ æ o m m
~ ~ o~ ~ O Co~
~ ~ ~; $ ~: p~
o ~s~ u~u ~u
r~ r-7 I r~ r~) ¦ r~ r~ r~- r~l ~ r~
_ _ o ~ ^ O ^ ^ ^ ^ ^
r~ ~ r~ r~r~l r~ m r~l r~ m r~ r~7 m r~
O m ~q u q u
P~ _ _ _ _ _ _, .... , ~ _ ~
~U
o
1- ~ rr~ ~ In ~o
rJ ,1 ~ ~ ~ ~ r
e o rd ~ X X X
~ ~ r~ ~ IL1 r~ ~
~ O
r~ :Z; o ~ ~ r
_ ~/
~5337
-22-
EXl~MPLE 1~1
A mixture of a solution of 6.3 g (0 01~ mole) of
4-[3,5-di-(tertiary-butyl)--4-llydroxyanilirlo]pllenylacetic
acid in 10 ml of N,N-dimetlly]formamide and 5 g (0.036 mole)
of potassium carbonate was heated on a steam bath until gas
evolution ceased. The mixture was al]owecl to cool to
ambient temperature, and 5 milliliters of methyl iodide
were then added. The mixture was lleated at its boiling
temperature and 5 ml aliquots of methyl iodide were added
at 20, 45 and 60 minutes. ~fter the mixture had
evaporated, the residue was taken up in water and 2N sodium
hydroxide solution was added. Tl-e mixt~1re was warmed, and
the insoluble residue of methyl 4-[3,5-di(tertiary-butyl)-
-4-hydroxy-N-methyl-anilino] phenylacetate was separated by
filtration. The residue was suspended and partially
dissolved in 50 ml of methanol, and 10 ml of 2.5N sodium
hydroxide solution was added. The mixture was stirred for
about 16 hours, diluted with 300 ml of water and 300 ml of
diethyl ether, and was then further diluted with about 100
ml of hexane. The mixture was acidified with dilute
hydrochloric acid and the aqueous phase was discarded. The
organic phase was washed first with 10~ sodium bicarbonate
solution and then with 5, sodium carbonate solution. The
product remained in the organic phase as the sodium salt.
The organic phase was acidified with 10~ aqueous
hydrochloric acid and washed with sodium chloride solution,
followed by drying. Evaporation provided a residue which
was extracted with 20 ml o~ boiling benr~etle. llexane (6 ml)
was added, and the llght yellow solid was recrystallized
first from ~06 aqueous ethanol, and then from benzene to
provide fine yellow needles of 4-t3,5-di(tertiary-butyl)-
4-hydroxy-N-methylanilinolphenylacetic acid, m.p.
lal-1~2.s~c. Analysis: Calculated for C23E131N03; ~C, 74.~;
%E~, ~.5; %N, 3.~; Found: sC, 74.~ .6; ~N, 3.6.
2 3 3 3~
EXAMPLE 1 5
-
A solution of 3.4g (~.010 mole) of
4-(3,5-di-tertiary-butyl-4-hydroxyanilino)benæoic acid
(prepared in Example 1) in 35 ml of N,N-dimetllylformamide
and 3.5 ml of methyl iodide was heated at 95~C for about 4~
hours under nitrogen. Ille reaction mixture was poured into
cold water and the resulting solid was collected and then
taken up in chloroform. The chloroform solution was
filtered, washed with water, dried with magnesium sulfate
and evaporated to give a tan solid. ~his material was
recrystalli~ed first from benzene and then from a mixture
of ethanol and water to give 1.5 g of white crystalline
4-(3,5-di-tertiary-butyl-4-hydroxy-N-methylanilino)benzoic
acid, m.p. 240-244C. Analysis: Calculated for C221l29N03:
%C, 74.3; %H, ~.2; %N, 3.9. Found: 6C, 7q.4; ~ .3; ~N,
3.5.
EXAMPLE 16
A mixture o~ 2.~ g (0.005~9 mole) of
2,6-di(tertiary-butyl)-4-(4-carboxyphenylimino)-2,5-
cyclohexadien-1-one and 2.5 g of sodium thiosulfite in 25
ml of lN sodium hydroxide solution and a few ml of diethyl
ether were stirred at 20nC. ~fter one hour o~ stirring the
mixture was heated on a steam bath while adding 1.5 g of
sodium thiosulfite and enough sodium llydroxide to make the
solution alkaline. After one hour the solution was
acidified with 6N hydrochloric acid. The preclpitate
separated by filtration to provide a llght oratlge solld was
about 50~ 3-[3,5-di(tertiary-butyl)-4-hydroxyanilino]-
ben70ic acid according to thin layer chromatographic andinfrared spectral analyses.
EX~MPLES 17 - 19
Using the general method of Example l, the
aminoben~ene starting materials o~ Formula III below were
reacted with 2,6-di~tertiary-hutyl)-p-ben7oquinone to
provide the imine products indicated in T~BLE III.
--2~ Z~533i';7
Tl~BLE I I I
Starting Product of Melting
Example Mater;al of Formula ]v Point
Number Formula III (m.~ in C _n C
Co2ll (C1~3)3C CO2ll
17 ~12N- ~ S ~ ~ / (2ql-245)
C~3(C113~3C C113
CO2H (CH3)~C CO2H
18 2 ~ Ol~ o ~ ~ N- ~ C)> - OH (249-259)
(Cll3)3C
CO2H (C~3)3 CO2H
l9 H2N ~ Cl ~ N ~ - Cl (195-199)
(C 3)3
EXAMPLE 20
To a mixture of 275 ml ethanol arltJ 13.6 9 (0.03
mole) of 2,6-di(tertiary-hutyl)-~-(5'-carboxy-2'-methyl-
30 phenylimino)-2,5-cyclohexadiene-1-one (from Example 18) was
added l g of 5% palladium on charcoal (50~ water wet). The
mixture was reduced by agitating on a Paar apparatus for 2
hours. The mixture was filtered througll celite to remove
tlle catalyst and the filtrate wa~ concentrated on a rotary
evaporator to give 13.0 g of a light orange solid, m.p.
229-233C. This material was recrystallized from aqueous
ethanol to give 10.B g orange crysta]line solid m.p.
-25-- ~Z9S33~
234-239C. This material w~s in turn recrystallized from
benzene to give 9.6 9 pale orange solid
3-(3,5-di-tertiary-butyl-4-hydroxyanilino)-~-metllylbenz.oic
acid, m.p. 234-239~C. ~nalysis: Calculated for C22ll29NO3:
%C 74.3; %1l n.2, 6N 3.9. Found: 6C, 7~.3; ~ .2; ~N 3.8.
EXAMPLE 21 - 22
Using the general method o Example 20, the imine
intermediates obtained in Examples 1~ and 19 were reduced
to provide compounds of Formula I shown in TAsLE IV below:
TAsLE IV
Sxample
Number Product of Formula I (m~p. in C)
- _ _ _ _ _ ___ _ __
~CH3)~C /CO2~}
21 HO ~ N~ Cl (200.5-201.5)
(Cil3)3C
(C1}3)/CO2ll
22 I}O- ~ Nll- ~ OEI (234-238)
(CH3)3C
X~MPLE 23
~ mixture of 22.0 g (0.10 mole) of
2,6-di(tertiary-butyl)-p-ben7oquinone, 19.0 9 (0.1~5 mole)
of (4-aminophenyl)thioacetic acid, 100 ml of
tetrahydrofuran and 1 ml of boron trifluoride: diethyl
ether complex was heated at gentle reflux, with stirring,
for 1.5 hours. The reaction mixture wa6 concentrated under
a nitrogen gas flow to a volume of 75 ml. rlle concentrate
was di.luted witll 250 ml ethanol and 1 g oE palladium on
-26- ~9533~
charcoal was added. This mixture was hyclrogel-ated on a
Paar apparatus for 12 hours, then filtered through celite
to remove the catalyst. The filtrate was concentrated to
give 33.7 g of an oil. The oil was taken up in diethyl
ether. Tllis solution was washecl with cl;]ute (about 10~)
hydrochloric acid, then dried and evapor~ted to give la.4 9
of a gummy solid. This materia] was recrystallized from
5:6 ben~ene:hexane to give 6.3 g of pink solid
4-(3,5-di-tertiary-butyl-4-hydroxyanilino)pllenylthioacetic
acid, m.p. 136.5-137.5C. ~nalysis! Ca]culated for
C22H29NO3S: %C, 68.2; ~Il, 7.5; %N, 3.6. Found: ~C, 6~.3;
~H, 7.7; ~N, 3.3.
EX~MPLE 24
A mixture of 22.5 g (0.105 mole) of
2,6-di-tertiary-butyl-p-benzoquinone, ll.B g (0.10 mole) of
anthranilonitrile, 50 ml of tetrahydrofuran and 1 ml of
boron trifluoride:dietllyl ether complex was heated at
gentle reflux for 2 hours. Heating was continued for an
additional 2 hours under a stream of nitrogen gas to
concentrate the mixture. The concentrated mixture was
diluted with 50 ml of ethanol, warmed to effect complete
dissolution, and then allowed to cool. The precipitate was
collected, rinsed with cold ~:1 methanol:water, and oven
dried to give 23.6 g of orange crystals of
2,6-di-tertiary-butyl-4-(2~-cyanophenylimino)-2,5-
cyclohexadien-1-one, m.p. 109~110.5C.
To a mixture of 15.0 9 (0.0~ mole) ok
2,6 di-tertiary-1~utyl~ (2'-cyanophenylimirlo)--2,5-
cyclohexadien-1-one (obtained above) and 200 ml of ethanol
was added 1 g of palladium on charcoal. The mixture was
hydrogenated on a Paar apparatus for 10 minutes. The
solvent was decanted off, then the residual solid was
dissolved in chloroform. The chlorokorm solution was mixed
with ethanol, filtered, and concentrated to give 1~.6 g of
tan so]id, m.p. 170-173C. ~ portion (].9 9) of this
material was recrystalli7ed from ethanol to give ~ale
-27- ~ 3~
orange prisms of the novel compound 2-(3,5-di-tertiary-
butyl-4-hydroxyanilino)benzonitrile, m.p. 171.5-172.5C.
Analysis: Calculated for C21H26N20: OC 7~.2; %ll 8-1; ~N
8.7. Eound: %C 78.3; ~H 8.3; ~N ~.6.
A mixture oE 5.~ g (O.OlR mole) 2-(3,5-di-
tertiary-butyl-4-hydroxyanilillo)ben70nitLile, 40 9 of 50~
sodium hydroxide and 100 ml of ethanol was heated at gentle
reflux for 3 hours. The reaction mixture was poured onto a
mixture of 100 ml of 6N hydrochloric acid and ice. The
precipitate was collected and dried in a vacuum oven to
give 5.8 g of yellow solid, m.p. 217-219C. This material
was recrystallized from a mixture of ethanol and water to
give 4.9 g of light orange needles of N-( 3,5-di-tertiary-
butyl-4-hydroxyphenyl)antll~anilic acid, m.p. 220.5-221.5C.
Analysis: Calculated for C2lll27N03: fiC 73.9; r~H 8.0; ~N
4.1; Found: fiC 74.1; ~ll 8.1; oN 4Ø
EX~MPLES 25-26
Using the general method of Example 1 the
starting materials of Formula VI shown in TAsLE V below
were reacted with 2,6-di(tertiary-butyl)-p-ben~oqllinone to
provide the imine products indicated in T~LLE V.
Starting
Example Material of
Number Formu a_ I_ Pro u t of Formula I m p. in ~C)
CN ( 3~ CN
N~2 ~ (102.5-103)
(Cl~3)3C
(C~l3)3c
26 NH~O~ CN O~N~O)-CN ( 140-142)
(C113)3C
-2
EX~MPLE 27
Using the method of Example 20, ~.0 g of
2,6-di-t-butyl-4-(3'-cyanoplleny]imino)-2,5-cyclohexadien-1-
one (from Example 26) was converted to 3-(3,5-di-t-butyl-
4-hydroxyanilino)hen70nitr;1e, m.p. ]50--153C.
EX~MPLE 28
Eight g (0.025 mole) of
3-(3,5-di-t-butyl-4-hydroxyanilino)benz.orlitrile (from
Example 27), 4.9 q (0.075 mole) of sodium azide, 4.0 g
(0.075 mole) of ammonium chloride, 1.06 g (0.025 mole) of
lithium chloride and 60 ml of N,N--dimetllylformamide were
combined under a nitrogen atmosphere and heated at 110C
for 4B hours. The reaction mixture was poured into cold 6N
hydrocllloric acid and a gummy solid precipitated out. The
supernatant was decanted off and tlle residue dissolved in
ethanol. The ethanol solution was diluted with water and a
pink solid was collected. Tllis material was recrystallized
from a mixture of ethanol and water to give 5.97 g of
5-[3-(3,5-di-t-butyl-4-hydroxyanilino)phenyl]tetrazole,
m.p. 231-233C. ~nalysis: C~].culatecl for C21l327N5O: ~C,
69.0, %H, 7.~; 6N, 19.2; Found: ~C, 6~.~; 6~1, 7.6; %N,
~ 3.
EX~MPLB 29
Using the metlloc3 of Example 20, 10 g o
2,6-di-t-butyl-~ 1'-cyanopllenyli~ o-2,5-cyc:l.ollexadie
one (~rom Example 27) was hydrogellate(l to give
4-(3,5-di-t-butyl-4-hydroxyanil;no)benzonitLile.
EX~MPLE 30
Seven g (0.0205 mole) of 4-(3,5-di-t-butyl-4-
hydroxyanilino)benzonitrile (from ~xample 29), 4.01 q
(0.0615 mole) of sodium azi(3e, 3.29 g (0.0615 mole) of
ammonium chloride and 50 ml of N,N--dimetllylformamide were
combined and heated first at 105~C for 20 hours and then at
150C for 9 hours. The re~ction mixture was diluted with
-29- ~ ;337
diethyl ether and water and acidified with 6N ~lCl~ The
ether phase was washed with sodium chloride solution, dried
over sodium sulfate, and concentrated to an oil under a
stream of nitrogen. The cr~lde product was treated with
5 chloroform and l-exane to convert it llrom an oil to a solid.
The solid was recrystalli7ed from a mixture of ethanol and
water to give 2.6 g of light orange needles of 5-14-(3,5-
di-t-butyl-4-hydroxyanilino)phenyl]tetraæole, m.p.
22~-225C. I~nalysis: Calculated for C211~27N5O-C2~l5OII:
10 %C, 67.1; %H, 8.1; %N, 17.0; Found: $iC, 67.1; ~iH, û.2; 7iN,
16.9.
EXI~MPLE 31
Preparation of N,N-Dimethyl-2-aminoethyl
15 3-(3,5-r)i-tertiary-butyl-4-hydroxyanilino)benzoate
Under a nitrogen atmosphere, 6.0 g (0.0176 mo]e)
of 3-(3,5-di-tertiary-butyl-4~hydroxyanilino)benzoic acid
(from Example 13) and 2.53 g (0.0176 mole) of
2-dimethylaminoethyl chloride hydrochloride were dissolved
20 in 17 ml of N,N-dimethylformamide. To this mixture was
added 4.9 ml (0.035 mole) of triethylamine and the reaction
was heated at l00C for 25 llours. Tlle reaction temperature
was then raised to 120C and lleating was continued for an
additional 2B hours. The reaction mixture was diluted with
25 10 ml of diethyl ether, then filtered to remove
triethylamine hydrochloride. The filtrate was diluted Witl
additional diethyl ether and then shaken with cold 10fi
hydrochloric acid. A middle layer contained most o tlle
prodùct hydrochloride. It was diluted witll water and
30 ether, and basified with solid sodium carbonate. The ether
extract was washed witll saturated aqueous sodium chloride
and then concentrated to give 1.7 g of a tan solid, m.p.
119-120C. This material was recrysta]li~ed first from 20
ml of cyclohexane and then from a mixture of 10 ml of
35 ethanol and 3 ml of water to give 1.4~3 g of light yellow
prisms of N,N-dimetl)yl-2-aminoetllyl 3-t3,5-di-t-butyl-4-
hydroxyanilino)benzoate m.p. 123-124C. Analysis:
~;29~3~37
-30--
Calculated for C2S1~36N203: %C, 72.~3; 611~ 8; ~N~ 6
Found: %C, 73.0; %H, û.8; ~iN, 6.7.
EX~MPLE 32
S Preparation of N-~cetyl-3-(3,5-di-t-butyl-4-
hydroxyanilino)benzoic ~cid.
A mixture of 5.6 g of 3-(3,5-di-t-butyl-~-
hydroxyanilino)ben~oic acid (from Example 13) and 15 ml of
acetic anhydride was heated under a nitrogen atmosphere at
10 70-120C for about 90 minutes. The mixture was cooled to
80C, 5 drops of pyridine were a~lde~l and the reaction was
reheated to 120C for 10 minutes. t~n additional 0.25 ml of
pyridine was added at about 80C, followed by the gradual
addition of 10 ml of water to hydrolyze the excess acetic
15 anhydride and the mixed anhydride of the product. Ileating
was continued until a precipitate formed. Tlle reaction
mixture was allowed to cool to room temperature, and the
precipitate was then collected, rinsed witll a cold mixture
of methanol and water, and dried to give 4.3 g of off-white
20 solid m.p. 204-205.5C. This material was recrystallized
first from a mixture of 30 ml of ethanol and 5 ml of water,
and then from a mixture of 30 ml of isopropanol and 5 ml of
hexane to give 3.3 g of white solid N-acetyl-3-(3,5-di-t-
butyl-4-hydroxyanilino)benzoic acid m.p. 214.5-215.5C.
25 ~nalysis: Calculated for: C23H29NO4 1/2(CM3)2CHO~ C,
71.2 %~l, 8.0; ~N 3.4. Found: 9~C, 71.4; lill, 8.1; %N, 3.2.
EXI~MPLE 33
- Preparation of N-T~ifluoroacetyl-3-(3,5-di-t-
30 butyl-4-hydroxyanilino)benzoic Acid.
2.96 g of 3-(3,5-di-t-butyl-4-1lydloxyanilino)-
benzoic acid ~from Fxample 13) was slurried in 10 ml of
trifluoroacetic anhydride. After several minutes, the
reaction boiled ('10C) and then became clear. The reaction
35 mixture was poured into a mixture of ice and water and the
resulting solid was col]ected and dried. This material was
recrystallized from a mixture of 40 ml of ethanol and 12 ml
-31- ~2~3~
of water to give 3.07 g of wI~ite crystalliIle N-trifluoro-
acetyl-3-~3,5 di-t-butyl-4-hydroxyanilino)-I~en70ic acid
m.p. 181C. ~nalysis: Calculated for C23II26~3NO~: ~C,
63.1; ~IT, 6.0; ~N, 3.2. Found: C, 63.0; %I~, 6.~; ~N, 2.8.
EXAMPLE 3~
~ mixture of 22.0 g (0.10 mole) of
2,6-di-t-butyl-p-benzoquinone, 15.2 g (0.10 mole) of
3,4-dia~inobenzoic acid, 50 ml of tetrahydrofuran and 1 ml
of boron trîfluoride etherate was heated at about 55c for
45 minutes. The reaction mixture was diluted with 100 ml
of ethanol and 40 ml of water and was then allowed to stand
overnight at 25C. The resu]ting precipitate was collected
and dried to give 31.~ g of deep red solid 4-amino-3-(3,5-
di-t-butylcyclohexadienon-4-ylideneamino)benzoic acid, m.p.
253-253.5C. Analysis: Calculated for C21II26N2O3: ~C,
71.2; ~II, 7.4, ~N, 7.9. Found: ~C, 71.0; 3II, 7.5; ~N, 7.8.
A mixture of 21.1 9 of 4-amino-3-(3,5-di-t-butyl-
cyclohexadienon-4-ylideneamino)- benzoic acid, 50 ml of
20 ethanol, 20 ml of water, 2.4 g of sodium hydroxide and 0.03
g of 5% palladium on charcoal catalyst was placed on a Paar
appartus. After 16 hours the hydrogen uptake was complete.
Under a nitrogen atmosphere, the reaction was filtered into
13 ml of 6N hydrochloric acid. The filtrate was diluted
with water and additional hydrochloric acid. The resulting
precipitate was collected, rinsed with a col~ mixture of
methanol and water and dried to give 15.5 ~3 of a lavender
solid, m.p. 261.5-262'~C. ~rwO g o tlIis materIal was
6tirred with 150 ml of warm ethyl acetate, and was tllen
filtered. The filtrate was diluted witll 25 ml of hexane.
The resulting precipitate was collectecl anc3 dried to give
0.3 g of light purple crystalline ~-amino-3-(3,5-di-t-
butyl-4-hydroxyanilino)benzoic acid m.p. 261-261.5"C.
Analysis: Calculated for C21II2~N2O3: ~C, 70.~; ~II, 7-9; -6N,
7.9. Found: ~C, 70.8; ~II, 7.9; ~N, 7.6.
liL`2~3~3~
-3~.-
EX~MPLE 35
Under a nitrogen atmosphere, a suspension of 2.0gof 5-[4-(3,5-di-tertiary-butyl-4-llydroxyanilino)phenyll-
tetrazole, prepared in Examp1e 30, and 2.0g of potassium
carbonate in 4 ml of N,N-dimetllylformamicle was warmed to
obtain a deep red solution of the potassium salt.
~pproximately 4g of methyl iodide was added and the
reaction was war~ed for several minute~ until the color
lightened. An additional qg of methyl iodide was added and
tlle reaction mixture was heated at a gentle reflux for
about five minutes. ~he reaction mixtul:e was cooled,
diluted with diethyl ether, and poured into dilute
hydrochloric acid. The ether phase was washed Witll water
and brine, dried with sodium sulfate, and evaporated to
give l.~g of a red-brown solid. L'llis material was
recrystallized from a mixture of ben7ene and hexane to give
0.53g of light yellow-tan Material ~ll, m.p. about 205C. A
solid precipitated from the mother liquor of Material ~1.
It was collected to give 0.73g of pink Material ~2, m.p.
172-175.5C. Material ~1 was recrystallized from a mixture
of 20 ml of ethanol and 5 ml of water to give 0.34g of
light brown granules, m.p. 21~-221C. Material ~2 was
stirred with 50 ml of ethanol, then filtered to remove some
undissolved material. The filtrate was diluted with 20 ml
Of water to give 0.56g of pale pink leaflets, m.p.
175.5-177C. By proton NMR analysis, Material ~1 is
believed to be the l-methyltetrazole an(l Matcrial l12 the
2-methyltetrazole. rhe delta-values are ~.15 and ~.36 ppm,
respectively, for the two products.
EX~MPLE 36
Using the method of Example 1, 4-aminobenzyl-
cyanide was reacted witll 2,6-di-tertiary-butyl-p-benzo-
quinone to give 2,6-di-tertiary-butyl-4-(4'-cyano-
methylphenylimino)-2,5-cyclollexadiene-1-olle, m.p.
126.5-127.5C. Following the reduction method of Example
1, Step B, the corre.sponding anilino phenylacetonitrile,
:~Z9~;337
--33--
m.p. 146-147C, was obtained. ~his was converted to
5-[4-(3,5-di-t-butyl-Q-hydroxyanilino)benzyl]tetrazole,
m.p. 212-214aC (dec), following the method of Example 30,
but carried out at 110C for 4~ hours.
EXAMPLE _
Example 3G was rerun using tin tetrachloride
instead of boron trifluoride as the catalyst. Thin layer
chromatography using two different systems showed that the
reaction mixture contained the desired 2,6-di-tertiary-
- butyl-4-~4~-cyanomethylphenylimino)-2,5-cyclohexadien-1-
one.
EXAMPLE 3B
Example 36 was rerun using titanium tetrachloride
instead of boron tri1uoride as the catalyst. Thin layer
chromatography using two diferent systems showed that the
reaction mixture contained the desired 2,6-di-tertiary-
butyl-4-(4~-cyanomethylphenylimino)-2,5-cyclohexadiene-1-
one.
EXAMPLE 39
A mixture of 1.70g (0.005 mole) of
3-( 3,5-di-tertiary-butyl-4-hydroxyanilino)benzoic acid
(prepared in Example 13) and 50 ml of hot isopropyl alcohol
was filtered to remove a small amount of insoluble
material. The resulting solution was deoxygenated witll a
stream of nitrogen gas. Uner a nitrogen atmosphere, a
solution o 0.44g (0.005 mole) o morpholine in 1.5 ml of
isopropyl alcohol was added with rapid stirring.
Evaporation provided a solid which was recrystallized from
a mixture of isopropyl alcohol and isopropyl ether to give
solid morpholinium-3-(3,5-di-tertiary-butyl-4-hydroxy-
anilino)benzoate, m.p. 147-l50~C. Analysis: Calculated for
C211127NO3C4EIgNO: ~C, 70.1; ~1l, n.5; %N, 6.5; Found: ~C,
69.8; ~ .5; ~,N, 6.4.
_3(~_ ~2~;33~
EXAMPLE 4 0
A suspension of 6.6~g (0 0196 mole) of
2,6-di-tertiary-butyl-4-(3'-carboxyphellylimino)-2,5-cyclo-
hexadien-1-one (prepared in Example 2) in 20 ml of benzene
5 and 3 ml of thionyl chloride was heated at reflux with
stirring until gas evolution had ceasec1. ~vapc)l-ation
provided an oil which was diluted with a sma]l amount of
tetrahydrofuran and added dropwise to a suspension of 3.7g
of anhydrous 5-aminotetrazole in 25 ml of tetrahydrofuran
10 containing 1.5 ml of pyridine. Tlle reaction mixture was
allowed to stand at room temperature under a nitrogen
atmosphere for 16 hours. ~he reaction mixture was diluted
to a volume of 500 ml witl diethyl ether, and was then
filtered. The filter cake was rinsed with diethyl ether
15 and resuspended in 300 ml of diethyl etller and filtered
again. The combined filtrates were evaporated to give 4.7g
of an orange solid, m.p. 241-244C. One g of this material
was recrystallized from ethanol to provide 0.3g of orange
3-(2,6-di-tertiary-butylcyclohexadienon-4-ylideneamino)-N-
20 (5-tetrazolyl)benzamide, m.p. 271.5C (dec.). Analysis:
Calculated for C22ll26N6O2
Found: %C, 65.1; 611, 6.4; %N, 20.5.
l~ of mixture of 3.1g 3-(2,6--di-tertiary-butyl-
cyclohexadienon-4-ylideneamino)-N-(5-tetrazolyl)benzamide,
25 200 ml of tetrahydrofuran, and 0.7g of palladium on
charcoal catalyst was placed on a Paar apparatus.
Hydrogenation was complete after two hours. Under a
nitrogen atmosphere, the reaction was filtered to remove
the catalyst. The filtrate was evaporated to give I.3g of
30 a sticky yellow solid which was recrystallied from a
mixture of 130 ml acetic acid and 20 ml of water to give
1.6g o~ yellow solid 3-(3,5-di-tertiary-butyl-~-hydroxy-
anilino)-N1-(5-tetrazolyl)benzamide, m.p. 2~2-2a3Oc.
I~nalysis: Calculated for C22ll2~3N6O2: %C, 6~.7; %H, 6-9; %N,
35 20.6; Found: %C, 6~.6; 9,ill, 6.~3; 6N, 20.2.
- 3 5 - ~Z9~33'7
EX~MPLE 4_
A mixture of 5.519 (0.0~5 mole) of 2,6-di-
tertiary-butyl-p-benzoquinone, ~.5~ g (0.027 mole) of
3-(~-aminophenyl)propionic acid, 50 ml of tetrahydrofuran
and 0.25 ml of boron trifluoride etherate was heatecl on a
steam cone under a slow stream of nitrogen for two hours.
The resulting so]id was triturated with hexane, collectecl,
rinsed with hexane and recrystallized from a mixture of
ethyl acetate and hexane to give 5.1g yellow 3~ (2,6-di-
tertiary-butylcyclohexadienon-4-ylideneamino)phenyl]--
propionic acid, m.p. 165-167~C. ~nalysi6: Calculated for
C231129NO3 %C, 75.2; ~ .0; 6N, 3.~; Found ~C, 74.~3; %1~,
7.9; %N, 3.7.
~ mixture of 4.0 g of 3-l4-(2,6-di-tertiary-
butylcyclohexadienon-4-ylideneamino)~henylIpropionic acid,
200 ml of ethanol of O.lg of 10~ palladium on charcoal
catalyst was placed on a Paar apparatus. Ilydrogen uptake
was complete after 30 minutes. Under a nitrogen
atmosphere, the reaction mixture was filtered to remove
catalyst. The filtrate was evaporated to give an oil which
was coevaporated with hexane to remove all traces of
ethanol and then triturated with hexane to give a light
orange crystalline solid. This solid was recrystallized
from a mixture of ethanol and water to give 3.lg
3-lN-~3~5-di-tertiary-butyl-4-hydroxypllenyl)-4-amino-
phenyl]propionic acid, m.p. 140-142C. Analysis:
calculated for C23ll31NO3: ~C, 74.~3~ , n-5: ~N~ 3-~3;
Found: ~C, 74.7; ~Il, n.3; N, 3.9.
CXAMPLE 42
~ mixture of 22.0g (0.10 mole) of
2,6-di-tertiary-butyl-p-benzoquinone, 10.99 (0.01 mole) of
m-aminophenol, 50ml of tetrahydrofuran and 0.5 ml of boron
trifluoride etherate was stirred ~t room temperature for
about one hour. The reaction mixture was diluted with
diethyl ether, and the ether solution was extracted ~lth
10~ hydroch]oric acid ancl dried over magnesium sulfate.
-36- ~29533~
Evaporation gave an orange-red solid. ~ is material was
dissolved in a mixture of dietllyl ether and methylene
chloride. The solution was filtered, and was then
evaporated to give 29.1g orange-red solid. This material
was recrystallized from a mixture of 50 ml of benzene and
100 ml of hexane to give 17.09 of orange-re-l crystals, m.p.
162-169C. This material (2.59) was recrystallized first
from a mixture of 15 ml ben~ene and 10 ml of hexane and
then from a mixture of 10 ml of isopropanol and 7 ml of
water to give 1.3g of orange crystalline 3-(2,6-di-
tertiary-butylchclollexadienon-~-ylideneamino)pllenol, m.p.
169-169.5C. Analysis: Calculated ~or C2o1~25No2: %C, 77.1;
.1; 6Ei, 4.5; Found: ~C, 77.2; 611, ~.0; fiN, 4.6.
1.36g (0.027 mole) of 506 sodium hydride was
added in portions to a solution of 7.06g (0.023 mole) of
3-(2,6-di-tertiary-butylcyclohexadienorl-4-ylidenamino)-
phenol in a mixture of 50 ml 1,2-dimethoxyethane and 10 ml
of dimethylacetamide. Three ml (0.027 mole) of ethyl
bromoacetate was then added in portions. The reaction
mixture was stirred at room temperature for about one hour,
and a solution of 1.3g of sodium hydroxide in 12 ml of
water was added. After about 30 minutes the reaction
mixture was acidified with hydrochloric acid and was
extracted with diethyl ether. The ether extract was washed
with a saturated sodium chloride solution and evaporated to
give an orange solid. T1lis solid was recrystalli%ed from a
mixture of ben~ene and hexane to g;ve 6.2g oE an orange
solid, m.p. 161-162DC. One g of this material was
recrystallizeci from a mlxture of 10 ml of etllarlol and 5 ml
of water to give 0.~g of orange crystalline 3-(2,6-di-
tertiary-butylcyclohexadienon-4-ylideneamino)phenoxyacetic
acid, m.p. 163-165C. ~nalysis: Calculated for C22~127NO4:
~C, 71.5; %~I, 7.4; ~N, 3.~; Found: 6C, 71.~ , 7.2; %N,
3.7.
~ mixture of 5.0g of 3--(2,6-di-tertiary-butyl-
cyclohexadienon-4-ylideneamino)phenoxyacetic acid, 250 ml
of ethanol and 10 mg of 5~ palladium on charcoal catalyst
_37_ ~Z~S337
was placed on a Paar apparatus. Ilydrogenation was complete
after five hours. The reaction mixture was filtered to
remove the catalyst and the filtrate was evaporated to give
a thick brown oil. The oil was dissolved in 20 ml of
benzene, filtered, diluted with 20 ml of cyclohexalle and
10 ml of hexane, and chilled to give 1.4g of light tan
crystalline 3-(3,5-di-tertiary-hutyl-4 hydroxyanilinoJ-
phenoxyacetic acid, m.p. 127-127.5C. ~nalysis: Calculated
for C22H29NO~; ~C, 71.1; ~H, 7.9; ~N, 3.8; Found: %C, 70.3;
%H, 7.6; ~N, 3.7.
EXAMPLE 43
A suspension of 6.0g (0.027 mole~ of
2,6-di-tertiary-butyl-p-benzoquinone, 3.3g ~0.020 mole) of
p-aminocinnamic acid, 15 ml of tetrahydrofuran and 0.3 ml
of boron trifluoride etherate was heated at reflux for one
hour. The reaction mixture was dissolved in a minimum
amount of methylene chloride, and was diluted to a final
volume of 700 ml with diethyl ether. The ether solution
was washed first with cold 10~ hydrochloric acid and then
with brine, and was then dried over magnesi~lm sulfate and
evaporated almost to dryness. The residue was diluted with
300 ml of hexane and evaporated almost to dryness before
being diluted with 500 ml of warm hexane. The mixture was
allowed to cool to room temperature before being filtered
to give 5.a9 of a bright red powder. One g of this
material was recrystallized from a mi.xture of 15 ml o
benzene and 3 ml of hexane to give 0.5g of red crystalline
4-(2,6-di-tertiary-butylcyclollexadienon-~-ylideneamino)-
cinnamic acid, m.p. 216-217.5C. Analysis: Calculated for
C23H27NO3 %C, 75.6; %H, 7.~; ~N, 3.~; Found: ~C, 75.9;
7.5; %N, 3.~.
A mixture of 2.0c~ of ~-~2,6-di-tertiary-butyl-
cyclohexadienon-4-ylideneamino)cirlnamic acid, 100 ml of
methanol, 0.5 ml of concentrater~ hy(lrocllloric acid and 2g
of zinc powder was stirred for ten minutes. I'he mixture
was filtered and the filtrate was evaporated to give a
" ~... ...
-3~ 2~35337
yellow gummy solid. This material was recrystallized from
a mixtu~e of 15 ml of l~enæene, 4 ml of hexane and 2 ml of
cyclohexane to give 0.4 9 of yellow granular 4-(3,5-di-
t:ertiary--butyl-4-hydroxyanilino)cinnamic ~cid, m.p.
199-200C. I~nalysis: Calculated for C231l29NO32/3C6ll6: ~C,
77.4; ~ill, 7.9; 6N, 3.3; Found: ~,C, 77.2; ~1l, 7.11, 6N, 3.3.
EXAMPLE 44
A suspension of 5.0g (0.0147 mole) of
10 2,6-di-tertiary-butyl-4-(3'-carboxyphenylimino)-2,5-cyclo-
hexadien-1-one (prepared in Example 2) in 15 ml of benzene
and 2.5 ml of tllionyl chloride was heated at reflux until
gas evolution ceased. Tlle solution was evaporated, and was
evaporated twice more following additions of benzene. The
15 resulting acid chloride was added dropwise to a solution of
5.5g sodium trifluorometllanesulfonamide in 25 ml of
1,2-dimethoxyethane. The solvent was evaporated with a
stream of nitrogen to give a yellow solid. This solid was
stirred with 200 ml of tetrahydrofuran and was then
20 filtered to remove insoluble material. The filtrate was
hydrogenated for 16 hours on a Paar apparatus using O.Sg of
5% palladium on charcoal as the catalyst. 5he catalyst was
removed by filtration and the filtrate was evaporated to
give a dark brown oil. rhe oil was dissolved in 25 ml of
25 water. This solution was adc3ed to a mixture of 5.0 ml of
10~; hydrochloric acid, water and ice to givc 6.7 g of a
white solid. l'his so]id was recrystallize(l llrom a mixture
of 70 ml of ethanol and 20 ml of water to give 3g of white
crystalline N-[3-(3,5-di-tertiary-hutyl-4-llydroxyanilino)-
30 benzoylltrifluoromethanesulfonamide, m.p. 234-234.5C.
Analysis: Calculated for C221127F3N20~15: ~;C, 55.9 ~1, 5-8;
6N, 5.9; Found: %C, 56.1; ~,11, 5.8; 6N, 5.9.
EXAMPLE 45
~ mixture of 13.2g (0.10 mole) of
3-amino-4-hydroxybellzoic acid, 22.0g (0.10 mole) of
2,6-di-tertiary-buty]-p-benzoguinone, 25 ml of
~ 295337
-39-
tetrahydrofuran and 1 ml of boron trifluoride etherate was
heated at a gentle reflux for about 20 minutes by which
time a thick precipitate had formed. ~he reaction mixture
was diluted witll 50 ml of ethanol and filtered to obtain
20.9g of an orange solid, m.p. 24~-250C. Six g of this
material was recrystallized from a mixture of 250 ml of
ethanol and 70 ml of water to give 3.4g of red granular
3-(2,6-di-tertiary-butylcyclohexadienon-4-ylideneamino)-4-
hydroxybenzoic acid, m.p. 275-276~C. ~nalysis: Calculated
for C21}E25NO4: %C, 71.0; 6El, 7.1; %N, q.0; Found: 6C, 71.4;
~}E, 7.1; 6N, 3.~.
~ mixture of 5.0g of 3-(2,6-di-tertiary-butyl-
cyclohexadienon-4-ylideneamino)-4-llydroxybenzoic acid,
0.05g of 5"6 palaldium on charcoal catalyst, 250ml of
ethanol and 50 ml of tetrahydrofuran was placed on a Paar
apparatus. Ilydrogen uptake was comp]ete in about 10
minutes. The reaction mlxture was filtered to remove
catalyst. The filtrate was evaporated to give a tan solid
which was recrystallized from a mixture of 40 ml of ethanol
and 15 ml of water to give 3.2g of reddish tan granular
3-(3,5-di-tertiary-butyl-4-hydroxyanilino)-4-llydroxybenæoic
acid, m.p. 254.5-255C. (dec). Analysis: Calculated for
C21lE27NO4: %C, 70.6; 61E, 7.6; 6N, 3.9; Found: 6C, 71.0; 61E,
7.6; 6N, 4.1.