Note: Descriptions are shown in the official language in which they were submitted.
3~
The present application is a divisional of Canadian
Patent application No. 359,647 filed on September 5, 1980 and
relates to new fungicidal compounds and more especially to new
fungicidal N-aryl-N-acyl-3-amino-oxazolidin-2-ones.
Fungicidal compounds of the class of N-phenyl-1,3-
oxazolidin-2,4-diones are described in, ~or instance, Dutch
Patent Application No. 68/17249 (Sumitomo?, in French Patent
No. 2,172,295 (BASF), and in Belgium Patent No. 874,406 to
Montedison, S.p.A.
Recently, there has also been described the bacteri-
cidal and fungicidal activity of some derivatives of aniline
and glycine which carry on the nitrogen atom a variously sub-
stituted phenyl group and an acylic group of varying nature.
More particularly, this acylic group may consist of
an ~- or ~-haloalkanoyl (German Patent Application DOS 2,513,789 -
Ciba Geigy = U.S. P. 4,025,648), or of an acetyl group substituted in
~-position with a sulphur or oxygen atom in its turn bound to
groups of varying nature (French Patent Application No. 7,510~722 -
Ciba - Geigy = British patent 1,500,581) or ~gain of a 2-furoyl, 2-thienoyl
or pyridyl-2-carbonyl group (German PatentApplications DOS 2,513,732
and 3,513,788 ~ Ciba-Geigy = U.S. P. 4,046,911~ . Methylalaninates are
.... ~
known which possess microbicidal activity; these methyl-
alaninates carry on the nitrogen atom a 2,6-dialkyl-phenyl and
one of the following groups: cyclopropanoyl, acryloyl,
crotonoyl. ~
Other derivatives of fungicidal acyl-anilines have
recently been described in Belgian Patent No. 863.615 (Ciba-
Geigy) and in German Patent Application DOS 2,745,633 (Chevron).
The interest in the search for new derivatives from
acylanilines having fungicida~l action originates from the
exigency of finding acyl~aniline derivatives that will have a
high fungicidal activity combined with a lack of phytotoxicity.
,,
``` ~ 63 ~
In fact, some of the already know.n products,
although developing an excellent fungicidal activi-ty, prove,
however, to be also toxic for the plants that one wishes -to
protect against infections by fungi.
The damages caused by the phytotoxicity to the
plants to be protected can hardly be avoided by using a dose
of a fungicidal compound that may be the best compromise
between the fungicidal activity of the compound and its phyto-
toxicity.
In fact, in the practical application in agricultural
cultivations, the quantity of product that actually remains on
the plant varies considerably depending on various factors such
as, for instance, the weather conditions, (particularly the
frequency of rainfalls), and the correctness and frequency of
the applications carried out by the farmer.
It would be advantages to have oxazolidin-2-ones
which are highly active fungicides and safe for use in fighting
fungi infestations of useful plants without damage to the
plants, even when used in large quantities.
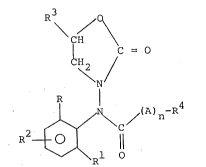
The present invention provides compounds of the
general formula ~I)
R3 /
\ CH
C = O
( 'H2
\ N (I)
R
J~/ ~ / (A ) n~ R
R ~ 1
wherein R and R equal to or different from each other, are
halogen., Cl-C4 alkyl or C1-C4 alkoxyJ
R2 is H, halogen or Cl-C4 alkyl;
r "~b i~ 2
~ ~63~
CH3
A is -CH2- or -CH-
n is 0 or l;
R4 is -OR or -SR5 (R5 is Cl-C5 alkyl or a heterocyclic
group of 5 or 6 atoms containiny from l to 3 hekero-
atoms) when n is l or
R4 is a heterocyclic group of 5 to 6 atoms containing
from l to 3 hetero-atoms or Cl-C5 alkyl substituted
with one or more halogen atoms, when n is 1 or 0
and R3 i 5 H or CH3:
and which are endowed with very effective fungicide
activity while having a very low phytotoxicity.
The heterocyclic groups may for example be furyl,
tetrahydrofuryl, thienyl, pyrimidyl, pyridyl, imidazolyl,
pyrazolyl or triazolyl.
N-phenyl-N-acetvl-3-amino-oxazolidin-2-one of
formula (II):
~> ~
~ (II)
N-C-CH
~ l 3
has been described~in the Journal of Organic Chemistry,
31, p. 968 (1966) byt it has not been recognized, however, as
possessing fungicidal activity.
In accordance with another aspect of the present
invention there is provided a method for figh-ting fungi
infections of plants consisting in distributing on the plants
or on the soil in which the plants live, an efective amount
of at least one of the compounds of the general formula (I)
-- 3 --
.
~63 ~
R3 O
\ / \
Cl C = O
~ N (I)
R ~ / ~A)n-R
~ '\ 1 11
wherein R and Rl equal to~or different from each other, are
halogenl Cl-C4 alkyl or Cl-C4 alkoxy;
R2 is H, halogen or Cl-C4 alkyl.;
CH3
A is -CH2- or -CH-
n is 0 or 1;
R4 is -oR5 or -SR (R5 is Cl-C5 alkyl or a hetero-
cyclic group of 5 or 6 atoms containing from 1 to 3
hetero-atoms) when n is 1 or
R4 is a heterocyclic group of 5 to 6 atoms containing
from 1 to 3 hetero-atoms or Cl-C5 alkyl substituted
with one or.more halogen atoms, when n is 1 or 0
and R3 is H or CH3.
The preparation of the compounds of general formuLa
(I) is carried out by means of processes known in the normal
practice of organic chemistry.
` For instance, an arylhydrazine (3) may be prepared
by reacting the corresponding aniline tl) wi-th sodium nitrite
.
(NaNO2)~ in hydrochloric.acid,.and by then reducing -the
diazonium salt (2) thus obtained:(scheme 1, equation 1 below),
as described for~ instance in Journal o~ American Chemical
Soclety~, 81, p~. 4673 ~1959).
The aryl-hydraz~ine is then caused to react with 2-
haloethyl or l-methyl-2-haloethyl-chloroformate (4) (in its
turn prepared by the action of phosgene on a halohydrin) in
'
-- 4 --
'
-
3 ~
the presence of a base, and the intermediate (5) thus obtained
is then cyclized in the presence oF bases, thereby obtaining
intermediate (6) (scheme 1, equation 2). This process has
been described in the jour. of Am. Chem, Soc. 48. 1951 (1926).
Intermediate (6) is then condensed by rneans of the
proper acylic halide (7), according to known techniques
(scheme l, equation 3). The condensation reaction between
intermediate (6) and acylic halide (7) may be substituted or
¦ replaced by analogous reactions known in the literature, which
allow the introduction of particular acylic groups. For
instance, the compounds of general formula (I), in which in
the acylic part: R4 = -oR5 or -SR5 may be prepared by reacting
intermediate (6) with phosgene or with haloacetyl or halo-
propionyl halid_s (such as, for instance, Cl-~-CH2Cl and
Cl-C-CH-CH3 respectively) and by then substi-tuting the halogen
O Cl
atom according to known techniques.
Scheme l:
G2~ ~lCI ~ ~ Re~l~~er~ \~
~1) (2) 3 (3)
- NH-NH-C-O-CH-CH2X
R3 R ~ lo
2) (3) ~ Cl-C-O- H-CH2X > ~ 2
R
(4) (5)
-- 5 --
R3
~>=
NH
5) -HX > ~Rl (6)
R2
1~ 0
3) (6) + X C- ~A) -R4 -HX ~ (I)
L~ = halogen; R, Rl, R2! R3, R4, A and n have the same
meanings as in general formula (1)7. Reaction 3 is carried
out in an inert solvent, in the presence of a halogenhydric
acid-accepting base, at reflux temperature.
The compo~mds of general formula (I) are endowed with
an èxcellent fungicide activity against phytopathogenous fungi
and their action has both a preven-tive character (i.e., they
hinder the inception of the disease?, as well as a curative
character (when, that is, the infection is already in progress).
.
The most important class of phytopathogenous fungi
which can be fought by using the compounds of the invention,
is that of Phycomicetes which comprises Plasmopara ~
Phytophtora ~., Peronospora ~., spp. and
Phythlum ~ .
The fungicidal compounds of the invention are
effective for fighting fungi infections of useul plants such
as vine, tomato, tobacco,~potato and other cultivations.
They~possess good systemic characteristics (l.e.,
they are carried into the ~various parts of the plant) where-
fore it is possible to apply these products both on the leaves
- ~ ,
6 --
,
-
3~q ~
as well as on the soil.
Moreover, the compounds of general formula (I)
proved to be compatible with the plants that are to be pro-
tected agains-t fungi attacks.
The majority of the compounds of general formula (I)
do not show any sign of phytotoxicity at the amounts tried out,
while the remainder showed only a low phytotoxicity, lower, at
any rate, than that of the previously known fungicides.
In practical agricultural applica-tions, the compounds
of formula ~I) can be used as such or in form of suitab-le com-
positions, consisting of the compounds of formula (I) as
active principle, an agriculturally acceptable carrier (e.g.
solid or liquid inert carriers) and, optionally, surfactants
and other additives. If desired, active compounds, such as
other funglcides, insecticides, plant-growth regulators and so
on, may be present in the compositions.
The compounds may be formulated according to the
normal agricultural practice, as dusts, powders, wettable
powders, emulsifiable liquids, granular formulates and so on.
2a The amounts of compounds of formula (I) to be dis-
tr~buted for fighting infections by fungi depends on various
factors, such as the active compound used, the type of com-
position or formulation, the kind of infection and its degree,
the kind of agricultural cultivation to be protected from
fungi attack, the cllmatic and weather conditions, and so on.
Generally, amounts of compounds of ormula (I) com-
prised between 10 and SOO g/ha are sufficient, the preferred
amount being from;100 to~250 g/ha. The followiny examples are
` given to i1lustrate the invention in more deta l, and are not
intended to be limitlng.
` ~ EXAMPLE 1
Preparation of N-(methoxyacetyl)-N-(2,6-dimethyl-phenyl)-3-
-- 7 --
`' ' ' ~ .
3 ~6~
amino-oxazolidin-2-one
(A) Preparation of 2,6-dimethylphenylhydrazine:
107 g of 2,6-xylidine were dripped into a solution
of 220 ml of concentrated HCl in 150 ml of water. After
cooling down to -5C, this mix-ture was thereupon additioned
with a solution of 66 ml of NaNO2 in 150 ml of H2O, over a
period of time of about 1 hour and under vigorous stirring.
To the yellow-orange colored suspension thus obtained
were added, at 0C and in about 4 hours, 450 g of SnC12.2H2O in
600 ml of a 5N aqueous solution of HCl.
The mixture was then maintained under stirring for
24 hours, allowing the temperature to rise up to ~20C. The
solid thus formed was filtered, dissolved in 700 ml of H2O and
then treated with a solution of 230 g of NaOH in 300 ml of
H2O, at a temperature of between 10 and 15C, after which the
product was extracted with diethylether (3 x 250 ml).
The etheric extract, after washing with H2O and
anhydrification on Na2SO4, was brought up to a volume of
1500 ml with diethylether and then was treated with anhydrous
~o gaseous HCl, until attaining the complete precipitation of
the chlorohydrate of 2,6-dimethylhydrazine.
The salt was then filtered and dried, thereby
obtaining 40 g of a white solid having a melting point (m.p.)
equal to 205-207C with decomposition.
By treatment with NaOH, from the chlorohydrate was
obtained 2,6-dimethyl-phenylhydrazine.
(B~ Preparation of 3-(2,6-dimethylaniline) oxazolidin-
2-one:
To 41.4 g of 2-bromoethyl-chloroformate, prepared
from phosgene and ethylenic bromohydrine, in 200 ml of benzene,
there were additioned, at 10C, the following reactants:
36.5 g of~2,6-dimethyl-phenylhydrazine (see point ~), and 18 g
-- 8 --
of pyridine in 100 ml of benzene. This addition once
completed, the temperature was allowed to rise up to 20C
under constant vigorous stirring.
The pyridine chlorohydrate was removed by washing
with water. The benzenic solution was further washed with HCl
and with water to a neutral pH, and was then dried on Na2SO4
and evaporated to yield 61 g of an oily product which, crys-
tallized from ligroin, gave 43 g of a light colored solid
having a m.p. of 58C-63C, and which consisted of 1-~2,6-
dimethylphenyl-2-(~-bromoethyl)-oxycarbonyl hydrazine. L~he
IR spectroscopy gave: v(C=O) = 1710 cm 1; v~NH-CO) = 3180 cm 1;
v(NH-Ar) - 3340 cm ~ .
` 40 g of this intermediate were dissolved in 500 ml
of toluene and the solution was treated with 16 g of tetra-
methylguanidine. This mixture was reflux-heated for 3 hours
under stirring. After cooling down, the mixture was washed
with 200 ml of H O and then with 100 ml of diluted HC1 and
finally again with 200 ml of H2O.
The aqueous phases, reunited, were extracted with
CH2C12 (2 x 200 ml).
The combined organic phases were anhydrified on
Na2SO4 and then the solvent was evaporated, thereby obtaining
a solid residue which was crystallized from ligroin-ethyl-
acetate (2:1).
` There were thus obtained 22.5 g of 3-(2,6-dimethyl-
aniline)-oxazolidin-2-one having a m.p. of 107-110C. L~he
IR spectrum showed: v(C=O) = 1770 cm 1; v(NH) = 3340 cm ~ .
The cyclization reaction was repeated, dissolving
the intermediate with m.p. of 58-62C in ethanol containing
sodium ethylate, and by then reflux-heating the solution.
After an analogous treatment of the reaction mixture, the same
intermediate was isolated; it had a m.p. of 107-110C.
_ g _
3 ~3
-
~ C) To 2 g of the intermediate, prepared as described
in (B?, in 70 ml of toluene and 0.2 ml of dimethylformamide,
there were added 1.1 g of methoxyacetyl chloride. The reaction
mixture was then reflux-heated for 8 hours. After cooling down,
the reaction mixture was subjected to complete evaporation of
the solvents. The residue, consisting of 2,9 g of a thick
oily product, was purified on a silica gel column, using as
eluent a mixture of benzene/ethylacetate, in a 1/1 ratio.
Thereby were obtained, after removal of the solvents,
1~ 1.1 g of a syrupy product which crystallized spontaneously and
which, after re-crystallization from ligroin/ethylacetate
(1:13 mixture, yields 1 g of the desired compound whose char-
acteristics have been recorded in Table 1 (compound No. 6).
Che IR spectrum gave: v(N-CO-CH2) = 1680 cm ; vtN-CO-O) =
1780 cm 1~7.
EXAMPLE 2
Operating analogously to Example 1, there was pre-
pared, in addition to compound No. 6, the other compound
reported in the following Table 1:
~0 TABLE 1( )
,
Com- R Rl R2 R n A R m.p. ( ) IR(C)
No. (~C) (cm
-
4 2 C 3 3 H ~ C 2 88-92 1635-1770
6 2-CH3 6-CH3 ~ H 1 CH2 OCH3 103-6 1680-1780
- .
Notes do Table 1:
(a) The elemental analysis of all the compounds is consistent
with the assigned structure.
(b) Melting points have not been corrected.
(c) Only the bands corresponding to vC=O are reported.
(d) vOH = 3300 1.
-- 10 --
,~
`:
\
XAMPLE 3
_rative activi-ty on vine Peronospora. ( _a o~ vitivola
(B et C) Berl et de Toni)
Vine leaves of Cv. Dolcet-to, grown in pots in a con-
ditioned environment stabllized at 25C and 60% of rela-tive
humidity, were sprinkled on the lower faces thereof with an
aqueous suspension of Plasmopara viticola conidia (200,000
conidia/cc). After 24 hours of dwelling in a humidity (mois-
ture) saturated environment, stabilized at 21C, the plants
were treated by sprinkling both faces of the leaves with the
products under examination in a hydroacetone solution at 20%
of acetone (vol./vol.).
At the end of the incubation period (7 days), the
degree of infection was assessed by sight on the basis of a
value scale with indexes going frorn:
0 : no control, infection equal to that of witness
plant (inEected but non-treated plants)
1 : 1-20% reduction of the infection;
2 : 20-60% reduction of -the infection;
3 : 60-90% reduction .of the infection;
4 : reduction of the infection greater than 90%.
The results ob-tained are recorded in the following
Table 2:
TABLE 2
Curative activity against vine Peronospora
by foliar application at the dose of 0.5%.
Compound No. Activi.ty
4 4
6 4
EXAMPLE 4
Curative activity on Peronospora of Tobacco (peronospora
-- 11 --
3~7
tabacina Adam).
The leaves of tobacco plants Cv. Burley, grown in
pots in a condi-tioned environment, were sprinkled, on the
lower faces of the leaves, with a Peronospora tabacina conidia-
suspension 1200,000 conidia/cc). After 6 hours of dwelling in
a humidity saturated environment, the plants were transferred
- to a conditioned environment stabilized at 20C and 70% of
relative humidity, for the incubation of the fungus. 24 hours
after the infection, treatment was carried out by sprinkling
1~ both leaf faces with the product under examination, in a hydro-
acetone solution of 20% in acetone (vol./vol.).
At the end of the incubation period (6 days) the
extent of the infection was assessed by sight according to a
value scale with an index range equal to that of Example 3.
The results of the test are recorded in the following
Table 3:
TABLE 3
Curative activity by foliar application
at doses of 0.5% on pl.ants infected with
Peronospora of Tobacco.
~0
Compound No. Activity
4 4 ~~.
6 4
.
EXAMPLE 5
Determination of the phytotoxicity degree
Leaves of Cv. DoIcetto vine plants, grown in pots in
a conditioned environment stabilized at 25C ar,d 60% relative
humidity, were treated by sprinkling both leaf faces with a
-hydroacetone solution at 20% o.f acetone (vol./vol.) of the
products under examinatlon.
- 12 -
`
~2.~3~
After 7 days, the extent or deyree of phytotoxic
symptoms were evaluated by sight according to a value scale
with indexes ranging from 100 (for fully damaged plant) to 0
(for a healthy plant).
The corresponding data are recorded in Table 4, in
comparison with the phytotoxicity indexes of the two compounds
known to be shortly marketed, i.e.: Furalaxyl (British
Patent No. 1,449,810 - Ciba-Geigy) and Ridomil (French Patent
Application No. 2,267,042 - Ciba-Geigy).
TABLE 4
Phytotoxity index for doses at 0.6%.
Compound No. Phytotoxicity index
4 0
6 0
Furalaxyl (1) 100
aRidomil (2) 60
(1) Furalaxyl = N-(2,6-dimethylphenyl)-N-(l'-carbomethoxy-
~0 ethyl)-2-furoyl-amide:
H3CO-b-CH \ j
H3C ~ CH3
~J
(2) aRidomil = N-(2,6-dimethylphenyl)-N-(l'-carbomethoxy-
ethyl)-methoxyacetamide:
CH3 1
3 ¦¦ ~ N/
H 3 C--~CEI 3
.
- 13 -
.