Note: Descriptions are shown in the official language in which they were submitted.
--1--
2-PYRIDINOL CO~IPOSITIONS AND MET~IODS
OF IJSE AS ANA13OL I C AGENTS
.
This invention relates to a method o~ pro-
duciny an anabolic response in animals by administering
to the animals an anabolic agent whereby s~ch response
is exhibited in one or more of the fo-lowing ways:
(a) an increased growth rate,
(b~ increased milk production,
(c) increased fibeL production,
(d) increased protei.n content,
(e) improved reproductive performance and
( f j increased feed conversion efficiency
in animals.
More particularly, the present inv~ntion xelates to a
method of obtaining the above anabolic responses by
administering to an animal an anabolically effective
amount o a substituted-2 pyridinol or a physiolGyi.c~
ally acceptable salt or ester thereo.
Briefly, in accordance with the present
invention,~ the anabolic response in animals, for
; : example, the feed conversion efficiency, milk and fiber
30,249A F -1- f ~
. -2-
production, protein content, reproductive performance,
and growth rate of meat-bearing animals, can be
increased by administering to said animals an anabolic~
ally effective amount of a compound corresponding to
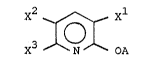
the following formula
X3 ~ X
wherein
X1, x2 and X3 independently represent halogen,
preferably chloro;
A represen-ts hydrogen, alkali metal, alkaline
earth metal, zinc, iron, manganese or
-CZ ; and
Z represents Cl-C11 alkyl, Cl C11 h y
Cl-C11 hydroxyalkyl.
Preerably, X1, x2 and X3 are chlorine. Also
preerred is that A is an alkali metal, more preferably
sodium.
For convenience, the compounds of Formula I
will be hereinafter referxed -to as the "pyridinol
compounds".
When used herein, the term "alkyl" is mean-t
to encompass straight and branched chain alkyl groups
and cycloalkyl groups of from 3 carbon at3ms up to the
30,249A-F -2
3~i
upper carbon atom limits as specifically set forth.
The term "Cl Cll" indicates that from one -to 11 carbon
atoms may be present in a group.
.
It is well known that pyridinols exist in the
S pyridinol and tautomeric pyridone form. When any
reference is made to present pyridinol compounds o~
Formula I where A is hydrogen, it is understood to
encompass both tautomeric forms.
The term "anabolic response" refers to the
effects eghibited by an animal in response to an anabolic
agent. ~len an anabolic agent is administered to an
animal the anabolic response is exhibi~ed by, for
example, an increase in weight gain, increase in fiber
production, increase in milk production in lactating
animals, increase in feed conversion efficiency,
increase in protein content or improved reproductive
performance when compared to animals not receiving the
anabolic agent.
An important objec-tive of this invention is
to produce an anabolic response in animals which is
maniested by, for example, one or more of the following:
increased weight gain, incxeased protein content,
increased wool production, improved reproductive per-
formance, increased milk production in lactating animals,
and increased feed conversion e~ficiency. Examples of
animals which exhibit increased grow-th rate when admin~
istered an anabolically e~fec-tive amount of one or more
of the present pyridinol compounds are commercial
meat-bearing animals such as cattle, sheep, swine, and
poultry.
30,249A-F -3-
~,...
~ ~3~3~:~
In commercial prac-tice, imma-ture sheep,
cattle and swine are commonly fed for maximum growkh
rate in feed lots and poultry, such as chickens and
turkeys, are raised in broiler pens, until -they reach a
marketable weight. When the desirecl weight of the
animals is achieved, they are then sold for slaughter.
It is important economically, that the animals achieve
market weight in as short a time as possible, while
consuming the least amount of food necessary to achieve
such gain. It has been unexpectedly found that when
anabolically effective levels of one or more of the
present pyridinol compounds are administered -to animals
of the classes described hereinabove, they gain weight
at a faster rate while consuming less feed per pound of
gain resulting in better overall economic efficiency,
and reflecting the fact that administration of the
present pyridinol compounds results in an anabolic
response.
The present pyridinol compounds can be admin-
istered to animals orally by dosage forms, such as, inadmixture with food, and additionally in the form of
boluses, capsules, tablets, susp~nsions or solutions
containing the present pyridinol compounds. The com~
pounds can also be adminis~ered paxenterally, such as,
for example, intramuscularly or inkrav~nous]y, or by
way of an implant which slowly releases the pyridinol
compound into the tissue or blood stream of the animal.
The anabolically effective amount of the
present pyridinol compounds exists as a range of from
0.01 to 20 milligrams per kilogram (kg) of body weight
of the animal per day. This effective range can vary
dep~nding on the size of the animal, the species of the
30,249A-F -4-
-5-
animal, the age of the animal, -the type of feed used,
the active compound used, or the rouke of administra-
-tion of the active compound. The optlmum range of an
effective amount, based on -the above-mentioned vari-
ables, can be found using conventionally knowntechniques, i.e., dose titration determinations.
An anabolically effec-tive amount of the
present pyridinols can be conveniently administered
substantially daily for at leas-t 7 days, preferably at
least 28, more preferably at least 90 days and even
more preferably throughout the life of the animal.
Lactating animals exhibit an increase in milk
production when administered an anabolically effectlve
am~unt of the present pyridinols. Lactating animals
seem to be more sensitive to the effects o the present
pyridinol compounds and thus may require a lower dose
of the present pyridinol compounds to produce an
increase in milk production when compared to an
anabolically effective dose in other animals to produce
an increase in body weight gain.
Animals raised for their fiber production,
such as sheep, exhibit an increase in fiber production
when administered an anabolically effective amount of
the present pyridinol compounds.
The present pyridinol compounds are conveni-
ently incorporated in a feed composition in an appropri-
ate amount to achieve the desired daily d.osage in the
amount of ration or supplement consumed regularly. For
example J one or more of the present pyridinol compounds
30,249A-F -5-
--6--
are conveniently incorporated in a feed composi-tion
from generally 0.5 to 1600 grams per ton [metric] o
- complete ration dependiny on the age and type of
animal. The term "ton", "tonne" or "ton [me-tric]" is
intended to mean, in khis speciEication, a metric ton
of 1000 kilograms. The "ton" in parenthesis associated
with "lb" or "pounds" uni-ts is intended to mean the
short ton of 907 kilograms. The present pyridinol
compounds may also be incorporated in a mineral, protein
or energy~type feed additive supplement in an appropri-
ate amount to provide anabolically effective daily
dosages.
For commercial use, it is convenient to
provide a feed additive premix or concentrate con-
taining one or more of the present pyridinol compoundsin a proportion such that a predetermined quantity of
the premix is to be added per ton [metric] of complete
ration, for example, from 0.2 to 454 kg (0.5 to 1,000
pounds) contains rom ~.5 to 16~0 grams of the present
pyridinol compounds. The feed additive premix or
concentrate comprises one or more of khe present
pyridinol compounds and a~carrier such as soybean meal
or ground corn or okher edible feed grade material or
innocuous diluent, such as, allohols, glycols or
molasses, suitable or the animal at han~. A concen-
trate may contain from 2 to 98 percent by weight of one
or more of the present pyridinol compounds in inkimate
admixture with an adjuvant there~or.
The present pyridinol compounds, when admin-
istered parenterally, are pref~rably dissolved insterile distilled water or other physiologically accept
able liquid media and compounded in accordance with the
known pharmaceutical art.
30,249A-F -6
~ i3~.~
The term "feed conversion efficiency" refers
to the total amount of feed consumed over a period of
time divided by the amount of body weight gain over that
period as seen in -the following formula:
feed consumed over time period
gain in body weight over time period
The term "increased feed conversion efficiency"
refers to a more efficient means of bringing animals to
market weight. An increase in feed conversion effici-
ency will be reflected by a lower numerical value of
the feed conversion efficiency number when compared to
a lower feed conversion efficiency.
The term "increa~ed protein content", when
referred to as being an anabolic response, means car-
15 cass alteration of an animal exhibited by a relativeincrease in body protein content and a relative
decrease in body fat con-tent.
One of the practical effects of this inven-
tion is to bring animals such as sheep, cattle, swine,
and poultry promptly to market weight with minimal feed
consumption. The present pyridinol compounds are most
conveniently dispersed uniformly throughout the normal
feed or feed additive supplement of the subject animal
in anabolically effective dosage levels. For example,
an anabolically effective amount of the present
pyridinol compounds is supplied in an animal feed
comprising from 0.0005 to 16 percent by weight of one
or more of the present pyridinol compounds and more
preferably from 0.005 to 0.10 percent by weight cor~
responding to 50 and 100 parts per million (ppm). Such
30,249A-F -7~
~3~
animal feeds should, when fed to an animal, provide ko
the animal from 0.01 to 20 milligrams per kilogram of
body weight per day of one or more of the present
pyxidinol compounds.
In further embodiments of the method o the
present invention, compositions containing -the present
pyridinol compounds can he advantageously employed in
combination with one or more additional feed additives
such as coccidiostats, antibiotics, minerals, vitamins
or the like.
The animal feeds most generally used in con-
junction with this invention are composed of various
grains and/or grain mixtures and/or roughage feeds such
as hay, cotton seed hulls, rice hulls, silage, or other
high fiber feedstuffs commonly fed to meat-, milk-,
and/or wool-producing animals, especially in cattle or
sheep feeds. The feeds for swine and poultry will
consist primarily of various grain mixtures plus the
usual additaments such as bran meal, soybean meal,
cotton seed meal, tankage or alfalfa meals suitable for
monogastric animals.
Examples oE carriers for premix or concentrate
composi.tions are soybean meal, corn oil, ground corn,
ground corn cobs, barlsy, wheat, mineral m:Lxtures con-
taining, e.g., vermiculite or diatomaceous earth, corngluten meal, corn distillers' solubles, soy flour or
other modestly priced edible ingredients. The active
ingredient will be in amounts to satisfy the criteria
set forth above for balanced feed rations. This premix
or concentrate is then in turn mixed uniformly with the
normal diet for the animal as desired by the grower or
30,249A-F -8-
,;
3~
g
the feed mixer. The above mentioned grains, grain mix-
tures, roughage feeds, usual additaments, carriers and
innocuous diluents constitute acceptabl~ adjuvants for
purposes of this invention.
-5 As indicated hereinabove, the amount of the
present pyridinol compounds added to such feeds will be
in the range of from 0.5 to ~600 grams per ton [metric]
of feed (dry matter basis), depending on the age and
type of animal. More prefercibly the amount added to
such feeds will be in the range of from 5 to 700 grams
per ton [metric]. Very young animals that have been
weaned or young poultry one or a few days old, will
have a lower feed consumption compared to an older
animal. However, as the animal goes through a growth
period to a fattening period, sometimes called finish-
ing, the feed consumption gradually increases, but
generally falls in proportion to body weight.
The daily dosage o a preferred compound,
3,5,6-trichloro-2~pyridinol or a physiologically accept-
able salt thereof, in swine falls in the range of from0.01 to 20 milligrams per kilogram of body weight. More
preferably, in the range of from 0.1 to 10 milligrams
per kilogram of body weight, and even more preerably
from 2.5 to 5 milligrams per kilogram of body weight.
Exarnples oP physiologically acceptable salts of
3,5,6-trichloro-2-pyridinol are the Na, K, Ca, Mg, Mn,
Zn and iron salts thereof.
Based on known inormation concerning the
average feed intake for various sizes and types of
animals, the followin~ amounts of one or a mixture of
two or more of the present pyridinol compounds are
30,249A-F -9~
,
3~
incorporated into each ton of animal feed in order to
provide an anabolically effective dose described herein.
For example, cattle on a growing diet will ordinarily
be fed a diet containing from 3 to ~00 grams of one or
a mixture of the present~pyridinol compounds per ton of
feed on a dry matter basis (DM), while cattle on a
fattening diet will be fed a feed containing ~rom 3 to
1000 grams of one or a mixture of the present pyridinol .
compounds per ton [metric] 5DM) (0.007 to 2.0 lb/ton).
Maintenance diets fed lactating dairy cattle should
contain from 5 to 1600 grams of one or a mixture of the
present pyridinol compounds per ton [me-tric] of feed
(DM) (.01 to 3.60 lbs/ton), depending on the size and
feed intake of the animal. Non-lactating dairy cattle
should receive a feed containing 5 to 1000 grams of one
or a mixture of the present pyridinol compounds per ton
of feed ~DM) (0.01 to 2.70 lbs/ton). Lambs on dry feed
will generally be fed a ration containing 2 to 600
grams of one or a mixture of the present pyridinol
compounds per ton of feed (DM) (0.004 to 1.4`1bs/ton).
Grower pigs may be fed a ration containing 1 to 400
grams of one or a mixture o the present pyridinol
- compounds per ton of feed (DM) while swine in the fat-
tçniny stage will generally be supplied a ration con-
taining 2 to 500 grams per ton (DM~ (0.002 to 1.20lbs/ton). Poultry such ~s very ~mall day-old or older
birds up through starter or grower s-taye will generally
be fed a complete ration of mash containing 0~5 to 400
grams of one or a mixture of the present pyridinol comr-
pounds per ton of feed (DM) while poultry on a fat-
tening diet will feed on a ration containing 1 to 400
grams per ton (DM) (0.001 to 0.8 lb/ton~.
30,249A-F -10-
36~
--11~
- The following examples further illustrate -the
practice of the present invention, but, as such, are
not intended to be limitations upon the overall scope
of the same.
Example 1: A typical growing ration for ruminants is
as follows:
Weight Percent
I d ents (D.M Ba6is)
10 Ground Yellow Corn 45.0
Soybean Oil Meal 7.0
Can Molasses 7.0
Dicalcium Phosphate 0.5
Trace Mineral Salt 0.5
100 . O
In addition to the above the following supplements are
added.
Vitami~ A 300 I~
Vitamin D 150 IU/lb
3,5,6-Trichloro-2~pyridinol 3.5 to 450
or a physiologically acceptable grams/ton of
salt thereof eed
Such a feed typically contains 8 to 15
percent by weight moisture.
30,249A-F -11-
~26~3~
-Example_2: A typlcal finishing ration for ruminants is
as follows:
Weight Percent
IncJredients (D.M. Basis)
5 Ground Shelled Corn 65.85
Mixed Ground EIay 20.00
Dried Molasses 6.00
Soybean Meal 6.00
Trace Mineral Salt 0.50
10 Dicalcium Phosphate 0.40
Ground Limestone 0.70
9g.45
In addition to the above the following supplements
are added.
Vitamin A (300,000 units/gms)66.7 grams/-ton
Vitamin D2 (16,000,000 um ts/lb)7.1 grams/ton
3,5,6-Trichloro-2-pyridinol 3.5 to 450
or a physiologically acceptablegrams/kon of
salt thereof feed
Such a feed typically contalns 8 to 15
percent by weight moisture.
30,249A F~12-
~6
-13-
Example 3
An example of a suitable feed addi~tive premix
is as follows:
3,5,6-Trichloro-2-pyridinol or 64 grams
5 a physiologically acceptable
salt thereof
Ground Yellow Corn (5-10% moisture) 390 grams
Example_4
For use in the field for animals on range,
the present pyridinol compounds may be administered by
means of salt or molasses blocks. A typical block is
prepared using the following compositions:
Weight Percent
Ingredients (D.M. Basis)
15 Dried Cane Molasses 35.00
Ground Soyhean Hulls 29.60
3,5,6-Trichloro-2 pyridinol or
a physiologically acceptable
salt thereof* 5.36
20 Granulated Salt 25.90
Trace Minerals and ~itamins 0.2g
Stabilized Animal Fat 1.30
Moisture 2.60
100 . 00
*Provided that the field animal ingests enough of
the block per day to provide an effective dose of
active ingredient hereinbefore mentioned.
30,249A-F -13-
p
Exam~le 5
If desired, the present pyridinol compounds
may be administered as a part of a liquid animal ~eed
supplement such as a supplement containing a non-protein
nitrogen source such as urea or ammonium sulfate in
: admixture with molasses and other feed ingredients. .
Such a liquid supplement is prepared using the fol-
lowing formula:
Weight Percent
10 In~edients (D.M. Basis)
Molasses 80.00
Water 16.25
Urea or Ammonium Sulfate 2.00
Trace Minerals .50
15 Vitamin A, D & E .05
Salt 1.00
: 3,5,6-Trichloro-2-pyridinol or
j a physiologically acceptable
: salt thereof* .20
*Provided the animal drinks enough o~ the li~uid per
day to provide an effective dose o~ active ingredien-t
hereinbefore mentioned.
The following examples demonstrate the ana~olic
ef~ects of the present pyridinols as seen by an incxease
in the average daily gain in weight, increased feed
conversion ef~iciency, or increased milk production in
feeding trials conducted in Europe and in the United
States.
30,249A-F -14-
~15-
I. Protocol and Results for European Trials
The process of the present invention was
tested in pig feed trials in 4 European countries as a
pig growth and as a feed eficiency promoting agent on
a total of 520 pigs, including controls. The cowntries
included Denmark, England, Germany and ~Iolland
(Netherlands~.
The use 2,3,5-trichloropyridinol (TriCP) in
feed was from weaning to the end of th~ fa-ttening
period. A s-tarter diet was fed to the pigs from the
beginning of the weaning period, which was 25 kg (in
Germany, 30 kg~ to a weight of 50 kg (in Germany, 65
kg~. ~ finisher diet was fed to the pigs from a weight
of 50 kg (in Germany, 65 kg) to the end of the fa-tten-
ing period which was 100 kg (in Germany, 105 kg).
Dosage rates of zero "0" (the control), 50, 75 and
100 ppm of TriCP in the feed were tes-ted. Protein
supply and energy content of the starter and finisher
diets was adapted to represent the most common nutri-
tional balance in each country, as represented in
Table 1.
30,249A-F 15-
6~i3~b
--16--
Table 1
Nutritional Data of Feed Used
in European Trials
Energy
In Feed ~ Lysine
Number % Protein (KCal/Kg in Feed
Country of Pi~s Diet By Weight Feed) b
Denmark128 Starter 21.4 2098 0.81
Finisher 16.6 2105 0.55
England64 Starter 17.0 2108 0.89
Finisher 15.7 2150 0.77
. _
Germany168 Starter 16.5 2210 0.90
Finisher 15.0 2225 0.70
. . . _ . .
Holland160 Starter 17.0 2190 0.96
Finisher 13.6 2185 0.70
:
30,249A-F -16-
.:
3~
-17-
- Individual protocols for each European country
are provided hereinafter.
IA. Denmark Trial
The ~enmark trialplan is shown below.
Feeding Trial Plan in Denmark
_ Group Number
1 2 3 4
Number of Pigs 3232 32 32
TriCP concentration 050 75 100
in feed in ppm
In each of the four groups there were 32
pigs, divided into 4 pens with 4 males and 4 pens with
4 female pigs. The trial was repeated 4 times. One
repetition consisted of 10 pens, with the pigs derived
mainly from the same livestock and the weight of the
individual pigs differed as little as possible. A11 4
groups were fed the same feed mixture; except that,
groups 2, 3 and 4 received 50, 75 and 100 ppm l~riCP,
respectively.
As agreed with the Ministry of Agriculture
and the Vetexinary Directorate, a withdrawal per:Lod of
10 days a~ter the latest supply of TriCP was follQwed.
To do this all pigs in every repetition received the
same feed mixture without additive as soon as -the first
pig had reached the time of 10 days before slaughter.
30,249A-F -17-
The following quantity of feed was given daily :
Weight, kg 20 30 40 50 60 70 80
Feed units* per pig
daily 0.9 1.5 1.9 2.2 2.5 2.7 2.8
* Feed units are calculated according to the Danish
Standard. In the feed composition used here it is
equal to 1.010 kg.
Table 2 shows the composition and contents of
the feed mixture used. The feed was in the forrn of
meal and a new lot was produced every month~ To the
basic feed the TriCP additives were added. The table
shows that up to 50 kg there was a percentage of 24
I5 percent soya in the mixture and after 50 kg this was
12 percent.
30,249A F 18-
.~.,;~t~ 6;~P
--lg--
Table 2
Feed Mixture Composition and Contents in Denmark Trial
Period, kg _ Up to 50 kg After 50 k~
Barley % 73.4 ~5.4
Soya % 24.0 12.0
Chalk % 0.8 0.8
Dicalciumphosphate % 1.2 1.2
Salt % * 0.4 0-4
Vitamine and microelemen~s % 0.2 0.2
Dry stuff % 86.8 87.2
In ~O of Dr~ Stuff
Raw protein 21.4 16.6
Raw fat 2.2 2.1
Fiber 5.8 5.5
NFE (Nitrogen Free Extract) 64.~ 70.6
Ash 5.8 5.2
Feed units per kg dry stuff ~ 1.16
Digestible protein, g/unit 154.0 118.0
Digestible lysin, g/unik 8.1 5.5
20 Diyestible Threonine, g/unit 5.~, 4.2
Cvntent per g: 1500 i.e., A-vitamin,~500, i.e.,
D3-vitamin, 0.01 mg B12-vitamin, 10 mg alpha-
-tocoferolacetat, 2.5 mg B2-vitamin, 7.S mg
pantothenic acid, 0.66 mg natriumse~enit, 50 mg
zincoxyde, 62.5 mg copper sulphate, 62.5 mg
manganese sulphate, 2.5 mg cobalt sulpha-te,
62.5 mg iron sulphate and 0.5 mg kaliumiodid.
:
30,249A-F -19-
-20-
IB. England Trial
The study was designed to evaluate the growth
promotion potential of TriCP as assessed in pigs reared
for bacon production. I'he test diets were off~red
continuously until the animals reached 100 kg. The
pigs were then slaughtered after a 2-week withdrawal
period. Weight gain, food consumption and feed utili-
zation were measured and at termina-tion carcass data
were obtained under commercial conditions.
Thirty-two ~32) castrated males and thirty-two
(32) female pigs (Sus scrofa) of the Large White type,
approximately 11 weeks old at the start of treatment,
were used in the study. They were obtained from
R. Beedles, Shadymoor, Dorrington, Shropshire.
The animals were housed throughout the study
in a building designed for hacon pig production in
~loor pens of concrete construction. Each floor pen
incorporated individual metal feeding cxates, a communal
lying area and a dung passage. The pens also contairled
automatic dxinkers and wheat straw was provided as
bedding material. The building was ventilated by mearls
of baffled inlets along the side walls and 2 extract
fans in the central roof ridge.
Four feed premixes were used to produce Eour
experimental diets, three containing TriCP at difexing
inclusion levels and one control diet without TriCP.
The feed premixes were not identified until after
termination of the study.
30,249A-F ~20-
3~
-21-
The animals were delivered -to Huntingdon
Research Çenter (HRC) at approxima-tely 8 weeks o age
and were maintained on basal diet for 3 weeks prior to
-the start of -the experimental period. All pigs were
inspected by a veterinary surgeon and were routinely
treated with an anthelmintic ('Thiprazole', ~erck,
Sharp and Dohme) and a swine erysipelas vaccine
(Wel-lcome Foundation Limited).
Immediately before the start of the study,
all pigs were allotted a temporary identification
number and were weighed and re-examined to assess
general health and condition. After discarding spare
animals, the pigs selected for use in the study were
allocated to treatment according to body weigh-t. In
practice this was achieved by stratification by weigh-t
(males and females separately) followed by systematic
allocation to feeder posi-tions in order star-ting in the
irst pen. This procedure minimized the variation in
size and weight within pens (blocks). The animals were
then tagged with permanent identifica-tion numbers to
keep track of each Animal's identity.
The pigs were offered one o 2 pelleted
concentrate rations throughout the study. The irs~
ration was a grower or starter ration with a higher
nutrien,t density. The grower ration was used initially
and up to 50 kg body weight, and was xeplaced by a pig
~inisher diet from 50 kg to termina-tion. The composi-
tion and specification of these 2 basal diets is given
in Table 3.
30,249A-F ~21-
-22
Table 3
Feed Mixture Composition and_Contents in England Trial
Pig grower Pig finisher
ration ration
~< 50 kg ~> 50 kg
body weight) body welght)
Composition Barley 40.51 40.51
(% w/w) Wheat 10.00 10.00
Maize 15.00 15.00
Extracted soya
bean meal 14.50 11.50
Provimi 66
fish meal 3.50 2.50
Weatings 10.00 15.00
Dicalcium
phosphate 0.44 0.44
Limestone flour 1.05 1.05
Salt 0.25 0.25
Molasses 2.50 2.50
Fat premix (50%) 2.00 1.00
Beta 114* 0.25 0.25
Pellet size
(mm diameter) 4 10
Theoretical Oil 3.25 2.85
25 analysis (%) Crude Protein16.95 15.66
Fiber 4.48 4.61
Total digestible
- nutrienks 71.81 70.93
Digestible energy
30 MJ/kg (approx.) 13.00 12.75
Lysine 0.89 0.77
Methionine and
cystine 0.56 0.51
Calcium 0.87 0.81
Phosphorus 0.60 0.5
Salt 0.47 ~ 0.46
* Mineral/vitamin premix ~B.P. Nutrition~ - formulated
without the standard inclusion of copper at 180 ppm
30,249A-F -22~
A restricted feedin~ regime was used in
accordance with normal commercial practice. The pigs
were given 2 e~ual feeds per da~, the feed allowance
based on body weight as follows:
5Body weight (kg)Feed allowance (g ~er feed~
< 25.0 500
25.5 to 30.0 600
30.5 to 35.0 700
35.5 to 40.0 800
1040.5 to 45.0 900
45.5 to 50.0 1000
50.5 to 55.0 1100
55.5 to 60.0 1200
60.5 to 65.0 1300
> 65.0 1400
Animals were weighed and feed allowances adjusted at`
weekly intervals.
All feed reusals were measured and recorded
and the total weekl~ food consumption for each pig was
derived.
Test diets wPre mixed and pelleted in batches
of 0.5 ox 1.0 tonne as follows: 1 tonne batches of
basal diet (2 or 4 as appropriate) were first mixed and
retained in meal form in 25 kg bags. These were then
divided into 4 lots of 0.5 or 1.0 tonne such -that each
lot consisted of an equal num~er of bags selected at
random from each basal 1 tonne mix. Test die-ts were
then prepared by returning each 0.5 or 1.0 tonne of
30,249A~F -23-
meal to the mixer together with the appropriate tes-t
premix (pre-weighed and supplied by HRC). The mix was
then pelleted to produce the finished diet.
Before the start of the study, 4 x 0.5 tonnes
of test diets were prepared using the test premixes at
the intended inclusion level of 500 g/tonne and samples
of the diets taken both be~ore and after pelleking were
submitted for assay. All samples were assayed at
40 percent below nominal levels and further test diets
were prepared using 750 g of premix/tonne. Analysis of
this second batch of diets showed recovery of target
levels of TriCP. This was the required premix inclu-
sion level throughout the -trial.
In all test diets used throughout the study,
premixes were included a-t a level of 750 g per tonne of
finished feed. The levels of test compound in each
diet (ppm) after termination of the study, are as shown
in Table 1.
On completion o~ its 2 week withdraw~l period
(basal diet only) each pig was sent for slaughter at an
approved commercial abatkoir. The eviscerated carcasses
were weighed a~d graded and this in~ormation was
re-turned to HRC.
All pigs were maintained on test diet from
the start of the test period until they reached 100 kg
live weight. They were then fed with basal diet only
for a period of 2 weeks before slaughter. All piy5
were observed daily and any clinically abnormal signs
were noted.
30,249A-F -24-
-25-
IC. Germany Trial
In a test on growing pigs, the effect of the
additive 3,5,6-trichloro-2-pyridinol (TriCP~ on the
body weight gain, feed consumption and feed conversion
was investigated. The aim of the investigations was to
determine the effectiveness of the additive from the
nutrition physiology point of view.
A total of 168 pigs consisting of ec~ual
numbers of castrated males and females were available
for the tests. The pigs were largely of uniform ~enetic
origin. The animals were numbered individually. At
the start of the test each pig had a body weight of
about 30kg. Fattening was continued until a final
weight of about 105 kg was achieved.
The 168 test pigs were divided into our
treatment groups at the start of the test, so th~t 42
animals were subjected to treatment in each case. Each
test group was subdivided into seven sub-groups with
six animals in each sub-group. These six pigs were
kept together in a pen.
The our test groups were trea-ted as follows.
One group was kept as a control group without addition
of the additive. Three further groups were used as
test groups, in which TriCP was administered in a
dosage of 5Q ppm, 75 ppm or 100 ppm in the feed.
. The composition of the test feed met the
requirements of the "Feed Regulations in the Federal
Republic of Germany". Table 4 gives details of the
nutrient content of the test feed.
30,249A F -25-
-2~
Table 4
Contents of the Test Mixtures in Percent in German~ Trial
Preparatory Feeding
Raw Protein 16.50
Lysine 0.90
Raw Fat 4.25
Starch 39.00
Sugar 4,50
Crude Fiber 3.~/5
Calcium 0.90
Phosphorus 0.60
Sodium 0.20
Final Fattenin~
Raw Protein 15.00
Lysine 0.70
Raw Fat 4.75
Starch 38.25
Sugar 4.70
- Crude Fiber 4.40
Calcium 0O85
Phosphorus 0.55
'I Sodium 0.18
. . ", . . .
I
30,249A-F -26-
$~
-27-
The test animals were kept in a closed house
on the experimental farm at Gottingen University. The
environmental condi-tions were largely standardized.
The feed was distributed twice daily a-t fixed times
(8 a.m. and 3 p.m.~. Water consumption ~as ensured by
automatic drinking bowls.
The following criteria were checked during
the test. The body weights of -the individual animals
were weighed every 28 days. This interval was reduced
to 21 days for some sub groups at the end of the test.
This was necessary, if the body weight trend with a
28 day interval between the last two weighings would
have resulted in excessive final weights.
The feed consumption of the o~uantity of feed
consumed daily was recorded. The feed conversisn per
kg gain was calculated from the feed consump~ion and
the gain in body weight.
.
ID. ~olland Trial
In this trial the effect of oral adminis-
tration of khree doshges of TriCP on we:Lyht gain and
eed conversion efficiency is described. The effect of
TriCP on the~e criteria is compared with that of a
control group without a growth promotor.
. The study was carried out with 160 pigs
distributed arnony four groups of 40 animals each. Each
group contained five repetitions of eight animals, (2
pens with 8 castrated males, 3 pens with 8 females~.
The trial was comprised of two periods. A first period
of 0 to 6 weeks, wherein the live weight of the pigs
30,249A-F 27-
~ ~9~i~3~
-28~
was 22 to 45 kg, in which pigs receiving 50, 75 or
100 ppm TriCP were compared with a control group
without supplementation. The second period was 6 to
16/18 weeks, wherein the live weight of the pigs was 45
to 100 kg, in which pigs receiving 50, 75 or 100 ppm
TriCP were compared-with a control group withou-t
supplemen-tation.
The pigs were of the cross Dutch Landrace x
Large White. For this experiment 64 castrated males
and 96 females of a known offspring were purchased.
The animals were selected in such a way that the varia-
tion in age and live weight was as small as possible.
At the beginning of the feed trial the aye of the
piglets was eight weeks; the average live weight of the
females was 21.5 kg and of the castrated males 21.9 kg.
Before the feed -trial commenced the pigs were
fed a commercial ration containing an antibiotic. Six
weeks after arrival of the animals at the Institute
they were treated with 0.25 g piperazine adipas per kg
body weight~
One day after arrival of the animals ak the
Institute they were distributed among the four treat-
ment groups in such a way, that the groups were com-
parable with respect to sex, parentage, age and live
weight. For both sexes ~h~ avexage initial live weight
and their standard deviation was equalized as well as
possible for each group. In general each group com-
prised no more than one animal of the same litter.
.
The pigs were housed in a piggery of the
Danish -type with partly slatted floors withou-t straw.
30,249A F ~28-
3~
~9
The piggery holds 24 pens. Eight animals wexe housed
in each pen. Ten pens held cas-tra-ted males and 14 pens
females. The piggery is lighted, heated and ventilated
ar-tificially.
The minimal and maximal temperature in the
piggery varied during the whole experimental period
- between 15 and 22C.
The experiment consisted of two treatment
periods of 6 and 10 weeks, respectively, during which
the experimental rations were fed.
The composition of the diets is given in
Table 5. The diets for the animals of groups 1 to 4
contai~ed no extra copper.
30,249A-F -29-
.30~3
Table 5
Feed Mixtu.re, Composition and Conten-ts in Holland Trial
Ingredient 0 - 6 week~ 6 - 16/18 weeks
Wheat 10.0 11.0
5 Barley 27.0
Corn germmeal feed ~ 15.0
Wheat middlings 5.0 20.0
Tapioca 23.2 32.7
Soya oil 1.5 2.5
10 Whey powder (delactosed) 2.0
Whey powder 5.0
Soybean oilmeal 23.0 15.6
Vitamins, minerals ** 3.3 3.2
Calculated contents ~in %)
Crude protein 17.0(16.6*) 13.6(14.4*)
Lysine 0.96 0.70
Methionine ~ cystine 0.55 0.45
Net energy (kcal/kg) (2190) (2185~
Calcium 0.95(0.96*) 0.80(0.89*)
Phosphorous 0.75(0.70*) 0.65(0.65*)
* Analyzed
** The vitamin premix supplied per 1 kg die-t: 6,000
I.U. Vitamin A, 1,200 I.U. Vitamin D3, 25 mg
Vitamin E, 2 mg Vitamin K, 4 mg Riboflavin, 20 mg
Niacin, 10 mg d-Pantothenic acid, 80 mg Choline
chloride, 0.030 mg Vitamin B12, 0.54 mg Kl and
4.65 mg COSOg-7H2O.
30,249A-F -30
-31-
After mixing ~he basal ra-tion, it was divided
into five sub-charges and then supplemented respectively
with carrier and 50, 75 and 100 ppm TriCP. The mixing
was done in several batches. Sampling of batches
followed by analysis of crude protein, calcium and
phosphorus were carried out.
The animals were fed in groups of eight,
twice a day, according to a fixed scheme based on the
mean body weight of the animals of each pen. If the
pigs of one or more pens grow faster than khose in
other pens, they were offered more feed. After each
feeding, the meal for the next feeding was mixed with
water in a 1:1 ra~io. The pigs had free access to
fresh water via automatic drinkers.
State of health of the pigs was observed
daily. Live w~ight of the pigs was determined every
three weeks. Feed conversion efficiency (kg feed/kg
live weight gain) was calculated per pen of eight
animal~ at the time oE each weighing. The animals were
slaughtered 16, 17 or 18 weeks after the start of the
experiment. The experimental rations were Eed -till 24
hours before slaughter, except for some castrated male
animals of group 4 (75 ppm): for one animal of this
group the withdrawal time was 0 days, for three animals
three days and for three animals seven days.
30,249A-F -31-
' ;
~3~
Summary of Results for European Trials
- After the end of the finisher diets, individual
pig weights and amount of feed consumed were ob-tained
in order to determine weight gains and efficiency o~
feed use. The results for swine average daily weighk
gain for pigs receiving TriCP are presented in Table 6.
The results for the feed conversion efficiency by the
pigs receiving TriCP are presen-ted in Table 7.
Table 6
SWINE AVERAGE DAILY WEIGHT GAIN (ADG~ IN GRAMS/DAY
DURING FINISHING PERIOD (50 TO 100 KG)
Number Concentration of
Country of Pi~s TrlCP in Feed in ppm
0 50 75 100
Denmark 12~696(0)~730(+34)1 712(+16) 729(+33)
~ngland 64777(0) 765(-12) 759(-la) 8~0(-~43)
Germany 168705(0)792(-~7) i732(~27) 697(~8)
Holland 160710~0)747(+37) 738(+2a) 738(~28)
~ Numbers in parenthesis ( ) represent ~he diffexence
in weight gain between those animals recei~ing TriCP
in their diets and those receiving "0" or n~ TriCP.
As can be seen in Table 6, TriCP was effective in
increasing the daily weight gain in swine receiving
~eed containing from 50 to 100 ppm TriCP.
30,249A-F -32-
-33-
Table 7
FEED CONVERSION EFFICIENCY BY PIGS RECEIVING TRICP
Concen-tration Feed Effici-
of TriCP ency of pigs
in feed 50-l00 kg
Country (ppm~ (%)
England Q l00.0
l0l.8
l0~.0
l00 93.3
Germany 0 l00.0
89.4
94.7
1 5 100 101 .
1 Measurements taken at pig weights 65 to 1.00 kg
As can be seen in Table 7, pigs receiving TriCP achieved
a feed conversion efficiency of 6 percent or more over
the pigs not receiving TriCP, where average daily
weight gains were also increased as in Table 6.
30,249A-F -33-
.3~ d~
-~4-
-II. Protocol for United States Trials
Example 6: The anabolic effect of sodium 3,5,6--tri.-
chloro-2-pyridinate as exhibited by an
increase in average daily gain and an
increase in feed conversion efficiency
for swine.
T~ree-hundred-forty (340) swine averaging 20
kg each, were allotted into 5 treat.ments of 6 replicates
of 8 animals and 2 replicates of 10 animals. The
animals were fed a two part basal diet consisting of:
~ of Diet
~ ient Grower Diet _ _ ?i n~ ~her DiY ~
Ground Corn79.60 (722 kg/ton)84.64 (767 kg/ton)
Soybean Oil Meal 17.85 ~162 kg/ton) 12.91 (117 kg/ton)
15 Defluorinated
Rock Rhosphate 1.80 (16 kg/ton) 1.80 (16 kg/ton)
Salt 0.50 (4.5 kg/ton)0.50 ~4.5 kg/ton)
Trace Mineral &
Vitamin Premix 0.25 (2.2 kg/kon) 0.25 (2.~ kg/ton)
whereby the grower diet was fed to animals until they
xeached a weight of 55 kg and the finisher diet was fed
to animals thereafter.
To these dlets, sodium 3,5,6-trichloro-2
pyridinate, hereinafter referred to as Na-TriCP, was
added at a rate so that the different trea~ment groups
received 1 mg/kg/day, 2 mg~kg/day, 3 mg/kg/day and 4
mg/kg/day of Na-TriCP in accordance with the invention.
30,249A-F 34~
3.~
-35-
The control group was fed the basal diet alone wi-th no
addition of Na-TriCP. After a 112 day total feeding
period, weight gains and feed consump-tion of -the animals
were determined. The results obtained are -tabulated as
follows:
Average Dose of Sodium 3,5,6-
-Trichloro-2-~yridinate, mg/kg/da~*
Treatment 0 1 2 3 4
Avg. Daily Feed
Consumption, lb. 5~32 5.32 5.43 5.68 5.59
Avg. Daily Gain, lb. 1.54 1.61 1.63 1.72 1.72
Feed Conversion
Efficiency 3.46 3.32 3.34 3.31 3.26
*The average daily dose of sodium 3,5,6~tri-
chloropyridinate in mg/kg was achieved by incorporatingNa-TriCP into the feed in 25 ppm, 50 ppm, 75 ppm and
100 ppm which correspond to an average dose of 1 mg/kg/day,
2 mg/kg/day, 3 mg/kg/day and 4 mg/kg/day respectively.
This is based on the average daily feed intake and the
average weight of the animals. The actual dose of
Na-TriCP may have varied from day to day.
The anabolic efec~ of 3,5,6-trichloro-2-
-pyridinol as e~hibiked by an increase in
milk production of lactating cows.
Six ~6) lactating Holstein-Friesian cows, in
their 4th to 10th week of lactation, were allotted into
2 treatments consisting of 3 cows each. All animals
were fed the same ration except the second group which
was fed the ration plus 3,5,6-trichloro-2-pyridinol,
hereinafter referred to as TriCP, at levels that were
increased at 14 day intervals for 6 feeding periods.
30,249A-F -35-
.; .
~36-
At the end of these feeding pexiods, a 14 day withdrawal
period was insti-tuted. The concentrations of TriCP
added to the ration were as follows:
Concentration of
5 Feeding Period TriCP in ppm
(14 days)Group 1 ~Control~ Group 2
1 (prefeeding) O O
2 0
3 0 3
4 0 10
0 30
6 0 100
7 0 O (withdrawal)
Milking and feeding were done on a morning and
evening schedule for each cow. The feeding was done
after the milking.
The average daily feed consumption in kg; the
average daily d.ry matter milk production in kg; and the
milk production efficiency are as follows:
G:roup 1
~Control~ Gro~
Avg. Daily Feed Conswmption in kg 18.52 18.1
Avg. Daily ~ry Matter Milk
Production per day in kg* 2.15 2.41
Milk Production Effic.iency 8.51 7.54
*the dry matter milk production is equal to 12% of the
actual milk production.
30,249A-F -36-
' :'
-37~
Example ~: The anabolic effect of sodium 3,5,6-tri-
chloro-2-pyridinate as exhibited by an
increase in average daily ga.in and an
increase in ~eed conversion efficiency
for beef cat-tle.
Two hundred fifty-six (256) steer calves
averaging 283 kg (625 lbs~ were allotted into four (4)
treatments of 8 replicates of 8 animals, i.e., 64
animals per treatment. The animals were assigned to
replicates in a randomized complete block design. The
animals were fed a two part basal diet consisting of:
% of Diet (by Weigh~
Intermedlate Finlshlng
_ In~redient _ Ration Ration
.
Dehydrated Alfalfa Meal 10.0 5.0
Cottonseed Hulls 15.0 10.0
Steam Rolled Corn 58.0 74.8
Soybean Meal (44%) 10.0 3.0
Calcium Carbonate 1.0 0.7
20 Na Tripolypho~phate 0.5 0.3
Cane Molasses 5.0 5.0
Trace Min0rali.zed Salt 0.5 0.5
Urea None 0.7
whereby the int~rmediate ration was fed to animals the
first 28 days o~ the e~periment and -the finishing
ration was ~ed to animals thereafter. All dieks and
water were available to the animals on an ad libitum
basis.
30,249A~F -37-
,,,
~38-
To the above diets, sodium 3,5,6~trichloro-
2-pyridirlate ~Na-TriCP) was added in amounts sufficient
to satisfy the dose requirements of Na~TriCP each
treatment was to receive, namely:
Concentration o~
TreatmentNa-TriCP in Diet (wt. percent)
1 0 tControl)
2 0.005 (50 ppm)
3 0.0075 (75 ppm)
4 0.01 (100 ppm)
The control diet contained no Na-TriCP while -the other
three treatments were fed diets containing ~a-TriCP in
concentrations listed above. After a 112 day total
eeding period, weight gains and feed conversion effici
ency improvements were determined. The average inikial
weight, average 112-day weight, average daily gain,
average feed intake per day and, average feed conver-
sion efficiency (feed consumed/body weight gain) are
tabulated as follows:
_ Treatment
~ 3 _ _ 4
Average initial weight (kg) 285 285 284 285
Average 112 day weight (kg) 424 433 432 429
Average daily gain (kg) 1.24 1.32 1.30 1.28
Average ~eed intake/da~ 9.40 9.50 9.40 9.40
Average feed conversion
efficien~y 7.52 7.23 7.14 7.39
All animal weights were calculated after a 16 hour
shrink off f~eed and water. The incorporation of Na-TriCP
into the feed in 50 ppm, 75 ppm and 100 ppm corresponds
approximately to an average daily dose of 1.55 mg/kg of
body weight/day, 2.33 mg/kg of body weight/day and 3.1
30,249A-F -38-
,i
~39
mg/kg of body weight/day respectively. This is based on
the average daily feed intake and the average weight of
the animals. The actual individual doses of Na-TriCP
may have varied from day to day.
~, , i
.
. 30,249A-F -39-
.,