Note: Descriptions are shown in the official language in which they were submitted.
DESCRIPTION 1'
METHOD AND SYSTEM FOR ELECTRONICALLY
CONTROLLING PELVIC VI~CERA VIA
~EURO-ELECTRICAL STIMULATION
Technical Field
This invention relates generally to a method for
controlling bodily functions and, more particularly, to the
utilization of one or more electrodes on selected nerve
bundles and the application of pulse trains to the
electrode(s) to control, regulate or treat such functions.
Background Art
Various medical patients exhibit involuntary control
over their bladder and/or bowel. Although vesicostomy or an
artificial sphincter implanted around the urethra are
commonly used to provide partial control over the evacuation
function of the bladder and to control continence, these
solutions have drawbacks well known to those skilled in the
medical profession and related arts. Other patients who
achieve a modicum of control over their bladder functions
are equally in need of a system to rehabilitate their nerve
and muscle dysfunctions. Similar problems arise in respect
to involuntary bowel control.
2.
The physioloyy of the bladder and bowel is closely
linked to the urethral muscle physiology of the pelvic
floor (levator ani muscle) and its related urethral and
anal sphineters. For the bladder to store urine and ~or
the bowel to serve as a reservoir for feces, two opposite,
but complementary, behaviors are found. In particular,
the bladder and rectum must relax and the urethral and
anal sphincters must remain contracted. The reverse is
true during evacuation of either urine or feces, i.e., the
urethral or anal sphincter will relax, along with the
pelvic floor, and subsequently the bladder and rectum will
eontract.
The sequence will reverse once voiding and
defecation is completed, i.e., the sphincters and pelvic
floor muscles will revert to their tonic closure states
and the bladder and rectum will revert to their storage
states. This behavior has been demonstrated by
simultaneous manometric (or EMG/pressure) reeordings of
this bladder/rectum, urethral/anal behavior during filling
and emptying of the bladder. This sequence of events is
well-established and is accepted universally.
Diselosure of Invention
Various proeedures are disclosed for controlling
bladder or bowel evacuation, twenty-one specific methods
are diselosed for modulating the symptoms resulting from
a loss of coordination between normally synchronized
functions of visceral organs and seven methods are diselosed
for treating incontinence by increasing sphincter tonus.
The term "controlling", '1modulatingt' and "treating" as used
herein not only include the selective control, modulation or tr~atment
of a particular organ on a continuous basis, but further include
isolated or periodic stirnulation of the organ for diagnostic or reha-
5 bilitation purposes, e. g., neurornodulation of muscular behavior torehabilitate muscular dysfunction in the pelvic floor without stimulat-
ing the pelvic nerve controlling the bladder's detrusor muscle. The
term "organ" as used herein broadly means an independent part of
the human body that performs a special function or functions, includ-
10 ing visceral organs, such as the bladder, bowel and colon, and asso-
ciated sphincters, cuffs and muscles.
In one aspect, the method of this invention comprises the
identification of the anatomical location and functional characteristics
of selected nerve bundles controlling the separate function of at least
15 one organ, including a person's bladder, bowel and/or associated
sphincters. Electrode means is then positioned on such nerve bun-
dles for electrically stimulating the nerve bundles while simultaneously
isolating adjacent nerve bundles therefrom. A hereinafter described
electronic control system is adapted to sequentially apply pulse trains
20 to the electrode means to separately control the function of the one
organ or a number of organs simultaneously.
Each method disclosed can be carried forth either bilaterally
or unilaterally, depending on a particular patient's needs.
,
~2~7~
Brief Description of the Dr~win~s
Other advantages and objects of this invention will become
apparent from the following description and accompanying drawings
wherein:
5Figure 1 schematically illustrates the pelvic plexus region in
a human, including the nervous system for controlling bladder evac-
uation and related functions, and further illustrates a first operative
procedure for controlling such functions;
Figure 2 schematically illustrates a stimulation~response
10curve of bladder contraction in response to stimulation of the S2, S3
and S4 sacral nerves;
Figures 3 and 4 are views similar to Figure 1, but illustrate
additional operative procedures for controlling bladder evacuation and
related functions;
15Figure 5 schematically illustrates the percutaneous implanta-
tion of an electrode adjacent to the S3 sacral nerve through the
dorsum for the purpose of selectively stimulating such nerve;
Figures 6-11 are views similar to Figure 1, but illustrate
additional operative procedures for controlling bladder evacuation and
2 0related functions;
Figure 12 illustrates a micturition control system adapted
for use in conjunction with an operative procedure for controlling
bladder and/or bowel evacuation and related functions;
.. . .
I
Figure 13 schematically illustrates a typical electronic circuit
for use in an implantable receiver of the Figure 12 micturition control
system;
Figure 14 diagramatically illustrates electronic signals and
5 their timed relationship for the Figure 12 micturition control system;
Figure 15 illustrates an electrode arrangement, including
pairs of electrodes attached to separate nerve bundles and adapted
for use with the Figure 12 control system;
Figure 16 is a view similar to Figure 15, but illustrates a
10 multiplicity of active electrode contacts on single èlectrodes;
Figure 17 diagramatically illustrates electrical impulses in
their timed relationship for the electrode arrangements illustrated in
Figures 15 and 16; and
Figure 18 is a view similar to Figure 1 s but illustrates the
15 positioning of electrodes on various nerve bundles to effect desired
results, as reflected in Charts I and II set forth hereinafter.
I~ESCRIPTION (FIGURES 1-11)
Figures 1 and 18 schematically illustrates the pelvic plexus
region of a human, including the nervous system for controlling blad-
20 der and bowel evacuat;on and related functions. The nervous systemincludes a somatic nerve system of fibers (or nerve bundles) S and
an autonomic nerve system of nerve bundles A, finding their immedi-
ate origin at sacral segments S2, S3 and S4 of the spinal cord and
sacrum , i. e., the triangular bone positioned below the lumbar verte-
25 brae and comprising five fused sacral vertebrae that are wedged
~2~37~
dorsally between the two hip bones. As illustrated in Figure 2, themain nerve supply to the detrusor muscle of a bladder B emanates
primarily from sacral segment S3, a lesser amount from sacral segment
S2, and a still lesser amount from sacral segment S4, i.e., "response"
5 refers to bladder response.
The operative procedures, methods flnd systems described
in this application are applicable for controlling the bladder or bowel
and related functions, modulating symptoms resulting from a loss of
coordination between the normally synchronized functions of the blad-
10 der and bowel and their associated sphincters and treating inconti-
nence by increasing sphincter tonus. Either permanent surgical
implantation or temporary percutaneous implantation for nerve stimu-
lation purposes is applicable. Also, electrodes can be implanted
either unilaterally or bilaterally.
As further illustrated in Figure 1, the main nerve supply
emanating from each sacral segment S2, S3 and S4 comprises two
components or roots, namely, a dorsal root D and a ventral root ~1.
The dorsal root is primarily sensory to transmit sensation to the
spinal cord whereas the ventral root is primarily motor to transmit
2 0 motor impulses from the spinal cord to bladder B and associated
sphincter. Although illustrated as being separated, the dorsal and
ventral roots for each nerve are, in fact, normally joined together
and their fibers or bundles mixed to progress as a single trunk.
Bundles of the nerve trunk are divided into somatic nerve
2 5 bundles S that connect to voluntary muscles and autonomic nerve
bundles A that connect to visceral organs, such as bladder B.
Dorsal root D can be separated from ventral root V since only stimu-
lation of the motor nerve bundles of a particular ventral root are
contemplated in many procedures. In this manner, the motor nerve
5 bundles can be stimulated without inducing pain and without generat-
ing impulses along the sensory passageway.
Somatic nerves S and autonomic nerves A can also be sep-
arated from each other. For example, in a particular procedure
wherein it is desireable to only drive muscles controlled by the
10 somatic nerve, the somatic nerve can be solely stimulated. Should it
prove desirable to control the muscles of only a visceral organ, such
as the detrusor muscle of bladder B, the autonomic nerve bundles
could be stimulated. Stimulation of the entire nerve trunk would
function to stimulate each of the dorsal, ventral, somatic and auto-
15 nomic nerve bundles.
However, such stimulation may prove undesirable for certainpatients since uncoordinated action would ensue, e.g., bladder B and
external sphincter E would each contract and no effective response to
stimulation would be realized. Thus, the ability to isolate the dorsal
20 and ventral roots from each other and to further isolate the autonomic
nerves from the somatic nerves enables a practitioner to alleviate pain
and to simultaneously achieve specific responses of the controlled
organ or organs.
For example, responses obtained with pre-operative eval-
2 5 uation of responses to stimulat;on recorded urodynamically could
~37~
indicate that the S2 sacral nerve constitutes the mairl rnotor supply toexternal sphincter E, whereas the S3 sacral nerve constitutes the
main motor supply to bladder B. Thus, the S3 sacral nerve would be
utilized to control the detrusor muscle and thus the contracting func-
5 tion of bladder B alone, whereas the S2 sacral nerve would beutilized to control the muscles controlling the continence function of
external sphincter E. Studies have shown that in certain patients,
only the nerves on one side of the sacrum provide the main motor
control over a particular organ , i . e ., unilateral control rather than
10 bilateral control. Pre-operative testing of a particular patient will
determine which variation will provide the best choice for a subse-
quent operative procedure. The ability of this invention to isolate
various components of the various nerves, with the combined ability
to test a patient intraoperatively and record responses, has enabled
15 the applicants herein to isolate and selectively stimulate the particular
nerve bundles that will effect the specific function or functions
required .
Figures 1 and 3-11 illustrate various combinations of opera-
tive procedures for effecting the desired neurostimulation for specific
20 case studies (male or female) wherein simultaneous control for coor-
dinating the synchronized functions of a bladder or bowel and its
associated sphincter is effected. For example, a quadriplegic who has
suffered a neck injury and damage to his spinal cord will normally
require an operative procedure wherein control of bladder B and
25 external sphincter E are of the utmost importance. In addition, the
~2~
qu~driplegic may suffer uncontrolled bowel evacustion, for example,
which can be concurrently controlled when bladder control is effected
by such operative procedure. In addition, it may prove desirable to
modulate other voiding dysfunctions that may occur as a result of one
5 or more of a multitude of other neurological reasons.
Thus, specific operative procedures herein described can be
combined with one or more of the other procedures described herein,
as dictated by pre-operative evaluation of responses to stimulation
recorded urodynamically. For example, when a particular procedure
10 (e.g., electrode implant, nerve separation, sectioning, etc.) is de-
scribed as being performed bilaterally, clinical testing may indicate
that in certain other patients, a unilateral procedure (or combination
of bilateral and unilateral) is necessary (and vice versa). Likewise,
the specific steps or procedures utilized in one operative procedure
15 (Figures 1 and 3-11) may be utilized in combination with one or more
steps utilized in other operative procedures, as will be appreciated by
those sl~illed in the arts relating hereto.
Although the operative procedures herein described are
primarily useful and applicable to coordinated control of bladder and
2 0 bowel and their sssociated sphincters, similar procedures hereinafter
more fully described are applicable to the control of other organs,
including the colon, associated sphincters and cuffs and to the
elimination or suppression of spastic detrusor activity, spastic urethal
and pelvic floor activity and spastic anal sphincter. In all of the
2 5 described operative procedures, it is assumed that pre-operative
10 .
~7~
evaluation of response to stimulation has been recorded urodynamically
to precisely locate the nerves requiring separation, neurostimulation
and/or isolation, such as by sectioning.
OPERATIVE PROCEDURE FOR CONTROLLING EVACUATION (FIGURE 1)
Although the following operative procedure shown in Fig-
ures 1 and 3-11 are primarily discussed in connection with the simul-
taneous control of the coordinated and synchronized functions of a
bladder and its sphincter, they ae also applicable to the control of a
bowel and its sphincter.
Figure 1 illustrates an operative procedure whereby
continenee and evacuation of bladder E~ is closely controlled in a
partieular patient, sueh as a quadriplegie. The particular operative
procedure utilized will depend upon a patient's ability to respond to
eleetrical stimuli at strategic locations on his or her nervous system
in the pelvic plexus region. For example, it is assumed in the Fig-
ure 1 operative procedure that the patient is unable to self-control
his or her bladder functions and that sueh locations have been evalu-
ated pre-operatively.
As shown in Figure 1, after the anatomical location of the
S3 sacral nerve is identified, such as by the percutaneous insertion
and electrical energization of an electrode placed at least in close
proximity to such nerve, as illustrated in ~igure 5, the dorsal ( sen-
sory) root D and ventral ( motor) root V are surgically separated
bilaterally on each side of sacral segment S3. An electrode 2 is then
1~ .
attached by sutures or otherwise implarlted on each ventral root V for
purposes of external excitation and stimulation, as hereinafter de-
scribed with ~eference to the micturition control system illustrated in
Figure 12.
After bilateral implantation of electrodes 2 on ventral com-
ponents or roots V, each superior somatic nerve S is sectioned at 3
bilaterally to eliminate any increase in the resistance normally provid-
ed by the levator ani muscles at least partially surrounding external
sphincter E and controlled by superior somatic nerve Ss. Superior
10 somatic innervation (Ss) is commonly described in anatomy books
(e.g., CIBA or Gray's) as part of the innervation to the levator ani
muscles, whereas inferior somatic innervation ( S I ) is classically de-
scribed as the pudendal nerve in Alcock's Canal. It should be noted
that an internal sphincter I will normally open automatically when the
15 bladder contracts and thus requires no artificial control.
The operative procedure in Figure 1 was preceded by iden-
tification of the S3 sacral nerve and confirmation that it controlled
bladder and related functions by use of intraoperative stimulation and
urodynamic recordings. Minimum requirements to effect bladder evac-
20 uation without sac~ificing continence, i.e., the ability to retain oon-
tents of the bladder until conditions are proper for urination, were
assumed to be confirmed. The subsequent bilateral separation of
ventral root V from dorsal root D and the bilateral implantation of
electrode 2 on the ventral root was found to minimize risk of pain or
25 other undesirable reflexoenic response. Outlet resistance through
7~
urethra U was insured by sectioning superior somatic nerve Ss at 3
whereby the various nerves controlling outlet resistance at external
sphincter E were totally isolated , i. e ., isolation of motor supply to
the levator ani muscle.
Pre-operative electrostimulation was achieved by the use of
a bipolar probe for stimulating the various nerve bundles. A nerve
stimulator was then used to deliver a DC square wave for stimulation
purposes. The nerve stimulator may be of the type manufactured by
Grass Medical Instruments of Quincy, Massachusetts, U.S.A., under
Model No. S-44. The electrodes are of the standard type. For
example, each electrode may constituté a bipolar cuff electrode having
an inside diameter approximating 3-5 mm. and provided with 1 mm. by
2 mm. platinum contacts having a 3 mm. separation placed outside
each other about the periphery of ventral nerve root V. This type
of electrode is manufactured by Avery Laboratories, Inc. under
Model No. 390.
As described more fully hereinafter by reference to Figures
12-17, suitable receivers in the form of implantable silastic-coated
units containing an antenna coil, adapted to receive RF (radio fre-
2 0 quency) pulses from an external transmitter, are implanted sub-
cutaneously in the patient to transmit pulses to the electrodes to
control bladder evacuation in a controlled manner.
OPTIONAL OPERATIVE PROCEDURE FOR
CONTROLLING EVACUATION (FIGURE 3)
Figure 3 illustrates optional variations to the Figure 1 oper-
ative procedure which will potentially enhance bladder evacuation.
$~
After the various critical nerves for controllin~ bladder evacuation
have been identified by intraoperative stimulation and urodynamic
recordings, each of the S2, S3 and S4 sacral nerves are separated to
isolate the respective ventral and dorsal roots thereof. Pudendal or
S inferior somatic nerve Sl is then sectioned unilaterally to isolate ex-
ternal sphincter E on one side. Dorsal root D of the S3 sacral nerve
is then sectioned at 2a to thus isolate the sensory function thereof.
Although illustrflted as being performed unilaterally, and as stated
above, in certain applications it may prove desirable to perform such
10 sectioning bilaterally.
An electrode 3a is then implanted on the entire S3 sacral
nerve unilaterally, with or without dorsal rhizotomies at other sacral
levels. The S3 sacral nerve is then sectioned at ~a unilaterally (or
bilaterally), downstream of pelvic nerve P to isolate this nerve's
15 contribution to inferior somatic nerve SI. It should be noted that
electrode 3a is thus positioned on the S3 sacral nerve to stimulate the
detrusor muscles of bladder B, via pelvic nerve P.
After appropriate separation of the dorsal and ventral roots
of the S2 sacral nerve, the dorsal root is sectioned at 5a unilaterally
20 (or bilaterally) and an electrode 6a is suitably implanted on the
ventr~l root V of the S2 sacral nerve. It should be noted that supe-
rior somatic nerve Ss is preferably sectioned bilaterally, as described
above in reference to the Figure 1 operative procedure, to eliminate
any additional increase in resistance from contraction of the levator
25 ani muscle when the bladder is contracting for evacuation purposes.
14 .
The above options will also tentJ to eliminate or rninimize a
response in the pelvic floor sphincter which would otherwise prevent
low resistance voiding of the bladder synchronous with stimulation.
These optional variations address the possibility that excessive resid-
5 ual sphincter activity remains with stimulation after the ~igure 1operative procedure has been attempted. Sphincter response may be
reflexly produced which suggests the need for dorsal sectioning at 2a
and 5a in Figure 3, or directly produced to suggest sectioning la of
inferior somatic SI, unilaterally or bilaterally. The above steps must,
10 of course, be carefully evaluated prior to the selected operative pro-
cedure so as not to compromise continence or the contraction of the
bladder or bowel or nerves controlling the erection process.
Additional optional procedures may include percutaneous
implantation of an electrode 7a' on sacral nerve S3 and/or S4, up-
15 stream of the point whereat the autonomic nerve roots forming pelvicnerve P separate from the respective sacral nerve proper, to aid in
bladder contraction through the pelvic nerve. A further option con-
templates implsntation of a cuff electrode 8a' around sacral nerve S4,
either unilaterally, as shown, or bilaterally, to assist in the control
20 of bladder evacuation. It should be understood that above sectioning
steps 2a and 5a, as well as the implantation of electrodes 3a, 6a and
8a', require laminectomy, i. e ., incision of the posterior arch of the
vertebrae .
15 .
NON-LAMINECTOMY PROCE~URE FOR CONTROLLING
VISCERAL ORGANS (FIGURES ~ AND 5)
Figure ~ illustrates an operative procedure wherein an
electrode lb is implanted onto the S3 sacral nerve through a sacral
5 foramen without excising the posterior arch of the vertebrae. A
second electrode 2b may be implanted in a like manner on the S4
sacral nerve, either in addition to or in lieu of electrode lb. These
electrode implants may be effected unilaterally, as illustrated, or
bilaterally, depending on the pre-operative test results. Optionally,
10 superior somatic nerve Ss is sectioned at 3', either unilaterally or
bilaterally as illustrated in Figure 4.
The system thus effected by the Figure 4 operative proce-
dure will normally provide means for selectively eliminating or sup-
pressing spastic detrusor activity, spastic urethral and pelvic floor
15 activity and spastic anal sphincter. Such system may further sup-
press or enhance erection.
Figure 5 illustrates the percutaneous implantation of elec-
trode lb through the dorsum and the sacral foramen of sacral segment
S3 for the purpose of selectively stimulating the S3 sacral nerve.
20 After the appropriate depth and location of the S3 nerve has been
verified by electrostimulation and recorded urodynamically, electrode
lb can be ;nserted through the hollow spinal needle used for such
stimulation with the wire lead connected to the electrode being
suitably sutured in place, as shown, for attachment to a receiver (not
2 5 shown), as will be described more fully hereinafter. This
percutaneous method can fllso be used to temporarily implant an
16 .
electrode on any one or more of the sacral nerves for testing pur-
poses , i. e ., to record activity in the bladder in response to stim-
ulation of one or more of the nerves by the electrodes to thus
determine which nerve or nerves are controlling the bladder
5 functions. This procedure can be conducted unilaterally or bilat-
erally .
For example, electrode lb could be percutaneously placed
on the S3 sacral nerve with the external extremity of the wire at-
tached to the electrode then being taped to the skin, along with a
10 receiver connected thereto. The patient could then resume his
day-to-day lifestyle and be allowed to stimulate the nerve or nerves
artificially via a transmitter compatible with the receiver. If the
response is positive and complete relief is achieved, the electrode or
electrodes could be permanently implanted or temporarily implanted for
15 the purpose of correcting any dysfunction by "retraining" the nerve
and associated muscles. Should little or no improvement result, the
same procedure could be followed to accurately ascertain which nerve
or nerves- require stimulation. Thus, this invention contemplates not
only the implantation of one or more electrodes in the sacral nervous
2 0 system for controlling evacuation of a visceral organ or the like, but
also contemplates use of such electrodes and procedures to rehabili-
tate muscle dysfunction by neuromodulation of muscular behavior, as
described more fully hereinafter with reference to Chart II.
~2~
ADDITIONAL OPTlONAL
OPERATIVE PROCEDURES (~IGS. 6-11)
Figure 6 illustrates an optional variation for cont-rolling
evacuation of bladder B . In particular, superior somatic nerve S s is
5 sectioned at 3, either unilaterally or bilaterally, as shown. In addi-
tion, electrodes lc are implanted on inferior somatic nerve SI, uni-
laterally or bilaterally depending on the response obtained from pre-
operative evaluation of responses to stimulation recorded uro-
dynamically .
In Figure 7, electrodes ld are implanted bilaterally on
inferior somatic nerve SI and electrodes 2d are implanted bilaterally
on the S3 sacral nerve percutaneously. In Figure 8, electrodes le
are implanted bilaterally on superior somatic nerve Ss and electrodes
2e are implanted bilaterally on inferior somatic nerve SI.
Figure 9 illustrates another operative procedure for control-
ling continence and bladder contraction. In the illustrated operative
procedure, electrodes 1f are implanted bilaterally on inferior somatic
nerve SI. Superior somatic Ss is sectioned bilaterally, as illustrated,
and a pair of second electrodes 2f are implanted bilaterally on the
20 separated ventral root V of the S3 sacral nerve.
Figure 10 illustrates an operative procedure particularly
adapted for achieving contmence due to muscle weakness of the blad-
der or bowel. Electrodes lg and 2g are implanted bilaterally on infe-
rior somatic nerve SI and on the S3 sacral nerve, as illustrated. As
25 an option to implantation of the electrode unilaterally on the S3 sacral
nerve, an electrode 2 g' could be implanted on the ventral root
thereof. In addition, an electrode 3g is impl~nted unilaterally on the
S3 sacral nerve percutaneously. Alternatively, an electrode 3g' could
be implanted on the S4 sacral nerve, also percutaneously.
As another option, illustrated in Fi~ure 10, another elec-
5 trode 2g could be implanted on superior somatic nerve Ss ~ eitherunilaterally or bilaterally, as illustrated. The Figure 10 operative
procedure illustrates the two components of sphinc~er contraction with
the number of implants and their locations being dependent on re-
cruitability of muscle activity in individual patients and/or the ability
10 of percutaneous technique to adequately couple the electrodes with
the appropriate nerve bundles.
Figure 11 illustrates an operative procedure particularly
adapted for controlling autonomic dysreflexia and bladder storage.
Superior somatic nerve Ss is sectioned bilaterally at lh and electrodes
15 2h are implanted on the inferior somatic nerve bilaterally. Alterna-
tively to the latter step, electrode 2h could be implanted unilaterally
with the opposite side of the inferior somatic nerve being sectioned at
2h' .
As indicated above, the operative procedures illustrated in
20 Figures 1 and 3-11 are suggestive of specific procedures applicable to
particular patients. Thus, the various steps described above in
connection with one particular procedure could be included with or
substituted in lieu of steps included in one or more of the other
procedures to meet a particular patient's needs. For e~cample, many
of the above steps could be performed bilaterally, although disclosed
unilaterally, and vice versa.
It follows that the claims appended hereto, when reciting
the method steps of "implanting" OI' "attaching" an electrode to a
5 particular nerve or "sectioning" a particular nerve , etc., intend to
cover both unilateral and bilateral procedures.
Selection of the various options described above would be
based upon evaluation of responses obtained from pre-operative stimu-
lation recorded urodynamically. The ability of a particular procedure
10 to be conducted percutaneously or surgically, or a combination there-
of, further expands application of this invention. Those skilled in
the medical arts relating hereto will also appreciate that the above
operative procedures may be utilîzed to control not only bladder
functions but also concurrently central functions of other organs,
15 such as the colon, bowel, anal sphincter, etc.
20 .
~2~
DESCRIPTION OF
MICTURITION CONTROL SYSTEM (FIGS. 12-19)
Figure 12 illustrates a micturition control system adapted to
transmit electrical current (radio frequency) pulses to electrodes
5 implanted on selected nerves of the above-described systerns, such as
the implantations illustrated in Figure 9 , i . e ., leads 26 and 27 con-
nected to electrodes lf and leads 47 and 48 connected to electrodes
2f. The control system comprises an external control-transmitter
system 10, and a receiver system 11 implanted on a patient for trans-
10 mitting electrical current pulses to the electrodes. The two-fold
purpose of this system is to efficiently ( 1 ) maintain urethral tone and
hence continence of urine until bladder voiding is desired; and (2)
provide bladder contraction and voiding on demand by a patient or
his attendant, for patients having bladder or pelvic problems or pa-
15 ralysis.
Figure 12 illustrates the electronic control, transmitter andreceiver components of the system whereas Figure 14 illustrates the
types of signals and their timed relationship in the electronic control
component of the system. The symbol SR as used herein depicts a
2 0 component of the control system connected to an electrode implanted
on a particular sacral nerve or root, whereas IS depicts connection to
inferior somatic nerve SI, controlling continence.
The ongoing control of continence (retaining the contents of
bladder B ) is provided by stimulation of a selected nerve or nerves
2 5 as described above . This control function is accomplished by the
ongoing stimulation produced by a stimulus pulse oscillator 12 (OSC)
and associated circuits. The oscillator, preferably operating at a r ate
within the range of from S to 40 pulses per second, emits a square
wave output signal that drives and IS stimulus pulse width one-shot
(O. S . ) 13 which produces signal pulses with widths within the rarlge
of from 50 to 500 microseconds, as illustrated in Figure 14. These
pulses are controlled by AND gates 14 and 15 to produce separate
trains of pulses, also shown in Figure 14. Gating is produced by an
IS pulse train duration oscillator 16. It should be noted in Figure 12
that the two phases of the square wave output of oscillator 16 can be
10 obtained by the illustrated inverter circuit.
The frequency of the square wave output of oscillator 16 is
preferably selected from within the range of from 0.1 to 0.5 Hertz
(cycles per second). This oscillator is controlled by an IS stimulus
control flip-flop 17. When the flip-flop is set, it allows oscillator 16
to run to enable gates 14 and 15 to transmit signal pulses (Figure 14)
to turn on and off radio frequency (RF~ generators or oscillators 18
and 19. Radio frequency amplifiers 20 and 21, whose output ampli~
tudes are adjustable by variable resistors 20' and 21', respectively,
receive the separate pulses to drive antennas 22 and 23.
The antennas are inductively coupled to receivers 24 and 25
which detect the separate sets of RF pulses and transmit such detect-
ed pulses to a particular nerve-implanted electrodes via electrical
leads 26 and 27, respectively. Receivers 24 and 25, as well as here-
inafter described receivers 45 and 46, are each subcutaneously im-
2 5 planted on a particular patient .
22 .
When it is desired to evacuate bladder B, and ~ssuming
that the four energized electrodes are properly implante(l for a par-
ticular patient in the manner described abs~ve, the patient or his
attendant will momentarily close a switch 28. Transmitter 1() and
5 attendant antennas 2~. and 23 are, of course, suitably housed as an
external unit, readily accessible to the patient. The closing of switch
28 will activate an SR stimulus timer 29 for a selected length of time,
preferably within the range of from 10 to 40 seconds.
During this time interval, the output Q of timer 29 goes
10 high to activate an SR delay oscillator 30 and resets IS stimulus con-
trol flip-flop 17 so that it turns off the IS pulse train duration
oscillator 16. As a consequence, stimulation of the urethral sphincter
closure, for example, is disabled. Simultaneously therewith, the Q
output of timer 29 goes low to allow flip-flop 31 to be set on a signal
15 from SR delay 30. After a predetermined and selected time delay of
from 2 to 20 seconds (Figure 14), for example, the output signal from
SR delay 30 sets a flip-flop 31 which then starts an SR stimulus pulse
oscillator 32 and SR pul~e train duration oscillator 33.
Oscillator 32 will generate a square wave signal that is
20 preferably selected from the range of from 15 to 50 Hertz, as dia-
gramatically illustrated in Figure 14. The output from oscillator 15
then drives an SR stimulus pulse width O . S . 34 which produces sig-
nal pulses at a selected width of from 50 to 500 microseconds. These
pulses are ANDED by gates 35 and 36 with the output of oscillator 33
25 to produce trains of pulses, as illustrated in Figure 14. It should be
noted in Figure 12 that the opposite phases of the square wave out-
put of oscillator 33 may also be obtained by the use of the illustrated
inverter circuit. ~ate control originates at oscillator 33 which has a
selected frequenc~ within the range of from 0.1 to 0.5 Hertz.
The pulse trains from AND gates 35 and 36 control RF
oscillators 37 and 38, respectively. These are turned on when the
pulses are high. The RF signals are amplified in RF amplifiers 39
and 40 wherein the output amplitudes are controlled by variable resis-
tors 39' and 40', respectively. The outputs of the amplifiers drive
10 antennas 43 and 44 which inductively couple the signal through the
patient's skin and to implanted receivers 45 and 46. The receivers
detect the 3~F pulses and transmit the stimulus pulses to the particu-
lar implanted electrodes, via electrical leads 47 and 48.
At the end of the selected time period , e . g., 10 to 40 sec-
15 onds, of timer 29, the output reverses, as illustrated in Figure 14.
The Q output goes low and enables flip-flop 17 to activate oscillator
16 when the "set" signal is transmitted from IS delay O . S . 49 . The
Q output of timer 29 goes high and activates 49. At the end of the
preselected time delay of from 2 to 20 seconds, the output (Figure
20 14) sets IS stimulus circuitry until bladder evacuation is again
required. The Q output from timer 29 also resets flip-flop 31 and
thus disables oscillators 32 and 33 to end the bladder voiding stimu-
lation.
The above oscillators (OSC) may be of the astable multivi-
25 brator type, manufactured by Intersil Corp., U.S.A., under Model
. .
No. lCM7556 . The one-shot (O . S . ) monstable multivibrators may also
be of the common type manufactured by Intersil Corp. The flip-~lops
(F . F . ) may constitute the type manufactured by RCA Corporation,
U . ~ .A., under Model No. CD~027B, whereas the gates may be of the
5 type manufactured by the same company under Model No. DC4081B.
Receivers 24, 25, 45 and 46 may constitute a standard
implantable silastic-coated unit containing an antenna coil adapted to
receive the "rf" pulses transmitted from their respective transmitter
antennas 22, 23, 43 and 44. ~or example, each receiver may be
10 similar to the type manufactured by Avery Laboratories , Inc .,
I~.S.A., under Model No. 1-110 (bipolar). Figure 13 illustrates a
typical circuit 55 for the receiver.
In particular, a coil 56 functions as an antenna that re-
ceives the inductive signal transmitted thereto by a respective trans-
15 mitter antenna which is placed externally of the patient, adjacent tothe location of the implanted receivers. A capacitor 57, in conjunc-
tion with coil 56, provides a tuned circuit that is tuned to one of the
four different frequencies of the transmitter. The other three re-
ceivers are tuned to their respective transmitting frequencies when
2 0 the system uses four separate radio frequencies . l'.lternatively, the
frequencies may be the same for all four transmitters with the four
receivers being tuned to the same transmitting frequency. In the
latter application, the four transmitting antennas and the four im~
planted receivers must be separated so that the signal in any one
25 .
, ~ .
transmitting antenna will not provide false signals in the other three
receivers .
Still referring to Figure 13, a diode 58 detects, by
half-wave rectification, the pulsed stimulus current from the RF
5 bursts. Resistors 59 and 60 and capacitors 61 and 62 function to
filter the RF out of the stimulus signal which is lead to the nerve
electrodes via electrical leads, described above. Maximum stimulation
of the nerves is achieved when the negative pole is attached to the
distal electrode contact on the nerve and the corresponding positive
10 pole is the proximal contact of the nerve electrode assembly.
Those skilled in the art will appreciate that the Figure 12
control system can be suitably modified to control the energization of
the electrodes used in the above-described procedures and variations
thereof. For example, only the portion of the system for controlling
15 pulse inputs to antennas 24 and 25 could be utilized to energize elec-
trodes 2 in Figure 1.
Figures 15 and 16 illustrate typical electrode implantations
for stimulation purposes. Figure 17 diagramatically illustrates the
timing of stimulus pulse trains to electrode pairs with each electrode
20 contact being activated essentially 100/n % of the time for n active
( cathodol) contacts .
One of the primary problems encountered with prolonged
and continuous electrical stimulation of a nerve and mus~le system to
achieve chronic on-going muscle contractions, is fatigue of the nerve
2 5 and associated muscles . One way to prevent or prolong the onset of
26 .
~2~
fatigue (the muscle being more susceptible to fatigue than the nerve)
is to stimulate the system in a non-continuous and time-modulated
format. Otherwise stated, alternate stimulation of different groups of
nerve bundles in a nerve system with short bursts of stimulus pulses
5 provides such desiderata. This method of stimulation allows the mus-
cles and nerves to recover between trains of stimuli while other
nerves and muscles are being activated to continue the desired
physiological effect.
Time-modulation of stimuli to nerves to achieve muscle con-
10 traction, for example, can be accomplished in at least two ways.
Figure lS illustrates a first approach wherein a multiplicity of elec-
trode pairs 63, 64 and 65, 66 are attached to separate nerve bundles.
Each pair of electrodes are activated so that each electrode is "on"
for only a portion of a particular stimulation cycle, e. g., with four
15 electrode pairs, each would be stimulating its nerve bundle approxi-
mately one-quarter of the time (in general, 100/n g6 of the time for n
electrode pairs). It should be noted in Figure 17, although shown in
coincidence, that an overlap or dead time can be affected between the
stimuli trains.
2 0 Figure 16 illustrates a second approach to accomplish time
modulation of nerve stimulation wherein a multiplicity of active elec-
trode contacts are employed on a single electrode. However, in order
to achieve this alternating stimulation on a single nerve bundle, the
intensity of the stimulus pulses must be sufficiently low so that all
nerve bundles are not stimulated by a single active electrode contact.
27,
Nonetheless, the amplitude of the stimulus pulses mus~ be sufficiently
high so that the desired physiological function can be achieved.
The active electrodes (cathodes) must be located on the
nerve bundle so that they stimulate different nerve bundles. Thus,
5 a single electrode is not acti~ating all of the nerve bundles in associ-
ate muscles, as illustrated in Figures 16 and 17. Ideally, each elec-
trode contact would function to activate the proper proportional num-
ber of fibers , e . g., in the case of two contacts each contact would
activate one-half of the nerve bundles and in the case of four con-
tacts each contact would active one-quarter of the nerve bundles,
etc. However, the desired physiological function must be achieved
when a single contact is used on that particular nerve bundle. An
exception, of course, would be in applications wherein other nerve
bundles are similarly connected to a similar time-modulated system.
In the latter application, partial nerve stimulation from the combined
group of stimulated nerve bundles would accomplish the desired
physiological results.
ELECTRICAL CONTROL OF VISCERAL, VISCER~-SOMATIC,
AND SOMATIC NEUROMUSCULAR DYSFUNCTIONS
The E~hysiology of the bladder and bowel is closely linked to
the urethral muscle physiology of the pelvic floor and its related
urethral and anal sphincters. The se~.uences for storage (continence)
and evacuation suggest that the somatic muscles of the pelvic floor
are principally responsible for both continence and evacuation. Dur-
ing the storage phase, the visceral organs, i.e., bowellbladder, are
either released from the reflex inhibition or are directly facilitated
into contractin~, or both. It has been determined that neural control
of the pelvic muscles largely determines the state of activity of the
pelvic viscera (bowel, bladder, and possibly erection). A sirnple
example is the "hold" reflex used to suppress a strong urge to void
5 or defecate at inconvenient times.
If the neural regulation of bladder and bowel activity is
directly tied to that of the pelvic muscles in the normal, then it is
most certainly tied to it in the abnormal. Just as the hold reflex is
used to suppress an inconvenient urge to empty either the bladder or
10 bowel, an electrically induced contraction of the pelvic sphincters can
be used to suppress an overly active bladder or bowel.
There is a broad spectrum of patients who experience a
multitude of symptoms resulting from dysfunctional behavior of the
bladder, bowel, urethra, anal and pelvic floor muscle systems. Not
15 uncommonly, the muscle dysfunction cannot be ascribed to any dis-
ease. The muscle dysfunction can, however, be very similar to that
found for other neural disorders (e.g., meningomyelocele, hydro-
cephalus, spinal injury, multiple sclerosis, stroke, etc. ) . The
visceral dysfunction can be demonstrated especially in the case of the
2 0 bladder--to be associated with pelvic muscle dysfunction, with the
behavior of the bladder being a direct result of excessive inhibition
(e.g., inability to void completely because of an inability to relax the
urinary sphincter completely), or excessive triggering of bladder
contractions (i.e., a precipitate urge to void one's bladder) because
2 5 of a breakdown in the efficiency of reflex coordination between the
29.
.... .
. .
bladder and pelvic muscles. A similar analogy can be made for prob-
lems affecting the bowel (as well as erection). Correction o~ the
pelvic muscle dysfunction can thus serve to correct the visceral mus-
cle dysfunction. Other effects desc~bed by patients have included
5 the reduction of severe neck spasm, back spasm and leg cramps.
Visceral muscle dysfunctions which can be considered a
result of overfacilitated activity include the spastic colon, interstitial
cystitis, detrusor instability, cardiovascular problems, such as mi-
graine headache or palpatations, and bladder retention syndromes.
10 Somatic muscle dysfunctions directly resulting from poor neural regu-
lation and overfacilitated behavior include: pelvic pain syndromes,
frequency syndromes (pelvic floor and/or urethral instability), incon-
tinency due to poor relaxation or instability of the sphincters ( either
bowel or bladder), and incontinence following prostatectomy.
Each of the above has been shown to respond to stimulation
of the somatic muscles of the pelvic floor lprimarily levator ani mus-
cle I in Figure 18). Neurostimulation of the pelvic muscles has a
stabilizing effect on their neuro-regulatory mechanisms. Behavioral
stabilization of pelvic muscles then affects the neuro control of the
2 0 viscera .
Because of the similarity in nervous control between the
bowel and bladder, the following bowel problems may also be treatable
by a sacral or pudendal nerve electrode implant, namely, "spastic
colon", and fecal incontinence either from spasticity or incompetence
30 .
` -
of the anal sphincter, and infrequent or too frequent bowel move-
ments .
A spinoff benefit that has been noted is the treatment of
foot drop. It appears that the planter flexion of the distal half of
5 the foot and toes gives added stability to the gait. It has long been
believed that foot drop was the result of a weakness in the muscles
controlled by the perineal nerve. Stimulation of this nerve has been
used to lift the foot using the foot dorsiflexors, but with limited
success. Foot drop has been shown to improve by stimulation of
10 sacral S3 nerve root N3 because of a better push the foot has as a
result of planter flexion.
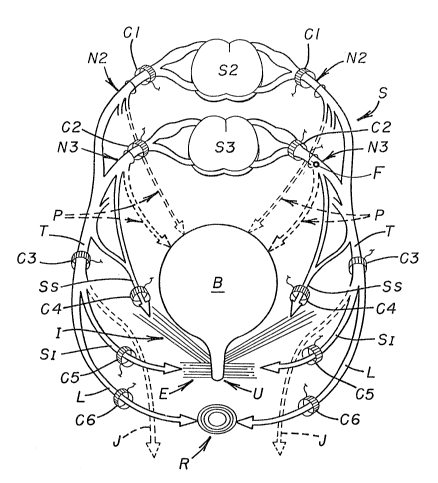
Figure 18 illustrates letters depicting various components of
the pelvic plexus region of a human that are common to those shown
in Figures 1-11. The following listing includes newly discussed com-
15 ponents, shown in Figure 18, as well as the common ones:
A: Autonomic nerve system.
B: Bladder.
C1-C6: Electrodes, shown as cuff electrodes for illustration
purposes (other types could be used).
D: Dorsal root of nerve (sensory).
E: External sphincter of bladder B.
F: Foramen electrode.
I: Internal sphincter or levator ani muscle (pelvis floor,
i . e ., the composite muscular structure that constitutes the outlet of
25 the boney pelvis and primarily consisting of the levator ani muscle).
J: Dorsal nerve of the penis.
L: Anal branch of pudendPl nerve T.
N2, N3: Sacral nerves originating at sacral se~ments S2 and
S3, respectively.
P: Pelvic nerves connected between the sacral nerves and
the detrusor muscle of bladder B.
R: Anal sphincter ( sphincter ani) .
SI: Inferior somatic nerve.
Ss: Superior somatic nerve.
10 T: Pudendal nerve.
U: Urethra.
V: Ventral root of nerve ( motor) .
Methods herein disclosed can be used to either modulate
symptoms resulting from a loss of coordination between the normally
15 synchronized functions of organs, including bladder B, rectum R and
associated bladder sphincters E and I and the anal sphincter for
rectum R (Chart I), or to treat incontinence by increasing sphincter
tonus (Chart II). Sacral nerves N2 and N3 originate at sacral seg-
ments S2 and S3, respectively, and form pelvic nerve P that controls
20 contraction of a detrusor muscle surrounding bladder B. The sacr~l
nerves also form somatic components that subdivide into: (1) superior
somatic nerve Ss; and (2) pudendal nerve T that includes
(a) inferior somatic nerve SI connected to muscles controlling external
sphincter E of bladder B, (b) anal branch L connected to the anal
25 sphincter for rectum R, and (c) dorsal nerve J connected to the
penis. The nerve bundles connected to the various sphincters are
controllable at a lower level of electrical stimulation than that required
to control the muscles for the blndder and rectum proper.
Figure 18 illustrates six cuff electrocles C1-C6 adapted to
5 be positioned on selected nerve bundles (while simultaneously isolating
adjacent nerve bundles) either individually or in combination with at
least one other electrode for stimulation purposes. Such positioning
step occurs after identifying the anatomical location and functional
characteristics of the selected nerve bundle or bundles. Pulse trains
10 are then applied sequentially to the electrode or electrodes to control
the function of the organ.
Individually, electrodes C1-C6 modulate or control the func-
tion(s) of the following organs:
C1: Bladder B, sphincter E, anal sphincter R and the
15 detrusor muscle for bladder E3.
C2: The detrusor muscle for bladder B and both bladder
and anal sphincters E and R.
C3: Both bladder and anal sphincters E and R,
C4: Internal sphincter I (pelvic floor).
C5: Bladder sphincter E.
C6: Anal sphincter R.
33 .
I'
C~IART l
The following chart indicates twenty-one different combina-
tions of electrode placement (unilaterally or bilaterally) for modulating
the above-discussed symptoms resulting from a loss of coordination
5 between a person's organs, including bladder B, rectum R and
associated sphincters:
C1 C2 C3 ~4 C5 C6
Superior Inferior
Organ(s) Sacral $acral Pudendal Somatics SaTEltisr Ana1 Branch
Affected Nerve N2 Nerve N3 Nerve T Nerves S Nerve I L
(1) B, E, R X X
(2) B, E, Rr I X X
(3) B, E, R X X
~4) B, E, R X X
(5) B, E, R, I X X X
15(6) B, E, R, I X X X
(7) B, E, R, I X X X
(8) B, E, R X X
(9) B, E, Rr I X X
(10) B, E, R X X
20 ~11) B, E, R X X
(12) B, E, R, I X X X
(13) B, E, R, I X X X
(14) B, E, R X X X
(15) B, E, R X X X
25 (16) B, E, R, I X X X
(17) B, E, R X X X
(18) B, E, R X X X
(19) B, E, R, I X X X X
(20) B, E, R, I X X X
30 (21) B, E, R, I X X X X
34 .
. . ~
CHART II
The following second chart indicates electrode placement
(unilaterally or bilaterally) for treatment of incontinence by increasing
sphincter tonus either by direct stimulation of a sphincter muscle or
5 by modulating reflex control mechanisms so that more effective
sphincter tonus results:
C3 C4 C5 C6
Organ(s)P~dendalSuperior S I f--or
Affected Nexve T Sc~natic Ne~es S Sanatic Nerve I Ana1 Branch L
10 (1) E, R, X
(2) I X
- (Pelvic F10~r)
(3) E X
(4) R X
15 (5) E, R, I X X
(6) I, E X X
(7) I, R X X
The term "reflex control mechanisms" means those nerve
bundles that control interrelated aetivity between bladder B and
~ pelvie floor musculature (primarily levator ani musele I) as they can
reflexively influenee eaeh other by either inhibition or facilitation.
It should be noted in the eharts that various eleetrode
eombinations may affeet the identical organs, but to different degrees
of intensity . For example, although eleetrode combinations ( 1)
25 and (8) in Chart I each affect bladder B, bladder sphincter E and
anal sphincter R, in combination (8) the bladder will be relatively
more responsive sinee the main pelvie nerve supply P emanates
35 .
primarily from sacral segment S3 and to a lesser amount from sacral
segment S2.
The site or sites chosen for implantation of an electrode is
determined by careful evaluation of a patient's problerns. Such eval-
5 uations consist of symptom analysis, physical deficits or variations inmuscle behavior of the lower extremities and pelvic muscles, or loss
of sensation, the results of urodynamic testing and the results of test
stimulation of the various sacral nerves. A temporary electrode is
normally inserted percutaneously into one or more of the sacral
lO foramena and specific nerve roots test stimulated for a response.
When a desired response is obtained, a temporary electrode can be
"floated" (e. g., Foramen electrode F in Figure 18) in the vicinity of
the nerve or nerves. This procedure allows the patient to have a
three to five day trial of stimulation to evaluate the therapeutic bene-
15 fits of stimulation.
Based on the response obtained with the test stimulation,the patient can be further evaluated for the response to be obtained
by percutaneously implanting an electrode on one or more of the
selected nerve bundles or an electrode can be permanently implanted,
20 either via sacral laminectomy and placement of an electrode directly on
a specific sacral nerve or by placing an electrode on the sacral
foramen without performing a laminectomy. Therapeutic beneffts are
thus obtained by stimulation of specific pelvic muscles.
The electronic control system and electrode arrangements
2 5 shown in Figures 12-17 can also be used to effect the operative
36 .
~25\~
procedures set forth in Charts I and Il, with appropriate modifica-
tions for each specific procedure.
37 .
'
.