Note: Descriptions are shown in the official language in which they were submitted.
~z97~9~1
This invention relates to a process for the
preparation o~ compounds which are uGe~ul ~s intermediate6
for herbicides.
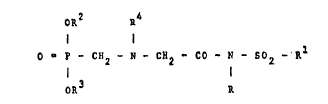
The present invention rela~tes to a process for
the preparation of a compound of forDnula:
.: OR2 a4
( I )
9 ' P ~ CH2 ~ N ~ CH2 ~ ~0 - N - S02 -
oR3 R
10 wherein
R represents an optionally substituted
hydrocarbon radical, pre$erably alkyl, aryl or cycloalkyl,
each of which may be substituted; suitable substituents
; include halogen atoms and phenyl, cyano, alkyl, alkoxy and
alkylcarboxylate groups in which the alkyl groups preferably
contain from 1 to 4 carbon atoms; Rl generally contains from
1 to 18 carbon atoms, preferably from 1 to 7 carbon àtoms,
and more particularly from 3 to 7 carbon atoms when R~ is a
cycloalkyl group; preferably Rl represents an alkyl group
containing from 1 to 4 carbon atoms, optionally ~ubstituted
by halogen, especially chlorine or fluorine, e.g. CF3;
R represents a hydrogen atom or has one of the
meanings given for Rl, and is preferably ~n alkyl group
containing from 1 to 4 carbon ato~s,
~5 R2 and R3 are such that oR2 and oR3, in which R2
and R3 may together ~orm a single optionally ~ubstituted
- ~2~49;~
--3--
divalent radical~ are hydrolysable groups; R~ and R3
preferably represent an optionally substituted alkyl~ aryl
or arylalkyl radical (suitable substituen~s are those
mentioned for Rl) or R2 and R3 may together form a single
optionally substituted divalent radical preferably
containing from 2 to 6 carbon atoms, e.g. an optionally
subsituted alkylene radical (e.g. an ethylene or propylene
radical); R2 and R3 generally each contain from 1 to 12
carbon atoms and preferably from 1 to 8 carbon atoms; and
R4 represents a radical of formula Ar(R5)(R6)C-
in which Ar is an optionally substituted aromatic group,
preferably phenyl, and R5 and R~ each represent a hydrogen
atom or an (aromatic) radical Ar or an alkyl group
preferably containing not more than 6 carbon atoms
15 (preferably R4 represents benzyl),
by reacting a compound of formula:
oa2
(Il)
O P CH2 N
I
OR
wherein R2, R3 and R4 are as hereinbefore defined with a
~o~pound of formula:
2 - CN~ - CO ~ ~ - sn2 - Rl
(III)
R
wherein
1~97493
--4--
X represents a halogen atom, e.g. chlorine,
bromine or iodine, preferably chlorine, ana R and Rl are as
hereinbefore defined.
Suitable substituents for the group Ar include
5 alkyl and alkoxy groups, preferably containing from 1 to 4
carbon atoms, nitro radicals and halogen atoms.
Such a reaction is known from European Patent
Application No. 0,189,725. The compounds of formula (I) are
intermediates for herbicides.
The present invention seeks to provide an
improved process for the preparation of the compounds of
formula ~I) for a more satisfactory implementation on an
industrial scale. The present invention also seeks to
provide a simplified preparation process.
It has been found that the molar ratio of the
compound~of formula II to the compound of formula I~I is
important in the reaction: in particular the use of a molar
ratio greater than 1.5 allows the presence of addi~ional
acceptor for acid to be avoided. This leads to two
20 important advantages: firstly, it enables any stage for the
removal of the said acceptor for acid to be omitted and
~econdly, it enables the yield to be increased.
Another important consequence of this total or
near total absence of additional accep~or Xor acid is that
it enables a recycling process to be carried out, further
improving significantly the yield and ~he implementation on
an industrial ~cale.
.
~2g~7~3
The present invention accordingly provides a
process for the preparation of a compound o formula (I)
wherein the various 6ymbols are as hereinbefore deined by
the reaction of a compound of formula ~ wherein the
5 various symbols are as hereinbefore defined with a compound
of formula (III) as hereinbefore defined, the molar ratio of
the compound of formula (II) to the compound of formula
(~II) being greater than l~5.
The reaction according to the invention may be
10 carried out in the absence, or more preferably in the
presence, of a solvent. In fact, the presence of the
~olvent enables the temperature to be controlled more
satisfactorily. The reaction is preferably carried out in
an inert organic solvent at an elevated temperature.
Suitable solvents include nitriles ~especially
acetonitrile), ketones (especially acetone, cyclohexanone,
~; methyl ethyl ketone and methyl isobutyl ketone), halogenated
or non-halogenated hydrocarbons (especially benzene,
toluene, xylenes and chlorobenzene), esters such as alkyl
20 alkanoates (especially ethyl acetate), and aprotic polar
~olven~s ~uch as dimethylformamide and N-methylpyrrolidone.
The reaction temperature is generally from 30 to
150C and preferably from 40 to l20C.
The reaction product of formula (I) may be
25 isolated by any means known per ~e.
Some compounds of formula (II) are known
.. . . . . .
~7493
.,
~Tetrahedron Letters no. 46, p. 4645~ 1973)o They can be
prepared conveniently by reacting tris(aralkyl)hexahydro-
triazines with diorganophosphites.
The compounds of formula (III) are also known~
The molar ratio of compound (II~ ~o compound
(III) i~ pre~erably greater than 1.8 and advantageously from
1.9 to 4.
According to a preferred feature, the process of
the invention is carried out in the total or near total
10 absence of additional acid acceptor. Near total absence
means that the reaction medium may contain traces of
acceptor for various reasons.
According to a further feature of the process
according to the invention, the process is carried out
15 continuously by recycling the compound of formula (II).
This recycling is accomplished at the end of the
reaction, by extraction of unreacted compound of formu}a
(II) into an acidic aqueous medium and then, after
neutralization, transferring the compound of formula ~II)
into an organic phase for reaction with further compound of
formula (III).
The process is more particularly suitable for
compounds in which R and Rl are alkyl groups containing from
1 to 4 carbon atoms, preferably methyl, and/or in which R2
and R3 are alkyl, phenyl or ~enzyl groups.
The following Examples illustrate the invention.
~2974~
Example 1
A mixture of diisopropyl N-benzylaminomethane-
pho~phonate ~M.W. 285; 11043 9; 4 x 10 2 moles) and
N-methyl-~-methylsulphonylchloroacetamide ~3~71 9; 2 x 10 2
5 moles~ is hea~ed for 2 h at 80C. The reaction mixture is
dilu~ed to 50 cc with toluene and treated with water and
with hydrochloric acid. The agueous phase and the ~oluene
phase are separated. The toluene phase is concentrated and
it is observed, by chromatography, that 8.025 9 of the
10 product is obtained, which corresponds to a yield of 92.44
relative to the N-methyl N-methylsulphonylchloroacetamide
employed.
The agueous phase containing diisopropyl
N-benzyl-aminomethanephosphonate hydrochloride is poured
15 into toluene (20 cc), the mixture is neutralized wi~h a
stoichiometric quantity of aqueous 2 N sodium hydroxide.
The aqueous phase and the toluene phase are separated. The
toluene phase contains diisopropyl N-benzylamino-
methanephosphonate (1.82 x 10 2 moles) in the ~orm of the ba~e
20 which oan be recycled,after the distillation of toluene if
required.
Example 2
The same trial carried out at 70C leads to a
yield, as determined by chromatography, of 72.5~ relative to
25 the N-methyl-N-methylsulphonylchloroacetamide employed.
Example 3
The same trial carried ou~ at 90C leads to a
IZ9749:3
~ 8--
yield, as determined by chromatography, of 86.7~ relative to
- the N-methyl-N-methylsulphonylchloroacetamide employed.
Comparative Example
Diisopropyl N-benzylaminomethanephosphonate (5.71
g; 2 x 10 2 mole) and N-methyl-N-methy]Lsulphonylchloro-
acetamide (3.71 g; 2 x 10 2 mole) are heated for 2 h at
80C. A solution of triethylamine (2.8 cc; 2 x 10 2 mole)
in toluene (15 cc) is poured gradually, in the course of 5
h, into the reaction mixture which is maintained at 80C.
10 The mixture is then cooled to 20C, poured into water (15
cc) at ambient temperature and the a~ueous phase ancl the
toluene phase are separated. The toluene phase is
concentrated under vacuum. It is observed, by
chromatography, that 6.6 9 of the expec~ed product was
15 obtained, which corresponds to a yield of 75.8% relative ~o
the 2 reagents charged.
Example 4
1,3,5-tribenzylhexa~ydrotriazine (59.5 9; 0.166
mole), diisopropyl phosphite (83 9; 0.5 mole) and toluene
2~ (100 g) are heated for 4 h 30 min at 120C. The mixture is
cooled to 20C. An analysis by chromatography shows that
the reaction yield of diisopropyl N-benzylaminome-
thanephosphonate is guantitative. N-methyl-N-methyl-
6ulphonylchloroacetamide (37.1 9; 0.2 mole) i5 added. The
25 reaction mixture is heated to 80C for 2 hours and then
cooled to ambient ~emperature. It is then poured into water
, , ~, , ~ ' ' .' ! , ` ' ' ' :1
,,, : ,, ,\ ~` . .`. ` ' "' `
1297~3
(150 9) and aqueous 374 ~Cl (12 cc) is added in order to
adjust the pH to 1. The phases are decanted and separated.
The organic phase has a weigh~ of 173 9. The aqueous phase
(265 g) is neutralized to pB = 6 with aqueous 30~ sodium
5 hydroxide (30 cc) after adding toluene (100 9). The phases
are decanted and separated. The second organic phase has a
weight of 195 y.
The first organic phase (173 9) contains
44.6% of the product expected, which amounts to 0.18 mole.
10 The ~econd organic phase (195 g) contains 42% o diisoprOpyl
N-benzylaminomethanephosphonate, which amounts to 0.287
mole.
1st recycling
The 2nd organic phase (195 9) is azeotropically
15 dried (67 g of toluene and water are removed) and a solution
(120 g) containing diisopropyl N-benzylaminomethane-
phosph~nate ~0.25 mole) and ~-methyl-N-methylsulphonyl-
chloroacetamide (37.1 g; 0.2 mole) in toluene is added and
the mixture is heated for 2 h at 80C. The reaction mass is
20 treated exactly as before.
2nd recycling
A ~nd recycling of diisopropyl N-benzylaminO-
methanephosphonate was carried out exactly as above 7
All the results are given in the table below:
25 A = diisopropyl N-benzylaminomethanephosphonate
B = N-methyl-N-methylsulphonylchloroacetamide
~;297493
C - condensat~on product expected
_
Raw materials A - recycled Yield of
empl oyed C/B
_ _ _ _
A - 0.5 mole ~9~6
B - 0. 2 mole
1st recycling A - 0. 25 mole 0 . 28 mole 100%
B - 0. 2 mole
. , . .
2nd recycling A - 0~25 mole 0.18 mole 100%
B - 0.2 mole _ _