Note: Descriptions are shown in the official language in which they were submitted.
1Z97631
HA362a
U9~
Jones et al. in WO 84/03044 published in
1984 disclose renin inhibiting tetra-, penta- or
hexapeptide analogues of the formula
X-D-E-A-B-Z-W
where X and W are terminal groups; D, E, B and Z,
of which any one or, except with reduced
analogues, two may be absent, are aromatic, lipo-
philic or (in the case of E) aromatic, lipophilic,or basic amino acid or amino acid analogue
: residues, and A is an analogue of a lipophilic or
aromatic dipeptide residue wherein the peptide link
is replaced by one to four-atom carbon or carbon-
nitrogen link which as such or in hydrated form is
an unhydrolyzable tetrahedral analogue of the
transition state of the peptide bond as given
above. In particula., A is defined as
R4 Rl R2
~;~ 25
-N - C - M - C -
R5 R6
',
' ":
1Z97631
-2- HA362a
wherein M can be -CH-OH.
Szelke et al. in European Patent Application
104,041 published in 1984 disclose renin inhibitory
polypeptides including the partial sequence
X- A -B - Z- W and
X-Phe-His-A-B-Z-W
10 wherein A is Rl R3 R2 0
-NH-CH - G- N - C~ - C-
and G is
OH
-CH - C~2
X is hydrogen, protecting group, or an amino acyl
residue, B is a lipophilic amino acyl residue, and
Z plu~ W are an amino alcohol residue or Z is
aminoacyl and W i8 hydroxy, ester, amide, etc.
Matsueda et al. in United States Patent
4,548,926 disclose renin inhibiting peptides
of the formula
~; 25
NH
2 But
Rl-- C~ - C~ X
~'
~,
, .~.. .. .. .
129763~
HA362a
--3--
wherein But represents an isobutyl or sec-butyl
group and X includes a group of the formula
-CH(R2)_y
Gordon et al. in IJ.S. Patent 4,514,391
disclose hydroxy substituted peptide compounds of
the formula
OH R Rl O
l l 11
R3- CH - CH -CH2- N -CH - C -X
10NH
R20
which possess angiotensin converting enzyme or
enkephalinase inhibition activity.
This invention is directed to new hydroxy
containing ureido renin inhibitors of formula I
including pharmaceutically acceptable salts thereof
(I)
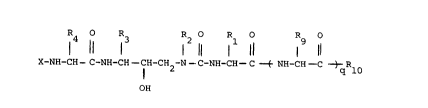
11 ~ R2 Rl Rg O
X-NH-CH--C-NH-CH--CH-CH2-N--C-NH-CH--C ( NH-CH--C ) Rlo
25OH
11
30 X is R6-~CH2)m-~ NH CH ~p
O R5 O
Il l 11
R6-(cH2)m-c-t ~H - CH -C ~-p ,
, .. ..
- 1297631
HA362a
--4--
O R5 O
R6 ( CH2 )m ~ C ~ NH--CH--C--
O R5 O
R6----(CH2)n C~ NH--CH--C tp
O R5 O
Il I 11
R6--(CH2)m NH--C~ NH--CH- C~
R5 O
l 11
6--(CH2 )m S02~ NH--CH--C
O R5 O
Il l 11
or R6-(CH2)m CH-C-(NH-CH--C ~p
I
(CH2)
R'
Rl, R3, R4, R5, and Rg are independently select-
30 ed from hydrogen, lower alkyl, halo substituted lower
alkyl, -(CH2)n-aryl, -(CH2)n-heterocyclo, -(CH2)n-OH,
( 2 )n H2, (CH2)n-SH~ -(CH2)n-S-lower alkyl,
-(CH2)n-0-lower alkyl, ~(CH2)n~0~(CH2)g~0H~
- ( CH2 ) ~- ( CH2 ) g~NH2 ~ ~ ( CH2 ) n ( 2
,.
~ ,~
~.. . . .
-~` 129763~
_5_ HA362a
NH
.~
(CH ) ~S~(CH2)g~NH2~ -(CH2)n
NH2
( 2)n C NH2 , ~(CH2)n ~ J~R7' (CH)n
P~8
and -(CH2)n-cycloalkyl.
R2 is hydrogen, lower alkyl, -(CH2)m-aryl,
-(CH2)m-cycloalkyl, or -(CH2)n-heterocyclo.
R6 and R'6 are independently selected from
lower alkyl, cycloalkyl, aryl and heterocyclo.
p is zero or one.
m and m' are independently selected from zero
and an integer from 1 to 5.
n is an integer from 1 to 5.
g is an integer from 2 to 5.
7 CH2 0 CH2 ~ or -CH2 ~ .
R8 is 2,4-dinitrophenyl, _1_0_CF72~ ,
-SO ~ H3 ~ or -CH -O-CH
q is zero or one.
R1o is hydroxy, -O-lower alkyl,
: -O-(CH2)m-cycloalkyl, -O-(CH2)m-aryl,
-O-(CH2)n-heterocyclo, -NH2, or -O-salt
forming ion.
..~
" .,~, . ,.~ ., .. ~ . . ,
1297631
HA362a
-6-
This invention in its broadest aspects
relates to the compounds of formula I above, to
compositions and the method of using such compounds
as antihypertensive agents.
The term lower alkyl used in defining
various symbols refers to straight or branched
chain radicals having up to seven carbons.
The term cycloalkyl refers to saturated
rings of 4 to 7 carbon atoms with cyclopentyl and
cyclohexyl being most preferred.
The term halogen refers to chloro, bromo and
fluoro.
The term halo substituted lower alkyl refers
to such lower alkyl groups described above in
which one or more hydrogens have been replaced by
chloro, bromo or fluoro groups such as trifluoro-
methyl, which is preferred, pentafluoroethyl,
2,2,2-trichloroethyl, chloromethyl, bromomethyl,
etc.
The term aryl refers to phenyl, l-naphthyl,
2-naphthyl, mono substituted phenyl, 1-naphthyl,
or 2-naphthyl wherein said substituent is lower
alkyl of 1 to 4 carbons, lower alkylthio of 1 to 4
carbons, lower alkoxy of 1 to 4 carbons, halogen,
hydroxy, amino, -NH-alkyl wherein alkyl is of 1 to
4 carbons, or -N(alkyl)2 wherein alkyl is of 1 to 4
carbons, di or tri substituted phenyl, 1-naphthyl
or 2-naphthyl wherein said substituents are
selected from methyl, methoxy, methylthio,
halogen, and hydroxy.
~. ~
-
1297631
HA362a
--7--
The term heterocyclo refers to fully saturated
or unsaturated rings of 5 or 6 atoms containing one
or two O and S atoms and/or one to four N atoms
provided that the total number of hetero atoms in
the ring is 4 or less. The heterocyclo ring is
attached by way of an available carbon atom.
Preferred heterocyclo groups include 2- and 3-thienyl,
2- and 3-furyl, 2-, 3- and 4-pyridyl, and
imidazolyl. The term heterocyclo also includes
bicyclic rings wherein the five or six membered
ring containing O, S and N atoms as defined above
is fused to a benzene ring. The preferred
bicyclic ring is indolyl.
The compounds of formula I wherein
O R5 O
Il l 11
X iS R6-(cH2)m-o-c-t NH- CH - C~p can be
prepared by coupling an alcohol of the formula
(II)
R3 R2 o R1 o Rg O
l 11 1 11 1 ll
H2N-CH- CH - CH2- N - C-NH-CH-C -(NH-CH-C ~ R1o
OH
with a peptide of the formula
(III)
O R5 R4
R6-(CH2)m-O-C-~-NH -CH - C ~-p NH -CH - COOH
~,~
.:..... .
~297631
HA362a
--8--
This reaction is preferably performed in a solvent
such as dimethylformamide and in the presence of
hydroxybenzotriazole, diisopropylethylamine, and a
coupling agent such as dicyclohexylcarbodiimide.
The corresponding compounds of formula I
wherein p is zero can be prepared by coupling
the alcohol of formula II with the amino acid of
the formula
(IV)
0 R4
Il I
R6-(CH2)m-0-C-NH-CH -COOH
to yield the products of the formula
(V)
ll 1 4 ll 1 3 1 2 ~ 9 11
R6-~CH2)m-O-C-NH-CH- C-NH-CH-CH-CH2-N- C-NH-CH-C-~ NH-CH-C ~- Rlo .
OH
When R6-(CH2)m~ is t-butyl or benzyl, then the product
of formula V can be treated so as to remove the
t-butoxycarbonyl or benzyloxycarbonyl group such
as by the use of hydrochloric acid when R6 is
t-butyl to yield the amine of the formula
(Vl)
R O R R O R O R O
14 11 13 12 ~ 19 11
H2N-CH-C-NH-CH-CH-CH2-N- C-NH-CH-C-~NH-CH-C ~ Rlo
OH
1297631
HA362a
Coupling with the amino acid of the formula
(VII)
O R5
Il I
S R6-(CH2)m~O~C - NH - CH -COOR
yields the products of formula I wherein
p is one.
The compounds of formula I wherein X is
O R5 O
other than R6-(CH2)m~O~C-~ NH -CH- C tp
can be prepared by treating the product of
formula I wherein R6 is
15 0 0
Il 11
C O C(CH3)3 or C O CH2 ~ and m is zero
to remove the t-butoxycarbonyl or benzyloxycarbonyl
group and yield the intermediates of the formula
(VIII)
R5 R4 1l 13 12 h 1 ll Rg O
H2N-CH-C-NH-CH C-NH-CH-CH-CH2-N- C-NH-CH-C-~-NH-CH-C ~ Rlo
OH
: The amine of formula VIII or formula VI is treated
with the halide of the formula
(IX)
R6 ( CH2 )m-halo
:~
, ., ~
~
.
~.,..... , ~ .. ......
.
1Z97631
HA362a
--10--
particularly where halo is Br to give the products
of formula I wherein X is
R5 0
1 11
R6-(CH2)m ( NH -CH- C tp
The amine of formula VIII or VI is treated
with the acid chloride of the formula
10 (X)
o
Il .
R6~CH2)m~C~Cl or
l5 (XI)
o
R6 (CH2)n C Cl
20 in the presence of triethylamine to yield the products
of formula I wherein
O R O
~ 5 11
X iS R6 (CH2)m C ~ NH CH C~p or
o R O
11 1 5
R6-0-(CH2)n-C ~ NH-CH - C-t-p
~ 30The amine of formula VIII or VI is treated
'~ with the substituted sulfonyl chloride of the formula
(XII)
~ ~ R6 ( CH2 )m-S02-C
,~'
.~
~'''''': '` ' ' , ~ ' .
: ' ' ' '
' ' , .
129763~
HA362a
--11--
to yield the products of formula I wherein X is
R o
R6-(CH2)m- So2-~ NH-CH - C-~ .
The amine of formula VIII or VI is treated
with phosgene and the resulting acid chloride is
treated with the substituted amine of the formula
(XIII)
R6-(CH2)m NH2
to yield the products of formula I wherein X is
O R5 O
R6-(CH2)m-NH-C-~ NH-CH C +p
The products of formula I wherein
O R5 O
Il l 11
X is R6-(CH2)m- G~-c-~NH--CH--C ~
I
(CH2)
R'6
can be prepared by coupling the carboxylic acid
of the formula
:
. ~.
'
;
...... ..
1297631
HA362a
-12-
(XIV)
o
Il
R6 (CH2)m CH C OH
S
( CH2 )
I
R~6
to the amine of formula VI or VIII in the presence of
dicyclohexylcarbodiimide and l-hydroxybenzo-
triazole hydrate. Alternatively, the acid of
formula XIV can be converted to the acid chloride
and this acid chloride can then be coupled to the
amine of formula VI or VIII in the presence of triethyl-
amine and tetrahydrofuran or water and sodium
bicarbonate.
The alcohol of formula II can be prepared by
reacting an alcohol of the formula
(XV)
R3 R2
Prot-NH-CH -CH - CH2-N -H
I
OH
preferably the hydrochloride salt thereof, with the
carbamoyl chloride of the formula
.,~
... .
1297631
HA362a
-13-
(XVI)
O Rl O
Il 1 ~1
Cl- C- NH - CH- C- 0 - CH
to give
(XVII)
R R2 I Rl
Prot-NH-C3-CH-CH2-N--C-NH-CH--C-O-CH
OH
wherein Prot is an amino protecting group such as
t-butoxycarbonyl. Removal of the benzyl group
from the intermediate of formula XVII such as by
hydrogenation yields the acid of the formula
(XVIII)
~ R R O R O
~ 25 13 l2 ~
Prot-NH-CH -FH-CH -N -C NH-CH-C-OH
OH
` ~ :
~: 30
.,~ 3S
.~
,::
.,.... ~
~297631
HA362a
-14-
Removal of the t-butoxycarbonyl amino protecting
group such as by treatment with hydrochloric acid
gives the alcohol of formula II wherein q is zero
and Rlo is hydroxy. When q is zero and Rlo is
other than hydroxy, the acid of formula XVIII is
treated to introduce the R1o group, for example,
treatment with diazomethane where R1o is methoxy,
followed by removal of the t-butoxycarbonyl
protecting group. Of course, where final products are
desired having Rlo as then the benzyl
-O-CH2~
group is not removed from the intermediate of
formula XVII.
When ~ is one, then the acid of formula XVIII
is treated with the amino acid of the formula
(XIX)
Rg 0
H2N CH -C Rlo
in the presence of a coupling reagent such as
dicyclohexylcarbodiimide to give
(XX)
R R O R O R O
13 12 ~ 19 11
Prot-NH-CH-CH-CH2-N-- C-NH-CH-- C-NH-CH-C-Rlo
OH
Removal of the t-butoxycarbonyl amino protecting
group as described above gives the alcohol of
formula II.
. . .
~ ,;
12g7631
HA362a
-15-
The alcohol starting compound of XV can be
prepared by treating the ketone of the formula
(XXI)
R3 R2
I
Prot- NH -CH - C -CH2- N - CH2 ~
with a conventional reducing agent such as sodium
borohydride followed by hydrogenation using
palladium hydroxide on carbon catalyst.
The ketone of formula XXI can be prepared by
treating the ketone of the formula
15 (XXI I )
R3
I
Prot-NH -CH -C- CH2- Cl
ll
o
with an amine of the formula
(~XIII)
R2-N-CH
H
in the presence of sodium iodide and sodium bicar-
bonate in a solvent such as dimethylformamide.
When R2 is hydrogen, the ketone of formula XXII is
reacted with dibenzylamine. After reduction to
the alcohol, both benzyl groups are removed by
"..;
~.
12g~631
HA362a
-16-
hydrogenation.
In the above reactions, if any of Rl, R2,
R3, R4, R5, and Rg are -(CH2)n-aryl wherein aryl
is phenyl, l-naphthyl, 2-naphthyl substituted with
one or more hydroxy or amino groups,
-(CH2)n-heterocyclo wherein heterocyclo
is an imidazolyl, -(CH2)n-NH2,
~ NH
-(CH2) -SH, -(CH2) -OH, or -(CH2) -NH-C
NH2
then the hydroxyl, amino, imidazolyl, mercaptan,
or guanidinyl function should be protected during
the reaction. Suitable protecting groups include
benzyloxycarbonyl, t-butoxycarbonyl, benzyl, benz-
hydryl, trityl, etc., and nitro in the case of
guanidinyl. The protecting group is removed by
hydrogenation, treatment with acid, or by other
known means following completion of the reaction.
The various peptide intermediates employed
in above procedures are known in the literature or
can be readily prepared by known methods. See for
example, The Peptides, Volume 1, "Major Methods Of
Peptide Bond Formation", Academic Press (1979).
Preferred compounds of this invention are
those of formula I wherein:
O R O
Il 15 1l
X is lower alkyl-O-C-NH-CH -C- ,
7~
HA362a
-17-
O R5 0
- CH2-0-C-NH -CH - C- , or
~ 11
~ ~ C~2,2 CH-C-
Rl is lower alkyl of 3 to 5 carbons
-(CH2)m-cyclopentyl, -(CH2)m-cyclohexyl,
-(CH2)m ~ , or ~(CH2)m- ~
wherein m is an integer from 1 to 3.
22 is hydrogen, lower alkyl of 3 to 5
carbons, -(CH2)m-cyclopentyl, -(CH2)m-cyclohexyl,
(CH2)m ~ or -(CH2)m ~ wherein m is an
integer from 1 to 3.
R3 is lower alkyl of 3 to 5 carbons,
-(CH2)m~cyclopentyl, -(CH2)m-cyclohexyl or
~(CH2)m ~ wherein m is an integer from
1 to 3.
~297631
HA362a
-18-
4 -CH2 ~ JNH , -CH2 ~ N-CH2-0-CH2 ~ ,
-CH2 ~ -CH2 ~ ' -CH2 ~ 0H
-CH2 ~ ' -CH2--@ N , CH2
-(CH2)2 ~ ' or -CH2
~ N02
N02
R5 and Rg are independently selected from
-CH2 ~ , -(CH2) ~ , -CH2-(~-naphthyl),
-CH2-(~-naphthyl), -CH2 ~ OH , -CH2-cyclopentyl,
,
. '
129763~
HA362a
--19--
-CH2-cyclohexyl, _[~) , -CH~N
5 --CH2~ , -CH2~ JNH , -CH2~JH
C -O -CH 2
and -CH ~ O
H
Rlo iS--o-CH3 ~ -0-C2H5, -0-CH2~>,
-0-CH2 ~ or hydroxy.
Moæt preferred are the above compounds
wherein
O R O
Il 15 11
X is (H3C)3-C-O-C-NH-CH- C-
Rl is -CH- CH2- CH3 or
CH3
-CH-(CH3)2, especially -CH - CH2- CH3
I
CH3
~2g763~
HA362a
-20~
R2 is hydrogen, -CH2-CH(CH3)2 or -CH(CH3)2 ,
especially hydrogen.
R5 is -CH2 ~
R4 is ~N J
R3 is -CH2-CH(CH3)2
q is zero or one, especially one.
R9 is -CH2 ~ TN~J
especially -CH2 ~ NH
Rlo is -O CH3
The compounds of formula I form salts with a
variety of inorganic and organic acids. The non-
toxic pharmaceutically acceptable salts arepreferred, although other salts are also useful in
isolating or purifying the product. Such
pharmaceutically acceptable salts include those
formed with hydrochloric acid, methanesulfonic
acid, sulfuric acid, acetic acid, maleic acid, etc.
The salts are obtained by reacting the product
'
,'~ `'
,
- ;.
~2g7~
HA362a
-21-
with an equivalent amount of the acid in a medium
in which the salt precipitates.
The compounds of formula I contain
asymmetric centers when any or all of Rl, R3,
R4, R5, and Rg are other than hydrogen and at
the carbon to which the -OH group is attached.
Thus, the compounds of formula I can exist in
diasteroisomeric forms or in mixtures thereof.
The above described processes can utilize
racemates, enantiomers or diastereomers as
starting materials. When diastereomeric products
are prepared, they can be separated by
conventional chromatographic or fractional
crystallization methods.
The compounds of formula I, and the pharma-
ceutically acceptable salts thereof, are anti-
hypertensive agents. They inhibit the conversion
of angiotensinogen to angiotensin I and therefore,
are useful in reducing or relieving angiotensin
related hypertension. The action of the enzyme
renin on angiotensinogen, a pseudoglobulin in
blood plasma, produces angiotensin I.
Angiotensin I is converted by angiotensin
converting enzyme (ACE) to angiotensin II. The
latter is an active pressor substance which has
been implicated as the causative agent in several
forms of hypertension in various mammalian
species, e.g., humans. The compounds of this
invention intervene in the angiotensinogen ~
(renin) ~ angiotensin I ~ (ACE) ~ angiotensin II
sequence by inhibiting renin and reducing or
~2g7~31
-22- HA362a
eliminating the formation of the pressor substance
angiotensin II. Thus by the administration of a
composition containing one (or a combination) of
the compounds of this i~vention, angiotensin
dependent hypertension in a species of mammal
(e.g., humans) suffering therefrom is alleviated.
A single dose, or preferably two to four divided
daily doses, provided on a basis of about 100 to
1000 mg., preferably about 250 to 500 mg. per kg. of
body weight per day is appropriate to reduce blood
pressure. The substance is preferably
administered orally, but parenteral routes such as
the subcutaneous, intramuscular, intraveneous or
intraperitoneal routes can also be employed.
The compounds of this invention can also be
formulated in combination with a diuretic for the
treatment of hypertension.
A combination product comprising a compound
of this invention and a diuretic can be
administered in an effective amount which
comprises a total daily dosage of about 1000 to
6000 mg., preferably about 3000 to 4000 mg. of a
compound of this invention, and about 15 to
300 mg., preferably about 15 to 200 mg. of the
diuretic, to a mammalian species in need thereof.
Exemplary of the diuretics contemplated for use in
combination with a compound of this invention are
the thiazide diuretics, e.g., chlorothiazide,
hydrochlorothiazide, flumethiazide, hydroflu-
methiazide, bendroflumethiazide, methyclothiazide,trichloromethiazide, polythiazide or benzthiazide
- , .
. ' :.' . ' '
`- 12g763~
HA362a
-23-
as well as ethacrynic acid, ticrynafen,
chlorthalidone, furosemide, musolimine, bumetanide,
triamterene, amiloride and spironolactone and salts
of such compounds.
The compounds of formula I can be for~.ulated
for use in the reduction of blood pressure in
compositions such as tablets, capsules or elixirs
for oral administration or in sterile solutions or
suspensions for parenteral administration.
About 100 to 500 mg. of a compound of formula I is
compounded with physiologically acceptable
vehicle, carrier, excipient, binder, preservative,
stabilizer, flavor, etc., in a unit dosage form as
called for by accepted pharmaceutical practice.
The amount of active substance in these
compositions or preparations is such that a
suitable dosage in the range indicated is obtained.
The following examples are illustrative of
the invention. Temperatures are given in degrees
centigrade.
lZ~7~9~
HA362a
-24-
Example 1
N-[[[(3S)-3-[[N-[N-[(l,l-Dimethylethoxy)carbonyl]-
L-phenYlalanyl]-L-histidyl]amino]-2-hydroxY-5-
methylhexvl](2-methYlpropyl)amino]carbonyl]-L-
valine, methyl ester, monohydrochloride (isomer A)a) (S)-[3-Methvl-1-[[(2-methylPropyl)(phenyl-
methyl)amino]acetyl]butyl]carbamic acid, 1,1-
dimethYlethyl ester,4-methylbenzenesulfonic acid
salt (1:1)
N-Methyl morpholine (16.5 ml., 150 mmole)
is added to a stirred solution of N-[(l,1-dimethyl-
ethoxy)carbonyl]-L-leucine (34.67., 150 mmole) in
dry tetrahydrofuran (135 ml.) at ~15 followed by
the dropwise addition of isobutyl chloroformate
(19.5 ml., 150 mmole). After stirring at -15 for
twenty minutes, it is filtered and diluted with
ether (4~0 ml.) kept at -20. This is added
dropwise over 15 minutes to an ethereal solution
of diazomethane (600 ml., generated from 60 g. of
N-methyl-N'-nitro-N-nitrosoguanidine). After the
addition is over the reaction is allowed to run at
; room temperature for two hours. Excess
diazomethane is blown off by a stream of
nitrogen. The ethereal solution is washed with
saturated aqueous sodium bicarbonate and saturated
sodium chloride solutions. The ethereal solution
is concentrated ln vacuo and the residue is
dissolved in hexane. On cooling, 27.7 g. of
(S)-[3-methyl-1-[(diazomethyl)carbonyl]butyl]-
carbamic acid, 1,1-dimethylethyl ester is obtained
as a crystalline material; m.p. (88) 89 - g0.
.
~,
, . . .
.
.
12g7631
HA362a
-25-
Hydrochloric acid in acetic acid (2.2 N,
94 mmole) is added dropwise to a stirred (ice-bath)
solution of the above diazo product (24 g.,
94 mmole) in ether (470 ml.). After stirring for
10 minutes the solution is evaporated ln vacuo.
The residue is dissolved in ethyl acetate:hexane
(1:3) and passed through a small column of silica
gel (400 g.) using ethyl acetate:hexane (1:3) for
elution. A homogeneous material is obtained
(23.2 g.). On crystallization from a mixture of
ether and hexane fine crystalline (S)-[3-methyl-
l-[(chloromethyl)carbonyl]butyl]carbamic acid, 1,1-
dimethylethyl ester is obtained; m.p. 66-68.
A solution of (phenylmethyl)(2-methylpropyl)-
amine (8.16 g., 50 ml.), (S)-[3-methyl-1-[(chloro-
methyl)carbonyl]butyl]carbamic acid, l,l-dimethyl-
ethyl ester (13.19 g., 50 mmole), sodium
bicarbonate (6.3 g., 75 mmole), sodium iodide
(3.74 g., 25 mmole) and dimethylformamide (100 ml.)
is stirred at room temperature for 4 hours. It is
then evaporated, taken into ethyl acetate and
washed with water. The ethyl acetate solution is
evaporated and the residue dissolved in ether.
The ethereal solution is filtered to remove a very
small amount of insoluble material. The ethereal
solution is evaporated and the residue is chromato-
graphed over a small column of silica gel (350 g.)
using the solvent system ethyl acetate:hexane
(1:4). The homogeneous fractions are pooled and
evaporated. The residue (17.62 g.) is dissolved
in ether and an ethyl acetate solution of
`",
1297631
HA362a
-26-
p-toluenesulfonic acid (8.6 g., 45.2 mmole) is
added. The crystallized salt is filtered to give
18.72 g. of (S)-[3-methyl-1-[[(2-methylpropyl)
(phenylmethyl)amino]acetyl]butyl]carbamic acid,
l,l-dimethylethyl ester, 4-methylbenzenesulfonic
acid salt (1:1); m.p. (140) 143; [~]D = -38.9
(c = 2.2, methanol). TLC (silica gel; n-propanol:
NH40H, 98:2) Rf = 0.8.
Anal. calc'd. for C30H46N26S C7H83
C, 64.03; H, 8.24; N, 4.98; S, 5.70
Found: C, 63.83; H, 8.00; N, 4.97; S, 5.78.
b) (S)-~l-[tRS)-l-HydroxY-2-[(2-methylpro~yl)
(~henvlmethYlLamino~ethyll-3-methylbutyl]carbamic
acid, 1,1-dimethYlethyl ester, monohydrochloride
The 4-methylbenzenesulfonic acid salt product
from part (b) (5.62 g., 10 mmole) is taken into
ethyl acetate and shook with saturated sodium
bicarbonate solution. The ethyl acetate layer is
dried over magnesium sulfate and evaporated in
vacuo. The resulting free base (3.9 g.) is
dissolved in ethanol (35 ml.). Sodium borohydride
(400 mg., 10.4 mmole) is added to the stirring
ethanolic solution at room temperature. After one
hour the solution is evaporated and the residue is
suspended in ethyl acetate and water and acidified
to pH 2.0 using dilute hydrochloric acid. Then
saturated sodium bicarbonate solution is added
until the ethyl acetate solution is slightly
basic. The ethyl acetate layer is dried over
magnesium sulfate and evaporated to give 3.91 g.
of alcohol product. An analytical sample of this
alcohol is prepared as the hydrochloride salt
as follows. Hydrochloric acid in dioxane
~1
...
, . " ~ , . .. .
lZg76~1
HA362a
-27-
solution (5N, 0.28 ml.) is added to an ethereal
solution of an aliquot of the above alcohol (O.S59 g.,
1.42 mmole). The solution is concentrated and
dried in high vacuum tc give (S)-[1-[(RS)-l-hydroxy-
2-[(2-methylpropyl)(phenylmethyl)amino]ethyl]-3-
methylbutyl]carbamic acid, l,1-dimethylethyl ester,
monohydrochloride as a crisp solid; m.p. 50 - 65
[a]D = -29.8 (c = 1.54, methanol). TLC (silica
gel, ethyl acetate:hexane, 1:4) Rf = 0.47.
Anal- calc'd- for C23H40N23 HCl 0-44 H2O
C, 63.21; H, 9.68; N, 6.41; Cl, 8.11
Found: C, 63.21; H, 9.49; N, 6.05; Cl, 7.88.
c) (S)-[1-[(RS)-l-HYdroxy-2-[(2-methylpro~yl)-
amino]ethyll-3-methylbutyl~carbamic acid,l,1-
dimethYlethyl ester, monohydrochloride
The alcohol product from part (b) (1.67 g.,4.25 mmole) is dissolved in methanol (50 ml.) and
aqueous hydrochloric acid (lN, 4.25 ml.) is added.
The solution is stirred under an atmosphere of
hydrogen in the presence of palladium hydroxide on
carbon catalyst (0.36 g.) for two hours. It is
filtered through hyflo and concentrated to dryness
ln vacuo to give 1.31 g. of (S)-[l-[(RS)-l-hydroxy-
2-[(2-methylpropyl)amino]ethyl]-3-methylbutyl]-
carbamic acid, l,l-dimethylethyl ester, monohydro-
chloride; m.p. 138 - 150; [~]22 = -29.0 (c = 1.2,
methanol). TLC (silica gel; chloroform:methanol:
acetic acid, 9:1:1) Rf = 0.73.
r C16H34N23 HCl 0.2 H2O:
C, 56.21; H, 10.41; N, 8.20; Cl, 10.37
Found: C, 56.21; H, 10.41; N, 8.14; Cl, 10.41.
1297631
HA362a
-28-
d) N-[[[(3S)-3-[[(1,1-DimethYlethoxy)carbonyl]-
amino]-2-hYdroxy-5-methylhexvl](2-methyl~ropyl)-
amino]carbonylL-L-valine, phenylmethyl ester
L-Valine, phenylmethyl ester, benzene sulfonic
acid salt (8.38 g., 23 mmole) is dissolved in
methylene chloride (100 ml.) and the solution is
cooled to -30. N-Methyl morpholine (6.33 ml.,
57.5 mmole) is added followed by a solution of
phosgene in benzene (12.5% solution, 27.4 ml.,
34.5 mmole). The reaction mixture is stirred at
-20 for 30 minutes. It is then evaporated
ln vacuo. A suspension of (S)-l-[l-hydroxy-2-[(2-
methylpropyl)amino]ethyl]-3-methylbutyl]carbamic
acid, l,l-dimethylethyl ester, monohydrochloride
(8.0 g., 23.6 mmole) in methylene chloride (50 ml.)
and N-methyl morpholine (5.05 g., 46 mmole) are
added to the above residue. The reaction mixture
is stirred in an ice-bath for 2 hours and then at
room temperature overnight. It is evaporated and
the residue is taken into ethyl acetate and washed
with water, saturated sodium bicarbonate solution,
and 10% potassium hydrogen sulfate solution. The
ethyl acetate extract is dried and evaporated.
The crude product is chromatographed over silica
gel (500 g.) using the solvent system ethyl
acetate:hexane (1:2) to give 7.7 g., of
N-[[[(3S)-3-[[(1,1-dimethylethoxy)carbonyl]amino]-2-
hydroxy-5-methylhexyl](2-methylpropyl)amino]carbonyl]-
L-valine, phenylmethyl ester as a gummy solid; m.p.
40 _ 49; [~]2D2 = -41.4 (c = 1.5, methanol).
TLC (silica gel; ethyl acetate:hexane, 1:2) Rf = 0.37.
129763i
HA362a
-29-
Anal. calc'd. for C29H49N3O6:
C, 65.01; H, 9.22; N, 7.84
Found: C, 64.64; H, 9.14; N, 7.91.
e) N-[[[(3S)-3-[[(1,1-Dimethylethoxv)carbonYll-
amino]-2-hYdroxY-5-methYlhexyl](2-methylproPyl)-
amino]carbonyl]-L-valine, methyl ester(isomer A)
The phenylmethyl ester product from part (d)
(2.2 g., 4.1 mmole) is dissolved in methanol (75
ml.) and stirred under an atmosphere of hydrogen
in the presence of palladium hydroxide on carbon
catalyst for 16 hours. The catalyst is filtered
through hyflo and the methanolic solution is
evaporated to give 1.8 g. of N-[[[(3S)-3-[[(1,1-
dimethylethoxy)carbonyl]amino]-2-hydroxy-5-
methylhexyl](2-methylpropyl)amino]carbonyl]-
L-valine.
N-[[[(3S)-3-[[(1,1-Dimethylethoxy)carbonyl]-
amino]-2-hydroxy-5-methylhexyl](2-methylpropyl)amino]-
carbonyl]-L-valine (3.0 g., 6.73 mmole) is
dissolved in an ethereal solution of diazomethane
generated from 2.67 g. (17.7 mmole) of
N-methyl-N'-nitro-N-nitrosoguanidine reagent.
After keeping the solution at room temperature for
one hour it is evaporated. The crude methyl ester
product is chromatographed over silica gel (300 g.)
using the solvent system ethyl acetate:hexane(2:5).
The earlier fractions (36 - 68, each 35 ml.
fractions) contain the faster moving isomer. They
; are pooled and evaporated to give 1.2 g. of
N-[[[(3S)-3-[[(1,1-dimethylethoxy)carbonyl]amino]-
2-hydroxy-5-methylhexyl](2-methylpropyl)amino]-
.~ ,
~Z97631
HA362a
-30-
carbonyl]-L-valine, methyl ester (isomer A);
m.p. 40 -49; [~]D22 = +7.2 (c = 1.5, methamol).
d. for C23H45N306 0-25 H20
C, 59.52; H, 9.88; N, 9.05
Found: C, 59.50; H, 9.78; N, 8.97.
f) N-~ L( 3S)-3-Amino-2-hydroxy-5-methylhexyl]-
(2-methylproPyl)amino]carbonyl]-L-valiner methyl
ester! monohydrochloride (isomer A)
The methyl ester (isomer A) product from
part (e) (0.4 g., 0.87 mmole) is dissolved in a
solution of hydrochloric acid in dioxane (5 ml.,
4.9 N) and allowed to stand at room temperature
for 40 minutes. It is then evaporated and re-
evaporated from methanol and ether. The residue
is dissolved in water, millipore filtered, and
lyophilized to give 0.29 g. of N-[[[(3S)-3-amino-
2-hydroxy-5-methylhexyl](2-methylpropyl)amino]-
carbonyl]-L-valine, methyl ester, monohydrochloride
(isomer A); m.p. 41 - 61; [a]22 = -2.94~
(c = 1, methanol). TLC (silica gel; chloroform:
methanol:acetic acid, 8:1:1) Rf = 0.61.
Anal. calc'd. for C18H37N3O4- HCl 0.7 H2O:
C, 52.88; H, 9.72; N, 10.28; Cl, 8.67
Found: C, 52.88; H, 9.42; N, 10.38; Cl, 8.41.
g) N-[N-[(1,1-Dimethvlethoxv)carbonyl3-L-phenvl-`
alanyl]-l'-~(Dhenylmethoxy)methyll-L-histidine
Thionyl chloride (27.2 ml., 375 mmole) is
added in drops to a stirred solution in an ice-
bath of L-histidine (38.75 g., 240 mmole) in
methanol (500 ml.). After 15 minutes the ice-bath
is removed and the reaction mixture is stirred at
..,~
.. . .
~7631 HA362a
-31-
room temperature for one hour. After refluxing
for 48 hours, it is concentrated ln vacuo. The
separated crystals are filtered using methanol for
washings to give 48.93 g. of L-histidine, methyl ester,
dihydrochloride. The methanolic solution on
dilution with ether affords an additional 10 g. of
product; m.p. 208 - 209; ~]2D2 = +10.1 (c = 1.8,
water).
Triethylamine (28 ml., 200 ml.) and di-tert-
butyl dicarbonate (48 g., 220 mmole) are added to a
suspension of L-histidine, methyl ester (24.2 g.,
100 mmole) in methanol (80 ml.). After 3.5 hours,
the mixture is filtered and the methanolic
solution is concentrated ln vacuo. The residue is
taken into chloroform and washed with 10% citric
acid. The crude product on crystallization from
isopropyl ether affords 23.1 g. of N,1'-bis[(1,1-
dimethylethoxy)carbonyl]-L-histidine, methyl ester;
m.p. (62) 88 _ 95; [~]2D2 = +25.4 (c = 1.1,
carbon tetrachloride).
Benzylchloromethyl ether (11.6 ml., 83.6 mmole)
is added to a solution of N,1'-bis[(1,1-dimethyl-
ethoxy)carbonyl]-L-histidine, methyl ester (24.7 g.,
66.9 mmole) in dry methylene chloride (156 ml.)
and the reaction mixture is stirred at room
temperature for 5 hours. After concentrating in
vacuo and on dissolution in ethyl acetate 17.85 g.
of N-[(l,1-dimethylethoxy)carbonyl]-1'-[(phenyl-
methoxy)methyl]-L-histidine, methyl ester, mono-
';
-`~ 1297631
HA362a
-32-
hydrochloride crystallizes out; m.p. (148) 152 -
153; [a]22 = -19.5 (c = 1.8, methanol). This
methyl ester product is dissolved in hydrogen
chloride in acetic acid solution (60 ml., 1.5 N)
and kept at room temperture for 15 minutes. It is
then evaporated ln vacuo and the residue is
dissolved in hot isopropanol. After cooling, the
separated crystals are filtered to yield 7.08 g.
of l-[(phenylmethoxy)methyl]-L-histidine, methyl
ester, dihydrochloride; m.p. (170) 173 - 174.
l-[(Phenylmethoxy)methyl]-L-histidine, methyl
ester, dihydrochloride (1.79 g., 4.94 mmole),
l-hydroxybenzotriazole (0.756 g., 4.94 mmole), and
N-[(1,1-dimethylethoxy)carbonyl]-L-phenylalanine
(1.31 g., 4.94 mmole) are dissolved in dimethyl-
formamide (16 ml.). While stirring the above
solution in an ice-bath, dicyclohexylcarbodiimide
(1.02 g~, 4.94 mmole) and N,N-diisopropylethylamine
(1.72 ml., 10 mmole) are added. After 3 hours the
ice-bath is removed and the relction mixture is
stirred at room temperature overnight. It is then
concentrated to dryness and the residue is tritura-
ted with ethyl acetate. The separated urea is
filtered off. The ethyl acetate solution is
washed with saturated sodium bicarbonate and then
it is evaporated. The residue upon crystallization
from ethyl acetate gives 1.97 g. of N-[N-[(1,1-
dimethylethoxy)carbonyl]-L-phenylalanyl]-1'-
[(phenylmethoxy)methyl]-L-histidine, methyl ester;
m.p. (165) 166 - lfi8.
., ~ .. . ..
1297631
HA362a
-33-
N-[N-[(1,1-Dimethylethoxy)carbonyl]-L-
phenylalanyl]-1'-[(phenylmethoxy)methyl]~L-
histidine, methyl ester (4.5 g., 8.4 mmole) is
dissolved in hot methanol (25 ml.~. After cooling
to room temperature aqueous sodium hydroxide
solution (9.24 ml., lN) is added and the mixture
is stirred at room temperature for 3 hours. It is
then concentrated in vacuo and water (60 ml.) is
added to the residue. After cooling the aqueous
solution in an ice-bath, it is acidified to pH 4.5
using aqueous hydrochloric acid. It is then
extracted with ~thyl acetate to yield 3.95 g. of
crystalline N-[N-[(1,1-dimethylethoxy)carbonyl]-L-
phenylalanyl]-l'-[(phenylmethoxy)methyl]-L-
histidine; m.p. 193 - 194; [~]22 = -4.8
(c = 1.1, dimethylformamide).
h) N-[[[(3S)-3-[[N-[N-[(l,l-Dimethylethoxy)-
carbonyl]-L-phenylalanyl]-1'-[(phenylmethoxy)-
methyl]-L-histidinyl]amino]-2-hydroxy-5-methyl-
hexyll(2-methylpropyl)amino]carbonyl]-L-valine~
methyl ester (isomer A)
N-~[[(3S)-3-Amino-2-hydroxy-5-methylhexyl]-
(2-methylpropyl)amino~carbonyl]-L-valine, methyl
ester, monohydrochloride (isomer A) (0.396 g.,
1 mmole~, N-[N-[(1,1-dime-thylethoxy)carbonyl]-L-
phenylalanyl]-1'-[(phPnylmethoxy)methyl] L-
histidine (0.522 g~, 1 mmole) and 1-hydroxybenzo-
triazole hydrate (0.153 g., 1 mmole) are dissolve~
in dimethylformamide (6 ml.). Diisopropylethyl
amine (0.21 ml., 1.3 mmole) is added and the
solution is cooled to -10. While stirring under a
,
` ~
..~
-` 1297631
HA362a
-34-
gentle flow of nitrogen, dicyclohexylcarbodiimide
(0.206 g., 1 mmole) is added. After stirring for
one hour between -10 and 0, the reaction mixture
is then stirred in an ice-bath for 2 hours and then
stirring is continued at ambient temperature overnight.
The separated dicyclohexyl urea is filtered off
and the dimethylformamide solution is evaporated.
The residue is taken into ethyl acetate and washed
with saturated sodium bicarbonate solution. The
ethyl acetate extract after concentration is
chromatographed over silica gel (45 g.) using the
solvent system chloroform:methanol:acetic acid
(9:0.5:0.5). Homogeneous fractions containing the
product are pooled, evaporated, and the residue
taken into ethyl acetate and washed with saturat~d
sodium bicarbonate. The ethyl acetate extract is
dried and evaporated to give 0.61 g. of N-[[[(3S)-
3-~[N-LN-[(1,1-dimethylethoxy)carbonyl]-L-phenyl-
alanyl]-1'-[(phenylmethoxy~methyl]-L-histidyl]-
amino]-2-hydroxy-5-methylhexyl](2-methylpropyl)-
amino]carbonyl]-L-valine, methyl ester (isomer A).
i) N-[~ f ( 3S)-3-[[N-[N-~(l,1-Dimethylethoxy)-
carbonyll-L-Phenvlalanyll-L-histidyllaminol-2
hydroxv-5-methylhexvll(2-methYlpropyl)amino]-
carbonYll-L-valine, methYl ester, monohvdrochloride
(isomer A~
The methyl ester product from part (h)
(0.3 g., 0.35 mmole) is dissolved in methanol
(25 ml.). Aqueous hydrochloric acid (lN, 0.35 ml.)
is added and the solution is stirred under an
atmosphere of hydrogen in the presence of palladium
:
;
,~"
1~
,
1297631
_35_ HA362a
hydroxide on carbon catalyst (0.1 g.) for 15
hours. It is then filtered through hyflo and
evaporated to dryness. The residue is triturated
with ether and filtered to give 0.22 g. of
S N- [ [ [ ( 3S ) -3-[[N-[N-[(l,l-dimethylethoxy)carbonyl]-
L-phenylalanyl~-L-histidyl]amino]-2-hydroxy-5-
methylhexyl](2-methylpropyl)amino3carbonyl~-L-
valine, methyl ester, monohydrochloride (isomer A);
m.p. 125 - 137; [a]2D2 = -2.9 (c = 1,
methanol). TLC (silica gel, chloroform:methanol:
acetic acid, 15:1:1) Rf = 0.23.
38H61N78 HCl- 1.1 H2O:
C, 57.02; H, 8.06; N, 12.25; Cl, 4.43
Found: C, 57.02; H, 7.89; N, 12.12; Cl, 4.99.
Example 2
N-~[(3S)-3-[[N-[~-[(l,l-DimethYlethoxY)carbonvl~-
L-~henYlalanYll-L-histidy~laminol-2-hYdroxy-5-
methylhexYl](2-methylpropyl2aminolcarbonYl]-L-
valine, methyl ester~ monohYdrochloride (isomer B)
a) N-[[ L ( 3S?-3 - r [ ( 1, 1 -DimethylethoxY ) carbonYl ] -
amino]-2-hydroxy-S-methvlhexYll(2-methylro~yl)
amino]carbonYll-L-valine, methYl ester (isomer B)
Following the chromatographic procedure
described in Example 1 (e), fractions 70 - 90
(50 ml. fractions) contain the slow moving isomer.
These fractions are pooled and evaporated to give
1.25 g. of N-~[[(3S)-3-~[(1,1-dimethylethoxy)-
carbonyl]amino]-2-hydroxy-5-methylhexyl](2-methyl-
propyl)amino]carbonyl]-L-valine, methyl ester
(isomer B); m.p. 44 - 55; [a]2D2 = -78 (c = 1,
1297~
HA362a
-36-
methanol). TLC (silica gel; ethyl acetate:
hexane 1:1) Rf = 0.38.
Anal- calc~d. for C23H45N3O6
C, 60.10; H, 9.87; N, 9.14;
S Found: C, 59.84; H, 9.82; N, 9.00.
b) N-[ r r ( 3S)-3-Amino-2-hydroxy-5-methylhexYll-
(2-methyl~ro~Yl)aminolcarbonyll-L-valine, methyl
ester, monohYdrochloride (isomer B)
The methyl ester (isomer B) product from
part (a) (0.35 g., 0.76 mmole) is dissolved in a
solution of hydrochoric acid in dioxane (4 ml.,
4.9 N) and allowed to stand at room temperature
for 50 minutes. It is then evaporated and
reevaporated from methanol and ether. The crude
residue is dissolved in water, millipore filtered,
and lyophilized to give 0.27 g. of N-[t[(3S)-3-amino-
2-hydroxy-S-methylhexyl]~2-methylpropyl)amino]-
carbonyl]-L-valine, methyl ester, monohydrochloride
(isomer B).
c) N-[[~(3S)-3-r[N-[N-[(1,1-DimethYlethoxv2-
carbonyl]-L-phenYlalanyl~ henylmethoxy)methyl]
L-histidYl]aminol-2-hvdroxy-5-methYlhexvll(2-methYl-
proyl)aminolcarbonvll-L-valine, methyl ester
(isomer Al
N-[[[(3S)-3-Amino-2-hydroxy-5-methylhexyl]-
(2-methylpropyl)amino]carbonyl]-L-valine, methyl
ester, monohydrochloride (isomer B) (0.36 g.,
0.91 mmole), N-[N-[(1,1-dimethylethoxy)carbonyl]-L-
phenylalanyl]-l'-[(phenylmethoxy)methyl]-L-histidine
(0.475 g., 0.91 mmole) and 1-hydroxybenzotriazole
~.
'.
-`` 1297631
HA362a
-37-
hydrate (0.139 g., 0.91 mmole) are dissolved in
dimethylformamide (6.5 ml.). Diisopropylethylamine
(0.21 ml., 1.3 mmole) is added and the solution
is stirred in an ice-bath. Dicyclohexylcarbodiimide
(0.188 g., 0.91 mmole) is added and the stirring
of the reaction mixture is continued at ambient
temperature overnight. Dicyclohexylurea is
filtered off and the dimethylformamide solution is
evaporated. The residue is taken into ethyl
acetate and washed with saturated sodium
bicarbonate solution. The ethyl acetate extract
after concentration is chromatographed over silica
gel (50 g.) using the solvent system chloroform:
methanol:acetic acid, 10:0.5:0.5. Homogeneous
fractions containing the product are pooled,
evaporated, and the residue taken into ethyl
acetate and washed with saturated sodium
bicarbonate. The ethyl acetate extract is dried
and evaporated to give 0.62 g. of N-[[[(3S)-3-
[[N-rN-[(1,1-dimethylethoxy)carbonyl]-L-phenyl-
alanyl]-1'-[(phenylmethoxy)methyl}-L-histidyl]-
amino]-2-hydroxy-5-methylhexyl](2-methoxypropyl)-
amino]carbonyl]-L-valine, methyl ester (isomer A).
d) N-~LL~3S)-3-rrN-[N-[(1,1-Dimethvlethoxy)-
carbonvll-L-phenvlalanYl]-L-histidyl]amino]-2-
hydroxy-5-methvlhexyll(2-methyl~ro~yl)amino]-
carbonvl]-L-valine, methyl ester,monohvdrochloride
(isomer B)
The methyl ester (isomer B) product from
part (c) (0.32 g., 0.37 mmole) is dissolved in
. ,~ . ..... . .. . . .
.
1297631
HA362a
-38-
methanol (25 ml.). Aqueous hydrochloric acid
(1 N, 0.37 mmole) is added and the solution is
stirred under an atmosphere of hydrogen in the
presence of palladium hydroxide on carbon catalyst
(0.1 g.) for 18 hours. It is then filtered
through hyflo and evaporated to dryness. The
residue is dissolved in isopropanol (3 ml.) and
while shaking vigorously isopropyl ether (35 ml.)
is added. The resulting precipitate is filtered
and dried to give 0.184 g. of N-[[[(3S)-3-[[N-[N-
[(l,l-dimethylethoxy)carbonyl]-L-phenylalanyl]-L-
histidyl]amino]-2-hydroxy-5-methylhexyl](2-methyl-
propyl)amino]carbonyl]-L-valine, methyl ester
monohydrochloride (isomer B); m.p. 115 - 140;
[a]2D2 = -36.9 (c = l.l, methanol). TLC (silica
gel; chloroform:methanol:acetic acid, 15:1:1)
Rf = 0.20.
38H61N708 HCl 1.34 H20:
C, 56.73; H, 8.10; N, 12.19; Cl, 4.41
Found: C, 56.73; H, 7.99; N, 12.28; Cl, 4.66.
Exam~le 3
N-[N-[r[(3S)-3-[[N-rN-[(1,1-Dimethvlethoxv)-
carbonyll-L-phenylalanvll-L-histidyl]aminol-2-
hvdroxY-5-methYlhexY1](2-methvl~ro~Yl)amino]
carbonyll-L-valvl]-L-~henylalanine, methyl
ester, monohvdrochloride
a~ N-[[[(3S)-3-[[(l,l-Dimethylethoxv)carbonyl]-
aminol-2-hvdroxv-5-methylhexyll(2-methYl~ropyl)-
amino]carbonvll-L-valine
N-[[[(3S)-3-[[(1,1-Dimethylethoxy)carbonyl]-
1297631
HA362a
-39-
amino]-2-hydroxy-5-methylhexyl](2-methylpropyl)-
amino]carbonyl]-L-valine, phenylmethyl ester
(2.0 g., 3.73 mmole), prepared as set forth in
Example l(d), is dissolved in methanol (100 ml.)
and stirred under an atmosphere of hydrogen for
16 hours in the presence of palladium hydroxide on
carbon catalyst (400 mg.). It is filtered and the
methanolic solution is evaporated to give 1.66 g.
of N-[[[(3S)-3-[[(l,1-dimethylethoxy)carbonyl]amino]-
2-hydroxy-5-methylhexyl](2-methylpropyl)amino]carbonyl]-
L-valine as a foamy solid.
b) N-[N- L[ [ ( 3S)-3-[[(l,1-DimethYlethoxY)carbonyl]-
amino]-2-hydroxv-5-methvlhexvl](2-methylDropYl)amino]
carbonyl]-L-valyl]-L-phenvlala-nlne~ methyl ester
N-[[[(3S)-3-[[(1,1-Dimethylethoxy)carbonyl]-
amino]-2-hydroxy-5-methylhexyl](2-methylpropyl)amino]-
carbonyl]-L-valine (1.62 g., 3.63 mmole), L-phenyl-
alanine, methyl ester, monohydrochloride (0.94 g.,
4.36 mmole) and N-hydroxy succinimide (0.42 g.,
3.63 mmole) are dissolved in dimethylformamide
(13 ml.). While stirring the above solution in an
ice-bath, dicyclohexylcarbodiimide (0.75 g.,
3.63 mmole) and diisopropylethyl amine (0.85 ml.
5.3 mmole) are added. The reaction is continued
in the ice-bath for two hours and then at ambient
temperture overnight. It is filtered and the
dimethylformamide solution is evaporated ln
vacuo. The residue is taken into ethyl acetate
and washed with saturated sodium bicarbonate,
:
, ,
- IZ97631
HA362a
-40-
water, and aqueous hydrochloric acid (0. 25 N)
solutions. The ethyl acetate solution is dried
and then evaporated. The crude product is
chromatographed over silica gel (100 g.) eluting
with ethyl acetate:hexane (1:1) to give 1.4 g. of
N-[N-[[[(3S)-3-[[(1,1-dimethylethoxy)carbonyl]amino]-
2 -hydroxy-5-methylhexyl](2-methylpropyl)amino]-
carbonyl]-L-valyl]-L-phenylalanine, methyl ester;
m.p. (52) 63 - 74; [a]2D2 = -26 4 (c = 1 2
methanol). TLC (silica gel, ethyl acetate:
hexane, 4:6) Rf = 0.4 and 0.44.
Anal. calc'd. for C32H54N4O7:
C, 63.34; H, 8.97; N, 9.23
Found: C, 63.04; H, 8.87; N, 9.17.
c) N-rN-[[[(3S)-3-Amino-2-hvdroxy-5-methylhexyll-
~methyl~ropvl)amino]carbonYl1-L-valYll-L-~henyl-
alanine, methYl ester, monohYdrochloride
The methyl ester product from part ~b) (0.92
g., 1.502 mmole) is dissolved in a solution of
hydrochloric acid in dioxane (4.9 N, 8 ml.).
; After keeping the solution at room temperature for
45 minutes it is evaporated. The residue is
chromatographed over silica gel (100 g.) eluting
with chloroform:methanol:acetic acid (12:0.9:0.9).
Fractions containing homogeneous material are
pooled and evaporated (0.49 g.). The impuxe
fractions are pooled, evaporated and
rechromatographed over silica gel (20 g.) eluting
with the solvent system chloroform:methanol:acetic
acid (12:1:1) to give an additional amount (0.15 g.)
of product resulting in a total yield of
,
_41_ HA362a
0.64 g. of N-[N-[[[(3S)-3-amino-2-hydroxy-5-methyl-
hexyl](2-methylpropyl)amino]carbonyl]-L-valyl]-L-
phenylalanine, methyl ester, monohydrochloride.
d) N-[N-[[[(3S)-3-LLN-~N-[(l,l-Dimethylethox~)-
carbonyll-L-~henylalanyl]-ll-[(phenylmethoxy)methyl]
L-histidyllamino]-2-hydroxy-5-methylhexYl](2-methYl-
proPyl)amino~carbonvll-L-valYl]-L-phenYlalanine,
methvl ester
N-[N-[[[(3S)-3-Amino-2-hydroxy-S-methylhexyl]-
(2-methylpropyl)amino]carbonyl]-L-valyl]-L-phenyl-
alanine, methyl ester, monohydrochloride (0.32 g.,
0.59 mmole), N-[N-~l,1-dimethylethoxy)carbonyl~-L-
phenylalanyl]-1'-[(phenylmethoxy)methyl]-L-histidine
(0.309 g., 0.59 mmole), and l-hydroxybenzotriazole
hydrate (0.09lg., 0.59 mmole) are dissolved in
dimethylformamide (3 ml.). A small amount of
benzene (3 ml.) is used for the washings and then
it is evaporated off. While stirring the above
solution at -10 under a gentle flow of nitrogen,
dicyclohexylcarbodiimide (0.122 g., 0.59 mmole)
and diisopropylethylamine (0.145 ml., 0.9 mmole)
are added. After stirring at -10 for two hours,
it is then stirred in an ice-bath for one hour,
and stirring is then continued at ambient
temperature overnight. The reaction mixture is
then evaporated, diluted with ethyl acetate, and
filtered to remove dicyclohexyl urea. The ethyl
acetate solution is washed with saturated sodium
bicarbonate and water and then evaporated. The
residue is chromatographed over silica gel (35 g.)
~297631
HA362a
-42-
eluting with the solvent system
chloroform:methanol:acetic acid (10:0:5:0.5). The
fractions containing the desired product are
pooled, evaporated, and the residue is dissolved
in ethyl acetate and washed with saturated sodium
bicarbonate solution. The ethyl acetate solution
is then evaporated to give 0.48 g. of N-[N-~[[(3S)-
3-~[N-[N-~(1,1-dimethylethoxy)carbonyl3-L-phenyl-
alanyl]-l'-[(phenylmethoxy)methyl]-L-histidyl]amino]-
2-hydroxy-5-methylhexyl](2-methylpropyl)amino]-
carbonyl]-L-valyl]-L-phenylalanine, methyl ester.
e) N-~N-[[[(3S)-3-~[N-[N-[(1,1-Dimethylethoxy)-
carbonvl]-L-phenvlalanvl]-L-histidYl]aminol-2-
hvdroxv-5-methYlhexyll(2-methylpro~Yl)amino]-
carbonyll-L-valYll-L-Dhenylalanine, methYl ester,
monohvdrochloride
The methyl ester product from part (d)
(0.195 g., 0.193 mmole) is dissolved in methanol
(25 ml.). Aqueous hydrochloric acid (lN, 0.2 ml.)
is added and the solution is stirred under an
atmosphere of hydrogen in the presence of
palladium hydroxide on carbon catalyst (0.1 g.)
for 18 hours. It is then filtered through hyflo
and evaporated to dryness. The residue (0.16 g.)
; 25 is stirred with ether and filtered. The solid
(0.145 g.) is dissolved in isopropanol (0.5 ml.)
and diluted with isopropyl ether (35 ml.). This
sample (120 mg.) is again precipitated from iso-
propanol-isopropyl ether to give 105 mg. of
N-[N-t[[(3S)-3-[[N-[N-[(1,1-dimethylethoxy)-
"` ,;
- 1~97631
HA362a
-43-
carbonyl]-2-phenylalanyl]-L-histidyl]amino]-2-
hydroxy-5-methylhexyl](2-methylpropyl)amino]-
carbonyl]-L-valyl]-L-phenylalanine, methyl ester,
monohydrochloride; m.p. (129) 135 - 145;
[~]D2 = -13.5 (c = 1, methanol). TLC (silica
gel; chloroform:methanol:acetic acid, 15:1:1)
Rf = 0.31.
Anal- calc'd- for C47H70N89 HCl 2.42 H2O:
C, 58.12; H, 7.87; N, 11.54; Cl, 3.65
Found: C, 58.12; H, 7.60; N, 11.50; Cl, 4.76.
Example 4
N-~N-~[(3S)-3-r[N-[N-[~1,1-DimethylethoxY)carbonyl]-
L-phenylalanyll-L-histidinYllamino]-2-hYdroxy-5-
methYlhexyl](l-methylethvl)aminolcarbonyl]-L-iso-
leucYll-L-histidine, methylester, dihYdrochloride
a) (S)-[3-Methyl-l-[[(1-methvlethYl)(~henYlmethvl)-
aminolacetYllbutyl]carbamic acid, l,1-dimethYlethYl
ester
A solution of (S)-[3-methyl-1-[(chloromethyl)-
carbonyl3butyl]carbamic acid, 1,1-dimethylethyl
ester (6.6 g., 25 mmole), N-isopropylbenzylamine
(4.182 ml., 25 mmole), sodium bicarbonate
(3.15 g., 37.5 mmole), sodium iodide (1.875 g.,
12.5 mmole), and dimethylformamide (80 ml.)
are stirred at room temperature for 7.5 hours.
The reaction mixture is then evaporated
ln vacuo and the residue is taken into ethyl
acetate and washed with water to give 9.4 g. of
(S)-[3-methyl-1-[[(1-methylethyl)(phenylmethyl)amino]-
acetyl]butyl]carbamic acid, l,l-dimethylethyl
`.~ ....
" ~Z97631
HA362a
-44-
ester.
b) (3S)-[l-[(R,S)-l-HYdroxy-2-[(1-methYlethyl)-
(~henylmethyl)amino]ethyll-3-methvlbutvl]carbamic
acid,l,l-dimethylethyl ester
Sodium borohydride (1 g., 26.3 mmole) is
added to a solution of (S)-[3-methyl-1-[[(1-methyl-
ethyl)(phenylmethyl~amino]acetyl~butyl]carbamic
acid, l,l-dimethylethyl ester (9.4 g., 25 mmole)
in ethanol (75 ml.). The solution is stirred at
room temperture for one hour. It is then concen-
trated to dryness ln vacuo. The residue is
suspended in ethyl acetate/water and acidified to
pH 2.0 by adding dilute hydrochloric acid. The
aqueous layer is then made basic by adding
saturated sodium bicarbonate. The aqueous layer
is then extracted with ethyl acetate. The ethyl
acetate solution on evaporation and redissolution
in a mixture of ethyl acetate:hexane (1:3)
deposits 2.526 g. of a crystalline material which
is found to be one of the isomeric alcohols. The
mother liquor is then chromatographed over silica
gel (300 g.) using the solvent system ethyl
acetate:hexane (1:3). The purified isomeric
alcohol derivatives are pooled to give 7.0 g. of
25 (3S)-[1-[(R,S)-1-hydroxy-2-[(1-methylethyl)(phenyl-
methyl)amino]ethyl]-3-methylbutyl]carbamic acid,
1,1-dimethylethyl ester.
c) (3S)-[1-[(R,S)-1-HvdroxY-2-[(1-methvlethvl)-
amino]ethyl]-3-methylbutyl]carbamic acid, 1,1-
dimethvlethvl ester, monohYdrochloride
.,
~297631
HA362a
-45-
A solution of the carbamic acid, 1,1-
dimethylethyl ester product from part (b) (2.27 g.,
6 mmole) in methanol (40 ml.) and aqueous hydro-
chloric acid (lN, 6 ml.) is stirred under an
atmosphere of hydrogen in the presence of palladium
hydroxide on carbon catalyst (460 mg.) for 3.5
hours. The mixture is then filtered through hyflo
and evaporated to give 1.87 g. of (3S)-l-[~R,S)-l-
hydroxy-2-[(1-methylethyl)amino]ethyl]-3-methylbutyl]-
carbamic acid, l,l-dimethylethyl ester, monohydro-
chloride.
d) N-[[t~3S)-3-[[(1,1-Dimethylethoxy)carbonyl]amino]-
2-hydroxY-5-methylhexyl](l-methylethYl)aminolcarbonYl]-
L-isoleucine, ~henylmethYl ester
N-Methylmorpholine (0.74 ml., 6.7 mmole) is
added to a stirred solution of L-isoleucine,
phenylmethyl ester, hydrochloride (1.06 g.,
2.7 mmole) in methylene chloride (12 ml.) at -30
followed by the dropwise addition of a solution of
phosgene in benzene (12.5%, 3.2 ml., 4.032 mmole).
After stirring for 30 minutes at -20, the mixture
is concentrated to dryness ln vacuo. A solution
of (3S)-[l-[(R,S)-1-hydroxy-2-[(1-methylethyl)amino]-
ethyl]-3-methylbutyl]carbamic acid,
1,1-dimethylethyl ester, monohydrochloride (0.9 g.,
2.77 mmole) in methylene chloride (6 ml.) is added
to the above residue. While stirring the above
solution in an ice-bath, N-methylmorpholine (0.59 ml.,
5.36 mmole) is added and the stirring is continued
for 2 hourc in the ice-bath and then overnight at
-
1297631
HA362a
-46-
room temperature. The reaction mixtur~ is then
evaporated to dryness, the residue is taken up in
ethyl acetate and washed neutral with saturated
sodium bicarbonate and 10% potassium bisulfate.
The ethyl acetate extract after drying is chroma-
tographed (silica gel, 75 g.) using the solvent
system ethyl acetate:hexane (1:1) to give 0.78 g.
of N-[[[(3S)-3-[[(1,1-dimethylethoxy)carbonyl]-
amino]-2-hydroxy-5-methylhexyl](l-methylethyl)-
amino]carbonyl]-L-isoleucine, phenylmethyl ester.
e) N-[[[(3S)-3-[[(1,1-Dimethylethoxv)carbonyl]-
amino]-2-hydroxy-5-methylhexYl](l-methylethyl)-
amino]carbonvl]-L-isoleucine
A solution of the phenylmethyl ester product
from part (d) (0.78 g., 1.45 mmole) in methanol
(40 ml.) is stirred under an atmosphere of
hydrogen overnight in the presence of palladium
hydroxide on carbon catalyst (0.15 g.). The
mixture is then filtered through hyflo and
concentrated to dryness to give 0.6 g. of
N-[[[(3S)-3-[[(1,1-dimethylethoxy)carbonyl]-
amino]-2-hydroxy-5-methylhexyl](l-methylethyl)-
amino]carbonyl]-L-isoleucine.
f) N-[N-[[1(3S)-3-[ r ( 1, 1-DimethYlethOxY ) carbonYl 1 -
amino]-2-hYdroxY-5-methYlhexY~ -methylethyl)amino]
carbonyl]-L-isoleucvl]-1'-[(DhenYlmethoxY)methyl]-
L-histidine, methYl ester
Dicyclohexylcarbodiimide (0.277 g., 1.35 mmole)
is added to a stirred (ice-bath) mixture of the
L-isoleucine product from part (e) (0.6 g.,
1297631
HA362a
-47-
1.35 mmole), N-hydroxy succinimide ~0.155 g.,
1.35 mmole), l'-[(phenylmethoxy)methyl]-L-histidine,
methyl ester, monohydrochloride (0.488 g.,
1.35 mmole), diisopropylethylamine (0.47 ml.,
2.76 mmole), and dimethylformamide (5 ml.).
Stirring is continued at 5 (cold room) overnight.
The reaction mixture is then evaporated and the
residue is taken up in ethyl acetate. The
separated dicyclohexyl urea is filtered off and
then the ethyl acetate solution is washed with
saturated sodium bicarbonate. After evaporation
of the ethyl acetate extract, the residue is
chromatographed (silica gel, 75 g.) using the
solvent system ethyl acetate:methanol (9:1) to
give 0.54 g. of N-[N-[[[(3S)-3-[[(1,1-dimethyl-
ethoxy)carbonyl]amino]-2-hydroxy-5-methylhexyl]-
(1-methylethyl)amino]carbonyl]-L-isoleucyl]-l'-
[(phenylmethoxy)methyl]-L-histidine, methyl ester.
g) N-~N-[~[(3S)-3-Amino-2-hYdroxv-5-methvlhexvll-
(1-methYlethyl)aminolcarbonvll-L-isoleucyl]-1'-
[(~henylmethoxY)methYll-L-histidine, methYl ester,
dihvdrochloride
The L-histidine, methyl ester product from
part (f) (0.545 g., 0.76 mmole) is dissolved in a
solution of hydrochloric acid in dioxane (2N, 12 ml.)
and kept at room temperature for 3.5 hours. It is
then concentrated to dryness in vacuo to give
0.53 g. of N-[N-[[[(3S)-3-amino-2-hydroxy-5-
methylhexyl](1-methylethyl)amino]carbonyl]-L-
isoleucyl]-l'-[(phenylmethoxy)me-hyl]-L-
-, .
, .
~297631
HA362a
-48-
histidine, methyl ester, dihydrochloride.
h) N-[N-[~[(3S)-3-[[N-[N-[(l,l-(DimethYlethoxy)-
carbonyll-L-PhenYlalanYl]-l'-~(phenylm-ethoxy)methyl]-
L-histidinyllamino]-2-hYdroxY-5-methylhexyl](1-
methylethyl-2amino]carbonyl]-L-isoleucyl]-ll-
[(phenYlmethoxy)methyll-L-histidine, methyl ester
Dicyclohexylcarbodiimide (0.156 g.,
0.76 mmole) is added to a stirred (ice-bath)
solution of the L-histidine, methyl ester, dihydro-
chloride product from part (g) (0.53 g., 0.76 mmole),
l-hydroxybenzotriazole hydrate (0.116 g.,
0.76 mmole), N-[N-[(l,l-dimethylethoxy)carbonyl]-
L-phenylalanyl]-l'-[(phenylmethoxy)methyl]-L-
histidine (0.396 g., 0.76 mmole), diisopropyl-
ethylamine (0.264 ml., 1.51 mmole) and dimethyl-
formamide (4 ml.). Stirring is continued at 5
(cold room) overnight. The reaction mixture is
then concentrated to dryness ln vacuo. The
residue is taken into ethyl acetate, separated
dicyclohexyl urea is filtered off, and the ethyl
acetate solution is washed with saturated sodium
bicarbonate. The ethyl acetate solution after
evaporation is chromatographed (silica gel, 75 g.)
using the solv~nt system ethyl acetate:methanol
(8.5:1.5) to give 0.48 g. of N-[N-[[[(3S)-3-
[[N-[N-[(l,1-dimethylethoxy)carbonyl]-L-phenyl-
alanyl]-1'-[(phenylmethoxy)methyl]-L-histidinyl]-
amino]-2-hydroxy-5-methylhexyl](1-methylethyl)-
amino]carbonyl]-L-isoleucyl]-l'-[(phenylmethoxy)-
methyl]-L-histidine, methyl ester.
lZg763i
HA362a
-49-
i) N-[N-[~[(3S)-3-[[N-[N-[(l,l-Dimethylethoxy)-
carbonYl1-L-phenYlalanyll~L-histidinyllamino~-2-
hydroxY-S-methYlhexyl](l-methYlethyl)amino]carbon~l]-
L-isoleucvl~-L-histidine, methyl ester,
dihvdrochloride
The L-histidine, methyl ester product from
part (h) (0.466 g., 0.415 mmole) is dissolved in
methanol (25 ml.) and aqueous hydrochloric acid
(lN, 0.79 ml.). The mixture is stirred under an
atmosphere of hydrogen in the presence of
palladium hydroxide on carbon catalyst (0.1 g.)
for 48 hours. The solution is filtered through
hyflo and concentrated to dryness ln vacuo. The
residue is chromatographed (silica gel, 39 ~.)
using the solvent system ethyl acetate:acetic
acid:water (4:1:1). The fractions containing the
product are pooled and evaporated. The residue is
dissolved in methanol and aqueous hydrochloric acid
(lN, 0.52 ml.) is added. The mixture is then
evaporated, the residue is dissolved in a minimum
amount of methanol, and passed through a column of
LH-20 using methanol for elution to give 0.224 g.
of product. 174 mg. of the above solid is
dissolved in methanol and aqueous hydrochloric
acid (lN, 0.047 ml.) is added and the solution is
evaporated to dryness to give 0.171 g. of
N-[N-[[[(3S)-3-[[N-[N-[(1,1-dimethylethoxy)carbonyl]-L-
phenylalanyl]-L-histidinyl]amino~-2-hydroxy-5-
methylhexyl](1-methylethyl)amino]carbonyl]-L-
isoleucyl]-L-histidine, methyl ester, dihydro-
.
~2~7631
HA362a
-50-
chloride; m.p. (115) 148 - 168; [~]22 = -7.8
(c = 1.2, methanol). TLC (silica gel; ethyl
acetate:acetic acid:water, 4:1:1) Rf = 0.45.
Anal. calc'd. for C44H6809N1o 2
C, 52.90; H, 7.57; N, 14.02; Cl, 7.10
Found: C, 52.86; H, 7.24; N, 13.73; Cl, 6.97.
Example 5
N-[[[(3S)-3-[[N-[N-[(1,1-Dimethylethoxy)carbonyl]-
L-Pheny~lalanYl]-L-histidYl]amino]-2-hydroxY-5-
methylhexyl~ methYlethYl)amino]carbonyl]-L-
isoleucine, methyl ester, isomer A, monohYdrochloride
a) N-r~[(3S)-3-[[(1,1-DimethYlethoxy)carbonYl]-
aminol-2-hYdroxy-5-methYlhexyll(l-methylethyl)-
amino]carbonYll-L-isoleucine, methyl ester,
isomer A
N-Methylmorpholine (0.79 ml., 7.1 mmole)
is added to a stirred solution of L-isoleucine,
methyl ester, monohydrochloride (0.442 g.,
2.43 mmole) in methylene chloride (10 ml.) at
-30 followed by the dropwise addition of a
solution of phosgene in benzene (12.5%, 2.94 ml.,
3.7 mmole). After stirring for 20 minutes at
-20, the mixture is concentrated to dryness ln
vacuo. An ice-cold solution of (3S)-[1-[(R,S)-1-
hydroxy-2-[(1-methylethyl)amino]ethyl]-3-methyl-
butyl]carbamic acid, 1,1-dimethylethyl ester,
monohydrochloride (0.79 g., 2.43 mmole) in
methylene chloride (10 ml.) and N-methylmorpholine
(0.54 ml., 4.86 mmole) is added to the above
residue. The reaction mixture is stirred in an
' '
....
~ ~ .
~297631
HA362a
-51-
ice-bath for 6 hours and at room temperature
overnight. It is then evaporated to dryness, the
residue is taken up in ethyl acetate and washed
with saturated sodium bicarbonate solution and 10%
potassium bisulfate solution. The ethyl acetate
extract after evaporation is chromatographed
(silica gel, 125 g.) using the solvent system
ethyl acetate:hexane (4:3). In this chromatography
the two diastereoisomers are separated giving
0.26 g. of N-[[[(3S)-3-[[(1,1-dimethylethoxy)-
carbonyl]amino]-2-hydroxy-5-methylhexyl](l-methyl-
ethyl)amino]carbonyl]-L-isoleucine, methyl ester,
isomer A and 0.448 g. of N-[[[(3S)-3-[[(1,1-
dimethylethoxy)carbonyl]amino]-2-hydroxy-5-
methylhexyl](l-methylethyl)amino]carbonyl]-L-
isoleucine, methyl ester, isomer B.
b) N- r [ ~ ( 3S)-3-Amino-2-hYdroxY-5-methylhexYll-
(l-methylethyl)aminolcarbonYll-L-isoleucine,
methvl ester, isomer A
The L-isoleucine,methyl ester, isomer A
product from part (a) (0.25 g., 0.544 mmole)
is dissolved in a solution of hydrochloric acid
in dioxane (2N, 2.25 ml.). After keeping the
solution at room temperature for 4 hours, it is
evaporated ln vacuo to give N-[[[(3S)-3-amino-2-
hydroxy-5-methylhexyl](1-methylethyl)amino]-
carbonyl]-L-isoleucine, methyl ester, isomer A.
c) N-[[[(3S)-3-[[N-[N-[(l,l-Dimethvlethoxy)-
carbonYl1-L-phenylalanY13-1'-[(phenYlmethoxv)-
methyl]-L-histidyllaminol-2-hYdroxv-5-methY1-
.' , . ~.
:
lZ97631
HA362a
-52-
hexyl](l-methYlethvl)amino]carbonYl]-L-isoleucine,
methyl ester' isomer A
Dicyclohexylcarbodiimide (0.109 g.,
0.59 mmole) is added to a stirred (ice-bath)
solution of N-[[[(3S)-3-amino-2-hydroxy-5-methyl-
hexyl](l-methylethyl)amino]carbonyl]-L-isoleucine,
methyl ester, isomer A from part (b), l-hydroxy-
benzotriazole hydrate (0.081 g., 0.53 mmole),
N-[N-[(l,l-dimethylethoxy)carbonyl]-L-phenylalanyl]-
l'-[(phenylmethoxy)methyl]-L-histidine (0.277 g.,
0.53 mmole), diisopropylethylamine (0.15 ml.,
0.885 mmole), and dimethylformamide (3 ml.).
Stirring is continued at 5 (cold room) overnight.
It is then concentrated to dryness in vacuo and
the residue is triturated with ethyl acetate and
the separated dicyclohexyl urea is filtered off.
The eth~l acetate solution is washed with
saturated sodium bicarbonate solution and water.
It is then evaporated in vacuo and the residue
chromatographed (silica gel, 75 g.) using the
solvent system chloroform:methanol:acetic acid
(10:0.5:0.5). The product containing fractions
are pooled, evaporated, and the residue is
dissolved in ethyl acetate and washed with
saturated sodium bicarbonate solution. The ethyl
acetate solution is dried over magnesium sulfate
and evaporated in vacuo to give 0.26 g. of
N-[[[(3Sj-3-[[N-[N-[(l,l-dimethylethoxy)carbonyl]-
L-phenylalanyl]-l'-[(phenylmethoxy)methyl]-L-
histidinyl]amino]-2-hydroxy-5-methylhexyl](1-
'
.~
,,"
!
129~631 HA362a
-53-
methylethyl)amino]carbonyl~-L-isoleucine, methyl
ester, isomer A.
d) N-[[[(3S)-3-[[N-[N-[(1,1-DimethYlethoxY)-
carbonYll-L-~henYlalanYll-L-histidyllaminol-2
hYdroxY-5-methYlhexYll(l-methylethyl)aminol-
carbonYl]-L-isoleucine, methYl ester, isomer A,
monohYdrochloride
The L-isoleucine, methyl ester, isomer A
product from part (c) (0.237 g., 0.274 mmole) is
dissolved in methanol (3 ml.). Hydrochloric
acid (0.1 N, 2.3 ml., prepared by diluting lN
aqueous hydrochloric acid with methanol) is added
and the solution is stirred under an atmosphere
of hydrogen in the presence of palladium hydroxide
on carbon catalyst (65 mg.) for 18 hours. The
mixture is then filtered through Celite and
evaporated. The residue is stirred with isopropyl
ether and filtered to give 0.165 g. of N-[~[(3S)-3-
[[N-[N-[(l,l-dimethylethoxy)carbonyl]-L-phenyl-
alanyl]-L-histidyl]amino]-2-hydroxy-5-methylhexyl]-
(l-methylethyl)amino]carbonyl]-L-isoleucine, methyl
ester, isomer A, monohydrochloride; m.p. 101 - 116;
[a]2D0 = ~11.65 (c = 1, methanol). TLC (silica
gel; ethyl acetate:acetic acid:water, 12:1:1)
Rf = 0.28.
Anal. calc'd. for C38H61N708 2
C, 56.52; H, 8.11; N, 12.14; Cl, 4.39
~ound: C, 56.65; H, 8.00; N, 11.73; C1, 4.48.
~ * Trade Mark
:
i
lZ97631
HA362a
-54-
Example 6
N-[[[(3S)-3-[[N-[N-[(1,1-Dimethylethoxy)carbonyl]-
L-Phenylalanyll-L-histidyl]amino]-2-hydroxy-5-
methylhexyl](1-methYlethyl)amino]carbonYl]-L-
isoleucine, methYl ester, isomer B, monohydrochloridea) N-[[[(3S)-3-Amino-2-hYdroxy-5-methylhexyl](l-
methylethYl)aminolcarbonyll-L-isoleucine, methyl
ester, isomer B
The L-isoleucine, methyl ester, isomer B
product from Example 5(a) (0.35 g., 0.76 mmole) is
dissolved in a solution of hydrochloric acid in
dioxane (4 N, 3 ml.). After keeping the solution
at room temperature for two hours, it is
evaporated in vacuo to give N-[[[(3S)-3-amino-2-
hydroxy-5-methylhexyl](1-methylethyl)amino]-
carbonyl]-L-isoleucine, methyl ester, isomer B.
b) N-[[[(3S)-3-[[N-[N-[(1,1-DimethYlethoxY)-
carbonYll-L-PhenYlalanyll-l'-[(Phenylmethoxv)-
methYll-L-histidyl]aminol-2-hydroxy-5-methylhexyl]
(1-methYlethYl)aminolcarbonYl]-L-isoleucine,
methYl ester, isomer B
Dicyclohexylcarbodiimide (0.161 g.,
0.783 mmole) is added to a stirred (ice-bath)
solution of N-[[[(3S)-3-amino-2-hydroxy-5-methyl-
hexyl](1-methylethyl)amino]carbonyl]-L-isoleucine,
methyl ester, isomer B from part (a), l-hydroxy-
benzotriazole hydrate (0.12 g., 0.783 mmole),
N-[N-[(1,1-dimethy7ethoxy)carbonyl]-L-phenylalanyl]-
1'-[(phenylmethoxy)methyl]-L-histidine (0.409 g.,
0.783 mmole), diisopropylethylamine (0.17 ml.,
''
~297631
HA362a
-55-
1.0 mmole), and dimethylformamide (2 ml.).
Stirring is continued at 5 (cold room) overnight.
It is then concentrated to dryness in vacuo and
the residue is triturated with ethyl acetate and
the separated dicyclohexyl urea is filtered off.
The ethyl acetate solution is washed with
saturated sodium bicarbonate solution and water.
It is then evaporated in vacuo and the residue
chromatographed (silica gel, 120 g.) using the
solvent system chloroform:methanol:acetic acid
(10:0.5:0.5). The product containing fractions
are pooled, evaporated, and the residue is
dissolved in ethyl acetate and washed with
saturated sodium bicarbonate solution. The ethyl
acetate solution is dried over magnesium sulfate
and evaporated ln vacuo to give 0.37 g. of
N-[[[(3S)-3-[[N-[N-[(1,1-dimethylethoxy)carbonyl]-L-
phenylalanyl]-1'-[(phenylmethoxy)methyl]-L-histidinyl]-
amino]-2-hydroxy-5-methylhexyl](1-methylethyl)amino]-
carbonyl]-L-isoleucine, methyl ester, isomer B.
c) N-[[[(3S)-3-[[N-LN-[(1,1-DimethvlethoxY)carbonYll-
L-phenvlalanyll-L-histidyl]amino]-2-hydroxy-5-methyl-
hexyl](1-methYlethyl)amino]carbonyll-L-isoleucine,
methYl ester, isomer B, monohYdrochloride
The L-isoleucine, methyl ester, isomer B
product from part (b) (0.363 g., 0.42 mmole) is
dissolved in methanol (10 ml.). Hydrochloric acid
(0.1 N, 3.6 ml., prepared by diluting lN aqueous
hydrochloric acid with methanol) is added and the
solution is stirred under an atmosphere of
~297631
HA362a
-56-
hydrogen in the presence of palladium hydroxide on
carbon catalyst (lO0 mg.) for 18 hours. The
mixture is then filtered through Celite and
evaporated. After reevaporation from benzene, the
residue is triturated and stirred with isopropyl
ether and then filtered to give 0.30 g. of
N-[[[(3S)-3-[[N-[(l,l-dimethylethoxy)carbonyl]-L-
phenylalanyl]-L-histidyl]amino]-2-hydroxy-5-
methylhexyl](l-methylethyl)amino]carbonyl]-L-
isoleucine, methyl ester, isomer B, monohydro-
chloride; m.p. 118-128; [~]2D0 = -50.5
(c = 1.1, methanol). TLC (silica gel; ethyl
acetate:acetic acid:water, 12:1:1) Rf = 0.2.
Anal. calc'd. for C38H61H7O8 2
C, 57.03; H, 8.06; N, 12.25; Cl, 4.43
Found: C, 57.03; H, 7.88; N, 12.04; Cl, 4.32.
Exam~le 7
N-[[[(3S)-3-[LN-[N-[(l,l-Dimethvlethoxy)carbonyl]-
L-~henvlalanvl]-L-histidvllaminol-2-hYdroxv-5-
methYlhexYllamino]carbonYl]-L-isoleucine, methYl
ester, monohydrochloride
a) (S)-[3-MethYl-l-[[bis(~henYlmethYl)amino]-
acetyl]butYllcarbamic acid, 1,1-dimethYlethyl
ester
A solution of (S)-[3-methyl-1-[(chloro-
methyl)carbonyl]butyl]carbamic acid, 1,l-dimethyl-
ethyl ester (3.165 g., 12 mmole), dibenzylamine
(2.31 ml., 12 mmole), sodium bicarbonate (1.52 g.,
18 mmole), sodium iodide (0.9 g., 6 mmole), and
30~ dimethylformamide (24 ml.) are stirred at room
temperature for one hour. An additional amount of
1~97631
HA362a
-57-
dibenzylamine (0.23 ml., 1.2 mmole) is added and
the stirring is continued for another 2.5 hours.
The reaction mixture is then concentrated to
dryness. The residue is taken into ethyl acetate
and washed with water. The ethyl acetate solution
is concentrated and on dissolution in benzene a
small amount of insoluble material separates. The
solution is filtered and the benzene evaporated.
The residue is dissolved in ethyl acetate and
passed through a small column of silica gel. The
ethyl acetate solution is evaporated and the
residue crystallized from hexane to give 3.603 g.
of (S)-[3-methyl-1-[[bis(phenylmethyl)amino]acetyl]-
butyl]carbamic acid, 1,1-dimethylethyl ester; m.p.
(72) 75 - 77.
b) (3S)-L1-[(R,S)-1-HYdroxv-2-~bis(~henYlmethYl)-
amino]ethY11-3-methYlbutYllcarbamic acid, 1,1-
dimethylethyl ester
Sodium borohydride (0.354 g., 9.3 mmole) is
added to a solution of (S)-[3-methyl-1-[[bis(phenyl-
methyl)amino]acetyl]butyl]carbamic acid, l,l-dimethyl-
ethyl ester (3.59 g., 8.45 mmole) in ethanol (25 ml.).
The solution is stirred at room temperature for one
hour and then concentrated to dryness ln vacuo.
The residue is suspended in ethyl acetate/water
and acidified to pH 2.0 using dilute hydrochloric
acid. The aqueous layer is then made basic using
solid sodium bicarbonate and it is extracted with
ethyl acetate to give 3.65 g. of (3S)-[l-[(R,S)-
1-hydroxy-2-[bis(phenylmethyl)amino]-
ethyl]-3-methylbutyl]carbamic acid, l,l-dimethyl-
ethyl ester.
~ 12~7631
HA362a
-58-
c) (3S)-[l-[(R,S)-2-Amino-l-hydroxyethyl~-3-
methylbutYl]carbamic acid, l,l-dimethylethyl
ester, monohydrochloride
(3S~-[l-(R,S)-l-~ydroxy-2-[bis(phenylmethyl)-
amino]ethyl]-3-methylbutyl]carbamic acid, 1,1-
dimethylethyl ester (4.25 g., 9.96 mmole) is
dissolved in methanol (80 ml.). Aqueous hydro-
chloric acid (lN, 9.96 ml.) is added followed by
palladium hydroxide on carbon catalyst (0.85 g.).
The mixture is then stirred under an atmosphere of
hydrogen for 24 hours. It is then filtered
through hyflo and the solution is concentrated to
dryness to give 2.62 g. of (3S)-[l-[(R,S)-2-amino-
l-hydroxyethyl]-3-methylbutyl]carbamic acid, 1,1-
dimethylethyl ester, monohydrochloride.d) N-[[[(3S)-3-[[(1,1-DimethylethoxY)carbonyl]-
aminol-2-hvdroxy-5-methvlhexyllaminolcarbonYl]-L-
isoleucine, methyl ester
N-Methylmorpholine (0.588 ml., 5.33 mmole)
is added to a stirred solution of L-isoleucine,
methyl ester, monohydrochloride (0.385 g.,
2.12 mmole) in methylene chloride (8 ml.) at
-30 followed by the dropwise addition of a
solution of phosgene in benzene (12.5%, 3.18 mmole,
2.52 ml.). After stirring for 30 minutes at -20,
the mixture is concentrated to dryness ln vacuo.
An ice-cold solution of (3S)-[1-[(R,S)-2-amino-1-
hydroxyethyl]-3-methylbutyl]carbamic acid,
1,1-dimethylethyl ester, monohydrochloride (0.6 g.,
2.12 mmole) in methylene chloride (6 ml.) and
N-methylmorpholine (0.47 ml., 4.24 mmole) are
~.,
1297~`3~
HA362a
-59-
added to the above residue. An additional 3 ml.
of methylene chloride is used for washings. The
reaction mixture is stirred in an ice-bath for
2 hours and at room temperature overnight. It is
then evaporated to dryness, the residue is taken
up in ethyl acetate and washed with saturated
sodium bicarbonate and 10% potassium bisulfate
solution. The ethyl acetate extract after
evaporation is chromatographed over silica gel
(60 g.) using the solvent system ethyl acetate:
hexane (2:1) to give 0.48 g. of N-[[[(3S~-3-
[[(l,1-dimethylethoxy)carbonyl]amino]-2-hydroxy-5-
methylhexyl]amino]carbonyl]-L-isoleucine, methyl
ester.
e) N-[[r(3S)-3-Amino-2-hvdroxv-5-methvlheXYll-
aminolcarbonvll-L-isoleucine, methYl ester, mono-
The isoleucine, methyl ester product frompart (d) (0.43 g., 1.03 mmole) is dissolved in a
solution of hydrochloric acid in dioxane (2N, 3ml.)
and kept at room temperature for 2.5 hours. The
mixture is then concentrated to dryness ln vacuo
to give N-[[[(3S)-3-amino-2-hydroxy-5-methylhexyl]-
amino]carbonyl]-L-isoleucine, methyl ester, mono-
hydrochloride.
f) N-[[~(3S)-3-[[N-[N-[(1,1-DimethylethoxY)-
carbonyl]-L-~henylalanvl]-1'-[(~henvlmethoxy)-
methvll-L-histidvllaminol-2-hvdroxv-5-methYlhexvll-
amino]carbonYl]-L-isoleucine, methyl ester
Dicyclohexylcarbodiimide (0.212 g., 1.03
mmole) is added to a stirred (ice-bath) solution of
;
'
,
1297631
HA362a
-60-
the L-isoleucine, methyl ester, monohydrochloride
product from part (e), l-hydroxybenzotriazole
hydrate (0.158g., 1.03 mmole), N-[N-[(1,1-
dimethylethoxy)carbonyl]-L-phenylalanyl]-l'-[(phenyl-
methoxy)methyl]-L-histidine (0.538 g., 1.03 mmole)
diisopropylethylamine (O.22 ml., 1.29 mmole), and
dimethylformamide (4 ml.). Stirring is continued
at 5 (cold room) overnight. The mixture is then
concentrated to dryness ln vacuo The residue is
triturated with ethyl acetate and the separated
dicyclohexyl urea is filtered off. The ethyl
acetate solution is washed with saturated sodium
bicarbonate solution and water. It is then
evaporated ln vacuo and the residue is
chromatographed over silica gel (65 g.) using the
solvent system chloroform:methanol:acetic acid
(10:0.75:0.75). The product containing fractions
are pooled, evaporated, and the residue is
dissolved in ethyl acetate and washed with
saturated sodium bicarbonate solution. The ethyl
acetate solution after drying over anhydrous
magnesium sulfate is evaporated 1n vacuo to give
0.57 g. of N-[[[(3S)-3-[[N-[N-[(1,1-dimethylethoxy)-
carbonyl]-L-phenylalanyl]-1'-[(phenylmethoxy)methyl]-
L-histidyl]amino]-2-hydroxy-5-methylhexyl]amino~-
carbonyl]-L-isoleucine, methyl ester.
g) N-[[[(3S)-3-[[N-[N-[(l,1-Dimethylethoxv)-
carbonyl]-L-phenvlalanyl]-L-histidvl]aminol-2-
hydroxv-5-methYlhexyl]aminolcarbonyl]-L-isoleucine~
methvl ester, monohvdrochloride
The L-isoleucine, methyl ester product from
part (f) (0.37 g., 0.45 mmole) is dissolved in
12~7631
HA362a
-61-
methanol (20 ml.). Hydrochloric acid (O.lN,
4.05 ml., prepared by diluting lN aqueous hydro-
chloric acid with methanol) is added and the
solution is stirred under an atmosphere of
hydrogen in the presence of palladium hydroxide on
carbon catalyst (125 mg.) for 18 hours. The
mixture is then filtered through Celite and
evaporated. The residue is dissolved in iso-
propanol and diluted with isopropyl ether. The
separated solid is filtered to give 0.27 g. of
N-[[[(3S)-3-[[N-[N-[(l,l-dimethylethoxy)carbonyl]-
L-phenylalanyl]-L-histidyl]amino]-2-hydroxy-5-
methylhexyl]amino]carbonyl]-L-isoleucine, methyl
ester, monohydrochloride, m.p. 133 - 158;
[~]D0 = -8.0 (c = 1.2, methanoll. TLC (silica
gel; ethyl acetate:acetic acid:water, lZ:l:l) Rf =
0.16.
Anal. calc'd. for C35H55N7O8 2
C, 54.93; H, 7.77; N, 12.82; Cl, 4.63
Found: C, 54.93; H, 7.66; N, 12.46; Cl, 4.82.
Examule 8
N-[N-[[[(3S)-3-[[N-[N-[(1,1-DimethYlethoxv)carbonYll-
L-~henYlalanYl]-L-histidYl]aminol-2-hYdroxy-5-meth
hexyl]amino]carbonyl]-L-isoleucyl]-L-histine, methyl
ester, dihydrochloride
a) N-[[[(3S)-3-[[(1,1-Dimethylethoxv)carbonyll-
amino]-2-hYdroxY-5-methYlhexyl]aminolcarbonYl]-L-
isoleucine, phenYlmethyl ester
N-Methylmorpholine (2.5 ml., 22.73 mmole) is
added to a stirred solution of L-isoleucine,
phenylmethyl ester, monohydrochloride (3.56 g.,
1297631
HA362a
-62-
9.264 mmole) in methylene chloride (38 ml.) at
-30 followed by the dropwise addition of a
solution of phosgene in benzene (12.5%, 10.8 ml.,
13.608 mmole). After stirring for 30 minutes at
-20, the mixture is concentrated to dryness ln
vacuo. A solution of (3S)-[l-[(R,S)-2-amino-
1-hydroxyethyl]-3-methylbutyl]carbamic acid, 1,1-
dimethylethyl ester, monohydrochloride (2.62 g.,
9.264 mmole) in methylene chloride (20 ml.) is
added to the above residue. While stirring th~
above solution in an ice-bath, N-methylmorpholine
(2.0 g., 18.18 mmole) is added and the stirring is
continued for 2 hours in the ice-bath and then
overnight at room temperature. The mixture is
then evaporated to dryness. The residue is taken
up in ethyl acetate and washed neutral with sodium
bicarbonate and 10% potassium bisulfate. The
ethyl acetate extract after drying is
chromatographed over silica gel (250 g.) using the
solvent system ethyl acetate:hexane (1:1) to give
1.92 g. of N-[[[(3S)-3-[[(1,1-dimethylethoxy)-
carbonyl]amino]-2-hydroxy-5-methylhexyl]amino]-
carbonyl]-L-isoleucine, phenylmethyl ester.
b) N-[[[(3S)-3-[[~1,1-Dimethylethoxy)carbonyl]-
aminol-2-hydroxy-5-methylhexyllamino]carbonyll-L-
isoleucine
~ A solution of the L-isoleucine, phenylmethyl
; ester from part (a) (1.92 g., 3.89 mmole) in
methanol (50 ml.) is stirred under an atmosphere
of hydrogen for 2 hours in the presence of
palladium hydroxide on carbon catalyst (0.4 g.).
1297~
HA362a
-63-
The mixture is then filtered through hyflo and
concentrated to dryness to give 1.51 g. of
N-[[[~3S)-3-[[(1,1-dimethylethoxy)carbonyl]-
amino]-2-hydroxy-5-methylhexyl]amino]carbonyl]~
L-isoleucine.
c) N-[N-[(l,1-DimethylethoxY)carbonyl~-L-
Phenvlalanyl]-3'-[(phenYlmethoxy)carbonyl]-L-
histidine
A solution of N-[N-[(1,1-dimethylethoxy)
carbonyl]-L-phenylalanyl]-l'-[(phenylmethoxy)-
methyl]-L-histidine in a mixture of methanol:
acetic acid (8:2, 200 ml.) is stirred under an
atmosphere of hydrogen overnight in the presence
of palladium hydroxide on carbon catalyst (2.6 g.).
The mixture is filtered through hyflo and
concentrated to dryness. The residue on crystalli-
zation from hot acetonitrile (150 ml.) gives
6.01 g. of N-[N-[(1,1-dimethylethoxy)carbonyl]-L-
phenylalanyl]-L-histidine; m.p. (192) 197 - 198.
To a stirred (ice-bath) solution of N-[N-
[(1,1-dimethylethoxy)carbonyl]-L-phenylalanyl]-L-
histidine (3.68 g., 10 mmole) in dimethylformamide
(20 ml.) and diisopropylethylamine (1.74 ml.,
10 mmole) is added N-carbobenzyloxysuccinimide
(3.0 g., 12 mmole). (This latter reagent is added
in 4 portions at intervals of 15 minutes.)
After stirring for 90 minutes, the mixture is
evaporated in vacuo. The residue is taken up in
ethyl acetate and washed with 10% citric acid.
After evaporation of the ethyl acetate solution,
the residue is chromatographed over silica gel
using the solvent system chloroform:methanol:acetic
1297~31
HA362a
-64-
acid (90:3:3) to give 3.2 g. of N-[N-[(1,1-dimethyl-
ethoxy)carbonyl]-L-phenylalanyl]-3'-[(phenyl-
methoxy)carbonyl]-L-histidine.
d) 3'-[(Phenylmethoxv)carbonyl]-L-histidine,
methyl ester, monohYdrochloride
N-[(1,1-Dimethylethoxy)carbonyl]-L-
histidine, methyl ester (2.42 g., 9 mmole)
[prepared as set forth by Hanford et al.,
J. Org. Chem., Vol. 33, p. 4251 (1968)] is
dissolved in tetrahydrofuran (40 ml.) and
diisopropylethylamine (1.74 ml., 9.9 mmole)
is added. While stirring the above solution at
room temperature, benzyloxycarbonyl chloride
(1.42 ml., 9.9 mmole) is added. After a period of
90 minutes, the mixture is concentrated to
dryness. The residue is taken up in ethyl acetate
and washed with water. The ethyl acetate extract
after evaporation is crystallized from ethyl
acetate:hexane (1:1) to give 2.07 g. of
N-[(1,1-dimethylethoxy)carbonyl]-3'-[(phenyl-
methoxy)carbonyl]-L-histidine, methyl ester.
This N-[(1,1-dimethylethoxy)carbonyl]-
3'-[(phenylmethoxy)carbonyl]-L-histidine, methyl
ester (1.09 g., 2.7 mmole) is dissolved in a
solution of hydrochloric acid in acetic acid
(2N, 24 ml.). After keeping the mixture at room
~ temperature for 10 minutes, it is concentrated
; in vacuo. The residue is reevaporated from
benzene and acetonitrile several times to give
; 30 3'-[(phenylmethoxy)carbonyl]-L-histidine, methyl
ester, monohydrochloride.
129763~
HA362a
-65-
e) N-[N-[[[(3S)-3-[[(1,1-Dimethylethoxy)carbonyl]-
aminol-2-hYdroxY-5-methylhexyl1amino]carbonyl]-L-
isoleucYl]-3'-[(phenYlmethoxy)carbonyl]-L-histidine,
methvl ester
Dicyclohexylcarbodiimide (0.556 g.,
2.7 mmole) is added to a stirred (ice-bath)
solution of N-[[[(3S)-3-[[(1,1-dimethylethoxy)-
carbonyl]amino]-2-hydroxy-5-methylhexyl]amino]-
carbonyl]-L-isoleucine (l.09 g., 2.7 mmole),
1-hydroxybenzotriazole hydrate (0.413 g.,
2.7 mmole), 3'-[(phenylmethoxy)carbonyl]-L-
histidine, methyl ester, monohydrochloride
(2.7 mmole), and diisopropylethylamine (0.47 ml.,
2.7 mmole) in dimethylformamide (12 ml.). The
reaction mixture is stirred at 5 (cold room)
overnight. It is then concentrated to dryness.
The residue is triturated with ethyl acetate and
filtered to remove dicyclohexyl urea. The ethyl
acetate solution is washed with saturated sodium
bicarbonate and then evaporated. The residue is
chromatographed over silica gel (200 g.) using the
solvent system chloroform:methanol (95:5) to give
1.022 g. of N-[N-[[[(3S)-3-[[(1,1-dimethylethoxy)
carbonyl]amino]-2-hydroxy-5-methylhexyl]amino]-
carbonyl]-L-isoleucyl]-3'-[(phenylmethoxy)carbonyl]-
L-histidine, methyl ester.
f) N-[N-[[[(3S)-3-Amino-2-hydroxy-5-methYlhexyl]-
aminolcarbonyl]-L-isoleucvll-3'-[(phenvlmethoxY)-
carbonvll-L-histine, methvl ester, monohvdrochloride
The L-histidine, methyl ester product from
part (e) (0.62 g., 0.9 mmole) is dissolved in a
. , ,. ,.... , .. ~ . . - .
. ' ' ' ,
' ' : '' ' .'
~Z9763~
HA362a
-66-
solution of hydrochloric acid in dioxane (4N,
10.2 ml.). After keeping the mixture at room
temperature for 20 minutes, it is concentrated to
dryness in vacuo to give N-[N-[~[(3S)-3-amino-
2-hydroxy-5-methylhexyl]amino]carbonyl]-L-iso-
leucyl]-3'-[(phenylmethoxy)carbonyl]-L-histidine,
methyl ester, monohydrochloride.
g) N-[N-[[[(3S)-3-[[N-[N-~(l,l-Dimethylethoxv)-
carbonyll-L-~henylalanyl]-3'-[(Phenylmethoxy)-
carbonyl]-L-histidyl]amino]-2-hydroxY-5-methyl-
hexyllaminolcarbonvl]-L-isoleucyl]-3'-[(phenYl-
methoxy)carbonYl]-L-histidine, methvl ester
Dicyclohexylcarbodiimide (0.185 g.,
0.9 mmole) is added to a stirred (ice-bath)
solution of the L-histidine, methyl ester, mono-
hydrochloride product from part (f) (O.9 mmole),
1-hydroxybenzotriazole hydrate (0.138 g.,
0.9 mmnole), N-[N-[(1,1-dimethylethoxy)carbonyl]-
L-phenylalanyl]-3'-[(phenylmethoxy)carbonyl]-L-
histidine (0.483 g., 0.9 mmole), and diisopropyl-
ethylamine (0.16 ml., 0.94 mmole) in dimethyl-
formamide (3 ml.). The reaction mixture is stirred
at 5 (cold room) overnight. It is then concen-
trated to dryness, triturated with ethyl acetate
2S and the separated dicyclohexyl urea is filtered off.
The ethyl acetate solution is washed with saturated
sodium bicarbonate and then evaporated. The
residue is chromatographed over silica gel (80 g.)
using the solvent system ethyl acetate:methanol
(95:5) to give 0.266 g. of N-[N-[[[(3S~-3-[[N-[N-
[(1,1-dimethylethoxy)carbonyl]-L-phenylalanyl]-3'-
.
~Z9763i
HA362a
-67-
[(phenylmethoxy)carbonyl]-L-histidyl]amino]-2-
hydroxy-5-methylhexyl]amino]carbonyl]-L-iso-
leucyl]-3'-[(phenylmethoxy)carbonyl~-L-histidine,
methyl ester.
h) N-[N-~[[(3S)-3-[[N-[N-[(1,1-DimethYlethoxy)-
carbonyll-L-DhenvlalanYl~-L-histidyl]amino]-2
hvdroxy-5-methylhexYllamino]carbonyl]-L-iso-
leucvll-L-histidine, methYl ester, dihydrochloride
The L-histidine, methyl ester product from
part (g) (250 mg., 0.23 mmole) is dissolved in
methanol (10 ml.). After adding aqueous hydro-
chloric acid (lN, 0.41 ml.) and palladium
hydroxide on carbon catalyst (50 mg.), the
solution is stirred under an atmosphere of
hydrogen for 4 hours. It is then filtered through
hyflo and evaporated to dryness to give 0.187 g. of
N-[N-[[[(3S)-3-[[N-[N-[(1,1-dimethylethoxy)-
carbonyl]-L-phenylalanyl]-L-histidyl]amino]-2-
hydroxy-5-methylhexyl]amino]carbonyl]-L-isoleucyl]-
Z0 L-histidine, methyl ester, dihydrochloride; m.p.
75 - 170; [~]2D0 = -7.8 (c = 1.7, methanol).
TLC (silica gel; chloroform:methanol:acetic acid,
6:4:2) Rf = 0.7.
Anal. cal'd. for C41H62OgNlo 2
C, 50.28; H, 7.36; N, 14.30; Cl, 7.24
Found: C, 50.30; H, 7.43; N, 14.00; Cl, 7.25.
" . ~
~,,
" 1297~31
HA362a
-68-
Exam~les 9 - 29
Following the procedure of Examples 1 to 8,
additional compounds within the scope of this
invention can be prepared having the formula
R4 R3 R2 o R1
I 11 I j I I i
X-NH-CH- C -NH - CH - CH ~ CH2--N -C-NH - CH - C-A
I
OH
wherein the substituents are as defined below.
~:;
... .
Z~7~3~
-69- HA362a
_~ Y
o=u O=~ ~z
N _N
O
_
-- I -- _, _
N
? I -. _
Z
t, ~, ~ N~
; I Y Y
O = U = ~J
U ~ -- I N
O=U = U ~--
X I _ _ ~
O ,_1 '
~'
', ~',,i
',
1297~31
..,
-7 O- HA362 a
"~ Z2
s ~ U ~Z-= $1
a,--a 'a-a aN
C I Z Z
~ I -~ ;~ ~
~N ¦ _ _ _
N N
~ _
~ I , _ _
~5-= Z~Z ~)
,' N N N
~c I ,a ,a ;.
O_~ ~
~~-, ~aN ~ o=$_~
( Z Z
~ o= U o=~ o
=~
~ o - ol ,o
., . , ~, ~,
~!' X I -- 2
'r
'l ~ ,D
',
`l ~
~1~
:r
1297~3i H~362a
~~
t~Z~ o=u ~
o = ~, o = t~ ~ o = ~. ~ _
z z
^r U ~
J
_~ O ~_ ~ U
X
.~ .
.,,j,.~,", .. . . .
~29763~
-72- HA362a
o=u ~ o=u ~ o=y~
c I z z z
,
~ ~. ^
,
z_~ ' z \\ I z
_ = _
8-- N _ ~ -- O ~ U
O = U Z U"
, ~ X ~\ ON ~
~ ,
.,1 .-- : '' '"'' ' '
1297~i31
~ .~
_73_ HA362a
0='~ ~ 0=~ ~ 0=o ~
V --;~
I Z Z Z
_
_
.'. `, `,
_
_
_
~ ~z-= z~z
q
o = ~
~ U--o = ~ U--y
: ' o~ " ~ N
~ . X' I
N N ~r
,1,
" i
'.'
~ * ~",.. ..... .. .
'
`~ 1Z97~31
74 HA362a
_~z ~ o ~ o ~
N
C ! Z _ _
^_^_ /~
_
Z 1~=-
'J
;~
I ~
O =t~ ~
O ~ ~ Z ~,~
U y U ~
W ¦ ~ N
.,
. ~ .
~, .
lZ97~31
HA362a
-75-
ExamPle 30
looo tablets each containing the following
ingrdients
N-[N-[[[(3s)-3-[[N-[N-[(~
5 Dimethylethoxy)carbonyl]-L-phenyl-
alanyl]-L-histidyl]amino]-2-hydroxy-
5-methylhexyl]amino]carbonyl]-L-
isoleucyl]-L-histidine, methyl
ester, dihydrochloride 250 mg.
10 Cornstarch 100 mg.
Gelatin 20 mg.
Avicel(microcrystalline cellulose) 50 mg.
Magnesium stearate 5 mg.
425 mg.
are preapred from sufficient bulk quantities by
mixing the N-[N-[[[(3S)-3-[[N-[N-[(1,1-dimethyl-
ethoxy)carbonyl]-L-phenylalanyl]-L-histidyl]amino]-
2-hydroxy-5-methylhexyl]amino]carbonyl]-L-isoleucyl]-
L-histidine, methyl ester, dihydrochloride and corn-
starch with an aqueous solution of the gelatin. Themixture is dried and ground to a fine powder. The
Avicel and then the magnesium stearate are admixed
with granulation. This mixture is then compressed
in a tablet press to form 1000 tablets each
containing 250 mg. of active ingredient.
In a simi}ar manner, tablets containing 250 mg.
of the product of any of Examples 1 to 7 and 9 to
29 can be prepared.
A similar procedure can be employed to form
tablets containing 500 mg. of active ingredient.
~'
;
~''
~.
A * Trade Mark
~'
~, ......
12~7~31
HA362a
-76-
Example 31
An injectable solution is prepared as
follows:
N-[[[(3S)-3-[[N-[N-[(l,l-Dimethyl-
5 ethoxy)carbonyl]-L-phenylalanyl]-
L-histidyl]amino]-2-hydroxy-5-
methylhexyl](2-methylpropyl)-
amino]carbonyl]-L-valine, methyl
ester,monohydrochloride (isomer A) lO00 g.
10 Methyl paraben 5 g.
Propyl paraben l g.
Sodium chloride 5 g.
The active substance, preservatives, and
sodium chloride are dissolved in 3 liters of water
for injection and then the volume is brought up to
5 liters. The solution is filtered through a
sterile filter and aseptically filled into pre-
sterilized vials which are closed with presteri-
lized rubber closures. Each vial contains 5 ml.
of solution in a concentration of 200 mg. of
active ingredient per ml. of solution for
injection.
In a similar manner, an injectable solution
containing 200 mg. of active ingredient per ml. of
solution can be prepared for the product of any of
Examples 2 to 29.
.. ... .
~zg7~3i
HA362a
-77-
Example 32
1000 Tablets each containing the following
ingredients:
N-[N-[[[(3s)-3-[[N-[N-~
5 Dimethylethoxy)carbonyl]-L-
phenylalanyl]-L-histidyl]amino]-
2-hydroxy-5-methylhexyl]amino]-
carbonyl]-L-isoleucyl]-L-histidine,
methyl ester, dihydrochloride 500 mg.
10 Avicel 300 mg.
Hydrochlorothiazide 14.5 mg.
Lactose 113 mg.
Cornstarch 15.5 mg.
Stearic acid 7 mg.
950 mg.
are prepared from sufficient bulk ~uantities by
slugging the N-[N-[[[(3S)-3-[[N-[N-[(l,l-dimethyl-
ethoxy)carbonyl]-L-phenylalanyl]-L-histidyl]amino]-
2-hydroxy-5-methylhexyl]amino]carbonyl]-L-isoleucyl]-
L-histidine, methyl ester, dihydrochloride, Avicel,
and a poriton of the stearic acid. The slugs are
ground and passed through a #2 screen, then mixed
with the hydrochlorothiazide, lactose, cornstarch,
and remainder of the strearic acid. The mixture
i~ compressed into 950 mg. capsule shaped tablets
in a tablet press. The tablets are scored for
dividing in half.
In a similar manner, tablets can be prepared
containing 500 mg. of the product of any of
30 Examples 1 to 7 and 9 to 29.
:,
. . ,