Note: Descriptions are shown in the official language in which they were submitted.
5012P/5011~ g S~ ~
- 1 - 17323IA
TITLE OF THE INVENTION
TETRAHYDRQCAR~AZOLE l-ALKANOIC ACIDS
BACKGROUND OF THE INVENTION
This invention relates to prostaglandin
antagonists useful in treating a variety of
conditions, such as allergic asthma where excessive
contractile activity of prostaglandins and
prostaglandin biosynthetic intermediates occur.
These compounds antagonize the actions of
; contractile prostaglandins, such as PGF2a,
: PGG2, PGH2, PGD2 and TXA2. The use of agents : :
: which act as prostaglandin antagonists offers new
: : approaches to therapy in a number of disease states.
; ~ 15 For example, certain prostaglandins, such as
PGF2a, PGD2, PGG2, and PGH2, are potent
bronchospastic agents. Indeed human asthmatics have
been shown to be especially sensitive to the
bronchial constricting action of PGF2~.
: 20
: .
;
~ ~ 25 : :
:: :
lZ~95~
5012P/5011A - 2 - 17323IA
The compounds of the present invention are
also antithrombotic agents. Thus, they are useful in
the treatment and/or prevention of thromboembolic
diseases such as arterial thrombosis and those
involving platelet deposition, e.g. prothesis.
In addition to the involvement of
contractile prostaglandins in asthma, prostaglandins
ar~ known to play a role in other allergic
conditions, as well as, diarrhea, hypertension,
~ 10 angina, platelet aggregation, cerebral spasm,
; cerebral ischemia, arrythmia, circulatory shock,
sudden death, atherosclerosis, myocardial ischemia,
premature labor, spontaneous abortion, dysmenorrhea,
glomerular nephritis, and systemic lupus erythem-
15 atosis. Consequently, the compounds of this
invention will alleviate the above mentioned diseases.
In addition to the prostaglandin antagonist
actions, the compounds of this invention are
inhibitors of the biosynthesis of 5-lipoxygenase
20 metabolites of arachidonic acid, such as 5-HPETE,
5-HETE and the leukotrienes. Leukotrienes B4,
C4, D4 and E4 are known to contribute to
various disease conditions such as asthma, psoriasis,
pain, ulcers and systemic anaphylaxis. Thus
25 inhibition of the synthesis of such compounds will
alleviate these and other leukotriene-related disease
states.
The compounds of the present invention may
be used to treat or prevent mammalian (especially,
; ~ 30 human) disease states such as erosive gastritis;
erosive esophagitis; ethanol-induced hemorrhagic
~ erosions; hepatic ischemia; noxious agent induced
'~ ~
,. .
~Z9~S77
5012P/5011A - 3 - 17323IA
damage or necrosis of hepatic, pancreatic, renal, or
myocardial tissue; liver parenchymal clamage caused by
hepatotoxic agents such as CC14 and D-galactosamine;
ischemic renal failure; disease-induced hepatic
damage; bile salt induced pancreatic or gastric
damage; trauma- or stress-induced cell damage; and
glycerol-induced renal failure.
Certain 9-benzyl-1,2,3,4-tetrahydro-
carbazole acetic acids or esters thereof are shown as
10 chemical intermediates in the preparation of
carbazoles that are known in the art as anti-
inflammatory, analagesic and anti-rheumatic agents
(see U.S. Patent 3,896,145 and British Patent
1,385,620). Certain 9-benzyl-1,2,3,4-tetrahydro-
15 carbazole carboxylic acids are known in the art asanti-inflammatory, analgesic and anti-rheumatic
agents (see U.S. Patents 3,868,387; 4,009,181;
3,905,998 and 3,758,496), and 9-benzyl-carbazole
carboxylic acids (U.S. Patents 3,956,295 and
20 4,057,640~ and 9-benzylcarbazole acetic acids and
esters thereof (U.S. Patent 3,896,145 and British
Patent 1,385,620) are known as anti-inflammatory,
analgesic and anti-rheumatic agents. None of these
compounds, however, are shown to be prostaglandin, or
25 thromboxane antagonists or inhibitors of leukotrine
biosynthesis.
~ .
.
~9~S77
5012P/5011A - 4 - 17323IA
DESCRIPTION OF THE INVENTION
The present invention relates to novel
compounds of Formula I:
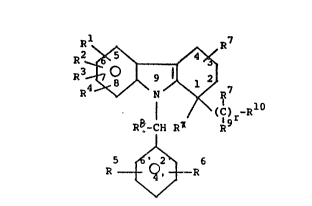
~ ~ R
(C) -R10
k8-CH R R9r
' 2 6
R t~i R
wherein:
Rl R2 R3 R4 R5 and R6 are
each independently selected from:
(1) hydrogen;
~ (2) alkyl having 1 to 6 carbon atoms;
: (3) alkenyl having 2 to 6 carbon atoms;
( CH2 ) nM
wherein n is O to 3 and M is
: 25 a) oRl3;
b) halogen;
c) CF3;
:
d) sR13;
~e) phenyl or substituted phenyl
~: wherein substituted phenyl is
: : ~ as de~ined below in the
~ : ~ definition of k 3;
, ~ ~
~' ' ,
9~;i7~
5012P/5011A - 5 - 17323IA
f) CooR14;
g) - C-RlS;
O
h) -NH-C-R16 wherein R16 is
Cl to C6 alkyl, benzyl or
phenyl;
i) NR14R14;
i) -N~SO2Rl wherein R
is Cl to C6 alkyl,
4-methylphenyl, phenyl, or
CF3;
o
k) -C-CH2OH;
1) -SOR 3;
m) coNR14R14;
n) -S2NR R
; 20 ) -So2Rl3;
p) N02;
1i 15
q) -O-C-R
o
S) -O- I-OR16;
t) N3;
u) CN;
R7 is H or alkyl of 1 to 6 carbons;
R8 is H or alkyl of 1 to 6 carbon atoms;
.
:
~12~;i77
5012P/5011A - 6 - 17323IA
each R is independently H, OH, Cl to
C4-O-alkyl or alkyl of 1 to 4 carbons;
R ~ is COOH; CH2OH; CHO;
NHSO2Rll wherein Rll is O~, alkyl or alkoxy of
1 to 6 carbons, perhaloalkyl of 1 to 6 carbons, phenyl
or phenyl substituted by alkyl or alkoxy groups of 1
to 3 carbons, halogen, hydroxy, COOH, CN, formyl or
acyl to 1 to 6 carbons; CONHSO2R11; hydroxy-
methylketone; CN; or CON(R932;
r is 1 to 6;
each R13 independently is H; Cl to C6
alkyl; benzyl; phenyl or substituted phenyl wherein
the substituents are Cl to C3 alkyl, halogen, CN,
CF3, CooR14, CH2CooR14, Cl to C3 alkoxy,
15 or Cl to C4 perfluoroalkyl;
each X is independently H, phenyl,
benzyl or Cl to C6 alkyl; and,
each R15 independently is H,
(CH2)mCooR14 wherein m is 0 to 4, Cl to C6
20 alkyl, CF3, phenyl, or substituted phenyl wherein
substituted phenyl is as defined above in the
definition of R 3;
or a pharmaceutically acceptable salt thereof.
As used herein, the terms "each
25 independently" or the equivalents thereof are employed
to describe a number of possible position isomers
and~or structural variations. For example, as
; described above, the following unit is attached to
position 1 of the tetrahydrocarbazole ring:
R7
(C)r R
R9
1299577
5012P/5011A - 7 - 17323IA
The letter r represents possible alkane
chains of from 1 to 6 carbon atoms, each having the
7 9
R and R substituent groups. On each carbon
atom of the alkane chain, the R7 and/or R9
S substituent may be different. The above description
therefore contemplates structures such as the
following for the segment -(CR R9)r~:
ICH3 IH H
(- C - C - C) , (- CH2 -)
OH H H
H H H H H H H H
~--C -- C -- C--) ,(--C -- C C -- C -- C--)
OH CH3 OH H OH CH3 H H
H H H
(-C - C - C-) , and the like.
H CH3 H
If an R9 is OH and R is CO2H, such
compounds may form a lactone, and such lactones are
to be regarded as part of the present invention.
The alkyl groups referred to above may be
25 straight chain or branched or may include cycloalkyl
groups. As used herein, the term "lower" as applied
to alkyl, acyl, alkoxy and the like, unless stated
otherwise refers to groups having 1 to 6 carbon
atoms. Halogen or halo means fluoro, chloro, bromo
30 and/or iodo.
Pharmaceutically acceptable salts of the
compounds described herein are included within the
~:
~Z9~57~7
5012P~5011A - 8 - 17323IA
scope of the present invention. Such salts may be
prepared from pharmaceutically acceptable non-toxic
bases including inorganic bases and organic bases.
Salts derived from inorganic bases include sodium,
potassium, lithium, ammonium, calcium, magnesium,
ferrous, zinc, copper, manganous, aluminum, ferric,
manganic salts and the like. Particularly preferred
are the potassium, sodium calcium and magnesium salts.
Salts derived from pharmaceutically acceptable organic
10 non-toxic bases include salts of primary, secondary,
and tertiary amines, substituted amines including
naturally occurring substituted amines, cyclic amines
and ~asic ion exchange resins, such as isopropylamine,
tri-methylarnine, diethanolamine, diethylamine,
15 triethylamine, tripropylamine, ethanolamine,
2-dimethylaminoethanol, 2-diethylamino-ethanol,
tomethamine, lysine, arginine, histidine, caffeine,
procaine, hydrabamine, choline, imidazole, betaine,
ethylenediamine, glucosamine, methylglucamine,
20 theobromine, purines piperazine, N,N-dibenzyl-
ethylenediamine, piperidine, N-ethyl-piperidine,
morpholine, N-ethylmorpholine, polyamine resins and
the like.
Preferred compounds of the present invention
25 comprise the compounds of formula I
wherein:
Rl R2 R3 R4 R5 and R6 are
each independently selected from:
(1) hydrogen;
(2) alkyl having 1 to 6 carbon atoms;
(3) alkenyl having 2 to 6 carbon atoms;
(4) -(CH2)nM
~a299S77
5012P/5011A - 9 - 17323IA
wherein n i5 0 or 1 and M is as defined
previously for Formula I;
R10 is COOH; CH2OH; CHO;
CONHSO2Rll wherein Rll is OH, alkyl or alkoxy
of 1 to 6 carbons, perhaloalkyl of 1 to 6 carbons,
phenyl or phenyl substituted by alkyl Ol- alkoxy
groups of 1 to 3 carbons, halogen, hydroxy, COOH, CN,
formyl or acyl to 1 to 6 carbons; hydroxymethylketone;
CN; or CON(R9)2;
r is 1 to 6; and the remaining substituents
are as defined previously for Formula I.
More preferred compounds of the present
invention comprise the compounds of Formula I.
wherein:
Rl R2 R3 R4, R5 and R6 are
each independently selected from:
(1) hydrogen;
(2) alkyl having 1 to 6 carbon atoms;
(3) alkenyl having 2 to 6 carbon atoms;
t4) M wherein M is as defined initially for
Formula I;
R10 is COOH; CH2OH; CHO;
hydroxymethylketone;
r is 1 or 2; and the remaining substituents
25 are as defined initially for Formula 1.
Most preferred compounds of the present
invention comprise the compounds of Formula I.
wherein:
Rl, R2, R3, R4, R5 and R are
30 each independently selected from:
~1) hydrogen;
(2) alkyl having 1 to 6 carbon atoms;
.
~z~1S~7
5012P/SOllA - 10 - 17323IA
~3) M wherein M is
a~ oR13;
b) ha logen;
c) CF~;
d) SR 3;
e) CooR14;
o
f) -C-R15;
g) -SoR13;
h) -CONR R
i) _so2NRl4Rl4;
o
k) -o-C-R15;
1) CN;
m) N3;
each R9 is independently H, or alkyl of 1
to 4 carbons;
R is COOH;
r is 1 and the remaining substituents are as
defined initially for Formula I.
In the above most preferred embodiment,
those compounds are particularly preferred wherein at
25 least one of R to R4 is not hydrogen; one of
R5 or R6 is not hydrogen; R7 is hydrogen; R9
is hydrogen, and the remaining substituents are as
defined in the most preferred embodiment.
A further embodiment of the present
30 invention are the following novel compounds (Table 1).
Among the resolved isomers in Table 1, the (-)
isomers, compounds 3,37,39 and 41, are preferred.
:~,
~zs~
501 2P/SO 1 lA - 11 - 1 73231A
Novel Tetrahydrocarbazole
A1 kanoi c Aci ds
R 5 4 R7
1~ R2 ~
R -CH R --C-CO2H
6' \ 2' \R9
R5~R6
4'
Compou~d Rl R2 R5 R6 R, R R R8
-
2Q 1 ( Ex . l ~ 6-F H 4 ' -Cl HH, H H H
2 (Ex. 4) 6-OMe H 4'-Cl H H, H H H
3 (Ex. 7) 6-F H 4'-Cl H H, H H H
(-) isomer
4 ~(Ex. 8) 6-F H 4'-C1 H H, H H H
(+) isomer
30 5 I Ex . 9) . 6-F H H H H, H H H
6 (Ex. 10) 6-F H 4'-OMe H H, H H H
.
~Z9~7
5012P/SOllA - 12 - 17323IA
Compound Rl R2 R5 R6 R , R R R8
7 (Ex. 11) 6-F H 3'-Cl 4'-Cl H, H H H
S 8 (Ex. 12) 6-F H H H H, H H Me
9 (Ex 13) H H 4'-Cl H H, H H H
1û (Ex. 14) 6-Cl H 4'-Cl H H, H H H
11 (Ex. 15) 8-Me H 4'-Cl H H, H H H
12 (Ex. 16) 6-Br H 4'-C1 H H, H H H
15 13 (Ex. 17) 6-Me H 4'-Cl H H, H H H
14 (Ex. l9) 8-F H 4'-Cl H H, H H H
:
15 (Ex. 20) 6-F H 4'-Cl H H, H 3-t-Bu H
_
16 (Ex. 21) 5-F H 4'-Cl H H, H H H
17 (Ex. 21) 7-F H 4'-Cl H H, H H H
25 18 (Ex. 22) 5-Cl 7-Cl 4'-Cl H H, H H H
19 (Ex. 23) 6-Cl 8-Cl 4'-Cl H H, H H H
20 (Ex. 18) 6-F H 4'-Cl H Me, H H H
3û
21 6-F H 4'-Cl H Me, Me H H
22 6-F H 4'-Cl H H, H l-Me H
~2~S~7
5012P/SOllA - 13 - 17323IA
Compound Rl R2 R5 R6 R ,R R R8
23 8-Br H 4'-Cl H H,H H H
S 24 (Ex. 24) 6-CH(Me)2 H 4'-C1 H H,H H H
25 (Ex. 25) 6-C(Me)3 H 4'-C1 H H,H H H
26 (Ex. 26) 6-CF3 H 4'-C1 H H,H H H
27 (Ex. 27) 6-SMe H 4'-C1 H H,H H H
28 (Ex. 28) 6-S(O)Me H 4'-C1 H H,H H H
29 (Ex. 29) 6-5(0)2Me H 4'-Cl H H,H H H
30 (Ex. 30) 8-CH(Me)2 H 4'-C1 H H,H H H
31 (Ex. 31) 8-SMe H 4'-C1 H H,H H H
32 (Ex. 32) 8-S(O)Me H 4'-C1 H H,H H H
33 (Ex. 33) 6-F H 4'-C1 H H,H 3-Me H
__
34 (Ex. 34) 6-F 8~F 4'-Cl H H,H H H
35 (Ex. 35) 6-Me 8-Me 4'-Cl H H,H H H
36 (Ex. 36) 6-OMe 8-Me 4'-Cl H H,H H H
37 (Ex. 37) 6-F(-)Isomer 8-F 4'-C1 H H,H H H
~29~57q
501ZP/SOllA - 14 - 17323IA
Compound Rl R R5 R6 R ,R R R~3
38 (Ex. 38) 6-F(+)Isomer 8-F 4'-C1 H H,H H H
S 39 (Ex. 39) 8-Me(-)Isomer H 4'-Cl H H,H H H
40 (Ex. 40) 8-Me(+)Isomer H 4'-C1 H H,H H H
.
41 (Ex. 41) 8-F(-)Isomer H 4'-Cl H H,H H H
42 (Ex. 4Z) 8-F(+)Isomer H 4'-C1 H H,H H H
__
43 6-F 8-F 3'-C1 4'-C1 H,H H H
15 44 6-F 8-F 2'-C1 4'-Cl H,H H H
45 6-F 8-F 4'-OMe H H,H H H
-
46 6-F 8-F 4'-OH H H,H H H
47 6-F 8-F 4'-SMe H H,H H H
48 6-F H 4'-S(O)Me H H,H H H
49 6-F 8-F 4'-NHCOMe H H,H H H
-
6-F H 4'-S(0)2Me H H,H H H
51 6-F H 4'-F H H,H H H
52 6-F H 4'-Br H H,H H H
_
~2~5~
5012P/5011A - 15 - 17323IA
Compound Rl R2 R R6 R ,R R R8
53 6-F 8-Me 4'-Cl H H,H H H
-
S 54 6-F H 4'-CO2H H H,H H H
6-F H 4'-CO2Me H H,H H H
56 6-F 8~F 4'-n-C3H7 H H,H H H
57 6-F 8-F 3'-I 4'-OH H,H H H
58 6-F 8-F 4'-I H H,H H H
59 6-N3 H ` 4'-Cl H H,H H H
_
6û 6-F H 4'~N3 H H,H H H
ZS
~%~9~;77
5012P/5011A - 16 - 17323IA
The following reaction schemes illustrate
the preparation of the compounds of the present
invention:
5 Scheme I Preparation of Formula I Compounds
R2 ~ ~ R lower
R3 ~ 0 ~ + 0 ~ J alkanoI
10R4 ~ / N-NH2 ~ reflux
¦ ~ HC1
~R ( C ) r-C02R -
15R5 R6 III
I I
Z OR~ R7 ~1
¦ R R hYdrolysis ~
~)\R8 R9 /~\ (C) r-C02H
2 5R ~ ~6 R5~0~;~ R6
IIIa I ;~
The reaction can be conveniently carried out
in an alcohol solvent such as t-butanol, i-butanol,
and the like. The hydrolysis of the ester inter-
mediates IIIa is conveniently carried out by using
129~57'7
5012P/5011A - 17 - 17323IA
NaOH or KOH ln aqueous ethanol or methanol followed
by acidification to obtain compounds of Formula I.
The following ketones ~1,2,4) of structure
III are known in the art, and ketone 3 is readily
prepared by procedures analogous to those for the
known ketones.
TABLE 2
ketones of Formula III
No.Structure Reference
Ethyl 2-cyclohexanone
1. ~ acetate; commercially
15 // ~ J available (Aldrich)
0 1'
C2Et
Methyl 2-cyclohexanone
2. ~ propionate; J.A.C.S. 85
~ ) 207 (1963)
O y G. Stork, A. Brizzolara,
~ H. Landesman,
~ J. Scmuszkovicz and
CO2ME R. Terrell
3. \ ~ Methyl 4-t-butyl-2-
~ cyclohexanone acetate
~ \
y
~CO2
3t'~31~7~7
5012P/5011A - 18 - 17323IA
TABLE 2 (Cont'd)
4. Methyl 2-(2-cyclohexanone)
~ propionate
~ ~ J.A.C.S. 85 207 ~1963)
G. Stork, A. Brizzolara,
~ H. Landesman,
/ CO2ME J. Scmuszkovicz and
R. Terrell
5. Ethyl 4-methyl-2-cyclo-
~ hexanone acetate
O y
C2Et
The sequence described above is an applic-
ation of the Fischer Indole Synthesis. Numerous-
indole syntheses are described in reviews, such as,for example "Heterocyclic Compounds" Volume 25, Parts
I, II, rII, W. J. Houlihan (Ed.~, Interscience, J.
Wiley & Sons, N. Y., 1979. Appropriate manipulations
of functional groups using sequences described in such
reviews will lead to the compounds of the present
invention.
~2~gs7~
5012P/5011A - 19 - 17323IA
Scheme II Preparation of Hydrazine Derivatives (II~
R 5HR8
R3 ~ ~ Et3 N
1, 1 ~ ~ toluene
R4 ~ \g-NH2 + R5 ~ R6 reflux
10HCl
(IV) (V~
Rl
R2 1 Z is a leaving group such as
15 R3 ~ ~ Cl, Br, I, mesylate or tosylate
R4 ~ \N-~H2
/\~\R8
R5 ~ R6
II
With regard to Scheme II, the preparation of
hydrazine starting materials is illustrated by the
preparation of 1-(4-chlorobenzyl)-1-t4-methoxyphenyl~-
hydrazine. A mixture of 10 g of p-methoxyphenyl-
hydrazine hydrochlorid~, 75 ml of toluene and 11.5 ml
of triethylamine was heated at reflux for 60 minutes.
Then, 7.1 g of p chlorobenzyl chloride was added.
After stirring 16 hours at reflux~ triethylamine
hydrochloride was filtered off and washed with ethyl
9S"~q
5012P/5011A - 20 - 17323IA
ether. The filtrate and washing were concentrated in
vacuo and chromatographed on a silica gel column
(hexane-ethyl acetate, 9:1) to give 6.64 g of 1-(4-
chlorobenzyl)-1-(4-methoxyphenyl)hydrazine. Other
~-- 5 hydrazines, similarly prepared, are also shown in
Table 3, below.
TABLE 3
H~drazines
4 ~ ~
X~ )
Y~.
Compound
No. X Y R Compound Name
1. 4-F 4-Cl H 1-(4-chlorobenzyl)-1-t4-
fluorophenyl) hydrazine
hydrochloride
: 30 2. 3,5-C12 4-Cl H 1-(4-chlorobenzyl)-1-(3,5-
dichlorophenyl)hydrazine
hydrochloride
~9~577
5012Pt5011A - 21 - 17323IA
_BLE 3 (Cont'd)
3. 4-OMe 4-Cl ~ 1-(4-chlorobenzyl)-1-(4-
metho~yphenyl) hydrazine
-- 5 hydrochloride
4. 2-Me 4-Cl H 1-(4-chlorobenzyl)-1-t2-
~ethylphenyl) hydrazine
hydrochloride
5. 4-Me 4-Cl H 1-(4-chlorobenzylj-1-(4-
methylphenyl) hydrazine
hydrochloride
6. 4-Cl 4-Cl H 1-~4-chlorobenzyl)-1-(4-
chlorophenyl) hydrazine
hydrochloride
: 7. H 4-Cl H 1-(4-chlorobenzyl)-1-(phenyl)
- hydrazine hydrochloride
8. 4-Br 4-Cl H 1-(4-chlorQbenz~fl)-1-(4-
:~ bromophenyl) hydrazine
hydrochlQride
9. 3-F 4-Cl H 1-(4-chlorobenzyl)-l-(3-
fluorophenyl) hydrazine
hydrochloride
: ~ 30 10. 2,4-C12:4-Cl H 1-(4-chlorQbenzyl)-1-(2,4-
dichlorophenyl)hydrazine
: hydrochloride
~ ~ '
:
~;~9~57~7
5012P/5011A - 22 - 17323IA
TABLE 3 (Cont'd)
11. 4-F H H l-(benzyl)-1-(4-f}uorophenyl)
hydrazine hydrochloride
12. 4-F 4-OMe H 1-(4-methoxybenzyl)-1-(4-
fluorophenyl~ hydrazine
hydrochloride
13. 4-F 3,4-Cl2 H 1-(3,4-dichlorobenzyl)-l-
(4-fluoro-phenyl) hydrazine
hydrochloride.
14. 4-F H CH3 1~ phenyl)ethyl]-1-(4-
fluorophenyl) hydrazine
hydrochloride
15. 2-F 4-Cl ~ 4-chlorobenzyl)-1-(2-
fluorophenyl) hydrazine
hydrochloride.
16. l-[2-(4-chlorophenyl)ethyl]-l-(4-fluorophenyl) hydrazine
hydrochloride.
17. 1-(2-propyl)-1-(4-fluorophenyl)hydrazine hydrochloride.
18. 4-CH(Me~2 4-Cl H 1-(4-chlorobenzyl)-1-~4-iso-
propylphenyl)hydrazine
hydrochloride
19. 4-C(Me)3 4-Cl H 1-(4-chlorobenzyl)-1-~4-tert-
butylphenyl)hydrazine)hydro-
chloride
;
lZ9~;77
5012P/5011A - 23 - 17323IA
TABLE 3 (Cont'd)
20. 4-CF3 4-Cl H 1-(4-chlorobenzyl)-1-~4-
trifluoromethylphenyl)-
~ydrazine hydrochloride
21. 4-SMe 4-Cl H 1-~4-chlorobenzyl)-1-(4-
methylthiophenyl)hydrazine
hydrochloride
22. 2-CH~Me)2 4-Cl ~ 1-(4-chlorobenzyl)-1-(2-iso-
propylphenyl)hydrazine
hydrochloride
.
9~77
5012P/501 lA - 24 - 17323IA
~kemQ~I Alterllative Prepartion of Formula I Comvounds
lower alkanol R--~jR7
III ~ IV reflux R3--~ I R~JR 16
H ~ C ) -CO R
R
lEster i ntermedi ate
Hydrol ys; s
~A 1) V ~ base ~R
r 2 R9
RS--~R6
Aci d i ntermedi ate
Scheme III illustrates an alternative
synthesis of the compounds of Formula I. In this
Scheme a Fischsr indole synthesis is carried out
using a phenylhydrazine IV and the ketone III,
followed by hydrolysis. The acid intermediate is
30 then N~benzylated with the reagent V, preferably
using a strong base such as potassium t-butoxide to
effect the reaction. Acidification of the reaction
mixture then yields the free acid of I.
~ 9577
5012P/SOllA - 25 - 17323IA
~beme~ ere~ara~ion of Sulfo)~ides and Sulfones ~f f~a ~ Cnmpounds
"` R = TC below.
R _ R is replaced by SR , 5(0)R or
~\N/R~R7 R -R~sR13 or s~o)Rl3
(C)~-
~ R9
10 R ~ R6
SR13
TC - C02R
VI
1.5 eg. of
mCPBA
~ S(0)2-R
S(O)R 2 eg. mCPBA ~ T~ C02R
TC - C02R
VIIa VIIIa
¦Hyd rol ys i s 1 Hyd rol ys i s
S(O)R S(0)2R
TC - C02H ~ TC - C02H
VII VIII
9577
5012P/5011A - 26 - 17323IA
In Scheme IY is illustrated a method of
preparing derivatives of Formula I in which one of
`~ the substituents among Rl-R4 is a sulfoxide or a
sulfone. It will be obvious to one skilled in the
5 art that a sulfoxide or sulfone derivative of R5 or
R6 could be prepared in the same way.
Ester VI (a representative of III a, Scheme
I) is prepared according to Scheme I or ~cheme III
followed by esterification of acid I. Treatment of
VI with a limited amount of an oxidizing agent such
as m-chloro-perbenzoic acid yields the sulfoxide
ester VIIa, which upon hydrolysis yields sulfoxide
acid VII. Further treatment of VIIa with the
- oxidizing agent, or treatment of VI with an excess
(>2 eq.) of the oxidizing agent yields the sulfone
ester VIIIa, hydrolysis of which yields the sulfone
acid VIII. Both VII and VIII are representatives of
Formula I compounds.
20 ~ch~me V Preparation of Furth~r sDmDallnds Df Formula I
~; = TIIC ~elow
19
/~\R8
R ~ R6
~Z9~57~
5012P/SOl lA - 27 - 17323IA
LiAlH4 PCC
~ ~1C - C~2R ~ THC - CH20H ~ THC - CHO
(IIIa, Sch~me I) IX X
Hydrol ysi s
' R 52NH2
SOCl 2 Et3N
THC - C02H > THC - COCl - ~ THC - CONHS02R
XI XIV
CH2~/ ~NH
THC - COCHN2 THC - CONR R
XII XV
15¦ ~12S04 ~ ~, R =R =H
lH2o
THC - COCH20H THC - CH2NH2 THC - CN
XIII XVI XVIII
J,R S02Cl lNaN3
N-NH
TH~ ~ CH2NHS2R THC~
N=N
XVII XIX
Other c~mpounds of Formula I can be prepared as
indicated in Scheme V. Thus the ester derivative
IIIa can be reduced to the alcohol IX by lithium
30 aluminum hydride or other suitabl~ reducing agents.
Alcohol IX can then be oxidized to aldehyde X by
pyridinium chlorochromate or other suitable oxidizing
agents. Carboxylic acids of Formula I can be
converted to the acid chloride XI (the acid bromide
~Z9~S77
5012P/5011A - 28 - 17323IA
or a mi~ed carbonate anhydride could a~lso be used~
which when reacted with diazomethane yields the
diazoketone XII. Compound XII, upon reaction with
aqueous acid, preferably a nonnucleopllilic acid such
S as sulfuric acid or p-toluenesulfonic acid, is
converted to the hydro~ymethyl ketone
Acid chloride ~I, upon react;on with
sulfonamide, RllS92NH2, in the presence of a
weak base yields the acyl-sulfonamide XIV. Reaction
of XI with an amine, R9R9NH, yields amide XV.
Amide XV can be sequentially reduced, to amine XVI,
with diborane or lithium aluminum hydride, and
sulfonylated with RllSO2Cl to produce sulfon-
amide XVII. Amide XV (when both R9 substituents
lS are hydrogen) can be dehydrated by standard reagents
to nitrile XVIII, which is converted to tetrazole XIX
by reaction with sodium azide, tri-n-butyltin azide
or other suitable methods. Compounds IX, X, XIII,
XIV, XV, XVII, XVIII and XIX are representatives of
Formula I compounds.
5~hem~YI Preparation ~,r Hvdrazine Derivatives IV
R ~ ) N~N3z ~
R NH2 ~ HCl R NHNH2 HCl
IV
~ s~.,!
gLZ95~i77
5012P/5011A - 29 - 17323IA
With regard to 5cheme VI, the preparation of
hydrazine starting materials is illustrated by pre-
-- paration of 4-methylthiophenyl hydrazine hydrochlor-
ide. 4-Methylthioaniline (13.9 9) was added dropwise
to cold HCl (6N) (50 mL~ and stirred for 5 min in an
ice bath. A solution of NaNO2 in water (7.25 9, 15
mL) was then added dropwise and stirred for 15 min.
The cold diazonium salt was then cannulated }nto a
stirred cold solution of Na2S2O4 in water ~50
g, 250 mL). After 20 min, ether ~200 mL) was added
and the reaction rnixture basified with NaOH(10N).
The ether layer was decanted, washed with brine,
dried over Na2SO4 and HCl gas was passed through
the ether solution to ~orm the hydrochloride salt
which precipitated out. After filtration, there was
obtained 7.0 g of pure final product. Other
hydrazines, similarly pr~pared, are also shown in
Table 3a, below.
TABLE 3a
HYDRAZINES
~5 ~3
NHNH2~HC
IV
No. _1 R2 ComPound Name
1 4-SMe H 4-methylthiophenyl hydrazine
hydrochloride
9~7
5012P/5011A - 30 - 17323IA
TABLE 4 (Cont'd)
.~ 2 2-CH(Me)2 H 2-isopropylphenyl hydrazine
hydrochloride
3 2-SMe H 2-methy:Lthiophenyl hydra~ine
hydroch:Loride
4 2-Me 4-Me 2,4-dimethylphenyl hydrazine
hydrochloride
2-Me 4-OMe 4-methoxy-2-methylphenyl
hydrazine hydrochloride
R3-R4-H
In those instances when asymmetric centers
are present, more than one stereoisomer is possible,
and all possible isomeric forms are deemed to be
included within the planar structural representations
shown. Optically active (R) and (S) isomers may be
resolved using conventional techniques known to the
skilled artîsan.
The prostaglandin antagonist properties of
the compounds of the present invention can be
demonstrated by a number of biological assays, two of
which, inhib~tion of platelet aggregation and
measurement of PA2 valves are described below.
~z~
5012P/5011A - 31 - 17323IA
Inhibition of Induced Threshold Aggregation of Human
Platelets _ _
Human platelet rich plasma (PRP) is prepared
from venous blood of male volunteers who have taken
no medication for ten days prior to test. Blood is
transferred into plastic centrifuge tubes containing
3.8% Trisodium Citrate in 0.9% NaCl (in a ratio of
blood to anticoagulant of 9:1), mixed by gentle
inversion, and centrifuged at room temperature for
ten minutes at 116 g. The supernatant (PRP) is
transferred into plastic tubes. Platelet poor plasma
(PPP) is obtained by centrifuging the residual blood
cells at 4000 y for ten minutes. PRP is left to
stand at least one half hour prior to testing.
Platelet Aggregation is measured using a
Payton Aggregometer and Recorder. Following
calibration of the instrument, a cuvette containing
PRP (225 microliters) is incubated for three minutes
at 37C. Drug vehicle (control), or a drug concentra-
tion is then added in a volume of 0.5 microliter.
After one minute, the aggregating agent (U44069,
9,11-dideoxy-9a,11a-epoxymethano PGF2a) is
added to the cuvette in a volume of 25 microliters.
Recording is continued until the maximal response is
obtained.
The threshold (approximately 20 - 30% of
maximum) aggregation concentration of the agonist to
be used is first determined in the presence of the
drug vehicle (control). Test compounds are then
assayed at 10 or 30 micrograms/ml initially, and if
active, are further tested in order to determine the
concentration range at which 20-80% of the threshold
~9~77
5012P/5011A - 32 - 17323IA
aggregatory response is inhibited. All drugs are
dissolved in dimethylsulfoxide.
The height of the aggregation response
(measured in divisions of the recorder paper, 1
~~ 5 division = 2.5 mm) in the presence of the drug is
recorded, and calculated as percent inhibition of the
mean height of the control threshold responses. The
- IC50 (drug concentration which inhibits 50% of the
aggregatory response) is obtained by regression
analysis.
Estimation of PA2 Values in Guinea Pig Tracheal
_ain
- Male albino Hartley strain guinea pigs
(300-350 gm) were sacrificed by a blow to the head
and exsanguinated. The trachea was removed, freed of
extraneous tissue and sectioned into rings of 1-2 mm
thickness. Five rings were tied together in series,
ensuring that the tracheal muscle lay in the same
vertical plane, and the cartilage of each ring then
separated at a point directly opposite the muscle.
The chains were suspended under 1 gm resting tension
in modified Rrebs solution (~aCl, 6.87; NaHCO3,
2.1; dextrose, 2.1; KCl, 0.32; CaC12, 0.28;
MgSO4, 7H2O, 0.11; KH2PO4, 0.16; gm/L:
equilibrated with 5% CO2 in 2 for 1 hour)
containing i~domethacin (1.4 x 10- M) to suppress
endogenous protaglandin synthesis, Organ bath
temperature was maintained at 37C and 5% CO2 in
2 diffused continously. Isometric tension changes
were recorded from a Gould Statham ~UTC 2) force
displacement transducer connected to a Beckman Type R
:~l29957~7
5012P/5011A - 33 - 17323IA
Dynograph. For assay purposes initial maximal
contractions were elicited with a high concentration
`~ of the contractile agonist [U-44069, 9.11~dideoxy-
9a,11a-epoxymethano PGF2a] and the tissue sub-
sequently washed at intervals until tension returned
to baseline. Agonist dose response curves were
obtained using a cumulative-dose schedule (4-8 doses)
and the preparations then washed at regular intervals
until baseline tension was recorded. After an
appropriate interval (1-1.5 hrs) agonist dose
response curves were repeated in the presence of
antagonist drug concentrations. Drug doses were
delivered in 10 ~1 volumes 5 minutes prior to the
second agonist challenge, and cumulative agonist
volumes did not exceed 100 ~1 per bath. EC50
values were obtained by regression analysis and used
to calculate 'apparent' and Schild Plot PA2 values
by the method of Tallarida and Murray 1981.
Compounds of Formula I can be tested using
the following assay to determine their mammalian
leukotriene biosynthesis inhibiting activity.
Rat Peritoneal Polymorphonuclear (PMN~
Leukocyte Assay
Rats under ether anesthesia are injected
(i.p.) with 8 ml of a suspension of sodium caseinate
~6 grams in ca. 50 ml water~. After 15 24 hr. the
rats are sacrificed (CO2) and the cells from the
peritoneal cavity are recovered by lavage with 20 ml
of buffer (Eagles MEM containing 30 mM HEPES adjusted
to pH 7.4 with NaOH). The cells are pelleted (350 x
g, 5 min.), resuspended in buffer with vigorous
~Z9~ i;77
5012P/5011A - 34 - 17323IA
shaking, filtered, through lens paper, recentrifuged
and finally suspended in buffer at a concentration of
10 cells~ml. A 500 ~1 aliquot of PWN suspension
and test compound are preincubated for 2 minutes at
37C, followed by the addition of 10 ~M A-23187.
The suspension is stirred for an additional 4 minutes
then bioassayed for LTB4 content by adding an
aliquot to a second 500 ~1 portion of the PMN at
37C. The LTB4 produced in the first incubation
causes aggregation of the second PMN, which is
measured as a change in light transmission. The size
of the assay aliquot is chosen to give a submaximal
transmission change (usually -70%) for the untreated
control. The percentage inhibition o~ I,TB~
formation is calculated from the ratio of transmission
change in the sample to the transmission change in
the compound-free control~
The cytoprotective activity of a compound
may be observed in both animals and man by noting the
increased resistance of the gastrointestinal mucosa
to the no~ious effects of strong irritants, for
example, the ulcerogenic effects of aspirin or
indomethacin. In addition to lessening the effect of
non-steroidal anti-inflammatory drugs on the gastro-
intestinal tract, animal studies show that cyto-
protective compounds will prevent gastric lesions
induced by oFal administration of strong acids,
strong bases, ethanol, hypertonic saline solutions
and the like.
Two assays can be used to measure cyto-
protective ability. These assays are; (A) an ethanol-
induced lesion assay and (B) an indomethacin-induced
ulcer assay and are described in EP 140,689.
1~99577
5012P/5011A - 35 - 17323IA
In Table 4 below are presented data
indicating the prostanoid antagonist activities of
compounds of the present invention indicated in Table
1. It is to be noted that PA2 values are on a
- 5 logerithmic scale, so that, for instance, a difference
between two PA2 values of 1 represents a difference
in potency by a factor of 10.
Compounds A, B, C and D in Table 4 are known
in the art: U.S. Patent 3,896,145 describes compounds
A and C, U.S. Patent 3,868,387 describes compound B,
and compound D is descxibed in U.S. Patent 3,905,998.
Compound A, which is a positional isomer of
the novel compound 2, is nevertheless almost 12 times
less potent as an inhibitor of platelet aggregation
and its PA2 is a factor of 14 (antilog of 1.6) less
than that of compound 2. The homolog B, with one
carbon less than A, shows no significant activity.
Also of interest is the fact that the fully aromatic
carbazole analog of the novel compound 2, compound C,
does not possess any significant activity. Compounds
D and E, isomeric with compound B further demostrate
that direct attachment of the carboxyl group to the
tetrahydrocarbazole nucleus results in severe loss of
activity.
Compound 8 indicates an interesting
differentiation of prostaglandin antagonist activity,
depending upon the tissue, with very weak action on
the guinea pig trachea ~pA2< 6), but with very good
potency as an inhibitor of human platelet aggregation
(IC50=O.O9~g~ml)-
Compound F demonstrates that a hydrogen atomattached to the 9-position (the nitrogen atom) results
5012P/5011A - 36 - 17323IA
in severe loss of activity. Likewise, compound G,
with a 9-alkyl substituted, has almost completely lost
activity. Compound H, with a 2-phenylethyl sub-
stituent at the 9-position has also suffered a serious
loss of activity by the addition of a CH2 group
compared to Compound 1. The last three compounds (F,
G and H) demonstrate the requir~ment for a benzyl or
substituted benzyl group at position-9 in the
compounds of the present invention.
Table 4
Prostanoid Antagonist ~ctivitles
15 Compound Inhibition of PA2
platelet aggregation
~IC50 in ~g/ml)
20 MeO ~
A ~ N ~ CO2H 3.5 6.8
Cl
~Z~95~7
5012P/5011A - 37 - 17323IA
Table 4 (Cont ' d3
~)\CO;~H >30 < 6
1~
10 C
MeO ~ 6 . 5 8
C '~) C02H
20 Cl
C02H
~5 `C`
D ~ ~6
Cl/~)
39S77
5012P/SOllA - 38 - 17323IA
Table 4 (Cont ' d)
Me~ 4.23 6.66
E ~f' C02H
1~ ~
Cl
F 1 0 3 . 41 < 6
H CO2H
13,5 <6
2 5 G J~ C02H
Me , Me
~L~9~3S77
5012P/5011A - 39 - . 17323IA
Table 4 ~Cont ' d~
l~o) Q
~ C2~t,
13 . 6 6 . 8:
10 (~
~1
C 03 8 . 6
C02H
20 ~/
Cl
~(~ D.30 8.4
3 0 2 ,) C02H
~: . 1 ~
C 1 \~
9577
5012P/SOllA - 40 - 17323IA
Table 4 _(Cont ' d~
~\~) D . 0 5 . 8 . 0
3 J L c02H
10 ~
Cl
~0 0.15 8.7
6 ) L CO~H
25 )~
lleO
~Z~D9S77
5012P/SOllA - 41 - 17323IA
Table 4 (Cont ' d)
~ ~ ; ~ \~ O . 09 3 . 3
7 ) C02H
C~
~ Q o . 09 < 6
,1~ C02H
9 ~ ISe
,(~ 0.05 ~9
g ) ~ CO H
Cl
~Z~5i77
5012P/5011A - 42 - 17323IA
Table a (Cont ' d)
Cl ~ 0.10 ~i.0
) C02H
,~ O J
Cl
'?`: ^` ' olo 9.5
2 5 11 Me ) C02
~,
J
C 1 ~/
~9~
5012P/5011A - 43 - 17323IA
Table 4 ~Cont ' d~
Br
- S ~ ~ ~ 0,O9 7.9
- 12 ) CO2H
~
Cl)~
~4)
J CO2H 0.08 7.6
20 13~
Cl~J
l 4 F J co2
~l~J 0.08 9 4
:~LZ9~S77
5012P/SOllA - 44 - 17323IA
Table 4 ~Cont ' d?.
(F)
- (F; 3\ N ~) O.17 1~.9
1 5 16 J C02H
Cl~
ClV~
19 Cl J C02~ 0 . 07 7 . 2
1~
C
~9S~7
5012P/5011A - 45 - 17323IA
Table 4 ~Cont'd)
^`:
~ ; 0 . 31 6 . 8
20 ¦ co~n
Me
lo 1J
The magnitude of a prophylactic or thera-
peutic dose of a compound of Formula I will, of
course, vary with the nature or the severity of the
condition to be treated and with the particular
compound of Formula I and its route of administration.
In general, the daily dose range for anti-asthmatic,
anti-allergic, or anti-thrombotic use lies within the
range of from about 0.01 mg to about 100 mg per kg
body weight of a mammal.
The exact amount of a compound of Formula I
to be used as a cytoprotective agent will depend on,
inter alia, whether it is being administered to heal
damaged cells or to avoid future damage, on the nature
of the damag~d cells (e.g., gastro-intestinal
ulcerations vs. nephrotic necrosis), and on the nature
of the causative agent. :An e~ample of use of a
compound:of Formula I to avoid future damage is co-
administration with a non~steroidal anti-inflammatory
drug ~for example, indomethacin).
77
5012P/5~11A - 46 - 17323IA
The effective daily dosage level for
compounds of Formula I inducing cytoprotection in
``~ mammals, especially humans, will generally range from
about 0.002 mg/kg to about 100 mg/kg, preferably from
about 0.02 mg/kg to about 30 mg/kg. The dosage may
be administered in single or divided individual doses.
Any suitable route of administration may be
employed for providing a mammal, especially a human
with an effective dosage of a compound of Formula I.
For example, oral, rectal, topical, parenteral,
ocular, nasal, buccal, intravenous and the like may
be employed. Dosage forms include tablets, troches,
dispersions, suspensions, solutions, capsules, creams,
ointments, aerosols and the like.
The pharmaceutical compositions of the
present invention comprise a compound of Formula I as
an active ingredient or a pharmaceutically acceptable
salt thereof, and may also contain a pharmaceutically
- acceptable carrier and optionally other therapeutic
~0 ingredients. The term "pharmaceutically acceptable
salts" refers to salts prepared from pharmaceutically
acceptable non-toxic bases including inorganic ~ases
and organic bases. The compositions include
compositions suitable for oral, rectal, ophthalmic,
pulmonary, nasal, dermal, topical or parenteral
~including subcutaneous, intramuscular and
intravenous)/administration, although the most
suitable route in any given case will depend on the
nature and severity of the conditions being treated
and on the nature of the active ingredient. They may
be conveniently presented in unit dosage form and
prepared by any of the methods well-known in the art
of pharmacy.
7~
5~12P/5011A - 47 - 17323IA
For use where a composition for intravenous
administration is employed, a suitable dosage range
" for anti-asthmatic, or anti-allergic use is from
about 0.01 mg to about 20 mg (preferably from about
0.1 mg to about 10 mg) of a compound of Formula I per
kg of body weight per day and for cytoprotective use
from about 0.002 mg to about 100 mg (preferably from
about 0.02 mg to about 30 mg and more preferably from
about 0.1 mg to about 10 mg) of a compound of Formula
I per kg of body weight per day. In the case where
an oral composition is employed, a suitable dosage
range for anti-asthmatic, or anti-allergic use is,
e.g. from about 1 to about 100 mg of a compound of
Formula I per kg of body weight per day, pre~erably
from about 5 mg to about 40 mg per kg and for cyto-
protective use from about 0.01 mg to about 100 mg
(preferably from about 0.1 mg to about 30mg and were
preferably from about 0.1 mg to about 10 mg) of a
compound of Formula I per kg of body weight per day.
For administration by inhalation, the
compounds of the present invention are conveniently
delivered in the form of an aerosol spray presenta-
tion from pressurized packs or a nebuliser, or a
powder which may be formulated as a cartridge from
which the powder composition may be inhaled with the
aid of a suitable device. The preferred delivery
system for inhalation in a metered dose inhalation
(~DI) aerosol, which may be formulated as a
suspension or solution in ~luorocarbon propellants.
Suitable topical formulations of compound I
include transdermal devices, aerosols, creams,
ointments, lotions, dusting powder, and the like.
~LZ995~q
5012P/5011A - 48 - 17323IA
In practical use, a compouncl o~ Formula I
can be combirled as the ~ctive ingred;ent in intimate
admi~ture with a pharmaceutical carri.er accordiny to
conventional pharmaceutical compounding techniques.
The carrier may take a wide variety of forms depending
on the form of preparation desired for administration,
e.g., oral or parenteral (including intravenous). In
preparing the compositions for oral dosage form, any
of the usual pharmaceutical media may be employed,
such as, for example, water glycols, oils, alcohols,
flavoring agents, preservatives, coloring agents and
the like in the case of oral liquid preparations,
such as, for example, suspensions, elixirs and
solutions; or carriers such as starches, sugars,
microcrystailine cellulose, diluents, granulating
agents, lubricants, binders, disintegrating agents
and the like in the case of oral solid preparations
such as, for example, powders, capsules and tablets.
Because of their ease of administrat-ion, tablets and
capsules represent the most advantageous oral dosage
unit form, in which case solid pharmaceutical carriers
are obviously employed. If desired, tablets may be
sugar coated or enteric coated by standard techniques.
In addition to the common dosage forms set
out above, the compounds of Formula I may also be
administered by controlled release means and/or
delivery devlces such as those described in U.S.
Patent Nos. 3,845,770; 3,91G,899; 3,536,809;
3,598,123; 3,630,200 and 9,008,719.
Pharmaceutical compositions of the present
invention suitable for oral administration may be
~;
~Z9~i7~
5012P/5011A - 49 - 17323IA
presented as discrete units such as capsules, cachets
or tablets each containing a predetermined amount of
~- the active ingredient, as a powder or granules or as
a solution or a suspension in an aqueous liquid, a
non-aqueous liquid, an oil-in-water emulsion or a
water-in-oil liquid emulsion. Such compositions may
be prepared by any of the methods of pharmacy but all
methods include the step of bringing into association
the active ingredient with the carrier which consti-
tutes one or more necessary ingredients. In general,the compositions are prepared by uniformly and
intimately admixing the active ingredient with liquid
carriers or finely divided solid carriers or both,
and then, if necessary, shaping the product into the
desired presentation. For example, a tablet may be
prepared by compression or molding, optionally with
one or more accessory ingredients. Compressed tablets
may be prepared by compressing in a suitable machine,
the active ingredient in a free-flowing for~ such as
powder or granules, optionally mixed with a binder,
lubricant, inert diluent, surface active or dispersing
agent. Molded tablets may be made by molding in a
suitable machine, a mixture of the powdered compound
moistened with an inert liquid diluent. Desirably,
each tablet contains from about 2.5 mg to about 500
mg of the active ingredient and each cachet or capsule
contains fro~ about 2.5 to about 500 mg of the active
ingredient.
The following are examples of representative
pharmaceutical dosage forms for the compounds of
Formula I:
~2~S77
5012P/5011A - 50 - 17323IA
Injectable Suspension ~I.M.) mq/ml
Compound of Formula I 2.0
Methylcellulose 5.0
Tween~ 80 0 5
5 Benzyl alcohol 9.0
Benzalkonium chloride 1.0
Water for injection to a total volume of 1 ml
Tablet mg~tablet
10 Compound of Formula I 25.0
Microcrystalline Cellulose415.0
Providone 14.0
Pregelatinized Starch 43.5
Magnesium Stearate 2.5
lS
Capsule mq~capsule
Compound of Formula I 25.0
Lactose Powder 573.5
20 Magnesium Stearate 1.5
600
In addition to the compounds of Formula I,
the pharmaceutical compositions of the present
2S invention can also contain other active ingredients,
such as non-steroidal anti-inflammatory drugs
(NSAIDs), pe~ipheral anaIqesic agents such as
zomepirac, diflunisal and the like, cyclooxygenase
inhibitors, leukotriene antagonists, leukotriene
biosynthesis inhibitors, H2 receptor antagonists,
antihista~inic agents, prostaglandin antagonists, ACE
inhibitors, and thrombo~ane synthetase inhibitors.
~ILZ9~3577
5012P/SOllA - Sl - 17323IA
The weight ratio of the compound of the Formula I to
the second active ingredient may be ~aried and will
depend upon the effective dose of each ingredient.
Generally, an effective dose of each will be used.
Thus, for example, when a compound of the Formula I
is combined with a second active ingredient the
weight ratio of the compound of ths Formula I to the
second ingredient will generally range from about
loOO:l to about 1:1000, preferably from 250:1 to
1:200. Combinations of a compound of the Formula I
and other active ingredients will generally be within
the aforementioned range, but in each case, an
effective dose of each active ingredient should be
used.
NSAIDs can be characterized into five groups:
(1) the propionic acid derivatives;
(2) the acetic acid derivatives;
(3) the fenamic acid derivatives;
(9~ the biphenylcarboxylic acid derivatives;
and
(5) the oxicams or a pharmaceutically
acceptable salt thereof. NSAIDS which are within the
scope of this invention are those disclosed ;n EP
140,684.
Pharmaceutical compositions comprising the
: Formula I compounds may also contain other inhibitors
of the biosynthesis of the leukotrienes such as are
disclosed in EP 138,481 tApril 24, 1985), EP 115,394
(August 8, 1984), EP 136,893 (April 10, 1985), and EP
140,709 (May 8, 1985).
1299S77
5012P/SOllA - 52 - 17323IA
The compounds of the Formula I may also b~
used in combination with leukotriene antagonists such
as those disclosed in EP 106,565 (April ~S, 1984) and
EP 104,885 ~April 4, 1984),
and others known in
the art such as those disclosed in European Patent
Application ~os. 56,172 and 61,800; and in U.K. Patent
Specification No. 2,058,785.
Pharmaceutical compositions comprising the
Formula I compounds may also contain as the second
active ingredient, other prostaglandin antagonists
such as those disclosed in European Patent Application
11,067 ~May 28, 19~0) or other thromboxane antagonists
lS such as those disclosed in U.S. 4,237,160. They may
also contain histidine decarboxyase inhibitors such
as a-fluoromethyl-histidine, described in U.S.
4,325,961. The compounds of the Formula I may also
be ad~antageously combined with an Hl or H2-receptor
antagonist, such as for instance benadryl, dramamine,
histadyl, phenergan, terfenadine, acetamazole,
cimetidine, ranitidine, famotidine, aminothiadiazoles
disclosed in EP 40,696 (December 2, 1981) and like
compounds, such as those disclosed in U.S. Patent
Nos. 4,283,403; 4,362,736; 4,394,508.
The pharmaceutical compositions may also
contain a K+/Ht ATPase inhibitor such as
omeprazole, disclosed in U.S. Pat. g,255,431, and the
like. Compounds of I may also be usefully combined
with most cell stabilizing agents, such as 1,3-bis~2-
carboxychromon-S-yloxy)-2-hydroxypropane and related
~, ''',;
~IZg~5~
5012P/5011A - 53 - 17323IA
compounds described in British Patent Specifications
1,144,905 and 1,144,906. Another useful pharma-
ceutical composition comprises the Formula I
compounds in combination with serotonin antagonists
such as methysergide, the serotonin antagonists
described in ature, Vol. 316, pages 126-131, 1985,
and the like.
When the second active ingredient in
compositions of this invention is a thromboxane
synthetase inhibitor, such inhibitor can be as
described in UK 2,038,821 ~e.g., UK 37248 and
dazoxiben hydrochloride), U.S.P. 4,217,357 (e.g., UK
34787), U.S.P. 4,444,775 (e.g., CGS 13080), U.S.P.
4,226,878 (e.g., ONO 046), U.S.P. 4,495,357 (e.g.,
U63557A) U.S.P. 4,273,782 (e.g., UK-3R485), or EP
98,690 (e.g., CV-9151).
An embodiment of the invention is a cardio-
vascular composition useful for treating arterialthrombosis which comprises an antithrombotic compound
of the Formula I.
A further embodiment of the invention is a
cardiovascular composition useful for treating
arterial thrombosis which comprises: (1) the
antithrombotic Formula I compound defined above; and,
(ii) an angiotensin converting enzyme tACE~ inhibitor
compound which is a member of the group: carboxyalkyl
dipeptide derivatives; captopril [1-(3~mercapto-2-
methyl-l-oxopropyl)-L-proline]; 2-[~-(S)-l-ethoxy-
carbonyl-3-phenylpropyl)-S-alanyl]-cis,endo-2-azabi-
cyclo~3,3,0~octane-3(S)-carboxylic acid; N-((S)-l-
~Z~S7~
5012P/5011A - 54 ~ 17323IA
ethoxycarbonyl-3-phenylpropyl~-L-alanyl-N-(2-indanyl)-
glycine; l-(N-[(S)-l-ethoxy-carbonyl-3-phenylpropyl]-
L-alanyl)-cis,sy~-octahydro-tH-indole-2-S)-carboxylic
acid; 2-(N-[(S)-l-ethoxy-carbonyl-3-phenylpropyl~ L-
alanyl)-1,2,3,4-tetrahydro-iso-isoquinoline-3(S)-
carboxylic acid; and, l-carboxy-methyl-3~S)-(l(S3-
ethoxycarbonyl-3-phenylpropylamino)-2,3,4,5-tetrahydro-
lH[l]-benzazepine-2-one.
In particular the class of ACE inhibitors
which have been found to have a potentiating effect
when used in combination with the Formula I compounds
are those disclosed in U.S. Patent 4,374,829, which
also discloses methods for their preparation.
Of
the carboxyalkyl dipeptides disclosed in U.S. Patent
4,374,829, those o particular interest in this
invention are N-~l(S)-ethoxycarbonyl-3-phenyl-
propyl]-L-alanyl-L-proline, a}so known and referred
to herein as enalapril; N-~l(S)-carboxy-3-phenyl-
propyl]-L-alanyl-L-proline, also know and referred to
herein as enalapril diacid; and, Na-[l(S3-carboxy-
3-phenylpropyl]-L-lysyl-L-proline, also known and
referred to herein as lisinapril.
The combination composition of the invention
can contain varying amounts of (i) the Formula I
antithrombotic compound and (ii) ACE inhibitor
antihyperten~ive compounds. The weight ratio of
(i):(ii) can range from about 25 to 1; preferably
; from about 10 to 1. In addition to the active
ingredients of (i) alone or of (i) and (ii) in
combination, the compositions of the invention can
also contain other conventional pharmaceutically
, ... .
9S~7
5012P/5011A 55 - 17323IA
acceptable compounding ingredients, as necessary or
desired. Such ingredients are generally referred to
as carriers or diluents. Conventional procedures for
preparing such compositions in appropriate dosage
forms can be utilized. Whatever the dosage form, it
will contain a pharmaceutically effective amount of
the present composition.
The combination compositions can be
administered orally or other than orally; e.g.,
parenterally, by insufflation, topically, rectally,
etc.; using appropriate dosage forms; e.g., tablets,
capsules, suspensions, solutions, and the like, for
oral administration; suspension emulsions, and the
like, for parenteral administration; solutions ~or
intravenous administration; and ointments, transdermal
patches, and the like, for topical adrninistration.
These compositions are formulated similarly to the
compositions discussed above.
Treatment dosage for human beings for
cardiovascular use can be varied as necessary.
Generally, daily dosages of the composition of the
invention can range from about 6000 to about 10 mg;
preferably, from about 3000 to about 20 mg.
The amount of active ingredient that may be
combined with the carrier materials to produce a
single dosage form for cardiovascular use will vary
depending upon the host treated and the particular
mode of administration. For example, a formulation
intended for oral administration may contain from 5
mg to 5 gm of active agents compounded with an
appropriate and convenient amount of carrier material
which may vary from about 5 to about 95 percent of
577
5012P/5011A - 56 - 17323IA
the total composition. Dosage unit forms will
generally contain between from about 20 mg to about
"~ 500 mg of active ingredients.
It will be understood, however, that the
~ 5 specific dose level for any particular patient will
depend upon a variety of factoxs including the
activity of the specific compound employed, the age,
- body weight, general health, sex, diet, time of
administration, route of administration, rate of
excretion, drug combination and the severity of the
particular disease undergoing therapy.
The composition of this invention inhibits
platelet accumulation at the damaged endothelial
~ surface via the Formula I compound. This inhibitory
effect is potentiated by the presence of the
antihypertensive compound.
Thus, the compositions of the invention are
useful in treating thrombosis and are also of value
in the management of acute and chronic congestive
heart failure, and limitation of myocardial infarct
damage.
In vivo testing of the composition of this
invention in test animals (rabbits) can be used to
demonstrate that this composition is pharmaceutically
effective in decreasiny platelet-related arterial
thrombic formation.
To demonstrate the potentiation of the
antihypertensive compound on the anti-thrombotic
Formula I compound comprising the combination
composition of the invention, the effect of these
compounds on test animals (rabbits) can be determined
separately and th~n in combination. The effect of a
~299577
5012PJ5011A - 57 - 17323IA
different class of antihypertensive agents singly and
in combination with the For~ula I compound of the
invention can also be determined for co~parative
purposes. The methods employed are described in U.S.
Patent 4,558,037
The following examples illustrate the
preparation of the compounds of the present invention
without, however, limiting the same thereto.
All temperatures are in degrees Celsius.
Reference ComPounds
6-fluoro-1,2,3,4-tetrahydrocarbazol-1-yl-acetic acid
Following the procedure of Example 1, but
using 1-(4-fluorophenyl) hydrazine hydrochIoride and
ethyl-2-cyclohexanone acetate as starting materials,
the title compound was prepared.
M.P. 124 -126C
~eference Compounds
9-[1-(2-p-chlorophenyl)ethyl]-6-fluoro-1,2,3,4-tetra-
hydrocarbazol-l-yl-acetic acid
Following the procedure of Example 2, but
using 1-[2-(4-chlorophenyl)ethyl~-1-(4-fluorophenyl)
hydrazine hydrochloride and ethyl 2-cyclohexanone
acetate as starting materials, the title compound was
prepared.
Empirical Formula: C22H2~NFClO2
C H N
Calculated 68.48 5.49 3.63
Found68.15 5.7~ 3.65
~;',~`1
,.
~2~ss~
5012P/5011A - 58 - 17323IA
Reference Compounds
6-fluoro-9-isopropyl-1,2,3,4-tetrahydrocarbazol-1-yl-
" acetic acid
Following the procedure of Example 1, but
using 1-~2-propyl)-1- (4-fluorophenyl)hydra2ine
hydrochloride and ethyl 2-cyclohexanone acetate as
starting materials, the title compound was prepared.
Empirical Formula: C17H20NFO2
M.P. 144-144.5 C
Example 1
9-p-chlorobenzyl-6-fluoro-1,2,3,4-tetrahydrocarbazol-
l-yl-acetic acid
SteP I
To 3.50 9 of 1-(4-chlorobenzyl~-1-(4-fluoro-
phenyl)hydrazine hydrochloride in 70 cc of iso_
propanol was added 2.23 g of ethyl 2-cyclohexanone
acetate. The reaction was refluxed under nitrogen
for 16 hours. The resulting reaction mixture was
then evaporated to dryness and the residue suspended
in ether. The solid material was then filtered. The
ether filtrate was washed with water, dried and
evaporated. The resulting syrup was chromatographed
on silica gel to give 2.8 g (42%).
Step II
To ~.59 g of ethyl ester from step I in 10
cc of methanol was added 10 cc of water and 420 mg of
potassium hydroxide. The resulting solution was
refluxed for 4 hours. Upon cooling the reaction
mixture was then acidified with HCl (lN). The
resultinq precipitate was filtered and washed with
~;~g~577
5012P/5011A - 59 - 17323IA
water. Analytically pure material was prepared by
trituration the solid with a mixture of hexane/ethyl
acetate S9:1~ followed by filtration and drying on a
high vacuum pump to give 1.24 g of the title compound
- 5 (89%)-
Analysis calculated for C21HlgNClFO2
C H N Cl F
- Calculated67.83 5.15 3.77 9.53 5.11
Found 67.B85.47 3.63 9.52 5.12
Example 2
3-[9-p-chlorobenzyl-6-fluoro-1,2,3,4-tetrahydro-
carbazol-l-yl]-propanoic acid
- Following the procedure of Example 1, but
using 1-(4-chlorobenzyl)-1-(9-fluorophenyl)hydrazine
hydrochloride and methyl-2-cyclohexanone propionate
as starting materials, the title compound was
prepared.
Empirical formula: C22H21ClFNO2
C H N
Calculated 68.48 5.49 3.63
Found 68.26 5.57 3.60
Example 3
25 3-[9-p-chlorobenzyl-6-methoxy-1,2,3,4-tetrahydro-
carbazol-l-yl]-proPanoic acid
Following the procedure o~ Example 1, but
using l-S4-chlorobenzylj-1-~4-methoxyphenyl)hydrazine
hydrochloride and methyl-2-cyclohexanone propionate
as starting materials, the title compound was
prepared.
577
5012P/SOllA - 60 - 17323IA
Empirical formula: C~2H22ClN03
C H N Cl
Calculated 69.436.0~ 3.52 8.91
Found 69.24 5.98 3.60 8.85
_ 5
Example 4
9-p-chlorobenzyl-6-methoxy-1,2,3,4-tetrahydrocarbazol-
l-yl-acetic acid
Following the procedure of Example 1, but
using 1-(4-chlorobenzyl~-1-(4-methoxyphenyl~hydrazine
hydrochloride and ethyl-2-cyclohexanone acetate as
starting materials, the title compound was prepared.
M.P. 152-153C.
Example 5
2-[9-p-chlorobenzyl-6-fluoro-1,2,3,4-tetrahydro-
carbazol-l-yl]ethanol
1.10 g of 9-p-chlorobenzyl-6-fluoro-1,2,3,4-
tetrahydrocarbazo-l-yl-acetic acid ethyl ester was
dissolved in 500-ml dry tetrahydrofuran and the
reaction was cooled at 0C and one equivalent of
lithium aluminium hydride (LAH3 was added portion
wise. The reaction was allowed to warm up to room
temperature and stirred for 16 hrs. The reaction
mixture was quenched with NH4Cl (aq.). Ethyl
acetate was added ~100 ml) and the organic phase
~eparated, washed with water and brine, dried and
evaporated. The product was isolated by column
chromatography.
M.P. 98.0 - 98.5 C.
~2~5~7
5012P/5011A - 61 - 17323IA
Example 6
3-[9-p-chlorobenzyl-6-methoxy-1,2,3,4-tetrahydro-
carbazol-l-yl]-propanol
Following the procedure of Example 5, but
using 3-[9-p-chlorobenzyl-6-methoxy-1,2,3,4-tetra-
hydrocarbazo-l-yl~-propanoic acid ethyl ester from
Example 3 as starting material, the title compound
was prepared.
Empirical formula: C23H26ClNO2
C H N
Calculated 71.95 6.83 3.65
Found 71.86 6.65 3.81
Example 7
15 (-)9-p-chlorobenzyl-6-fluoro-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic acid
SteP I
10.0 g of 9-p-chlorobenzyl-6-fluoro-1,2,3,4-
tetrahydrocarbazo-l-yl-acetic acid from Example 1 was
dissolved in a mixture of hot (refluxing) aceto-
nitrile (150 cc), and ethanol (25cc) and 4.4 g of
d(~) ephedrine wa~s added. The reflux was continued
for 15 min. and the hot solution was filtered and
allowed to cool to room temperature. Crystals
separated from the solution and were separated by
filtration. After three recrystallizations from
acetonitrile 3.9 g of the pure salt was obtained.
Step II
3.9 g of pure salt from step I was dissolved
in 200 cc of methanol and acidified using 1 N hydro-
chloric acid. Water was added and the crystals were
~L2~15~
5012P/5011A - 62 - 17323IA
separated by filtration and dryed under vacuum. Upon
trituration with hexane ethyl acetate mixture (9:1)
"~ the title compound was prepared.
aD = -42.5 (methanol) M.P. 151 - 15105C.
_ 5
Example 8
(+~9-p-chlorobenzyl-6-fluoro-1,2,3,4-tetrahydro-
carbazol-l-Yl-acetic acid
Following the method of Example 7, but using
10 1(-) ephedrine and 9-p-chlorobenzyl-6-fluoro-1,2,3,4-
tetrahydrocarbazo-l-yl-acetic acid as starting
material, the title compound was prepared.
aD = ~43.0 (methanol) M.P. 150 - 150.5C.
Example 9
9-benzyl-6-fluoro-1,2,3,4-tetrahydrocarbazol-1-yl-
acetic acid
Following the procedure of Example 1, but
using l-(benzyl)-l- (4-fluorophenyl)hydrazine
hydrochloride and ethyl 2-cyclohexanone acetate as
starting materials, the title compound was prepared.
Analysis calculated for C21H20NFO2
C H N
Calculated 74.76 5.98 4.15
25 Found 74.95 6.07 3.90
, Example 10
9-p-metho~ybenzyl-6-fluoro-1,2,3,4-tetrahydrocarbazol-
l-yl-acetic acid
Following the procedure of Example 1, but
using 1-(4-methoxybenzyl)-1-~4-fluorophenyl~hydrazine
hydrochloride and ethyl 2-cyclohexanone ac0tate as
stating materials, the title compound was prepared.
~29~5~7
5012P/5011A - 63 - 17323IA
Analysis calculated for C22H22NFO3
C H N
~`~ Calculated 71.92 6.04 3.91
Found 71.70 6.22 4.05
_. 5
Examp1~_11
9-(3,4-dichloro)benzyl-6-fluoro-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic acid
Following the proceduré of Example 1, but
using 1-(3,4-dichlorobenzyl~- 1-t4-fluorophenyl)
hydrazine hydrochloride and ethyl 2-cyclohexanone
acetate as starting materials, the title compound was
prepared.
Analysis calculated for C21H18NC12FO2.
15 C H N
Calculated 62.08 4.47 3.95
Found 61.96 4.71 3.67
Example 12
9-[1-(1-phenyl)ethyl]-6-fluoro-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic acid
Following the procedure of Example 1, but
using l-[l-(l-phenyl)ethyl]-1-~4-fluorophenyl)-
hydrazin~e hydrochloride and ethyl 2-cyclohexanone
acetate as starting materials, the title compound was
prepared.
C H N F
Calculated 75.196.31 3.99 5.41
Found 75.18 6.054.11 5.51
.
~2995~7
5012P/5011A - 64 - 17323IA
Example 13
""~ 9-p-chlorobenzyl-1,2,3,4-tetrahydrocarbazol-1-yl-acetic
acid
Following the procedure of Example 1, but
using 1-(4-chlorobenzyl3~1-(phenyl)hydrazine hydro-
chloride and ethyl 2-cyclohexanone acetate as starting
materials, the title compound was prepared.
Empirical Formula: C21H20NClO2
C H N Cl
Calculated 71.28 5.70 3.96 10.02
Found 70.7n 5.90 3.82 10.23
xample 19
9-p-chlorobenzyl-6-chloro-1,2,3,4-tetrahydrocarbazol-
l-yl-acet~c acid
Following the procedure of Example 1, but
using 1-(4-chlorobenzyl)-1-(4-chlorophenyl)hydrazine
hydrochloride and ethyl-2-cyclohexanone acetate as
starting materials, the title compound was prepared.
Empirical Formula: C21H19NC12O2
C H N Cl
Calculated 69.96 4.93 3.61 18.26
Found 65.20 5.16 3.38 18.04
Example 15
9-p-chlorobe~zyl-8-methyl-1,2,3,4-tetrahydrocarbazol-
l-yl-acetic acid
Following the procedure of Example 1, but
using 1-(4-chlorobenzyl~-1-(2-methylphenyl)hydrazine
hydrochloride and ethyl-2-cyclohexanone acetate as
starting materials,~the title compound was prepared.
~9~577
5012P/5011A - 65 - 17323IA
Empirical Formula: C22H22NC1O2
C H N Cl
Calculated 71.83 6.03 3.81 9.64
Found 72.196.23 4.12 9.84
Example 16
6-bromo-9-p-chlorobenzyl-1,2,3,4-tetrahydrocarbazol-1-
yl-acetic acid
Following the procedure of Example 1, but
using 1-~4-chlorobenzyl)-1-(4-bromophenyl)hydrazine
hydrochloride and ethyl 2-cyclohexanone acetate as
starting materials, the title compound was prepared.
Empirical formula: C21HlgBrClNO2
~ C H N Br Cl
15 Calculated 58.29 4.43 3.24 18.46 8.19
Found 58.49 4.60 3.44 18.50 8.27
Example 17
9-p-chlorobenzyl-6-methyl-1,2,3,4-tetrahydrocarbazol-
l-yl-acetic acid
Following the procedure of Example 1, but
using 1-(4-chlorobenzyl)-1-(4-methylphenyl)hydrazine
hydrochloride and ethyl 2-cyclohexanone acetate as
starting materials, the title compound was prepared.
Empirical formula: C22H22ClNO2
C H N Cl
Calculated ~1.836.03 3.81 9.64
Found 71.72 6.14 3.77 9.88
~ '
~LZ~ 5~C7
5012P~5011A - 66 - 17323IA
E~ample 18
2-[9-p-chlorobenzyl-6-fluoro-1,2,3,4-tetrahydro-
`~ carbazol-l-yl-]propanoic acid.
Following the procedure of Example 1, but
using 1-~4-chlorobenzyl)-1-(4-1uorophenyl) hydrazine
hydrochloride and methyl 2-(2-cyclohexanone) pro-
pionate as starting materials, the title compound was
prepared.
M.P. 203-205C
Example 19
9-p-chlorobenzyl-8 fluoro-1,2,3,4-tetrahydrocarbazol-
l-yl-acetic acid.
Followiny the procedure of Example 1, but
using 1-(4-chlorobenzyl)-1-(2-fluorophenyl) hydrozine
hydrochloride and ethyl Z-cyclohexanone acetate as
starting materials, the title compound was prepared.
M.P. 228-229C.
Example 20
3 [(a and B)-t-butyl-6-fluoro-9-(p-chlorobenzyl)-
1,2,3,4-tetrahydrocarbazol-1-yl]acetic acid.
Following the procedure of Example 1 but
using 4-t-butyl-2-carbomethoxymethyl cyclohexanone
and 1-t4-chlorobenzyl)-4-fluorophenylhydrazine hydro-
chloride as the starting materials, the title compound
was prepared~
M.P. 210-211C.
,n
~ g ~
5012P~5011A - 67 - 17323IA
Example 21
9-p-chlorobenzyl-5-fluoro-1,2,3,4-tetrahydrocarbazol-
`-~ l-yl-acetic acid and 9-p-chlorobenzyl-7-fluoro-
1,2,3,4-tetrahydocarbazol-1-yl-acetic acid (mixture).
Following the procedure of Example 1, but
using 1-(4-chlorobenzyl)-1-(3-fluorophenyl)hydrazine
hydrocloride and ethyl 2-cyclohexanone acetate as
starting materials, the title compounds were prepared.
Empirical Formula: C21HlgNClFO2
M.P. 211-219C
Example 22
9-p-chlorobenzyl-5,7-dichloro-1,2,3,4-tetrahydro-
carba~ol-l-yl-ac_tic acid.
Following the procedure of Example 1, but
using l-(4-chlorobenzyl)-1-(3-5 dichlorophenyl)-
hydrozine hydrochloride and ethyl 2-cyclohe2anone
acetate as starting materials, the title compound was
prepared.
Empirical formula: C21H18NC13O2
M.P. 204-206C
Example 23
9-p-chlorobenzyl-6,8-dichloro-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic acid.
Following the procedure of Example 1, but
using 1-(4-cnlorobenzyl)-1-(2-4-dichlorophenyl)-
hydrozine hydrochloride and ethyl 2-cyclohexanone
acetate as starting materials, the title compound was
prepared.
Empirical formula: C21H18NC13O2
M.P. 203-204C.
~Z9~57~7
5012P/5011A - 68 - 17323IA
Example 24
9-p-Chlorobenzyl-6-isopropyl-1,2,3,4-tetrahydrocar-
-~ bazol-l-Yl-acetic acid
Following the procedure of Example 1, but
using 1(4-chlorobenzyl)-1-(4-isopropylphenyl~-
hydrazine hydrochloride and ethyl 2-cyclohexanone
acetate as starting material, the title compound was
prepared.
Empirical Formula: C24H26ClNO2
C H N Cl
Calc: 72.81 6.62 3.5~4 B.95
Found: 72.83 7.09 3.61 9.21
Example 25
9-p-Chlorobenzyl-6-tert-butyl-1,2,3,4-tetrahydrocar-
bazol-l-yl-acetic acid
Following the procedure of Example 1, but
using 1-(4-chlorobenzyl)-1-t4-tert-butylphenyl)
hydrazine hydrochloride and ethyl2-cyclohexanone
acetate as starting materials, the title compound was
prepared.
Empirical Formula: C25H28ClNO2
C H ~ Cl
Calc. 73.24 6.88 3.42 8.65
25 Found: 73.21 7.32 3.10 8.26
~ Example 26
9-p-Chlorobenzyl-6-trifluoromethyl-1,2,3,4-tetra-
hydrocarbazol-l-yl-acetic acid _
Following the procedure of Example 1, but
using l-(4-chlorobenzyl)-1-(4-trifluoromethylphenyl)
hydrazine hydrochloride and ethyl 2~cyclohexanone
~Z~5~7
5012P/5011A - 69 - 17323IA
acetate as starting materials, the title compound was
prepared. m.p. 167-168C.
""
_ Example 27
9-p-Chlorobenzyl-6-methylthio-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic acid
Following the procedure of Example 1, but
using l-(4-chlorobenzyl)-1-(4-methylthiophenyl)
hydrazine hydrochloride and ethyl 2-cyclohexanone
acetate as starting materials, the title compound was
prepared.
Empirical Formula: C22~22ClNO2S
C H N S Cl
Calc: 66.07 5.54 3.50 8.02 8.86
15 Found: 66.27 5.83 3.38 8.00 8.70
ExamPle 28
9-p-Chlorobenzyl-6-methylsulfinyl-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic acid
Step I
To 498 mg of ethyl 9-p-chlorobenzyl-6-methyl-
thio-1,2,3,4-tetrahydrocarbazol-1-yl-acetate from
~xample 27, Step I, in 10 cc of methylene chloride
was added 300 mg of m-chloro perbenzoic acid. The
resulting mixture was stirred for 1.5 hours at room
temperature. The reaction mixture was diluted with
ether and washed consecutively with a solution of
sodium bicarbonate, water and brine. The crude
product obtained after evaporation of the organic
layer was purified on silica gel by flash chromato-
graphy eluting with 20% hexane/ethyl acetate and
yielded 420 mg (82%) of the pure sulfoxide derivative.
~ 99~77
5012P/5011A - 70 - 17323IA
.~ Step XI
Following the procedure of Example 1, Step
-- II, but using the ethyl Pster from Step I, there was
obtained the title compound. m.p. 105-107C.
Empirical Formula: C22H22ClNO3S
C H N S Cl
Calc: 63.53 5.33 3.37 7.71 8.52
Found: 63.31 5.03 ~.94 7.69 8.48
Example 29
9-p-Chloro~enzyl-6-methylsulfonyl-1,2,3,4-tetrahydro-
carbazol-l-Y1-acetic acid
steP I
To 439 mg of ethyl 9-p-chlorobenzyl-6-methyl-
sulfinyl-1,2,3,4-tetrahydrocarbazol-1-yl-acetate from
Example 28, Step I, in 10 cc of methylene chloride
was added 3~3 mg of m-chloro perbenzoic acid. The
resulting mixture was stirred for 18 hours at room
temperature. The reaction mixture was diluted with
ether and washed consecutively with a solution of
sodium bicarbonate, water and brine. The crude
product obtained after evaporation of the organic
layer was purified on silica gel by flash chromato-
graphy eluting with 30% hexane/ethyl acetate and
yielded 200 mg (92%) of the pure sulfone derivative.
.
Step II
Following the procedure of Example 1, Step
~ I, but using the ethyl ester from Step I, there was
obtained the title compound. ~.p. 101-102C.
:
lZ~'$7~
5012P/5011A - 71 - 17323IA
ExamPle 30
9-p-Chlorobenzyl-8-isopropyl-1,2,3,4-tetrahydrocar-
~- bazol-l-yl-acetic acid
Following the procedure of Example 1, but
using 1-(4-chlorobenzyl)-1-(2-isopropylphenyl)
hydrazine hydrochloride and ethyl 2-cyclohexanone
acetate as starting materials, the title compound was
~ prepared.
Empirical Formula: C24H26ClNO2
C H
Calc: 72.81 6.62
Found: 72.59 6.90
Example 31
9-p-Chlorobenzyl-8-methylthio-1,2,3,4-tetrahydrocar-
bazol-l-yl-acetic acid
Following the procedure of Example 34, but
using 1-(2-methylthiophenyl) hydrazine hydrochloride
and ethyl 2-cyclohexanone acetate as starting
materials, the title compound was prepared. m.p.
141-142C.
Example 32
9~p-Chlorobenzyl-8-methylsulfinyl-1,2,3,4-tetrahydro-
carbazol-l-Yl-acetic acid
Following the procedure of Example 28, but
using the et~yl ester from Esample 31, Step II, there
was obtained the title compound. m.p. 119-120.5C.
~L2~'~S77
5012P/5011A - 72 - 17323IA
Example 33
9-p-Chlorobenzyl-6-fluoro-3-methyl-1,2,3,4-tetrahydro-
`~- carbazol-l-yl-acetic acid
Following the procedure of Example 1, but
using 1-(4-chlorobenzyl)-1-(4-fluorophenyl) hydrazine
hydrochloride and ethyl 4-methyl-2-cyclohexanone
acetate as starting materials, the title compound was
prepared. m.p. 205-206C.
Empirical Formula: C22H21FClNO2
C H N Cl
Calc: 68.48 5.49 3.63 9.19
Found: 68.80 5.50 3.30 9.47
Example 34
9-p-Chlorobenzyl-6,8-difluoro-1,2,3,4-tetrahydro-
carbazol-l-Y1=acetic acid
Step I
To 114 g of 1-(2,4-difluorophenyl) hydrazine
hydrochloride in 350 cc of 2-propanol containing 40
cc of acetyl chloride was added 138 g of ethyl
2-cyclohexanone acetate. The reaction was refluxed
under nitrogen for 2 days. After cooling, 200 cc of
ether was added and the precipitate filtered. The
filtrate was evaporated to dryness. The resulting
residue was dissolved in a (1:1) mixture of
ether/ethyl acetate and consecutively washed with
water, sodiu~ bicarbonate solution and brine. The
organic layer was dried over sodium sulfate and
evaporated to dryness. The crude product was passed
through a silica gel bed eluting with 5% ethyl
acetate/hexane to yield 84 g of a 1:2 mixture of
ethyl and isopropyl esters.
~Z~ 77
5012P/5011A - 73 - 17323IA
Step II
84 g of esters from Step I was dissolved in
250 cc of methanol and 400 cc of sodium hydroxide
(lN) was added and reflu~ed 4 hours. After cooling,
the reaction mixture was washed with a (1 1~ mixture
of ether/hexane and the aqueous layer was acidified
with HCl (lN). The resulting precipate was filtered,
washed with water and air dried to afford 50 g of
6,8-difluoro-1,2,3,4-tetrahydrocarbazol-1-yl~acetic
acid.
Step III
A solution of 11:1 g of acid from Step II in
100 cc of THF was added portionwise 10.3 g of
potassium tert-butoxide. The resulting mixture was
stirred for 45 min. at room temperature and 10.3 g
p-chlorobenzyl bromide was added portionwise. The
reaction mixture was stirred 18 hours at room
- temperature. The resulting mixture was diluted with
lOQ cc of water and washed with hexane. The aqueous
layer was acidified with HCl (lN) and the resulting
precipitate filtered washed with water and air-dried
to afford 9.4 g of the title compound. m.p.
168.5-170C.
Example 35
9-p-Chlorobenzyl-6,8-dimethyl-1,2,3,4-tetrahydro-
arbazol-l-yl-acetic acid
Following the procedure of Example 34, but
using 1-(2,4-dimethylphenyl) hydrazine hydrochloride
and ethyl 2-cyclohexanone acetate in Step I as
starting materials, the title compound was prepared.
m.p. 187-188C.
99~77
5012P/5011A - 74 - 17323IA
Example 36
~-p-Chlorobenzyl-6-methoxy-8-methyl--1,2,3,4-tetrahydro-
`~ carbazol-l-yl-acetic acid
Following the procedure of Example 34, but
using 1-(4-methoxy-2-methylphenyl) hydrazine
hydrochloride and ethyl 2-cyclohexanone acetate as
starting materials, the little compound was
prepared. m.p. 188-188.5C.
Example 37
(-)-9-p-Chlorobenzyl-6,8-difluoro-1,2,3,4-tetrahydro-
carbazol-l-Yl-acetic acid
Following the method of Example 7, but using
d(~)ephedrine and 9-p-chlorobenzyl-6,8-difluoro-
15 1,2,3,4-tetrahydrocarbazol-1-yl-acetic acid from
Example 34 as starting materials, the title compound
was prepared. from Example 34 [a]D = -6g.3
(methanol) m.p. 130-131C.
Example 38
(+)-9-p-Chlorobenzyl-6,8-difluoro-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic_ac;d
Following the method oE Example 371 but
using l~-)ephedrine, the title compound was prepared.
25 [a]D = +61.5 ~methanol) m.p. 129.5-130C.
Example 39
~ 9-p-Chlorobenzyl-8-methyl-1,2,3,4-tetrahydro-
carbazol-l-Yl-acetic acid
Following the method of Example 7, but using
d(~)ephedrine and 9-p-chlorobenzyl-8-methyl-1,2,3,4-
tetrahydrocarbazol-l-yl-acetic acid from Example 15
lZ~9577
5012P~5011A - 75 - 17323IA
as starting materials, the title compound was
prepared. [a]D = -51.6 (methanol) m.p.
196-198C.
_
Example 40
~ 9-p-Chlorobenzyl-8-methyl-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic acid
Following the method of Example 39, but
using l(-~ephedrine, the title compound was prepared.
10 [a]D = +45.9 (methanol) m.p. 135-197C.
Example 41
(-)-9-p-Chlorobenzyl-8-fluoro-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic acid
Following the method of Example 7, but using
d(~)ephedrine and 9-p-chlorobenzyl-8-fluoro-1,2,3,4-
tetrahydrocarbazol-l-yl-acetic acid from Example 19
as starting materials, the title compound was
prepared. [a]D = -62.1 (methanol) m.p. 74-75C.
Example 42
~ 9-p~Chlorobenzyl-8-fluoro-1,2,3,4-tetrahydro-
carbazol-l-yl-acetic acid
Following the method of Example 41, but
25 using l(-)ephedrine, the title compound was prepared.
[a]D = f65.2 (methanol) m.p. 94-94.5C.
ExamPle- 43
2-(9-p-Chlorobenzyl-6,8-difluoro-1,2,3,4-tetrahydro-
carbazol-l-yl)ethanol
Following the procedure of Example 5, but
using a mixture of 9-p-chlorobenzyl-6,8-difluoro-
g577
5012P~5011A - 76 - 17323IA
1,2,3,4-carhazol-1-yl-acetic acid ethyl and isopropyl
esters from Example 34 as starting materials, the
title compound is obtained.
- 5 Exam~le 44
(-) or t+) 2-(9-p-Chlorobenzyl-6,8-difluoro-1,2,3,4-
tetrahYdrocarbazol-l-yl)sthanol
Following the procedure of Example 5, but
using (-) or (+) 9-p-chlorobenzyl-6,8-difluoro-
10 1,2,3,4-tetrahydrocarbazol-1-yl-acetic acid from
Example 37 or 38 as starting material, the title
compounds are obtained.