Note: Descriptions are shown in the official language in which they were submitted.
BIOLOGICAL FLUID MEASURING DEVX OE
_ _ _
Technical Field
The present invention relates to devices having
replaceable membranes which cooperate with an electrode
assembly to determine the amount of a substance in a
biological fluid.
Bac~ nd of the Invention
The continuous measurement of substances in
biological fluids is of interest in the study and control of
metabolic disorders. Electrode systems have been developed
for this purpose whereby an enzyme-catalyzed reaction is
monitored by an electrochemical sensor. In such electrode
systems, the electrochemical sensor comprises an electrode
with potentiometric or amperometric function in close
contact with a thin layer containin~ an enzyme in dissolved
or insoluble form. The thin layer may also include a co-
enzyme.
In conventional practice, a semipermeable membrane
separates the thin layer of the electrode containing theenzyme ~rom ~he sample of biological ~luid that includes the
substance to be measured. The electrochemical sensor
measures the concentration of the substance involved in the
enzyme reaction. For example, the concentration of a co-
enzyme or a reaction product can be determined. Thisconcentration may be related to the substrate concentration
in the sample by its stoichiometric relationship and by
calibration of the eleotrode system.
.
:
:
- ' . ' '
, ~ - " :, .. . . . ..
.
- , , - , . .
.
A number of enzyme electrodes have been developed,
and the operation of those electrodes varies depending on
the nature of the enzyme reaction and the particular
substance being measured. For example, enzyme electrodes
include those that measure: (1) a reactant or product of
the enzyme reaction; (2) the consumption of a co-enzyme
based on the decrease of its initial concentration and (3)
the amount of the reduced or oxidized form of a co-enzyme
produced during the enzyme reaction.
The operation of a particular enzyme electrode
depends on a number of parameters including diffusion
processes, kinetics of the enzyme reaction and the type of
electrochemical sensor. In particular, the operation of the
electrode can be affected by the diffusion of substances
through the semipermeable membrane.
Electrode systems that include enzymes have been
used to convert amperometrically inactive substances into
reaction products which are amperometrically active.
Specifically, in the analysis of blood for glucose content,
glucose (which is relatively inactive amperometrically) may
be catalytically covered by the enzyme glucose oxidase into
the presence of oxygen and water to gluconic acid and
hydrogen peroxide. Hydrogen peroxide is anodically active
and produces a current which is proportional to the
concentration of hydrogen peroxide in the blood sample and
thus to the concentration of glucose in the sample.
In a sample of undiluted whole blood, however, a
molar excess of plasma glucose is present relative to the
amount of plasma oxygen. As a result, if a semipermeable
membrane is not included over the enzyme, the concentration
of glucose in the sample relative to the concentration of
oxygen will be so high that the glucose oxidase-catalyzed
.
~L2~
reaction of glucose and oxygen to gluconic acid and hydrogen
peroxide will be oxygen limited.
The effect of an oxygen limited reaction is that
the range of glucose concentrations that can be measured
with such an electrode is very limited. In particular,
linearity is not achie~ed above minimal concentrations of
glucose. In a clinical setting, linear glucose levels must
be obtained at glucose concentrations of at least up to
about S0~ milligrams per deciliter (mg/dl). Without a
semipermeable membrane over the enzyme, linear glucose
levels can be obtained only up to about 40 mg/dl. Thus, the
purpose of the membrane over the enzyme in a glucose
sensing electrode system is to limit the amount of glucose
that passes or diffuses through the membrane. This extends
the upper limit of linearity of glucose measurement from a
low value without the membrane to a high value with the
membrane.
The two fundamental diffusion processes by which
a semipermeable membrane can limit the amount of a substanc~
that passes therethrough are diffusion through the
semipermeable membrane as a monolithic, homogeneous
structure and diffusion through the semipermeable membrane
as a porous structure. The processes of diffusion of
substances through these different types of membranes
2~ differ considerably~
~ semipermeable membrane can comprise a porous
structure consisting of a relatively impermeable matrix that
includes a plurality of "microholes" or pores of molecular
dimensions. Transfer through these membranes is primarily
due to passage of substances through the pores. In other
words, the membrane acts as a microporous barrier or sieve.
Examples of materials that may be used to form
such membranes include polyethylene, polyvinylchloride,
.
.
.
6~
--4--
tetrafluoroethylene~ polypropyl~ne, cellophane,
polyacrylamide, cellulose acetate, polymethyl methacrylate,
silicone polymers, polycarbonate, cuprophane and collagen
Selectivity in such a membrane can be explained on
the basis of the molecular size of the diffusing
substances. For substances much smaller than the diameter
of the pores, passage of the substance through the membrane
is relatively unimpeded. As the effective molecular
diameter of the substance approaches the diameter of the
pore, the pore will exert a drag on the diffusing substance,
reducing its permeability to a value lower than that
expected on the basis of the membrane porosity. If the
molecules of the substance are too large, they will not pass
through the membrane at all.
Since transfer is due primarily to passage of the
substance through pores, the permeability is directly
related to the size of the pores and to the molecular volumo
of the diffusing substance. As a result, there is little
selectivity in the separation of two chemically or
structurally related molecules, except when their molecular
size is approximately the same as the size of the pore.
When this occursl there is the possibility that forces
acting between the substance and the surface o~ the pore
channel may influence the rate of transfer.
Also, the upper size limit to diffusion will be
determined by the largest pore diameter, and the overall
diffusion rate will depend on the total number of pores for
movement of the substance.
Passage of a substance through a monolithic,
~0 homogeneous membrane, on the other hand, depends upon
selective dissolution and diffusion of the substance as a
solute through a solid, non-porous ~ilm. As used herein,
the term "monolithic" means substantially non-porous and
53
having a generally unbroken surface. The term
"homogeneous", with reference to a membrane, means having
substantially uniform characteristics from one side of the
membrane to the other. However, a membrane may have
heterogeneous structural domains, for example, created by
using block copolymers, and still be characterized
functionally as homogeneous with respect to its dependence
upon dissolution rather than sieving to effect separation of
substances. A monolithic membrane can thus be used to
selectively separate components o~ a solution on the basis
of properties other than the size, shape and density of the
diffusing substances. The membrane acts as a barrier
because of the preferential diffusion therethrough of some
substance (a solute).
Despite advances in membrane technology, devices
that include semipermeable membranes which have been used to
detect and measure the presence of a substance in a
biological fluid have generally been restricted to
laboratory environments. This is because the devices are
generally large and complex and require extensive training
to operate. In addition, these devices have been somewhat
limited because of the difficulty in replacing a membrane
used with the electrode.
A need exists for an improved device that
selectively measures the presence and the amounts of
particular substances in biological fluids. Such a device
should accurately measure the amount of substance in a
sample without dilution or pretreatment of the sample. In
addition, a basis for selecting appropriate membrane
3~ materials for use in such devices is needed. The device
should also be easy to use and provide a means for replacing
the membrane as necessary.
Summary o~ the_Tnven~ion
The present invention relates to a biological
fluid measuring device which permits rapid and accurate
determination and measurement of the amount of a particular
substance in a biological fluid such as blood.
Generally, the device includes a main housing
carrying electronic circuit means and at least one
electrode. In a preferred embodiment, at least two
electrodes are carried by the housing. A disposable
cartridge is removably mounted on the housing. It is, of
course, possible to design a device wherein one electrode is
carried by the housing and a second electrode is carried by
another component of the device, as by the cartridge. For
ease of description, however, the present device will be
described as including at least two electrodes carried by
the housing.
The cartridge includes a membrane which is
operably associated with the electrodes when the cartridge
is mounted on the housing. The cartridge also includes
means for protecting the membrane from the ambient
surroundings when the device is not in use. In addition,
means is provided for maintaining the membrane in operative
contact with the electrodes by osmotic pressure.
In a preferred embodiment, the housing includes
an instrument case having an upper portion and a lower
portion which together define a cavity. The electronic
circuit is contained within the cavity. The electrode is
carried by a post which extends upwardly from a base surface
defined by the upper portion of the case.
The cartridge preferably includes a body portion
which is releasably mounted on the upper portion oP the case
and a cover which is movably mounted as by a hinge on the
body portion. The body portion preferably defines a
2~
sidewall which together with the membrane defines a well.
The well receives the biological fluid such as a droplet of
blood. Because of the particular design of the present
invention, the well can be particularly small thereby
S minimizing the amount of biological fluid sample needed for
analysis. In the case of blood, this minimizes both the
emotional and physical trauma to the patient.
The body portion preferably includes a collar
which extends about the post such that, when the cartridge
is mounted on the case, the membrane is placed in contact
with the electrodes and is stretched over the surface of the
electrodes. This ensures good operative contact between the
electrodes and the membrane at the electrode-membrane
interface.
In a preferred embodiment, a liquid means for
maintaining the membrane in operative association with the
electrodes at the electrode-membrane interface by osmotic
pressure is received in the well above and in contact with
the membrane. This liquid includes an osmotic agent which
does not permeate the membrane and is capable of applying an
osmotic pressure across the membrane. This osmotic pressure
ensures constant stable proximity of the membrane to the
electrode to maintain stable contact during use, and thus
enhances sensor stability. In effect, the osmotic pressure
maintains stability of the diffusion path from sample to the
electrode by gently forcing the gel-like membrane to
maintain contact with the electrode surface. This prevents
the accumulation of additional unwanted electrolyte solution
between the membrane and the electrode surface. The osmotic
pressure effect withdraws solvent molecules from the
hydrated membrane and from the electrode-membrane interface
that might otherwise mechanically destabilize the ~iffusion
path from sample to electrode surface. This diffusion path
--8--
must maintain constant length in order for the sensor to
exhibit analytical stability.
The electrodes, the supporting structure for the
electrodes such as the post, the pressure means and the
membrane together form an electrode assembly . The membrane
is a multilayered structure including layers formed of
materials such as polyethylene, polyvinylchloride,
tetrafluorethylene, polypropylene, cellophane,
polyacrylamide, polymethyl methacrylate, silicone polymers,
polycarbonate, cuprophane, collagen, polyurethanes and block
copolymers thereof. The membrane prevents direct contact of
the fluid sample with the electrodes, but permits selected
substances of the fluid to pass through the membrane for
electrochemical reaction with the electrodes. To ensure
electrochemical reaction, the surface of the membrane layer
nearest the electrode is preferably coated with a water-
swellable film to maintain electrolyte at the electrode-
membrane interface, and thereby improve the sensitivity of
the measurement.
In a preferred embodiment, the membrane is a semi-
permeable multilayered membrane having at least one layer
formed of a nonporous block copolymer having hydrophobic
segments and hydrophilic segments that limits the amount of
a substance passing therethrough and a second layer
including an enzyme that reacts with the substance to form a
product.
~ n a more preferred embodiment, the electrode
assembly comprises an electrode, a first (outer) layer of a
block copolymer that limits the amount of a hydrophilic
substance passing therethrough, a second ~intermediate)
layer of a block copolymer including an enzyme bound to the
first layer and a third (inner) layer of a block copolymer
bound to the second layer and covering the surface of the
$~i~i3
electrode. The third layer is permeable to relatively low
molecular weight substances, such as hydrogen peroxide, but
restricts the passage of higher molecular weight substancss.
In a particularly pre~srred embodiment, the
unbound surface of the third (inner) layer is coated with a
semipermeable, substantially solid water-swellable gel-like
film. The film comprises the aqueous reaction product of a
polyurethane having anionic carboxyl functional groups and
non-ionic hydrophilic polyether groups crosslinked in the
presence of polyvinylpyrrolidone. The coating, which
preferably has a dry film thickness of about 0.1 mil to
about 0.5 mil, enhances and maintains the selectivity of the
molecular separation of the inner layer and thereby improves
the sensitivity of the measured amount of product.
The preferred polymers which form the above-
described membrane layers and the coating are selected and
based on permeability and water swelling. An accepted
industry test procedure for determining the permeability of
a coating or membrane is ASTM E 96 which measures the
moisture-vapor transmission rate of a material. tAmerican
Society for Testing and Materials, Philadelphia, PA).
As used herein, the moisture-vapor transmission
rate (MVTR) of a membrane material is expressed in grams per
square meter per 24 hours and is one means of defining the
water resistance of a material.
The MVTR of a material ma~ be expressed by the
equation: ~
MVTR = Q
at
wherein the letter "Q" represents the amount of water vapor
(in grams) that permeates the film; the letter "a"
represents the ~ilm area (in square centimeters) and the
653
- 1 O-
letter "t" represents the time (in hours at a designated
thickness). This value can be convexted to grams of water
per square meters per 2~ hours. The MVTR values identified
herein are for membranes tha~ are about 1 mil thick.
The MVTR of the first (outer) layer described
herein should be greater than about 4,000 grams per square
meter per 24 hours, preferably greater than about 5,000
grams per square meter per 24 hours.
The MVTR of the third (inner) layer of the
assembly should be from about 500 to about 4,Q00 grams per
square meter per 24 hours, preferably from 1,000 to about
3,500 grams per square meter per 24 hours~
It will, of course, be understood that the above
MVTR values for each layer can be varied or optimized
depending on the substance to be measured and the enzyme
that is employed.
In a preferred embodiment, the enzyme is glucose
oxidase and the substance to be measured is glucose. The
amount of glucose, for e~ample, in an aliquot of undiluted
whole blood, is determined by measuring the amount of
hydrogen peroxide produced during the oxidation of glucose
to gluconic acid by the enzyme.
Preferred polymers for the membrane layers may
also be selected by studying water uptake or the swelling of
the polymer. This is normally measured by soaking the
polymer sample in water at a controlled temperature and
exposure conditions until equilibrium is achieved followed
by rapid drying of surface water and weighing of the polymer
sample. Subtracting the dry weight from the swelled weight
and then dividing by the dry weight and multiplying the
value obtained by 100 provides the swell rate as a percent
of dry weight. The swell rate of the first (outer) layer
described herein should be greater than about 5 percent and
iS3
preferably greater than about 10 percent. ~1he swell rate of
the third (inner) layer should be less than about 5 percent
preferably less than about 3 percent.
The swell rate of the coating should be greater
than about 5 percent and preferably greater than about 10
percent.
The present invention, however, is not limited to
the measurement of glucose concentrations, and other enzyme-
substrate systems can be used. Examples of other enzymes
include galactose oxidase, uricase, cholesterol oxidase,
alcohol oxidase, lactose oxidase, L-amino acid oxidase, D-
amino acid oxidase, xanthine oxidase and ascorbic acid
oxidase.
Nonetheless, to demonstrate the improvement of
this invention over other membxane systems, the invention
will be described in terms of measuring glucose
concentrations based on the production of hydrogen peroxide
by the action of glucose oxidase.
The membrane systems currently available are based
on semipermeable membranes with microholes or pores. With
these membranes there is little selectivity in the
separation of substances that are rather close in size,
except when the molecular diameters of the substances
approach the diameters of the pores. When this occurs,
forces between the substance and the surface of the pore
channel may influence the rate of transfer.
The layers of the preferred multilayered membrane
described herein each comprise homogeneous, monolithic
membranes and differ in compos1tion, structure and
operation from conventional microporous membranes. This
represents a substantial improvement over current membrane
systems in terms of ease of manu~acturingr lifetime of
~Z~6~
enzyme activity, and the ability to measure the
concentrations of substances in undiluted samples.
In addition, the water~swellable coating on the
layer of the membrane closest to the electrode represents a
substantial improveMent in sensor sensitivity by maintaining
electrolyte in the electrolyte space at the membrane-
electrode interface. ~his improvement also provides a more
stable operation of the device by overcoming electrode
start-up problems and drifting problems caused by inadequate
electrolyte and the excessive hydrophobicity of the
interface environment. Also, by coating the membrane in the
above manner, the yield of usable membrane manufactured
increases.
Thus, the sensitivity of the device of this
invention is improved by the use of a multilayered membrane
having the unbound surface of its inner layer coated
intermediate to and covering the electrode and by
maintaining the membrane in contact with the electrode by
osmotic pressure during use. This improvement represents a
substantial advantage over current membrane devices in terms
of sensor sensitivity, stability of operation, overcoming
electrode start-up problems and overcoming interference from
mechanical or osmotic disturbances at the electrode~membrane
interface.
In summary, passage of substances through the
membranes described herein depends upon dissolution and
diffusion of the substance through a solid, non-porous film.
Components of a solution can be separated on the basis of
properties other than the size, shape and density of the
3~ diffusing substance.
~13-
Brief Description of the Drawinqs
FIGURE 1 is a perspective view of biological fluid
measuring device of the present invention showing a
cartridge received on a housing;
FIGURE 2 is an exploded perspective view of the
device of FIGURE l showing the cartridge above and separated
from the housing;
FIGURE 3 is a top plan view of the device of
FIGURE l showing the cover of the cartridge open and the
1Q membrane exposed;
FIGURE 4 is a side elevational view taken in
section along the plane 4-4 of FIGURE l;
FIGURE 4a is an enlarged view of the portion of
FIGURE 4 that i5 outlined in phantom;
FIGURE 5 is a top plan view of a second embodiment
of the electrode assembly;
FIGURE 6 is a side elevational view showing a
device including the electrode assembly of FIGUR~ 5 taken in
section along a plane similar to that shown as plane 4-4 of
FIGURE l; and
FIGURE 7 is an electronic circuit diagram in block
form.
Detailed Description of the Invention
The present invention relates to a biological
fluid measuring device which permits rapid and accurate
measurement of the amount of particular substance in a
biological fluid. One particular use of the present
invention is to determine the level of glucose in blood
using only a small sample. This is a particularly important
measurement for individuals having diabetes, and the device
is a substantial development over devices that are now being
used by individuals with diabetes to determine glucose
levels.
5;3
-14-
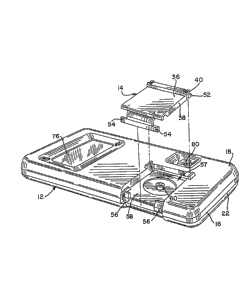
~ eferring to FIGURES 1 and 2, the measuring device
comprises a main housing 12 and a cartridge 14 which is
removably mounted on the housing (see FIGURE Z ) . This
permits the cartridge 14, which can be made disposable, to
be easily replaced as needed. The construction of the
cartridge will be described in detail with reference to
FIGURES 4 and 4a. The housing 12 includes a case 16 having
an upper portion 18 and a lower portion 22. The upper
portion 18 and lower portion 22 are connected together by
any particular fastening means such as several screws which
are not shownO
Referring also to FIGURES 3 and 4, the main
housing 12 also includes electronic circuit means which can
be carried in part on a circuit board 24. The electronic
circuit means is preferably maintained in a cavity 26 which
is defined by the case 16. The housing also includes at
least one electrode. In the embodiment shown in FIGURE 4,
three electrodes 28, 30 and 32 are shown.
The operation of these electrodes is discussed in
more detail below. The cartridge 14 includes a membrane 34
which is operably associated with the electrodes 28, 30, and
32 when the cartridge is removably mounted on the housing
12. In addition, the cartridge 14 can include means for
maintaining osmotic pressure across the membrane 34 during
use as also discussed in more detail below. The cartridge
14 also includes means for protecting the membrane when not
in use. The protection means is preferably a cover 36 which
is movably mounted on a body portion 38 of the cartridge 14.
Alternatively~ the cover 36 may be mounted on the case 16.
In the illustrated embodiment, the cover 36 is movably
mounted on the body portion 38 by a hinge assembly 40.
Generally, the cover 36 has a ~irst position such
as shown in FIGU~ES 1 and 4 in which it protects the
~2~
membr~ne 34 and a second position such as shown in FIGURE 3
which permits access to the membrane. Access to the
membrane 34 is necessary to place the biological fluid
sample on the membrane for analysis.
S As is more clearly shown in FIGURE 4a (which is an
enlarged view of the area outlined in phantom in FIGURE 4),
the body portion preferably defines an opening having a
sidewall 42 which together with a portion of the membrane 34
defines a well 44 having a bottom 45. The bottom 45 of the
well is defined at least in part by ~he membrane 34. For
use, a liquid comprising an osmotic agent is received in the
well 44 above and in contact with the membrane 34 in an
amount sufficient to apply osmotic pressure across the
membrane. The biological fluid sample is then placed in the
well 44 for analysis~
An osmotic pressure of about 30 to about 90
millimeters, preferably about 70 millimeters, mercury (Hg~
column height is exerted across the membrane at ambient room
temperature.
Generally, the sidewall 42 defines an opening of
less than 4 millimeters in diameter and the well 44 has the
depth of less than 2 millimeters. As a result, the well has
a volume of less than about 0.1 to about 0.2 ~ubic
centimeters. This substantially minimizes the size of the
biological fluid sample necessary for analysis down to
sample sizes as small as about five microliters. Because
the size of the sample can be particularly small,
compensation for temperature changes during analysis which
was often necessary with previous devices can be avoided.
For purposes of providing osmotic pressure across
the membrane, prior to placing the biological fluid sample
in the well 44, the surface of the membrane is first
"cleaned" by blotting with an absorbent tissue. ~hen a drop
6~3
~16-
of aqueous buffered cleaning and storage solution
containing an osmotic agent is placed in the sample well.
Preferably, the osmotic agent is a water-soluble nonionic
polymer that is substantially solid at room temperature.
Suitable osmotic agents have a weight average molecular
weight of between over about 800 and about 20,000 molecular
weight, preferably between about 1,500 and about 15,000,
more preferahly between about 3,000 and about 12,000. A
particularly preferred aqueous cleaning and storage
solution applies an osmotic pressure across th~ membrane of
about 70 millimeters mercury column height at ambient room
temperature and pressure. The volume of this liquid
pressure means present in the well 44 is substantially
minuscule. Nevertheless, the liquid received in the well 44
and in contact with the membrane 34 during storage or
storage/use, enhances the measuring sensitivity and
stability of the electrodes 28, 30 and 32 by 1) holding the
membrane 34 against the electrodes 28/ 30 and 32 by a
relatively consistent osmotic pressure thereby maintaining
optimal and stable contact at the electrode-membrane
interface 64.
A preferred liquid pressure means comprises, as
the osmotic agent, a homopolymer of polyvinylpyrrolidone
dissolved at about ~ weight percent (about 4 millimolar) in
water. An exemplary homopolymer is sold under the trademark
BASF K-17PF by BASF Wyandotte Corporation (Parsippany, NJ)
which is stated to have a number average molecular weight of
about 2,500. Each millimole of concentration difference
applies about 17 millimeters Hg column height pressure, so
the foregoing liquid pressure means prepared from BASF K-
17PF applies about 70 millimeters Hg column height pressure
across the membrane.
Alternatively, a copolymer of N-vinylpyrrolidone
and vinyl acetate or like water-soluble copolymer of N-
vinylpyrrolidone can be used.
Other suitable osmotic agents include water~
soluble linear ethylene oxide polymers, such as polyethylene
glycols having a terminal hydroxyl group or terminal methoxy
group having a weight average molecular weight distribution
above about 800 to about 20,000, preferably about 900 to
about 4,000 and being substantially solid at ambient room
temperature.
Exemplary polyethylene glycols are commercially
sold under the family trademark CARBOWAX as a PEG and MPEG
series by Union Carbide Corporation, Industrial Chemicals
Division (Danbury, CT). A detailed description of the
properties of these polymers can be found in the CARBOWAX
Polyethylene Glycols, Product Information Bulletin F-4772M,
published in 1986 bv the Union Carbide Industrials Chemical
Division~
Particularly preferred is CARBOWAX 3350, a
solid polyethylene glycol having a molecular weight average
distribution of about 3000 to about 3700, a melting point of
about 54 to about 58 degrees C (about 129.2 to about 13604
degrees F) and a water solubility of about 67 weight percent
at 20 degrees C (about 68 degrees F).
The protection means of the cartridge 14
preferably also includes means for sealing the well 44 and
hence the operative portion of the membrane 34 at the bottom
45 of the well 44 from the ambient surroundings. This can
include a flexible gasket 46 which extends about the well 44
and cooperates with the body portion 38 and cover 36. The
gasket 46 is preferably mounted in a groove 48 defined by
the body portion 38 and is engaged by a ring 50 carried on
the cover 36.
When the cover is in its second or closed position
such as shown in FIGURE 4, the ring 50 engages the ~asket 46
to seal the well 44 and membrane 34 from the ambient
surroundings and to prevent dehydration of the membrane.
This also prevents damage to the membrane by physical
intrusion or dirt. The ring 50 is preferably provided with
a edged surface which bites into the gasket to provide a
particularly effective seal.
A retaining means is also provided for releasably
retaining the cartridge 14 and its body portion 38 on the
housing 12. The retaining means preferably includes a
detent 52 on the cartridge 14 which is received in a recess
53 defined by the upper portion 18 of the case 16. The
retaining means also pre~erably includes at least one, and
optimally, two wings 54 on the body portion 38 of the
cartridge 14 which are received in one or more slots 56 on
the case 16. (See, in particular, FIGURE 2). The slots 56
are generally perpendicular to the cover 36 so that opening
the cover will not disengage the wings 54 from the slots 56.
The upper portion 18 of the case 16 pre~erably
defines a recessed cell 57 (see FIGURE 2) into which the
cartridge 14 is received. The bottom portion of the cell
57 is defined by a base surface 58. The electrodes 28, 30,
and 32 preferably extend upwardly from the base surface 58.
The electrodes are preferably mounted within a post 60 which
supports the electrod~s as they extend upwardly of the base
surface 58~ The post is preferably generally annular in
design with the interior portion thereof filled with an
electrically nonconductive support material 62 such as a
hardened polyepoxide-containing resin. The electrically
nonconductive support material 62 and the top portions of
the electrodes define a membrane contact surface 64. The
membrane contact surface 64 is preferably generally dome-
- ~2~653
- 1 9-
shaped such that the membrane 34 can be stretched over the
contact surface to more e~fectively place the membrane in
operative association with the electrodes.
In order for the sensitivity of the electrode to
function properly, electrolyte must be present and
maintained between the membrane 34 and the electrodes at the
membrane contact surface 64. In prior devices, variations
in electrolyte volume from inconsist~nt osmotic pressure
above the membrane could result in loss of full sensitivity
or changes in sensitivity owing to variations in the
relatively thin electrolyte layer at the membrane-electrode
interface 64. Also, mechanical disturbances could cause
changes in the electrolyt~ media at the membrane-electrode
~nterface 64, where the surface of the membrane and that of
the electrode support material (i.e., epoxy resin) were both
substantially hydrophobic. In the present device, however,
this problem is overcome by including a water-swellable
coating on the surface of the membrane layer nearest to and
covering the electrode as discussed in more detail below.
Alternatively, this coating can be applied to the membrane-
electrode interface 64, but for convenience, the coating is
preferably applied to the disposable and more easily
renewable membrane.
The water-swellable coating on the surface of the
membrane layer at the membrane contact surface 64 provides a
substantially consistent electrolyte volume. This improves
the sensitivity of the measurement by about 2:1 over that of
prior devices. In addition, less sensitivity drift is seen
providing a more stable operation. Unlike prior devices
using standard membranes, the device of this invention using
the coated membrane provides adequate signals to the sensory
microcomputer during start-up procedures
~2~ 3
-20-
The body portion 38 preferably also includes a
collar 66 which extends opposite of the well 44 with
respect to the membrane 34 where it defines the bottom 45 of
the well. As shown in FIGURE 4, the collar 66 extends
about the post 60. The membrane 34 is preferably attached
to a retaining surface 65 by an adhesive at the edge of the
collar 66 with the portion of the membrane within the collar
being free to move. As the cartridge 14 is mounted on the
housing 12, the membrane is then stretched over the post 60
providing continuous contact between the membrane 34 and the
contact surface 64.
The co~er 36 is preferably provided with a closure
means 72 such as one or more latches which enga~e the body
portion 38. Generally, the force necessary to disengage the
closure means 72 from the body portion 38 should be less
than that necessary to disengage the wings 54 from the slots
56. In this manner, the operator can easily open the cover
36 without accidentally disengaging the cartridge 14 from
the main housing 12.
The electrodes 28, 30 and 32 together with a
support assembIy such as the post 60 and the membrane 34
comprise the electrode assembly. In addition, during use the
electrode assembly includes means for maintaining osmotic
pressure across the membrane 34 as discussed earlier. It is
this assembly which is contacted with the body fluid sample
for analysis. The electrode assembly 74 is operably
associated with the electronic circuit means which analyzes
the current from the reaction of the components in the body
fluid with the electrodes. The electronic circuit means is
in turn operably associated with display means such as a
liquid crystal display 76 to indicate amount of glucose in
the fluid sample.
~9~6X3
Referring to FIGURE 5, another embodiment of the
electrode assembly 74 i5 shown wherein the three electrodes
28, 30 and 32 are deposited onto a ceramic surface 66. An
electrically nonconductive material 62 is applied as a
coating over the electrodes to form an insulating barrier.
portion of each electrode, however, is not coated to form
a membrane contact surface 64 so that a membrane can be
applied over the electrodes in operative contact therewith.
FIGURE 6 shows the electrode assembly 74 of FIGURE
S in the device. In particular, the electrode assembly
including the membrane 34 is positioned within a recess 78
in the base surface 58 of the recessed cell 57. The
cartridge 14 is then positioned within the recessed cell as
described above whereby the bottom 45 of the well 44 in the
body portion 38 of the cartridge contacts the membrane 34.
A cover 36 (as shown in FIGURE 4) can be attached to the
body portion 38 to protect the membrane when the device is
not in use.
The three electrode configuration in combination
with the osmotic pressure across the membrane and the
chemical reactions occurring in the multilayered membrane,
its coating and on the electrode make possible consistent
electrode behavior and, in partlcular, performance of the
reference electrode that is stable with time. It is well
know in the art that silver/silver chloride electrodes
provides a stable reference system for electrochemical
sensors.
A silver/silver chloride electrode is typically
formed by treating a silver surface with an oxidant and
chloride ions (such as by treatment with ferric chloride or
a neutral hypochlorite solution), by electrochemical plating
of chloride ions onto a silver surface or by the mechanical
~2~53
-22-
forming of silver and silver chloride by sintering or
similar processes.
When this type of electrode is used in a two
electrode configuration with the reference cathode,
chloride ions will be lost from the reference electrode
which e~entually leads to unstable electrode behavior.
According to the present invention, permanent stable
reference electrode behavior is achieved when the hydrogen
peroxide produced in the membrane oxidizes the silver metal
to silver oxide which is then converted to silver chloride
by chloride ion. Advantaqes include ease of manufacturing
of the electrode, self-forming and self-maintaining
electrode behavior and long-term reference electrode
stability.
The relatively low power needs of the present
electrode system, as compared to the relatively high power
needs of conventional light reflectance-based methods,
permit use of a very compact, lightweight device having an
extended battery life. CMOS circuitry is used throughout
the device and provides a use-dependent battery life of one
to two years.
A representative electronic circuit for the device
is shown in FIG~RE 7, but other circuits may also be
employed. See, for example, Implantable Sensors for Closed
2S Loop Prosthetic Systems, edited by Wen H. Ko, ch. 12, pages
167-175, Futura Publishing Co., Mount Xisco, N.~. (1985)~
During operation of the device, glucose rom the
blood sample produces a current flow at the working
electrode 28. Equal current is provided by a counter
electrode 30 in a reference circuit 82. The current is
converted in an analog section ~4 by a current to voltage
129~5~
converter to a voltage which i9 inverted, level-shifted and
delivered to an Analog/Diyital (A/D) conver~er 8~ in the
microprocessor 88. As part of the calibration circuit
means, the microprocessor can set the analog gain via its
control port 90. The A/D converter is activated at one
second intervals. The microprocessor looks at the converter
output with any number of pattern recognition algorithms
known to those skilled in the art until a glucose peak is
identified. A timer is then activated for about 30 seconds
at the end of which time the difference between the first
and last electrode current values is calculated. This
difference is then divided by the value s~ored in the memory
during instrument calibration and is then multiplied by the
calibration glucose concentration. The glucose value in
milligram percent or millimoles per liter is then displayed
on the LCD display screen 94~
During this operation sequence, prompts or
messages may be displayed on the LCD screen to ~uide the
user through the calibration and sample measurement
procedures. In addition, prompts may be displayed to inform
the user about necessary maintenance procedures, such as
"Replace Sensor" or "Replace Battery." An on/off button
80 initiates the operation and calibration sequences.
As indicated above the membrane is a monolithic
homogeneous, multilayered structure including layers formed
of materials such as polyethylene, polyvinylchloride,
tetrafluoroethylene, polypropylene, cellophane,
polyacrylamide, polymethyl methacrylate, silicone polymers,
polycarbonate, cuprophane, colla~en, polyurethanes and block
copolymers thereof.
The layer o~ the multilayered membrane that is
intended to be nearest to and cover the electrode can be
coated w~th a semipermeable water-swellable, su~stantially
-24-
solid gel-like film to maintain hydrophilicity at the
electrode-membrane interface. This coating also enhances
the stability of the third layer o~ this invention by
protecting and supporting the third layer. Tha electrolyte
between a hydrophobic membrane and electrode may experience
a large pH qradient due to the electrochemical activity of
the electrode, thus damaging the third layer. The buffered
electrolyte solution contained in this additional
hydrophilic coating adjacent to the third layer protects
against such pH-mediated damage. In addition, higher
manufacturing yields of usable membranes are achieved by
coating the membrane as disclosed herein.
Preferably the coating comprises a flexible water-
swellable film having a "dry film" thickness of about 0.l
mil to about 0.5 mil, preferably about 0.25 mil. "Dry film"
thickness means the thickness of a cured film cast from a
coating formulation onto the surface of the membrane by
coating techniques known in the coating arts. The coating
formulation comprises a premix of film-forming polymers and
a crosslinking agent and is curable upon the application of
moderate heat.
Suitable coatings are formed of a curable
copolymer of a urethane polymer and a hydrophilic film-
forming polymer. Particularly preferred coatings are formed
of a polyurethane polymer having anionic carboxylate
functional groups and non-ionic hydrophilic polyether
segments, which is crosslinked in the present of
polyvinylpyrrolidone and cured at a moderate temperature of
about 50 degrees C ~about 122 degrees F).
Particular~y suitable for this purpose arè aqueous
dispersions of fully reacted colloidal polyurethane
polymers having cross-linkable carboxyl functionality sold
under the trademark BAYBOND by Mobay Corporation, a Bayer
653
-25~
U.S.A., Inc. Company, Coatings Division (Pittsburgh, P~).
These polymers are supplied in dispersion grades having a
polycarbonate - polyurethane backbone containing carboxylate
groups identified as XW-121 and XW-123; and a polyester-
polyurethane backbone containing carboxylate groups,identified as XW-110-2. A detailed description of the
properties of these aqueous polyurethane dispersions can be
found in the Technical Summary publication saybond Aqueous
Polyurethane Dispersions, published by the Coatinq Division
of Mobay Corporation (undated)J
Particularly preferred is BAYBOND 123, described
as an aqueous anionic dispersion of an aliphate
polycarbonate urethane polymer and sold as a 35 weight
percent solution in water and cosolvent N-methyl-2-
pyrrolidone. A description of the properties of BAYBOND 123
is found in the Product Data sheet dated 9/87 and Material
Safety Data Sheet dated 9/7/87 published by the supplier
and incorporated herein by reference.
Polyvinylpyrrolidone is also particularly
preferred as a hydrophilic water-soluble polymer and is
available commercially in a range of viscosity grades and
range of number average molecular weights from about 18,000
to about 500,000, under the trade designation PVP K
homopolymer series by ~ASF Wyandotte Corporation
(Parsippany, NJ) and by GAF Corporation (New York, NY).
Particularly preferred is the homopolymer having a number
average molecular weight of about 360,000 identified as
PVP-K90 by the suppliers, and sold as a powder.
Also suitable are hydrophilic, film-forming
- copolymers of N-vinylpyrrolidone, such as a copolymer of N-
vinylpyrrolidone and vinyl acetate, a copolymer of N-
~2~ 653
-26-
vinylpyrrolidone, ethylmethacrylate and mekhacrylic acid
monomers, and the like.
The polyurethane polymer is crosslinked in the
presence of the polyvinylpyrrolidone by preparing a premix
of the polymers and adding a cross-linking agent just prior
to the production of the membrane. Suitable cross-linking
agents can be carbodiimides, epoxides and
melamine/formaldehyde resins. Carbodiimide is preferred. A
suitable and preferred carbodiimide crosslinker is sold
under the trademark UCARLNK XL-25 by Union Carbide
Corporation, Solvent Division (Chicago, IL). The properties
of this crosslinking agent are found in the product
specification brochure titled "UCARLNK XL-25SE in UCAR PM
ACETATE."
The flexibility and hardness of the coating can
be varied as desired by varying the dry weight solids of the
components in the coating formulation. The term "dry weight
solids" means the dry weiyht percent based on the total
coating composition after the time the crosslinker is
included. A preferred useful coating formu]ation can
contain about 6 to about 20 dry weight percent, preferably
about 8 dry weight percent, polyvinylpyrrolidone; about 3 to
about 10 dry weight percent preferably about 5 dry weight
percent cross-linking agent; and about 70 to about 9l weight
percent, preferably about 87 weight percent of a
polyurethane polymer, preerably a polycarbonate~
polyurethane polymer. The reaction product of such a
coating formulation is referred to herein as a water-
swellable copolymer of polyurethane and
polyvinylpyrrolidone.
In a particularly preferred embodiment, the
membrane is a semi-permeable multilayered membrane having at
least one layer formed of a nonporous block copolymer having
~2~ 3
-27-
hydrophobic segments (such as silicone polymer segments,
aromatic and aliphatic polymer segments, polypropylene oxide
segments, polytetramethylene oxide sagments and the like)
and hydrophilic segments (such as polyoxyethylene segments,
polyvinylpyrrolidone segments, polyvinyl alcohol segments
and the like) that limits the amount of a substance passing
therethrough and a second layer including an enzyme that
reacts with the substance to form a product.
The first layer limits the amount of a substance
in a fluid that can pass therethrough. The substance can
react with the enzyme in the second layer to produce one or
more reaction products. A third layer that is permeable to
one of the reaction products, but which restricts the
passage of other materials can also be used.
1S The ability of each layer to limit the amount of a
molecule that can pass therethrough may be expressed in
terms of the moisture-vapor transmission rate ~MVTR) and
water swelling of the material that forms the layer. As
used herein, the MVTR of a material is measured as described
in ASTM E 96, the procedure of which is incorporated herein
by reference.
The MVTR of the block copolymer of the first layer
should be greater than about 4,000 grams per square meter
per 24 hours, preferably greater than about 5,000 grams per
square meter per 24 hours. The water swelling of this layer
should be greater than about 5 percent.
The MVTR of the block copolymer of the third layer
should be from about 500 to 4,000 grams per square meter per
24 hours. The above values relate specifically to layers
that are employed to measure the amount of glucose in a
biological sample. It will be understood that block
copolymers having different MVTR values can be used to
measure the amounts of other substances in a biological
,
6S3
sample and the description o~ glucose measurement is only
illustrative.
The most pr~ferred membranes of this invention are
formed of polyurethanes which, of course, include urethane
groups and the polyurethane ureas which also include urea
groups. The polyurethanes and the polyurethane ureas of
the present membrane system are based on poly(oxyalkylene)
glycols including poly(oxyethylene) glycol. In accordance
with conventional usage, both types of polymers will be
referred to herein as polyurethanes.
Membranes of polyurethanes based on
poly(oxyalkylene) glycol display no predictable relationship
between molecular weight and permeability. The unique
separation observed with the present membranes may be
explained on the basis of substance-membrane or solute-
membrane interactions which tend to affect the partitioning
is not due only to the hydrophilic poly~oxyalkylene) glycol
or "soft" segment, but the hydrophobic or "hard" segment of
the block copolymer also contributes to the overall
selectivity.
Thus, by changing the structure of the
hydrophobic segment of the block copolymer and/or increasing
or decreasing the molecular weight of the poly(oxyalkylene)
glycol, the selecti~ity of the membrane sys~em can be
modified. In the membrane system of this invention, for
example, the use of two different membranes of block
copolyether urethanes based on poly(oxyalkylene) glycol
produces the desired selectivity for glucose and hydrogen
peroxide.
The preferred poly(oxyalkylene) glycols of this
invention include poly(oxyalkylene) glycols,
poly(oxytetramethylene) glycols and poly(oxypropylene)
glycols. A particularly preferred poly(oxyalkylene) glycol
is a poly(oxyethylene) glycol having a weight average
molecular weight in the range of about 1,000 to about
4,000.
.
,
.
-29-
The organic diisocyanates suitable for use in the
preparation of the polyurethanes of the present membranes
include 2,4-toluene diisocyanate, 2,6-toluene diisocyanate
and 4,4'-diphenylmethane diisocyanate. The use of 4,4'-
diphenylmethane diisocyanate is preferred~
Diols useful herein include ethylene glycol,
propylene glycol, l,5-dihydroxypentane, l,6-dihydroxyhexane,
l,l0-dihydroxydecane, l,4-cyclohexanediol, l,3-
dihydroxyneopentane and alpha, alphal-dihydroxy-p-xylene.
Diamines useful in the preparation of the
polyurethanes described herein include ethylene-diamine,
l,2- (and l,3-) propanediamine, and methylene-bis-o-
chloroaniline.
Example l
The polyurethanes are preferably prepared as block
copolymers by solution polymerization techniques as
generally described in Lyman, D.J., J. Polymer Sci., 45, 49
(1960). Specifically, a two-step solution polymerization
technique is used in which the poly(oxyethylene) glycol is
first "capped" by reaction with a diisocyanate to form a
macrodiisocyanate. Then the macrodiisocynate is coupled
with a diol ~or diamine) and the diisocyanate to form a
block copolyetherurethane (or a block copolyurethaneurea).
The resulting block copolymers are tough and elastic and may
be solution-cast in N,N-dimethylformamide to yield clear
films that demonstrate good wet strength when swollen in
water.
In particular, a mixture of 8.4 grams (0.006 mole)
poly(oxyethylene) glycol (CARBOWAX 15~0, Union Carbide
Corp., New York, NY) and 3.0 grams ~0.0l2 mole) 4,4'-
diphenylmethane diisocyanate in 20 milliliters (ml) dimethyl
sulfoxidet4-methyl-2-pentanone l50/50) is placed in a three-
3653
-30-
necked flask e~uipped with a stirrer and condenser and
protected from moisture. The reaction mixture is stirred
and heated at 110 degrees C (230 degrees F) for about one
hour. To this clear solution is added 1.5 grams (0.014
mole) 1,5-pentanediol and 2.0 grams (0.008 mole) 4,4'-
diphenylmethane diisocyanate.
After heating at 110 degrees C for an additional
two hours, the resulting viscous solution is poured into
water. The tough, rubbery, white polymer precipitate that
forms is chopped in a Waring Blender, washed with water and
dried in a vacuum oven at about 60 degrees C (about 140
degrees F). The yield is essentially quantitative. The
inherent viscosity of the copolymer in N,N-dimethyl
formamide is 0.59 at 30 degrees C (at a concentration of
about 0.05 percent by weight).
Example 2
A membrane formed of a homogeneous, nonporous
block copolymer may be prepared as follows. Polymerization
is carried out in a 2-liter glass flask with a detachable
top containing five inlets. The inlets provide for nitrogen
passage, condenser attachment, stirring, thermometer
placing, and ingredient addition. A regulated flow of
oxygen-free nitrogen passes from a cylinder, throuqh the
apparatus, into a water trap, and to the drain. The
contents of the reaction flask are stirred by a Teflon blade
connected to an electric motor running at 350 rpm. Air is
excluded by a mercury seal. Heat is supplied by an electric
mantle and temperature recorded by placing a thermometer in
the flask contents. A dropping funnel is used for the
addition of ingredients during the reaction.
Thirty grams of dimethylaminoethyl methacrylate
and 170 grams of acrylonitrile are used. Potassium
.
6~3
-31-
persul~ate is dissolved in 40 milliliters distilled water
and portions -of the solution are added in sequence with the
foregoing monomers as described in Muier et al., J Biomed.
Mater. Res., 5, 415-445 (1971),
s
The temperature of the mixture in the flask is
maintained at 45-50 degrees C (113~122 degrees F) for about
6 hours. The reaction product is an off-white plasticized
polymer. The product is washed with water, filtered and
dried in a desiccator under vacuum to provide an off-white
powder. A typical yield is about 28 grams with a
dimethylaminoethyl methacrylate content (as determined from
oxygen content analysis) of about 97 percent and an
intrinsic viscosity in dimethylformamide at 25 degrees C (77
degrees F) of 1.13 dl/g.
The polymer is dissolved in DMF to provide a 10
percent solution by weight. The solution is filtered under
vacuum through a Porosity Gl sintered glass funnel and is
stored in a desiccator over phosphorus pentoxide for at
least 16 hours. The polymer solution is poured onto a
glass plate and is spread as a film by passing a doctor
blade across the plate. Solvent evaporation is achieved by
maintaining a temperature of 45-50 degrees C for 8 hours in
the region of the plate, while solvent vapor is removed by
an extractor fan. The membrane is removed from the glass
plate by stripping dry or after being soaked with water.
In the enzyme electrode assembly, the membrane
layer nearest the anode (the inner layer) comprises a block
copolymer, as described above, which is permeable to
hydrogen peroxide but which restricts the passage of higher
molecular weight substances. This layer has a preferred
thickness of less than about 5 microns, more preferably in
~ 2~X3
-32-
the range of about 0.1 to about 5 microns and most
preerably in the range of about 0.5 to about 3 micron6.
The membrane layer nearest the sample (the outer
layer) functions as a diffusion barrier to prevent the
passage of high molecular weight substances. This layer,
also formed of a block copolymer, when used in an electrode
assembly to monitor glucose concentrations in a fluid
sample, limits the amount of glucose that passes
therethrough. This layer has a preferred thickness of less
than about 45 microns, more preferably in the range of about
15 to about 40 microns and most preferably in the range of
about 20 to about 35 microns.
The second (intermediate) layer that binds the
inner and outer layers together includes glucose oxidase,
galactose oxidase, uricase or the like combined with a bloc~
copolymer of this invention.
The second layer is applied as a thin uniform
layer on either the inner or outer membrane layer and the
other membrane layer is brought into contact with the second
layer to form a multilayered membrane (also referred to as a
laminate). The laminate is then dried to cure the enzyme-
containing second layer and to bind the layers together.
E am~le 3
The unbound surface of the inner membrane layer
intended to be closest to the electrode and to cover the
electrode of a multilayered monolithic membrane formed
according to the procedure of Example 2 can be coated with a
water-swellable film. This example illustrates a coating
comprising a polyurethane having anionic carboxylate
functional groups and hydrophilic polyether groups and
polyvinylpyrrolidone (PVP) that can be cross linked by
carbodiimide as follows.
S3
-33-
A coating preparation is prepared comprising a
premix of a colloidal aqueous dispersion of particles of a
urethane polymer having a polycarbonate-polyurethane (PC-PU)
backbone containing carboxylate groups and the water-soluble
hydrophilic polymer, PVP, which is crosslinked by the
addition of the cross-linking agent just before production
of the coated membrane. Example coating formulations are
illustrated in the following table.
_ A B C
DRY DRY DRY
WEIGHr WEIGHr WEIGHT
PERCENT PE~NT PE~NT
PREMIX WEIGHT SOLIDSwEIGHr SOLIDS W$IGRT SOLIDS
1. PVP 48 6 64 8 160 20
~Note 1)
2. PC-PV 260 91 248 87 200 70
(Note 2)
CROSS-L ~ ING A ~ T
253. Carbodi- 6 3 10 5 20 10
imide
(Note 3) _ _ _ _ _
Ib~als 314 100 322 100 380 100
Note 1: Aqueous solution containing 12.5 weight
percent PVP prepared from Polyvinylpyrrolidone having a
number average molecular weight of about 360,000 sold as a
powder under the trademark BASF K-90 by BASF Wyandotte
Corporation (Parsippany, NJ).
Note 2: Colloidal dispersion of a polycarbonate
-polyurethane ~PC-PU) polymer at about 35 weight percent
solids in a cosolvent mixture of about 53 weight percent
water and about 12 weight percent N-methyl-2-pyrrolidone
sold under the trademark B~YBOND 123 (or XW-123) by Mobay
Corporation, Coatings Division (Pittsburgh, PA)~ As
supplied, the dispersion has a pH of ahout 7.5-9Ø
-34-
Note 3: Carbodiimide sold under the trademark
UCARLNK XL-25SE by Union Carbide Corporation, Solvent
~ivision (Chicago, IL) supplied at about 50 weight percent
solids in a solvent solution of propylene glycol
monomethylether acetate.
The viscosity and pH of the premix can be
controlled and maintained during processing and to prolong
the pot life by adding water or adjusting the pH with dilute
ammonia solution or an equivalent base prior to adding the
crosslinker.
For production, the coating is applied with a
Mayer rod into the unbound surface of a multilayered
membrane that constitutes the inner layer described in
Example 2. The amount of coating applied should cast a film
having a "dry film" thickness of about 0.1 mil to about 0.5
mil, preferably about 0.25 mil. The coating is dried above
room temperature preferably at about 50 de~rees centigrade.
This coating dries to a substantially solid gel-
like film that is water swellable to maintain electrolytebetween the membrane covering the electrode and the
electrode in the electrode assembly during use.
In cextain applications, for ease of application
in the electrode assembly, an appropriate carrier or frame
made of cardboard, rubber or plastic can be secured to the
surface of the laminate or multilayered membrane. The frame
includes an opening, for example, in the central portion
thereof whereby the outer layer of the membrane may be
exposed to the electrode.
The electrode assembly of this invention may also
be used in the manner commonly employed in the makin~ of
amperometric measurements. A sample of the fluid belng
analyzed is placed in contact with a reference electrode,
eOg., silver/silver-chloride, and the electrode of this
invention which is preferably formed of platinum. The
1~299~i3
-35~
electrodes are connected to a galvanometer or polarographic
instrument and the current is read or recorded upon
application of the desired voltage between the electrodes.
The ability of the present device assembly to
accurately measure the concentration of substances such as
glucose over a broad range of concentrations in fluids
including undiluted whole blood samples enables the rapid
and accurate determination of the concentration of thosë
substances. That information can be employed in the study
and control of metabolic disorders including diabetes.
The foregoing is intended as illustrative of the
present invention but is not limiting. It should be
understood that numerous variations and modifications can be
made without departing from the spirit and scope of the
novel concepts of the invention.
:
.~