Note: Descriptions are shown in the official language in which they were submitted.
~ lZ99942
. .
SY-1868
TW0 PART MAS,~TIS CANNU~A CAP
r ieid of the ;nvention
The present invention is dlrected generally to a two
part cap for a cannula. More particularly, the present invention
,s directed to a two part cap for the cannula of a mastitis
infusion syringe. Most specifically, the present invention is
directed to a two part, separable mastitis infus on cannula cap
which is useable to limit the cannula's insertion aepth into the
teat canal of a dairy cow. The two part mastitis~cannula ca?
includes an outer or overcap which is positionable at a free or
distal end of an elongated inne~ cap. The outer cap is
dimensioned to expose only a portion of the mastitis syringe
cannula when this outer cap is removed .rom the inner ca?. This
insureC that the cannula's insertion depth can be ef'ectively
l mi'ed to less than the length of the .eat canal when only 'he
outer czo is removed. 3acteria thus cannot be carried into the
teat cistern by ~he mast'tis .reatment cannula eau-?ped with the
two part cap when only the outer cap is removed.
~esc,i~tion o' ~he Prior Art
30vine mastit~s is a serious problem which af'licts
large number~of dairy cows. This mast~tis, or inflammat.ion of
~q~1 the cow's mammary sland, strikes substantial percentages of cows
in dairy herds and has a detrimental effect on milk production
and herd pro'itabllity. The generally followed method of
~; treatment for bovine mastitis has been the administration of
::
dk
~qr`
12999~2
various antibiotic preparation into the cow's udder through the
teat canal. A mastitis infusion syringe, which carries the
antibiotic preparation, typically is equipped with an insertion
cannula having a length of 20 to 25 mm. Thl~ cannula and syringe
assembly is provided from the antibiotic supplier as a molded
plastic, disposable unit which is prefilled with the treatment
antibiotic. A single piece plastic cover, which typically snap
fits onto the hub of the syringe at the base of the cannula, is
used to cover the cannula prior to use. At the time o'
treatment, the protective cap is removed from the mastitis
treatment syringe cannula and the cannula end is inserted into
the cow's teat end, passed through the teat canal, and positioned
within the teat cistern. Once the cannula has been so
pos~ioned, the syringe is utilized to deposit the treatment
antibiotic directly into the cow's teat cistern.
~ ecent studies have sugoested tha~ the previously
practiced 4ull cannula insertion techniaue may actuaily reduce
rather than enhance the ef'ectiveness Oc the treatment. This
research has indicated that in some instances infection in the
teat canal are carried into the teat cistern by the mastitis
cannula during full cannula insertion. Lhe cow`s teat canal is
approximately 1 cm in length and has a very narrow lumen. This
canal helps to prevent bacteria Crom entering the cow's udder.
Some bacteria may survive in secretions in the distal teat canal
but are prevented from traveling the full length Oc the canal.
These bacte-ia may be aided in their travel up the teat canal by
:: ~
~: ~
lZ99942
the cannula as it passes through the canal during full cannula
insertion. It has also been found that the teat canal or duct
kera~in layer, which helps control bacterial penetration lnto the
udder, may be damaged by full cannula insertion. This full
length cannula insertion may also cause the distal lumen to
become larger than normal thus allowing increased bacterial
travel and penetration. ~acteria which might otherwise exist for
months in teat canal keratin without causing mastitis might enter
the teat cistern area during .ull cannula insertion.
As a result of these above-discussed studies, there is
now being utilized a partial insertion technique wherein the
mastitis cannula is inserted into the teat end and up the teat
canal only to a depth of generally about 3 mm. This technique
appears to be beneficial in the treatment of mastitis but has
made treatment procedures more time consuming for the dairyman.
It is necessary that the cannula insertion àepth be limited to
generally about 3 mm to avoid the teat canal ~erat~n damage and
transport of bacteria from the teat canal along into the teat
cistern which was caused by the full insertion techniaue.
There presently exists no commercially prepared,
readily useable yet disposable mastitis infusion syringe assembly
which will allow the user to ~uickly and easily control the depth
of cannula insertion thus rendering this partial insertion
treatment effective; Individual measurements of each insertion
depth are time consuming and are apt to be inaccurate. ~ere
guessing is even less accurate and may make the treatment Oc
1299942
little value. It will thus be seen that a need exists for a
mastitis treatment cannula assembly whlch will accurately,
positively, and reproducably limit the depth of cannula insertlon
while not increasing treatment time, cost or the rlsk of
contamination. The two part mastitis cannula cap assembly of the
present invention provides a very satisfactory solution to the
problem.
Sum~arv o' the Invention
It is an object of the present invention to provide a
mastitis treatment cannula and syringe assembly.
Another object of the present invention is to provide a
mastitis cannula cover having a depth of insertion limiting
capabili~y.
A further object of the present invention is to provide
a two part mastitis cannula cap.
Yet another ob~ect o' the present invention is to
provide a two part mastitis cannula cap having an insertion depth
limi'ing outer cap.
Still a further object of the present invention is to
provide a two part mastitis cannula cap which will allow full
cannuia insertion.
Yet still another ob~ect of the present invention is to
provide a two part mastitis cannula cap usable with existing
treatment syringes.
Even yet a further object of the present invention is
to ?rovide a two part mastitis cannula cap that is eas~ to
~299942
handie, does not leak, is sterile, and will not harm the teat
end.
As will be discussed in greater detall ln the doscrip-
tion of the preferred embodiment which is set forth subsequently,
the two part mastitis cannula cap assembly in accordance with the
present invention includes an inner cap which snaps onto the base
of the cannula at a first end, and which has a relatively wide
outer or distal end; and an outer cap which is removably carried
on the distal end of the inner cap and which includes an outer
rim or flange to facilitate removal. The outer cap covers
generallv about the outer 3 mm of the mastitis cannula which
; extends through an aperture at the distal end of the inner cap
I and, when removed, allows only partial depth insertion of the
, cannula into the teat canal. This depth o' insertion is limited
j by tbe relat'vely large diameter of the dlstal end of the ~nnor
cap which also stabilizes the cannula against the teat end and
prevents leakage during infusion of the treatment material during
utilization of the partial insertion technique.
¦ The two part mastitis cannula cap in accordance with
.
the present invention includes an outer circumCerential .im or
''ange which is formed as a part of the outer cap and which
facilitates easy removal of this first cap The herdsman or the
i li~e who ~s responsible for the treatment Oc the cattle can
; readily remove the outer cap, e~pose only that length of cannula
required for part,al insertion, and effect ~n,usion o' ~he
treatment ma~erial in an ef'icien~, predictable manner. Since
~;''' -
.
~299942
the depth of cannula insertion is controlled by the abutment of
the wide diameter distal end of the inner cap agalnst the teat
end, there is no chance for insertion of the cannula to an
improper depth. Thus the partial insertion process is done to
the same depth every time.
Should a full insertion procedure be desired, the
complete cap assembly can be removed from the cannula by
separation of the first or proximal end of the inner cap from the
base of the cannula generally as has been accomplished in the
past. Once the inner cap has been removed to expose the full
lens~h of the cannula, full insertion can be done in the
conventional manner.
The two part mastitis cannula cap in accordance with
the present invention provides the user with a choice. He can
remove only the outer cap and utilize the cannula in a part
inserSion treatment procedure, or he can remove both the inner
and outer caps to expose the full length o. the mastitis control
cannula for a full insertion procedure. The two par~ cap is
usable with existing mastitis treatment cannula and syringe
assemblies, is not expensive to manufacture and is thus
disposable, and provides treatment flexibility not previously
available. The two part mastitis cannula cap of the present
nvention is a significant advance in the art and is an effective
tool in the control of bovine mastitis.
~- -
1299942
Brief Descr~tion of the Drawinas
While the novel features of the two part mastitis
cannula cap in accordance with the present invention are set
forth with particularity in the appended claims, a ull and
complete understanding of the invention may be had by referrlng
to the detailed description of the preferred embodiment as is set
forth hereinafter and as illustrated in the accompanying drawings
in which:
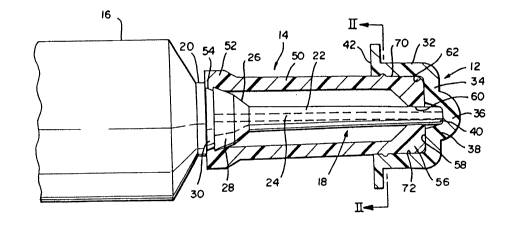
Fig. 1 is a side elevation view, partly in section, of
a mastitis treatment syringe and cannula utilizing the two part
cap o' the present invention;
Fig. 2 is a cross sectional view oS the cannula and cap
assembly taken along line II-II of Fig. ; and
Fig. 3 is an enlarged cross sectional side view o. a
portion of the outer and inner caps of the present invention.
Descri~t on o, the Preferred Embodiment
Turning initially to Fis. 1 there may be seen a two
part mastitis cannula cap, generally at 10, in accordance with
the present invention. Two part cap assembly 10 includes an
outer or overcap ;2 and an inner cap 14. .his two part mastitis
cannula cap 10 is shown in Fig. l in conjunc~ion with a typical
mastitis in'usion syringe 16 that conventionally is a lO ml
disposable plastic syringe which is intended Cor a one time
usage. A proximal or cirst end of an insertion cannula 18 is
fused to a reduced diame~er end 20 of syringe 16. Insertion
cannula 18 typically is 20 to 25 mm in length and has a generally
., ~ .
12999~2
cylindrical hollow body 22 with a through bore 24. A generally
conical shaped hub 26 is formed at first or distal end 28 of
cannula 18 and this hub 26 is 'used to the reduced diameter end
20 of syringe 16. An annular groove 30 ls formed ln the end 20
of syringe 16 adjacent cannula hub 26. This syringe 16 and
cannula 18 assembly is a typlcal configurat~on in which a bovlne
mastitis treatment material is supplied. While the two piece
mastitis cannula cap 10 ln accordance with the present invention
will be discussed for use with this syringe and cannula assembly,
it will be understood that the size of the syringe, the overall
length of the cannula and other similar structural
characteristics of this syringe and cannula assembly, which form
no part of the subject invention, could be changed.
Two part mastitis cannula cap 10 includes, as was
ndicated above, an outer cap 12 and an inner cap 14. As may be
seen in Fig. 1, outer cap or overcap 12 is somewhat cup-shaped
and has a general y cylindrical siaewall 32. A somewhat planar
end wall 34 of outer cap 12 is joined to a first end of
cylindrical sidewall 32 and has a cent~al projection 36 which
forms an ~nterior concavity 38 that receives a free end or distal
tip 40 of the insertion cannula 18. Concavity 38 has a depth of
generally about 3 mm, depending on the length of free end ~0 o'
cannula 22 which extends beyond inner cap 14, in a manner to be
discussed shortly. Concavity 38 and cannula tip 40 are
cooperatively sized to ,orm a snug, leak resistant in~erfit. An
enlarged annu'ar outer flznge ~2 is joined to outer cap i2 zt the
. ~ , .,,: ' ' . . ` .
1299942
second end of sidewall 32 opposite planar endwall 34; This outer
flange 42 should be sufficiently large to facilitate grasping of
outer cap 12 when this outer cap 12 is to be removed from ~nner
cap 14. In the preferred embodiment, this annular outer flange
42 may have a diameter of generally about 10 to 20 mm.
Inner cap 14, as may be seen most clearly in Fig. 1, is
generally in the shape of an elongated cylindrical sleeve having
a tubular sidewall 50. A snap end 52 of inner cap 14 has a
radially inwardly extending lip 54 that is receivable in annular
groove 30 formed at the ~unction of syringe end 20 and hub 26 of
cannula 18. Detachment of the snap end 52 of inner cap 18 from
syringe 16 is achieved by bending inner cap 14 to unseat lip 54
from groove 30.
Inner cap 14 terminates at its distal end 56 in an
outer end face 58 that has a central aperture 60 through which
the free end 40 of insertion cannula 18 passes. Lhe outer end
face 58 of inner cap 14 has a relative;y wide diameter, generally
in the range of about 5 to ~ mm and is smooth and somewhat
rounded at its peripheral ed~es 62.
Outer cap 12 overlies the distal end 56 of inner cap
14, as mzy be seen in Figs. 1 and 3. An inner surface 70 of
outer cap sidewall 32 slidingly cooperates with an outer surSace
72 of tubular sidewall 50 of inner cap 14 to effect retention of
outer cap 12 on the distal end 56 of inner cap 14. Securement of
inner cap 12 to outer cap 14 is enhanced by the cooperation of a
~ pa r of opposed protrusions 76, formed on the outer surface ~2 of
,,
~,
lZ99942
tubular inner cap sidewall 50, with a circumferentlal recess 80
cooperatively located in the inner surface ~0 of outer cap
sidewall 32. Alternatively, the number of protrusions ~6 could
be increased or a continuous rim (not shown) could be
substituted. These protrusions ~6, or rim, and circumferential
recess 80 are sized so that a pulling or pushing force exerted on
outer cap annular flange 42 will effect separation of outer cap
12 from inner cap 14 and not separation of inner cap 14 from
syringe end 20. Separation of inner cap 14 from syringe end 20
is more easily accomplished by grasping the tubular sidewall 50
of inner cap 14 and by utilizing a bending force to unseat lip 54
from groove 30. Thus the two separating forces are of differing
~ypes so that separation will occur at the desired point.
In use, ~he two part mastitis cannula cap assembly in
accordance with the present invention allows the dairy farmer,
veterinarian, herdsman, or the like to practice whichever
n'usion procedure he feels will be more effective. Should
partial insertion be desired, the outer cap's outer flange 42 is
srasped and the outer cap 12 is removed. This exposes the free
or distal end of insertion cannula. In accordance with present
procedures, generally about 3 mm of the cannula free end 40
projects beyond the relatively wide diameter smooth end face 58
of inner cap 14. Since the teat canal o' a cow is approximately
1 cm in length, the 3 mm projection o, cannula free end 40 limits
cannula insertion depth to a point within the teat canal and not
into the teat cistern. Thus partial cannula insertion, to the
1 0
,
.
- 129994Z
correct depth can be quic~ly accomplished. The relatively large
dia...eter outer end face 58 of the inner cap serves to stabilize
the infusion cannula against the teat end while the free end 40
of the cannula is inserted partially lnto the teat canal. This
wide end face also minimizes leakage of the material being
infused. Since this end face 58 is smooth with gently rounded
corners it will not harm the teat end. Additionally, since the
teat canal is ~uite small in diameter, there is no possibility of
the generally wide, large diameter end face 58 of the inner cap
being inserted into the teat canal.
If full insertion of the mastitis treatment cannula is
desired, this can readily be accomplished by removal of both the
outer and inner caps as a single assembly. As discussed
previously, this is accomplished by grasping the tubuiar sidewall
50 of the inner cap i4 and by exerting sufficient bending force
to unseat the lip 54 on the snap end or proximal end 52 of the
inner cap 14 'rom its cooperating groove 30 at the Juncture of
the syringe body 16 with the attached cannula 18. This exposes
.he entire length o' cannula 18 so that full insertion of ~he
cannula into 'he teat cistern through the teat canal can be
accomplished.
T~e overall length of the two part mastitis cannula cap
of the present invention will be generally in the ~ of 35 to
7~
87 40 mm. This dimension is determined by the length o- the cannula
and is not particularly critical in itself. The length of the
' inner cap 14 wi~h .espect ~o the length Oc the cannula 18 is
1 1
1299942
important because the difference in lengths between the shorter
inner cap 14 and the longer cannula 18 determines the length of
cannula free end tip 40 protrusion beyond the end face 53 of
inner cap 14. As discussed above, a pro~ection of generally
about 3 mm is believed to be proper for optimal infusion of the
mastitis treatment antibiotic preparatlon into the teat canal.
The length of cannula tip 40 pro~ectlon, in turn, dictates the
depth of interior concavity 38 of projection 36 on the end wall
34 of the outer cap 12. The outer end of the distal tip 40 o'
cannula 18 should bear against the inner surface Oc this
projection 36 to minimize any possible treatment material loss
during shipment or handling and before the outer cap 12 is
removed, either by itself during partial insertlon, or with inner
cap 1~ during full insertion.
.he two piece mastitis cannula cap in accordance with
the psesent invention provides a safe, easy to use, accurately
controilable and re?roducable, ,nexpensive means to ?ractice the
partial cannula insertion techniaue which recent studies have
suggested may be effective in the treatment of mastitis in dairy
cows. At the same time, the two part cap structure affords the
user an assembly which can be utilized for conventional full
cannula insertion treatment, if desired. Thus the two part
mastitis cannula cap of the present invention provides the
freedo~ to select and use whichever of the two treatment
procedures is deemed more desirable without sacrificing ease of
use, d'sposability, and package integrity.
1299942
While a preferred embodiment of a two part mastitis
cannula cap in accordance with the present invention has been set
or~h 'ully and completely hereinabove, it will be obvious to one
of skill in the art that a number of changes in, Lor example the
size and shape of the syringe, the materials used for the
syringe, cannula and cap, the overall length of the cannula and
hence the overall length o' the two part cap and the li~e may be
made without departing from the true sp~rit and scope of the
67 present invention which is accordingly to be limited only by the
following claims.