Note: Descriptions are shown in the official language in which they were submitted.
-- 1 --
TITLE OF THE INVENTION:
PURIFICATION PROCESS OF METHACRYLIC ACID
BACXGROUND OF THE INVENTION
1) Field of the Invention:
This invention relates to a process for removing
impurities from crude methacrylic acid obtained as an
aqueous solution by vapor-phase catalytic oxidation of
isobutylene or the like.
2) Description of the Prior Art:
Regarding the production of methacrylic acid by
catalytic oxidatIon of isobutylene, tertiary butanol,
methacrolein or isobutyl aldehyde with molecular oxygen
in the presence of steam i.n accordance with a single-
stage or two-stage reaction, a number of proposals have
been made including, for example, catalytic reactions
disclosed in ~.S. Patent Nos. 4,001,317, 4,301,031 and
so on, purification and recovery methods and processes
disclosed in various publications, etc. A reaction gas
obtained in such a manner contains byproducts in :
addition to methacrylic acid as the target product, for
example, carboxylic acids such as formic acidt acetic
acid, propionic acid, maleic acid, citraconic acid,
: benzoic acid, toluic acid and terephthalic acid, and
: aldehydes such as formaldehyde, acetaldehyde, propion-
.
,
:::
.
~L3~ 3
aldehyde, methacrolein, benzaldehyde, tolualdehyde and
furfural. Most of these impurities can be removed by a
conventional puri~ication method such as extraction or
distillation. It is however difficult to remove
impurities which are contained in trace amounts. ~or
example, it is difficult to remove maleic acid,
citraconic acid and aldehydes completely. When
aldehydes are contained in particular, absorption is
observed in the ultraviolet range, and inconvenient
problems arise in many instances such that at the time
of a polymerization reaction, the polymerization is
suppressed to require longer reaction time and the
resulting polymer is tinged.
As a method for removing aldehydes from meth-
acrylic acid in which the aldehydes are contained, it
has been known to add an amine such as hydrazine,
ethylenediamine, aniline or polyamine (Japanese Patent
Laid-Open No. 23017/1977), ethylene glycol (Japanese
Patent Laid-Open No. 128336/1983), a bisulfite
(Japanese Patent Laid-Open No. 252446/1985), a
mercaptan (Japanese Patent Laid-Open No. 6635/1985),
resorcin, pyrogallol or ~-naphthol (Japanese Patent
Laid-Open No. 130546/1985), or-the like.
However, the use of the above-described amine
cannot bring about sufficient effects for the removal
o~ aldehydes and moreover, tends to induce
:~ :
::
: . :
~3(~4(~3
polymerization in a distillation step to be performed
after the treatment. Glycols, bisulfites and mercaptans
cannot exhibit strong effects for the removal and must
hence be added in a large amount, whereby such additives
cause secondary contamination or reaction loss of
methacrylic acid. On the other hand, phenols such as
resorcin can exhibit removal effscts only in the
co-presence of a strongly acidic substance such as
sulfuric acid or hydrochloric acid. Accordingly, they
render the operation complex and requires the selection
of high-quality materials for actual facilities.
SUMMARY OF THE INVENTION
A first aspect of this invention is to provide
a process for the purification of methacrylic acid, which
permits easy removal of dibasic acids and aldehydes,
which are contained in trace amounts in crude methacrylic
acid obtained by vapour-phase catalytic oxidation of a
compound having 4 carbon atoms such as isobutylene,
without secondary contamination due to substances added
and without need for the selection of high-quality
materials for facilities.
A second aspect of this invention is ~o provide
a purification stage which is more effective than the
above mentioned purification step of the crude
methacrylic acid.
A third aspect of this invention is to provide
a purification process which features less coloration of
distilled methacrylic acid and less formation of
insoluble matter in a bottom.
The first aspect of this invention can be
attained by treating crude methacrylic acid, which has
been obtained by vapour-phase catalytic oxidation of
isobutylene, tertiary butanol, methacrolein or isobutyl
aldehyde, with at least one compound selected from the
group consisting o~ m-aminophenol, m-phenylenediamine,
2,4-diaminotoluene and 2,4-diaminodiphenylamine and then
.
~3~ 3
subjecting the thus-treated methacrylic acid to
distillation.
The second aspect of this invention can be
achieved by subjecting the crude methacrylic acid
obtained by the oxidation to distillation in advance in a
distillation column, treating with said at least one
compound a fraction drawn out from an intermediate stage
of an enriching section of the distillation column, and
then subjecting the thus treated fraction to
distillation.
The third aspect of this invention can be
fulfilled by subjecting the crude methacrylic acid or
fraction, which has been treated with said at least one
: L3~44Q3
-
compound, to distillation in the presence of at least
one p-phenylenediamine represented by the following
general formula (I):
Rl-NH- ~ -NH R2 (I)
wherein Rl means a hydrogen atom or a C3-C6-alkyl,
phenyl, p-tolyl, p-methoxyphenyl or ~-naphthyl group
and R2 denotes a hydrogen atom or a phenyl, p-tolyl
or B-naphthyl group.
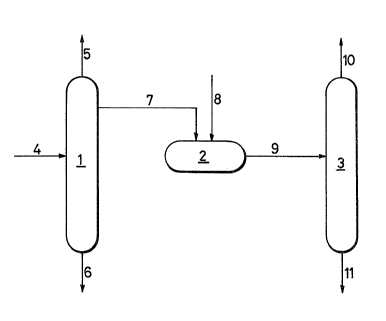
BRIEF DESCRIPTION OF THE DRAWING
10FIG. l illustrates one example of apparatus
suitable for uses in the practice of the purification
process of this invention, in which there are shown a
first distillation column l, a treating tank 2, a
second distillation column 3, a crude methacrylic acid
feed line 4, an overhead discharge line 5, a discharge
line 6 for high boiling products, a draw out line 7
from an enriching section of the distillation column, a
compound feed line 8, a treating product guide line 9,
a distillation llne 10 for purified methacrylic acid,
and a discharge line ll for high boiling bottoms. The
; process of this invention will hereinafter be described
in detail on the basis of the illustrated apparatus.
: :DETAILED DESCRIPTION OF THE INVENTION
Methacrylic acid is usually obtained by
. .. , ~
: ~ ,' ' . - ; .:
catalytically oxidizing isobutylene, tertiary butanol,
methacrolein or isobutyl aldehyde in a single catalyst
layer or two catalyst layers. In such a process,
methacrylic acid is extracted out with a solvent from
an aqueous solution, which has been collected by
cooling and condensing a reaction product gas which
contains methacrylic acid, and is then purified by a
distillation procedure which comprises a step for the
separation of the extracting solvent, another step for
the separation of light distillates, and a further step
for the separation of heavy distillates.
The crude methacrylic acid to be processed in
accordance with the process of this invention may be
crude methacrylic acid obtained from any one of the
above-described steps. Namely, the present invention
may be applied to any crude methacrylic acid such as
the aqueous solution of methacrylic acid, the extract
containing methacrylic acid, the methacrylic acid
obtained after the removal of the extracting solvent
and light distillates, and the methacrylic acid
obtained subsequent to the removal of the heavy
distillates. In order to obtain maximum xemoval
effects by an additive in a smallest proportion, it is
however desirable to apply this invention to the
methacrylic acid obtained after the removal of the
light distillates. Namely, it is desirable to apply
:
... . . . . .
: :
:
. '
~3~ 3
this invention to methacrylic acid obtained subsequent
to the removal of low boiling products such as acetic
acid, acrylic acid, propionic acid and isobutyric acid
or to methacrylic acid which has been obtained after
the subsequent separation of a portion of maleic acid,
polymerization inhibitor, polymer and other high
boiling products as bottoms and is to be subjected to
final superfractionation. As a further preferable
embodiment, it is preferable to use a methacrylic acid
fraction containing less aldehydes in order to obtain
maximum removal effects. For this purpose, it is
desirable to apply this invention to a methacrylic acid
fraction drawn out from an intermediate stage of an
enriching section of a distillation column which, for
example, serves to remove ~he heavy distillates from
the methacrylic acid obtained after removal the
extracting solvent and light distillates. The fraction
drawn out from the intermediate stage of the enriching
section of the distillation column is generally reduced
by 50~ or so in terms of aldehyde contents compared
with a fraction obtained from the top of the
distillation column and is most suitable as crude
methacrylic acid to be processed by the process of this
invention. Methacrylic acid obtained from the top of
distillations column may be used, as is, as a raw
material for the production of methyl methacrylate.
: :
.. - .
.. , . ~ :
-
n~lL 3 ~ ? 3
-- 8 --
Regarding the term "intermediate stage of the enriching
section of the distillation column" as used herein, a
section above a feeding port of the distillation column
is called "enriching section" and the intermediate
stage is located between a top of the distillation
column and the feeding port, for example, feeding line
4 at a height 1/4-3/~ the way down from the top of the
distillation column toward the fee~ding port, for
example, feeding line 4 of the distillation column,
preferably, about 1/2 the way down from the top.
In the present invention, m-aminophenol,
m-phenylenediamine, 2,~-diaminotoluene or 2,4-diamino-
diphenylamine may be used in at least an e~uimolar
amount, preferably in at least a twofold molar amount,
most preferably in at least a threefold molar amount,
all, based on carboxylic acid groups derived from
unsaturated dibasic acids and aldehyde groups
containing in methacrylic acid. It is difficult to
precisely analyze various impurities contained in trace
amounts in an actual solution in a production step of
methacrylic acid. Although the amount of the additive
varies depending what separation and purification steps
methacrylic acid to be processed in accordance with
this invention has been subjected to, the additive may
2~5 generally be used in a molar amount 3-10 times based on
~ carboxylic acid groups, derived from unsaturated
: ~
- :
.
,
~3~ ?3
_ 9 _
dibasic acids, and aldehyde groups which can both be
analyzed quantitatively.
The treatment of methacrylic acid with
m-aminophenol, m-phenylenediamine, 2,4-diaminotoluene
or 2,4-diaminodiphenylamine may be achieved by simply
mixing them together at room temperature. It is
however preferable to heat them to a temperature in a
range of 50-100C in order to complete the reactive
treatment in a short period of time. Although the time
of the treatment may usually be from 1 minute to 60
minutes, such a separate heating step may be omitted
because the methacrylic acid added with the above
compound is usually heated in the next distillation
step. The treatment may therefore be effected by a
simple procedure, for example, by adding the above
compound in a predetermined amount to a feed liquid to
be fed to a distillation column for methacrylic acid,
to a still liquid or to the interior of the column.
Upon distillation of methacrylic acid, it is
widely practiced to use a polymerization inhibitor in
order to prevent polymerization. In particular,
hydroquinone, hydroquinone monomethyl ether,
phenothiazine and the like are known well. In the
process of this invention for the removal of
impurities, polymerization may still tàke place in some
instances during distillation when these conventional
.
': "' .
-` ~3~
-- 10 --
polymerization inhibitors are used. Depending on a raw
liquid to be processed, a polymerization inhibitor to
be used, the structure of a distillation column,
operational conditions for the distillation column and
the manner of operation, polymerized substances may
occur inside the distillation column and/or the still
liquid so that long-term continuous distillation may be
rendered in~easible. The present inventors have
carried out a further investigation in this regard. As
a result, it has been found that the combined use of at
least one p-phenylenediamine represented by the general
formula ~I) can exhibit outstanding effects for the
prevention of polymerization upon treatment of
methacrylic acid, which has been obtained by vapor-
phase catalytic oxidation, with the compound specifiedin this invention.
As specific examples of the p-phenylenediamine
of the general formula (I) useful as a polymerization
inhibitor in this in~ention, may be mentioned N,N'-
diphenyl-p-phenylenediamine, N,N'-ditolyl-p-phenylene-
diamine, ~,N'-di-~-naphthyl p-phenylenediamine,
N-phenyl-N'-tolyl-p-phenylenediamine, N-phenyl-N'-
isopropyl-p-phenylenediamine, N-phenyl-N'-tl,3-
dimethylbutyl)-p-phenylenediamine, N-(p-methoxyphenyl)-
p-phenylenediamine r 4-aminodiphenylamine and
p-phenylenediamine. The amounts of these polymeriza-
~: i
.
.
- ~L3~4~?3
tion inhibitors to be used vary depending on conditions
under which they are used. They may generally be used
in an amount of 0.005-1.0 wt.%, preferably 0.01-0.5
wt.%, both, based on methacrylic acid or its solution
to be fed to a distillation column. In addition, no
problem or inconvenience will arise from its combined
use with a conventional polymerization inhibitor such
as hydroquinone, hydroquinone monomethyl ether or
phenothiazine.
The stage of treatment between crude methacrylic
acid and the specified compound, which stage is adopted
in a preferred embodiment of this invention, will
hereinafter be described in further detail with
reference to FIG. 1.
Crude methacrylic acid, from which light
distillates, extracting solvent and the like have been
removed, is fed via the feeding line 4 to the irst
distillation column 1 which serves to remove high
boiling components. In the first distillation column
1, a portion is distilled out from the top of the
column, another portion is drawn out from an
intermediate stage of an enriching section, and the
remaining portion is discharged as high boiling
components from the bottom oE the column. The fraction
from the top is delivered to a next esterification step
~ia the line 5, while the fraction drawn out from ~he
~3~ 3
intermediate stage of the enriching section of the
column 1 is fed to the treating tank 2 via the line 7
and after subjecting it to treatment with the compound
specified in this invention and charged, e.g., in a
form dissolved in high-purity methacrylic acid by way
of the line 8, the resulting mixture is fed to the
second distillation column 3. In the second
distillation column 3, purified methacrylic acid from
which aldehydes and the like have been removed is
distilled out from the top, while the specific compound
reacted with the aldehydes and the like, such as
m-aminophenol, and unreacted substances are discharged
as bottoms along with high boiling components from the
bottom.
Example 1:
Isobutylene was subjected to vapor-phase
catalytic oxidation. Distillates were cooled,
condensed and collected. An aqueous solution of
methacrylic acid, thus obtained, was processed by a
thickener and a byproduced solid was separated.
Thereafter, methacrylic acid was extracted with hexane.
Hexane and low boiling components were distilled off
; from the extract, thereby obtaining crude methacrylic
acid described in Table 1. m-Aminophenol, m-phenylene-
diamine, 2,4-diaminotoluene and 2,4-diaminodiphenyl-
~3~
13 -
amine were added separately in a molar amount 7 times
the sum of carboxyl groups derived from maleic acid in
the crude methacrylic acid and aldehyde groups derived
from furfural and benzaldehyde in the crude mathacrylic
acid. Batch distillation was conducted under the same
conditions. The contents of impurities in each
methacrylic acid distilled are also shown in Table 1.
It has been confirmed that substantially 100~ removal
has been achieved with respect to maleic acid and
furfural and at least 97% removal has also been
attained regarding benzaldehyde.
;:: : : ::
:: :
:
: . : , .
. .
~ .
~3~44~3
-- 14 --
~ 9 ~ 1
,c~ ~
E c: o o o _ _ _ ~ o o o o
a ~ u~ o oo
g . _ _
u~ ~ 3 o u~ o 1 1 ~ o o o o
ê c e ~ o oo
e
~1 C ~ , .................. ~
C 0 v~0 --I --I 11~ U~ ~ o o O o
u E
.~ o
e z u~ a~ ~ ~ a~ u~ ~ o o O o
r
:~
O O O ~ ~ ~ O co O O O O
~ ~8 ~ ~
~ ~ ~ ~ . ~ O
O rl _ C U U _~
r~ ~J~J ~ ~ o U C r-l ~_
.~ U O :~ D ~ rl rl U
8 ~ r~ ~d C
.~ rJ U O ~ D
r/~ r~ I e ~ ~ ~
OJ ~ O r~ C ~ oi~ I ~ O
¢ U~ ~ O O D¢
: __ _. ~
-
:: ` : :
.
~ 3 ~ 3
Coloration of distilled methacrylic acid:
The absorbance of each distilled methacrylic
acid (as measured in terms of -log T at 350 nm, using a
10 mm thick glass cell) is also shown in Table 1. The
table indicates that the coloration has been improved
significantly by the treatment with each of the
compounds specified in this invention.
Example 2:
Tertiary butanol was subjected to vapor-phase
catalytic oxidation, and an extract of methacrylic acid
was obtained in the same manner as in Example 1. Crude -
methacrylic acid, which has been obtained by distilling
off the extra~ting solvent from the extract, contained
50 ppm of maleic acid, 110 ppm of furfural and 140 ppm
of benzaldehyde. Ten kilograms of the crude meth-
acrylic acid were added with 10 g of 2,4-diamino-
toluene, and the resultant mixture was distilled under
reduced pressure to remove low boiling components,
thereby to obtain a . 7 kg of bottoms. Since the bottoms
formed a little solid matter, the solid matter was
filtered off. The filtrate was then continuously
distilled at a reflux ratio of 0.5 in a 20-stage
Oldershaw column, whereby 7.6 kg of methacrylic acid
was obtained as a final product~ The contents of
impurities contained in the final product were lower
than the detection limits of gas chromatograph and
~3~4~3
- 16 -
liquid chromatograph. In contrast, methacrylic acid
obtained by following the above procedure without any
additive still contained 25 ppm of maleic acid, 90 ppm
of furfural and 100 ppm of benzaldehyde.
Example 3:
An aqueous solution of methacrylic acid was
obtained by subjecting tertiary butanol and a molecular
oxygen containing gas to a vapor-phase catalytic
reaction in the presence of an oxidizing catalyst and
then quenching the resultant high-temperature reaction
product gas. After removing light distillates such as
methacrolein from the aqueous solution, methacrylic
acid was extracted with hexane. Hexane and low boiling
components were distilled off from the extract, so that
crude methacrylic acid shown in Table 2 was obtained.
The crude methacrylic acid was purified in accordance
with the flow depicted in FIG. 1. Incidentally, the
outlines of the distillation columns and treating tank
are summarized in Table 3.
The crude methacrylic acid was fed at 20 ky/hr
to the first distillation column 1 which was operated
at a top temperature of 95C, a pressure of 60 mmHg
and a reflux ratio of 0.5. Methacrylic acid which
distilled out at 12 kg/hr from the top was delivered to
a subsequent esterification step (not shown in the
drawing) as a raw material for the production oE methyl
::
. :
~3~4~J3
methacrylate. A fraction was drawn out at a rate of 6
kg/hr from the intermediate stage of the enriching
section and was then supplied to the treating tank 2.
A solution, which contained 1 wt.% of m-phenylene-
diamine and l wt.~ of N-phenyl-N'-isopropyl-p-
phenylenediamine in high-purity methacrylic acid and
was charged at 0.3 kg/hr to the treating tank 2 which
had been controlled to 60C. Thereafter, the treating
mixture was guided at 6.3 kg/hr to the second
distillation column 3 so that the treatment of the
fraction from the intermediate stage of the enriching
section was performed for about 30 minutes. Likewise
the first distillation column l, the second
distillation column 3 was operated at a top temperature
of 35C, a pressure of 60 mmHg and a reflux ratio of
0.5 so that purified methacrylic acid was obtained at
4.3 kg/hr from the top. On the other hand, m-
phenylenediamine which reacted with aldehydes and the
like, and high boiling components, were obtained at 2.0
kg/hr from the bottom. Table 2 shows the compositions
of the crude methacrylic acid, the overhead of the
first distillation column, the fraction drawn out from
the intermediate stage of the enriching section and the
overhead of the second distillation column as well as
the absorbances of the overheads and fraction.
. .
'
~L3~44~'3
- 18 -
. .
The methacrylic acid drawn out from the
intermediate stage of the enriching section of the
first distillation column contained aldehydes at the
low concentrations, and was hence treated successfully
with the small amount of m-phenylenediamine so that its
coloration was improved significantly.
Example 4:
The procedure of Example 3 was repeated except
that 1 wt.% of m-phenylenediamine dissolved in high-
purity methacrylic acid was changed to l wt.~ of
m-aminophenol. Absorbance was measured with respect to
the overhead of the second distillation column. As a
result, purified methacrylic acid having 0.03 as -log T
and improved in coloration was obtained.
' ': '
13~ 3
- 19 -
~ _ _
e c
C O ., ~
.,~ c ~d e O O O ~ O O O O O
.~ O~ ~ ~`l ~ \~l `/1 ~/1 `D 00 u~ O
~_
O
-.~
~a
~0
a ~,
~ U ~ O O O ~ ~ O O O O O O
O ~ 0 00 ~ ~ U~ U~ ~ O
o .e ~ _ ~ O
.~ U V ~
o~ .e o O o U~ O O O O O O O O
~ ~n o o ~ ~ ~ ,~ u~ O O .
o U~ o
_
.,
C~ ~
~, ~ O O O U~ O O O O O O O
1 ~ u~ 1~ ~ ~ r~ oo r~ ~ O O
r ~a ~ _ _ ~ n
E
_
o~
C ,~ C
~ O O ~
'~ 0~ ~ o~
U ~ OC U t)
L~ ~ C
:~ ~ 0 1 C ~ o
a u ~, a u c ~ N _~ u U
~1 la 1, rd _ Q) (d ,1 r~ td C
~ U ~ ~ U ~ ~d
U U ~ ~1 U .~ ~ D g D
u,~ ~ 1 N ~ ~ '- I U O
a~ o ~ c ~c_- ~o c u u~
u ~ u O ~r~ O~a ~
~: ~- ¢ ~ ~ ~ o P~ ~: æ 3 ¢
::
' , ~ ' : -
~, . .
. . :
,
~3~ `3
- ZO -
, . s ~.~
~ o ~ ~o
JJ X X
~0 V ~D ~.3C e.~
:~ __ _
~ ~ ~D ~1 1
~ 1 h m
I f __ _
o . ~,a) .c
O ~ ~ 3 0 ~ ~
._1 ~ ~D G~ 1~1 a1
~ O ~ ~ !C~
_~ ~ X U~ ~ Q~
._, a) t~ u~ b~
~ rY m ~n
,~n U ~ ~ ~C C ~ _I c
~ P~ E3 (~ o~ ~
h ~ 0~o ~ .
O
: .~ ~ .
h C ~.,~
~ ~ ~ ~ ~ O JJ
Q~ a1 ~J h t) U O
Q~ ~ c a) ~ a)
_. .. E~ ~n ~: .~ ~ .
'
.
~ .. :' ' ~ '
, ~ '- ' ' "' ' '
r~
~3~ 3
- 21 -
Comparative Example 1:
The procedure of Example 3 was repeated except
for the replacement of m-phenylenediamine by aniline.
Absorbance was measured with respect to the overhead of
the second distillation column. As a result, -log T
was found to be 0.15 so that no improvement was
observed in the coloration.
Example 5:
In a process as in Example 3, m-phenylenediamine
was charged directly to the second distillation column
without the treatment in the treating tank. Results
... ..
similar to those obtained in Example 3 were obtained.
Examples 6-10:
Portions of the intermediate-stage fraction of
the enriching section of the first distillation column,
which had been obtained in Example 3, were added with
their corresponding additives shown in Table 4 in a
amount 5 times in mole the sum of maleic acid, furfural
and benzaldehyde. The resultant mixtures were
separately distilled at an operation pressure of 60
mmHg and a reflux ratio of 0.5 by means of a 20-stage
Oldershaw column. The absorbances of methacrylic acid
samples thus obtained were measured. Results are also
shown in Table 4.
:
. .
: .
:: : - , '~ '' ' ' ~.. . .
- 2 2
a. =~ o o o o o v~
C o _ o o o o o o
~ ' o~ _
~ ~ `~1 ~ Vl vl vl o
O'
C ~ o
I ,. IV~ I vl~
E~ ,'Co .'
u vl Vl `~I Vl vl u~
S
~ .~ ~
~1 c: ~: c 5 ~ ~
E E .~: u
'~ -I ~ O r~ ~
c ~ O " 1 a a ~ cl
:~ ~ ~ o o ~ o o
o~ C C ~ C C ~ .1
E ~ D. a a ~ a ~ .,
e ~ C ~
X ~ O C W
.:
:; ' ' ' '
' ' ' : ~ ' ' '
..
~3~ 3
- 23 -
Example 11:
Crude methacrylic acid, which had been obtained
by vapor-phase catalytic oxidation of isobutylene, was
purified to obtain methacrylic acid containing 300 ppm
of hydroquinone monomethyl ether. Portions of the
methacrylic acid were added with 100 ppm of their
corresponding compounds specified in the present
invention and 200 ppm of tbeir corresponding polymeri-
zation inhibitors of various kinds. Fifty grams of
each of the resultant mixtures were placed in a 100-m~
eggplant-type flask fitted with a packed column of
20 cm tall. Distillation was then conducted by a
conventional method while continuously feeding the same
methacrylic acid at ~0 g/hr. After continuously
conducting the distillation at a pressure of 50 mmHg
and a flask liquid temperature of 92-95C for 1.5
hours, insoluble matter in the distillation residue
~about 125-130 g) was filtered off.
Effects of the additives:
The coloration (-log T) of each distilled
methacrylic acid at 340 nm was measured by using a
10 mm thick-glass cell. Results are shown in Table 5,
in which circle ~ indicates 0.01 or smaller while
letter X shows 0.05 or greater.
Effects of the polymerization inhibitors:
The c~ncentration of insoluble matter in each
:
~: ~' : ' , . . . .
- , .
.- : . . ~ . .
, ~
~ ' . ~ ' ' : ' ' '
~3~
- 24 -
distillation residue was measured. Results are also
shown in Table 5, in which circle O indicates 100 ppm
or lower, triangle ~ shows 100-200 ppm and letter X
designates 1000 ppm or higher. In the table,
Experiment Nos. 1-9 are each directed to an illustra-
tive example of the combined use of one of the
compounds specified in the present invention and a
polymerization inhibitor, whereas Experiment Nos. 10-lS
are individually directed to an illustrative example in
which a conventional polymerization inhibitor was used
in combination with one of the compounds specified in
this invention or neither any one of the compounds
specified in the present invention nor any polymeriza-
tion inhibitor were employed. Incidentally, Experiment
Nos. 11 and 13 are directed respectively to examples inwhich the polymerization inhibitors were both increased
to 500 ppm.
.~. '
`' ~ ' : "
: ~
'
- 25 -
.
o ~ ~ O ~ O O O O O O ~C ~ X ~ X o
.. H
.__
O
O O O O O O O O O O O O O O X
O
~ ~,
O _ _ ~ :'
E C ~ a ~ a ~ ~ ~ o E~ o E~ ~
U~ ~C .'~
~ P~ ~1 a
E~ _ . C~a)
~ ~ s~,C
a
~a ~ ~ .c ~ c .c
J O O ~:! O o O O C) O ~ o ~ .~
O Q. ~ .L~ J O ~ 0~ J~ a) ~ O ra ~1 ~.C Q C
O O 1~ ~C J~ ~ C ~ ~ O ~ C J~ C t~ a) ~ c Q ~
~ ~ . ~ O
ra ~ c ,~ ~ c ~1 z
a I au a c ~ r~
c I ~ s~ l c I oQ~ c
~`J e, ~I Q, Q' ~ o ~ C 3 ~ ~
Q~ CD~-/
~ ! ' X ~ c ~
~ ~ ~ ~i I Z Z O ~
C S OCQ I ~ C ~ ~C
I I I a) ~
. ~ ~ o ,~ - - z C S ~-~' ~S
~Q. Z~;ZZZZ P~ol
_ ~ a w E
:
~:
:
~r
~3~
26 -
Example 12:
Crude methacrylic acid obtained in the same
manner as in Example 3 was continuously distilled at a
reduced pressure of 50 mmHg and a reflux ratio of 0.5
in a 20-stage Oldershaw column, thereby separating
methacrylic acid from high boiling components. The
methacrylic acid contained 50 ppm of maleic acid, 30
ppm of furfural, 50 ppm of benzaldehyde, and 100 ppm of
hydroquinone monomethyl ether as a polymerization
inhibitor. Portions of the methacrylic acid were each
added with fivefold moles of one of m-aminophenol,
m-phenylenediamine and 2,4-diaminotoluene as additive
compounds in combination with 300 ppm of one of N,N'-
diphenyl-p-phenylenediamine, N-phenyl-N'-isopropyl-p-
phenylenediamine and N-phenyl-N'-(1,3-dimethylbutyl)-p-
phenylenediamine. Each of the resultant mixtures was
continuously distilled in the above-described 20-stage
Oldershaw column similarly. In all the 9 combinations
of the additive compounds and polymerization inhibi-
tors, there was no precipitation of polymerizedsubstance so that continuous distillation was feasible
- for 10 hours or longex. The -log T of each methacrylic
acid fraction thus obtained was 0.05 or smaller. In
contrast, precipitation of polymerized matter started
immediately after the initiation of distillation when
'
~3n'~4~3
- 27 -
hydroquinone monomethyl ether or phenothiazine was used
in a proportion of 300 ppm.
;
.