Note: Descriptions are shown in the official language in which they were submitted.
HOECHST AKTIENGESELLSC~AFT HO~ 87/F 178 Dr~ SW/sch
Description
An immunometric determination method
The invention relates to an immunometric determination
method in which, in order to avoid non-specific increases
or decreases in the values for the antigen which is to be
determined, an excess of a substance (suppressor substance~
~hich, by reason of its great similarity to the reagent
antibodies used ancl by reason of its great excess, selec-
tively traps the substance which interferes with the anti-
bodies is added to the unlabeled and/or the labeled anti-
body, and thus rules out an ;nfluence on the assay.
It is known that immunometric methods are be;ng employedto an ever increasing extent for the qualitative and quan-
titative detection of antigens. These methods are based on
the formation of a complex of the antigen with one or more
antibodies, with one of the partners in the binding being
labeled in such a way that it can be qualitatively and/or
quantitatively measured using physical or chemical detec-
tion methods~ Th;s makes it possible to establish whether,
and in what amount, a complex has formed from the antigen
and one or more antibodies. Crucial improvements in the
immunometric determination methods were achieved with the
introduction of monoclonal antibodies (Milstein and
Kghler, 1975), the use of which in ;mmunometric assays is
described in detail in German Offenlegungsschrift
3,130,834.
The immunometric determination methods can be divided into
two main types depending on whether the antigen or the
antibody is labeled~ The labeLed partner in the binding is
always used in excess.
Particular interest has been attracted to ;mmunometric
methods in which one of the antibodies which is used i
~3~83
labeled. These entail the antigen being bound in the form
of a ternary complex resembling a sandwich and, after the
incubation, the unbound labeled antibody be;ng removed by
decantation or washing out. These embodiments are called,
depending on the nature of the labeling, 3 two-s;ded
;mmunoradiometric assay (IRMA), an immunoenzymonletric
assay (IEMA) or an immunochemiluminometric assay (ICMA).
As a rule, these entail the unlabeled antibody being bound
to a solid phase.
Different var;ants of embodiments of the abovementioned
sandwich assays are possible, these differing by the reac-
tion steps resulting in the formation of the ternary com-
plex. Either the antigen can be mixed s;multaneously with
the labeled and the unlabeled antibody in a one-step
method, or a sequential procedure is possible and the
antigen is reacted initially with the unlabeled antibody
and, after a sufficient incubation time, then also with
the labeled antibody. Finally, it is also possible for
these reaction steps to be carried out in the reverse
sequence.
The sandwich assays based on the use of labeled antibodies
have crucial analytical advantages compared with the
assays carried out using labeled antigens:
It is possible to employ the antibodies in excess and,
owing to the increase in their concentration, to displace
the equilibrium in the direct;on of formation of the
ternary complex. It is possible in this way for even small
amounts of antigens to be bound and coupled to the label.
Since, moreover, the signal emitted by the label is direc-
tly proportional to the antigen concentration, and the
signal/dose plot has a steep gradient, it is possible even
for the weak signals caused by low antigen concentrations
still to be clearly recognized. For these reasons, the
sandwich assays which operate with labeled antibodies are
considerably more sensitive than assays which are based on
the use of labeled antigens.
:~3~ 3
FinaLly, another point in favor of the sandw;ch assays
which operate with labeled antibod;es is the considerably
larger dynamic measurement range, i.e. there is a larger
range in which the strength of the signal em;tted by the
ternary complex changes in a sens;tive manner when there
is also a change ;n concentrat;on of the ant;gen. Another
important point for the routine use of commercial ;mmuno-
metric determination methods is that they allow the incu-
bation times to be m;nimized. This is ensured when ;t ;s
possible to employ h;gher reagent concentrations than is
the case when labeled ant;bodies are employed in sandwich
assays.
aoth types of assay (those with labeled antibodies and
those with labeled antigens) may be subject to interfer-
ence by serum constituents wh;ch ;ntervene in the primary
antigen/ant;body react;on and/or separat;ng react;on. The
meaning of separating reaction in this connection ;s the
separat;on of bound and free tracer. Th;s may result,
depending on the nature of the ;nterference and the prin-
ciple of the assay, in either falsely high or falsely low
values. Separations based on non-specific precipitation
reactions such as, for example, IgG prec;pitation by
alcohols such as polyethylene glycol (PEG) have, of course,
the greatest degree of robustness in respect of interfer-
ing immunological effects, but, on the other hand, as a
consequence of the ~ide variation in the;r NSB (non-
specific binding) (misclass;f;cation of free tracer) they
frequently give results which are analyticallytdiagnosti-
cally unreliable. The double-ant;body separating tech-
niques, which are distingu;shed by low and precise NSB,
are subject to interference by endogenous heteroph;lic
antibodies which are directed against the primary (antigen-
binding) antibodies in such a way that the separating
antibody can no longer attack the ant;gen/antibody complex.
The consequence of this in competitive assays is that the
antigen content ;n the pat;ent's sample is overestimated
(A. Sain et al. 71, 540 (1979)).
~3~
Interference in the primary antigen/antibody reaction is
often observed when auto-antibodies against the anaLyte
occur. In doubLe-antibody RlAs this effect gives a faLseLy
high result~ ~hereas in the PEG assays falsely low vaLues
are obta;ned. Sample preparation by preliminary PEG precip-
;tat;on avo;ds th;s type of interference.
Very recentLy interference has also been observed in the
sandw;ch assays, this being manifested by falsely h;gh
measurements on pat;ents' sera due to an ;ncrease ;n the
NSB. The cause is supposed to be ant;bod;es aga;nst the
reagents in the assays (R.J. Thompson et al., Clin. Chem,
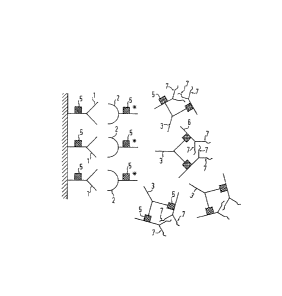
_ , 476 (1986)) (Figs. 1 and 2). A representation of this
is shown in Figure 1, using the way of representing anti-
bodies customary in the literature. An antibody (1) boundto a solid phase is bound via a non-analyte-specific bind-
ing (NSB), for example by an ;nterfer;ng substance (3), to
the epitope (5) of a labeled antibody (2). The diagram in
Figure 2 represents how such non-specific b;nd;ng (NSB)
adds to the spec;fic binding (SB) between ant;body (1)
bound to a solid phase, antigen or analyte (4) and labeled
antibody (2) to s;mulate excess;vely high, often patholog;-
cal analyte concentrations. Since the interfering substan-
ces are spec;es-spec;fic ant;bodies, they cannot be bound
by just any IgG (as is the case with rheumatoid factors),
but species-specific carrier gamma-globulin must be
employed.
In the present state of the art, non-specific mouse or rat
serum is added to assays in which monoclonal anti-bodies
from mice are used (EP-A 0,174,026 or RIA-gnostR hCG,
Behring-Werke, Marburg, since 1.9.1984). However, despite
this measure, it is repeatedly founcl that sera have false-
ly high values because of the high titer of antl-mouse
lgG. If these samples are tested again ind;vidually with
addit;on of a larger amount of mouse or rat serum, gener-
ally a lowering of the ;nitial value occurs. The large
amounts necessary for this purpose rule out employment as
a prophylactic measure in the assay because an effect on
~3 1)~
the assay is to be expected.
It is possible according to the invention to eliminate the
disadvan~ages described above by using as carrier (sup-
pressor) substance (6) a protein which has great ;mmuno-
logical similarity to the IgGs used in the assay and, in
the ideal case, di~fers from the latter only in the anti-
gen binding structure (7) and thus does not b;nd the
analyte (Fig. 3). A large excess of these substances can
be admixed to the labeled reagent antibody without inter-
vening in the analyte reaction.
Another prerequisite for this is immunological inertness
to all the other serum constituents~ Non-compliance with
this requirement might result in network formation in the
serum sample and reduce the suppressive action of the sub-
stance. Moreover, it might not be possible to rule out an
effect on the kinetics and position of equilibrium of the
analyte reaction.
~ence the invention reLates to an immunometric determina-
tion method for an antigenic substance which has at least
two antibody-binding sites, in which a liquid sample con-
taining the antigenic substance (a) is incubated, sequen-
t;ally or in one step, with an unlabeled antibody (b)which is immobilized on a solid phase and is specific for
(a)~ and another, labeled antibody (c) which is specific
for (a), there being formation of a ternary complex of
(a), (b) and (c) which is bound to a solid phase and emits
a detectable signal corresponding to the amount of (a),
which comprises addition, in the first and/or second incu-
bation step in the sequential method and to the reaction
mixture in the one-step method, of protein which is immu-
nologically related to the antibodies used and which is
thus able to bind components which are present in the
patient's serum and which would enter into undesired bind-
ing w;th the assay reagents, in order in this way to rule
out an adverse effect on the assay.
33
- SA _
The invention will now be described in relation ko the
drawings, in which:
Figure 1 is a schematic diagram showing binding of
antibody via non-analytic specific binding (NSB) to a
labeled antibody;
Figure 2 is a schematic diagram showing how NSB adds to
the specific binding (SB) between antibody bound to a solid
phase, antigen or analyte and labeled antibody;
Figure 3 is a schematic diagram showing the use of a
carrier (suppressor) substance to block ~SB in accordance
with the present invention;
Figure 4 is a graph showing the binding affinity of an
interfering substance (goat anti-mouse IgG) for the reagent
antibodies;
lS Figure 5 is a graph showing the binding affinity of an
interfering substance (rabbit anti-mouse IgG) for the
reagent antibodies;
Figure 6 is a graph showing percentage binding of
reagent antibodies to an interfering substance (goat anti-
mouse IgG) as a function of varying amounts of suppression
medium ~rat IgG);
Figure 7 is a graph showing percentage binding of the
reagent antibodies to an interfering substance (rabbit anti-
mouse IgG) as a function of varying amounts of suppression
medium (rat serum);
Figure 8 is a graph showing percentage binding of the
reagent antibodies to an interfering substance (patient's
Serum A) as a function of varying amounts of suppression
medium (mouse IgG); and
Figure 9 is a graph showing percentage binding of the
reagent antibodies to an interfering substance (patient's
Sexum B) as a function of varying amounts of suppression
medium ~monoclonal anti-idiotype antibodies to monoclonal
antibody against carcinoembryonic antigen).
, .
~l3~4~i83
-- 6
The proteins which are employed are pre-ferably one or more
different antibodies, in particular one or more from the
same species (animal species) as that of the reagent anti-
bodies used~ Particularly pre~erred are monoclonal anti-
bodies, and very particularly preferred are ant;idiotypemonoclonal antibodies. The meaning of protein hereinbefore
and here;nafter is a macromolecular organic compound which
is constructed of amino acid units and has antigenic prop-
erties.
1û
Anti-idiotype antibodies are defined as those directed
against the idiotypic region of the antibody which has
elicited their formation.
The preparation of the suppressor substances according to
the invention preferably starts from the reagent antibodies
which are used, and entails modification of the latter in
such a ~ay that although they still retain their specifi-
city agains~ the interfering substance they no longer react
ZO - or at least react d;stinctly less - with the antigen
which is to be analyzed. The large excess of the suppressor
substances which is expediently added to the immunometric
assay means that of the two competing reactions - reagent
antibody + ;nterfering substance and suppressor substance
;nterfering substance - the latter is given far greater
preference, so that the interfering substances are virtu-
ally quantitatively removed from the reaction equilibrium.
Examples of possible ways of generating these suppressor
substances - starting from the reagent antibodies - are
the following:
a) Selective elimination of the antigen-binding structures
from the reagent antibody used
b~ Blockade, by a complementary antigenic structure, of
the antigen-binding structures of the reagent antibody
used
c) Alteration of the antigen-binding structure of the
reagent antibody used, by external intervention, for
~l3~i83
-- 7
example by mutation in the cell culture
d) Preparation of anti-idiotype antibodies (see US Patent
4,536,479) which belong to the same IgG subclass as the
reagent antibodies.
s
Antibodies can be cleaved for variant a) by methods known
from the literature, for example using enzymes. It is pos-
sible in this connection to eliminate individual fragments
from the light and the heavy chains. As is known, the
antigen-binding structures are located on the N-terminal
end of the polypeptide chains so that the elimination
thereof means almost complete loss of the epitope-recogniz-
ing constituents of the antigen. It is likewise possible
to separate the two Fab parts from the Fc part. If, for
example, the epitopes which are recognized by the interfer-
ing substance are located on the Fc part of the assay
antibody, then an ideal suppression medium would be the
pure Fc part of this antibody since, although it reacts
~ith the interfering substance, it does not react with the
antigen (analyte) because of the absence of the Fab parts.
The blockade, by a complementary antigenic structure, of
the ant;gen-binding structures of the reagent antibody
used, as in b), can be effected in general by every sub-
stance ~hich is directed against the idiotypic region ofthe reagent antibody and whose binding affinity is suf-
ficiently high, for example by an anti-idiotype antibody.
The alteration by mutation in the cell culture as in c)
can be carried out, for example, as follows:
Somatic mutants of monoclonal antibodies are isolated from
cell cultures and then the effects of these mutations on
antibody function are examined. lsolation of such mutants
~;th an altered Ig structure is made possible by the
instability of the Ig genes in cultivated hybridoma cells
(Morrison et al., CRC Crit. Rev. Immunol~ 3: 1 - 2Z, 1981).
3 Types of structural mutants of particular importance for
hybridoma technology are known: (1) class and subclass
- 8 -
switch variants (Cebra et al., Ann~ Rev. Immunol~, 2: 493
- 548, 1984; Sublitzky et al., Immunol. Rev., 67: 59 - 72,
1982; Shimizu et al., Cell~ 36: 801 - 803, 1984; Tilley et
ai. Proc. Natl. Acad. Sci., USA, 80: 6967 - 6971, 1983);
(2) deletion or point mutations in the constant reg-
ion with changes in effector function (Yelton et al.~ J.
Exp. Med., 156: 1131 - 1148, 1982; Kenter et al., Science,
206: 1307 - 130~, 1979; Teillaud et al., J. Immunol, 1984
in press); and ~3) mutants with altered antigen-binding
propert;es (Dildrop et al., EM~0 J., 1: 635 - 640, 1982;
Cook et al., Proc. Natl. Acad. Sci. USA, 74: 5687 - 5691,
1~77). Mutations which finalLy result in complete loss of
the ability to bind a specific antigen have a very high
spontaneous occurrence in some clones and derive from the
replacement of individual amino acids ;n the heavy chain
of the V region (Rudikoff et al., Proc. Natl. Acad. Sci.
USA, 79: 197 - 1983, 1982).
Variant 3 is suitable and preferred for employment as
suppression medium~ To choose the most su;table mutants
it is merely necessary to carry out a fe~ e%periments
to elucidate and establish ;n each case ~hether
1. the antigen-binding properties are still present and
2. the interfering substance still recognizes, and thus
binds, "its" epitopes on the mutant.
For d) it is possible to generate anti-idiotype ant;-
bodies by immunization ~ith a monoclonal antibody direc-
ted against a non-analyte antigen. These substances have
epitope homology in respect of the constant regions of
the reaoent antibody and comply ~ith the requirement of
non-reaction ~ith the other serum constituents.
The suppressor substances prepared as in a-d can be
employed in immunometric determination methods ~ith the
aim of selectively trapping interfering substances (for
example antibodies or the like). They can be employed ;n
immunoassays for the determination of a very wide
variety of antigenic substances, for example for human
thyroid-stimulating hormone (hTSH) or for the oncofetal
~3~ 3
proteins such as alpha-fetoprotein (AFP), human chori-
onic gonadotropin (hCG) or carcinoembryonic antiyen
(CEA). Use is particuLarly advantageous in the case of
analytes (antigens) which occur in low concentrations or
S are virtually absent ;n healthy people and ~here even
low concentrat;ons ind;cate a pathological state. In
these cases, even smaLl increases ;n concentration
brought about by ;nterference would then ;nd;cate a
patholog;cal state.
The immunometric determ;nat;on method according to the
invention is rel;able, techn;cally stra;ghtforward,
rapid and robust. It can be used in a large number of
commercial sandwich kits. It is also possible for sup-
pressor substances with the properties described aboveto be introduced as supplements to existent commercial
assay kits.
For this purpose, it ;s exped;ent for them to be added
to the convent;onal assays ;n concentrations which are
at least 10, preferably 50, particularly preferably 80,
and not more than 1000, preferably S00, particularly
preferably 120, times those of the reagent antibodies.
The commercial assay kits are preferably those in wh;ch
the polyclonal, but preferably monoclonal, antibody
which is bound to the solid phase is unlabeled. This
antibody is preferably adsorbed onto or covalently bond-
ed to the soLid phase, with the solid phase preferably
be;ng composed of a plastic tube, a microtiter plate,
plastic articles such as plastic beads or plastic pro-
pellers~ or else of microscopically small plastic beads
which, for example, are suspended in a liquid. The
labeled antibodies are likewise polyclonal, but prefer-
ably monoclonal, antibodies. The labeling is effected bymethods known from the literature, us;ng a radioactive
isotope, an enzyme or a fluorescent or chemilum;nescent
group.
~ 3 ~
- 10 -
~he invention is explained in more detail by the
examples which follou.
Example 1
Preparation of somatic mutants of monoclonal antibodies
Hybridoma ~ell clones ~hich produce antibodies of kno~n
specificiey were recloned in microtiter plates using
a single-cell manipulator or the limiting dilution
method. The culture supernatants from the wells with
positive cell growth ~ere then tested first for their
Ig content and then for their antigen-binding properties.
~hen the cloning rate was sufficiently high the cell
clones obtained were distinguished by a high production
of Ig molecules with, at the same time, antigen-binding
;nsufficiencyO
xample 2
Preparat;on of monoclonaL anti-idiotype antibodies
A monoclonal antibody ~as used as immunization antigen
for the preparation of monoclonal anti-idiotype anti-
bodies. The monoclonal antibody used in this case was
one directed against carcinoembryonic antigen (CEA) and
of syngeneic origin, and ~as administered as the whole
antibody to female 8ALP/c mice 6 - 8 weeks old. This
entailed about 10 ~9 per mouse of the whole antibody,
emuLsified in complete Freund s adjuvan~, being injected
subcutaneously and, in a second case, intraperito-
neally. A second and a third immunization followed 4 and8 weeks~ respectively, later. Immediately before the
actual fus;on, the experimental animals were addition-
ally intravenously boosted on 4 consecutive days. On the
day of fusion, the spleens were removed under sterile
conditions and converted into suspensions of single
cells. Hybrid cells were produced by fusion of 108
spleen cells with 2 x 1~7 cells of a myeloma cell line
(SP 2/0) and were then inoculated on 24-well plates
(Costar~ in a concentration of 106 cellstwell in a
.. r ~ * Denotes trade-mark
Ç;83
select;on medium (DMEM (Dulbecco's minimal essential
nedium) + 20% FCS (fetal calf serum); 0.1 mM hypo-
xanth;ne; 0.4 mM am;nopterin; 16 mM thym;dine). 2 - 3
~eeks later ;ndiv;dual cell colonies were isolated from
the wells and each was transferred into another weLl of
ne~ culture plates t24-well, Costar). After a further
2 - 3 days~ these culture supernatants were examined
in an en~yme immunoassay, using the immun;zation anti-
body conjugated with peroxidase (POD), for the presence
of anti-idiotype antibodies, and these in turn were
exam;ned in a further enzyme immunoassay for their
antigen inhibitability. Hybrids producing antigen-
inhib;table anti-idiotype antibodies ~ere selected and
cLoned using a single-cell manipulator~
Example 3
In analogy to Example 2, anti-idiotype antibodies were
prepared using for the immunization in place of the
uhole antibody the Fab' fragment of the ant;-CEA ant;-
body, coupled to 8SA.
Example 4In analogy to Example 2, ant;-id;otype antibodies were
prepared using for the immun;zat;on ;n place of the
~hole antibody the Fab' fragment of the anti-CEA anti-
body, coupled to KLH.
The following comparison experiments were carried out to
detect the suppressor properties of the substances
accord;ng to the ;nvention:
Example 5
The b;nd;ng aff;nity of interfer;ng substances (Fig. 4:
goat anti-mouse IgG; F;g~ 5: rabb;t ant;-mouse IgG) for
the reagent ant;bod;es used - expressed in % b;nd;ng
(1û0% b;nding - 100% of reagent antibodies bound to the
interfering substance) - was determined with a constant
amount of suppress;on med;um as a funct;on of the amount
of interfer;ng substance added~ The RIA-gnost~R) hTSH
~3~ 3
- 12 -
assay kit (~ehringwerke, Marburg) was used. The folLow-
ing compar;sor, suppression media were used:
Curve No. (in Figs. 4 and 5)
Mult;ple *
rat serum 1 43004300
rat IgG 2 100 100
subs~ance according to 3 100 100
the invention (from
1û Example 2)
* based on the specific tracer IgG used
Two other curves are drawn in Figures 4 and 5 for com-
parison, one (curve No. 4) describing the RIA-gnost hTSH
assay kit currently on the market (contains 4400 times
the amount of a non-specific suppressor IgG relative to
the tracer IgG used) and the other describing a RIA-
gnost assay kit which contains no suppress;on medium
whatever (curve No. 5, multiple = 0; standard).
The binding (in %) to the reagent antibody increases
with increasing concentration of interfering substance
(the following were used: goat anti-mouse IgG (Fig. 4)
and rabbit anti-mouse IgG (Fig. 5)). Analyte is simula-
ted. The increase in non-specific binding is least when
the substances according to the invention from Example 2
are used (curve No. 3 in Figs. 4 and 5).
xample 6
The percentage binding of the reagent antibodies to an
;nterfer;ng substance whose concentration (titer) was
constant was determined as a function of varying amounts
of suppression medium. The following ;nterfering sub-
stances were used:
~30~
- 13 -
Titer
__
Goat anti-mouse IgG 1.5 ~g/ml (Fig. 6)
Rabbi~ anti-mouse IgG 1.5 ~g/ml (Fig. 7)
Patient's serum A (Fig. 8)
Patient's serum a (Fig. 9)
The fol~owing comparison suppression substances were
used:
Curve No. in Figs. 6 to 9
10 rat IgG 6
rat serum 7
mouse IgG 8
substance according 9
to the invention
15 (from Example 2)