Note: Descriptions are shown in the official language in which they were submitted.
~3~5~5~
5-162~7/1~2
Substituted dioxolan and dioxan derivatives
The present invention relates to novel substituted 1,3-dioxol~n and
1,4-dioxan derivatives, to processes for their preparation, and to
their use in pest control.
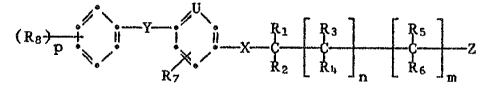
The compounds according to the inventlon have the formula I
(~ p~,;~ Y~ Z (I)
in whlch
R1, Rz, R3, R4, Rs and R6 each independently of the others
represents hydrogen or C1 C4-alkyl or, if n ~ 1,
Rz and R4 together represent one of the radicale -~CH2~- or
~~CHz~- ;
R7 represents hydrogen, halogen, methyl, ethyl, C1-Cz~alkyl sub-
stitut~d by 1 to 5 halogen atoms, methoxy, ethoxy or Cl-Cz-alkoxy
substituted by 1 tG 5 halogen atoms;
Rg represents halogen, C1-C3-alkyl, C1-C3-alkyl substituted by 1 to
7 halogen atoms, C1-C3-a1koxy, C1-C3-alkoxy substituted by 1 to
7 halogen ato~s, or cyano;
represents a grouping -CH- or -N=;
X represents -O-, -S- or -N(R9)-;
Y represents -O-, -S-, -S(O)-, -S(Oz~-, -CH2-, -CO- or -N(Rg)-,
wherein
Rs represents hydrogen, C1-CI,-alkyl or alkylcarbonyl having a total
of from 2 to 4 earbon atoms;
31 3~ 159~
-- 2 --
n is a number O or 1;
m is s number 0, 1 or 2;
p is a number 1, 2 or 3; and
Z represents one of the radicals
~0\ / R~
or
\0/ \R \o R14
wherein
R1~ represents hydrogen, methyl or ethyl;
Rll represents hydrogen, C1-C6-alkyl, C3-C6-cycloalkyl, Cz-C4-
alkenyl, C2-C4-alkynyl, alkoxyalkyl having a total of 2 to 6 carbon
atoms, CI~C6 alkyl substituted by 1 to 7 halogen atoms, benzyl or
benzyl substituted in the nucleus by a halogen atom, by a C1-C3-
alkyl radical or by a C1-C3-alkoxy radical, or represents cyano-
methyl, ~CI-C2-alkoxy)-carbonyl or the radical -CH2-NH-COO-
( C I -C 2 -alkyl);
Rlz repre3ents hydrogen, Cl-Cs-allcyl~ phenyl, phenyl substituted by
1 to 3 substituents from the group consisting of halogen, Cl-C4-
alkyl, Cl-C3-alkyl substituted by 1 to 7 halogen atoms, Cz-CI,-
alkenyl, Cz-C4-alkynyl, C1-C3-alkoxy, Cl-C3-alkoxy substituted by 1
to 7 halogen atoms, alkoxyalkyl having a total of 2 to 5 carbon
atoms, C3-C4-alkenyloxy, C3-C4-alkynyloxy and C1-C3-alkylthio, or
represents pyridyl, furan-2-yl, thien-2-yl or the radical
/0\
Il ; or
~ /-\ /
Rlz and Rll together represent one of the groups -~CH2~-,
-~CHz~- or -~H2~-;
and
Rl3 and R14 each independently of the other represents hydrogen,
halogen or Cl-C3-alkyl; or
Rl3 and Rl4 together represent the group -~CHz~-.
-
-- 3 --
In the context of the present invention, halogen is fluorine,chlorine, bromine or iodine, preferably fluorine or chlorine.
The invention also relates to the pGssible isomers, including the
diastereoisomers and the enantiomerg, of the compounds of formula I.
The alkyl, alkoxy, haloalkyl, haloalkoxy, alkoxyalkyl, alkylthio-
alkyl, alkenyl and alkynyl groupg may be straight-chain or branched.
Examples of such groups are, inter alia, methyl, methoxy, methoxy-
methyl, difluoromethoxy, ethyl, ethoxy, 2-fluoroethoxy, 171,2,2-
tetrafluoroethyl, pentafluoroethyl, 1,1,2,2-tetraEluoroethoxy,
pentafluoroethoxy, propyl, isopropyl, n-butyl, n-pentyl, n-hexyl and
their isomers, vinyl, 1-propen-3-yl and 1-propynyl.
Preferred cycloalkyl groups for Rl1 are cyclopentyl and cyclohexyl.
According to the invention, preferred compounds of formula I are
those in which
R1, R2, R3, Rli, Rs and R6 each independently of the others repre-
sents hydrogen or Cl-C4-alkyl or, if n ~ 1,
R2 and R4 together represent one of the radicals -~CH2t- or
-~CHz~-;
R7 represents hydrogen, halogen, methyl, ethyl, C1-Cz-alkyl substi-
tuted by 1 to 5 halogen atoms, methoxy, ethoxy or C1-C2-alkoxy sub-
stituted by 1 to 5 halogen atoms;
R8 represents halogen, C1-C3-alkyl, C1-C3 alkyl subst~tuted by 1 to
7 halogen atoms, C1-C3-alkoxy, C1~C3-alkoxy substituted by 1 to
7 halogen atoms, or cyano;
~ represents a grouping -CH~ or -N-;
X represents -0-, -S- or -N(Rg)-;
Y represents -0-, -S-, -S(0)-, -S(02)-, -CH2-, -C0- or -N(Rg)-,
wherein
Rg represents hydrog~n, C1-C4-alkyl or alkylcarbonyl having a total
of 2 to 4 carbon atoms;
n is a number 0 or 1;
m is a number 0, 1 or 2;
p ls a number 1, 2 or 3; and
Z represents one of the radicals
_i \./ ~ /0~ ~ l3
O Rl2 R1l,
wherein
Rlo represents hydrogen, methyl or ethyl;
Rl1 represents hydrogen, C1-C6-alkyl, C3-C~-cycloalkyl, C2-C4-
alkenyl, C2-C4-alkynyl, alkoxyalkyl having a total of 2 to
6 carbon atoms, C1-C6-alkyl substttuted by 1 to 7 halogen
atoms, benzyl, or benzyl substituted at the nuclsus by a halogen
atom, by a Cl-C3-alkyl radical or by a C1-C3-alkoxy radical;
Rl2 represents hydrogen, Cl-C6-alkyl, phenyl, phenyl substltuted by
1 to ~ substituents from the group consisting of halogen, C~-C4-
alkyl, Cl-C3-alkyl substltuted by 1 to 7 halogen atoms, C2-CIl-
alkenyl, C2-CI,-alkynyl, Cl-C3--alkoxy, Cl-C3-alkoxy substltuted by 1
to 7 halogen atoms, alkoxyalkyl having a total of 2 to 5 carbon
atoms, C3-CI,-alkenyloxy, C3-C4-alkynyloxy and Cl-C3-alkylthio, or
represents the radical
~ \
o/
R12 and Rl1 together represent one of the radicals -tCHz~- or
-~CH2~~;
and
Rl 3 and Rl4 aach independently of the other represents hydrogsn,
halogen or C1~C3-alkyl; or
Rl3 and Rl4 together represent the group -~CH2~-.
Attention ls drawn also to compounds of formula I that are
characterised in that
R1 and R3 each independently of the other represents hydrogen,
methyl or ethyl;
R2, R4, Rs and R6 represent hydrogen or, if Jl ' 1,
~3~5~
-- 5 --
Rz and R4 together repregent one of the radicals -~CH2-~- or
-~CHz~-;
R7 represents hydrogen or halogen;
RB rep}esents methyl, ethyl, halogen or Cl-Cz-alkyl substituted by
1 to 5 halogen atoms;
U represents a grouping -CH~ or -N~;
X represents -O-, -S- or -N(Rg)-;
Y represents -O-, -S-, -CH2- or -CO-; wherein
Rg represent~ hydrogen, methyl or acetyl;
n and m independently of each other ls a number O or l;
p is a number 1 or 2, and
Z represents one of the radicals
~0\ / Rll /0\ ~R13
! / \ oder
O Rlz R
wherein
Rll represents hydrogen, C1-C~I-alkyl, C3-C6-cycloalkyl, Cz-C3-
alkenyl, Cz-C3-alkynyl, alkoxyalkyl having a total of 2 to 4 carbon
atoms, Cl-C4-alkyl substituted by 1 to 7 halogen atoms, or benzyl;
R1z represents hydrogen, Cl-C~-alkyl, phenyl, phenyl substituted by
1 or 2 substituents from the group con~isting of halogen, Cl-C3-
alkyl, Cl-Cz-alkyl substituted by 1 to 5 halogen atoms, Cz-C4-
alkenyl, Cz-C4-alkynyl, C1-C3-alkoxy, methoxy substituted by 1 to
3 halogen atoms, alkoxyalkyl having a total of 3 to 4 carbon atoms,
C3-C4-alkenyloxy, C3-C4-alkynyloxy and Cl-C3-alkylthio, or repre-
sents the radical
_.~ \./\
'! ~-; o~
~./ \o
Rlz and Rl1 together represent one of the radicals -~CHz~- or
-~CHz~-; and
Rl 3 and R14 each independently of the other represents hydrogen or
methyl.
~^3~'J~
- 6 -
Also preferred are those compounds of formula I in whlch
Rl represents hydrogen or methyl;
Rz, R3, R4 9 Rs, R6 and R7 represent hydrogen;
Rg represents fluorine, chlorine, methyl or trifluoromethyl;
U represents the grouping -CH~;
X represents -0-;
Y represents -0-;
n and m are the number O;
p is a number 1 or 2; and
Z represents one of the radicals
~0\ ~Rl 1 ~0
or i t
\0/ \Rl Z \0/
wherein
Rll represents hydrogen, Cl-C~I-alkyl, C3-C6-cycloalkyl, C2-C3-
alkenyl, Cz-C3-alkynyl, alkoxyalkyl having a total of 2 to 4 carbon
atoms, or benzyl;
Rlz represents hydrogen, Cl-C4-alkyl, Cl-C2-alkyl substituted by
from 1 to 5 halogen atoms, phenyl~ phenyl substituted by 1 or 2 sub-
stituents from the group consisting of halogen and C1-C3-alkyl, or
repre~ents the radical
~ \ /\
o/
R1z and Rl1 together represent one of the radicals -~CHzt- or
-~CH2~-;
those compounds of formula I in which
Rl represents hydrogen or methyl;
Rz, R3, R4, Rs, R6 and R7 represent hydrogen;
Rg represents halogen or trifluoromethyl;
U represents the grouping -CH~;
X represents -O- or -S-;
~3~5~
Y represents -O-, -S-, -CH2- or -CO ;
n and m are the number O;
p i9 a number I or 2; and
Z represents one of the radicals
/o~ ~ 13
i . or
\ ~ 12 ~/ ~14
wherein
Rl~ represents hydrogen or methyl;
Rll represents hydrogen, Cl-C6-alkyl, C3-C6-cycloalkyl, C2-C4-
alkenyl, C2-C4-alkynyl, alkoxyalkyl having a total of 2 to 4 carbon
atoms, Cl-C4-alkyl substituted by 1 to 5 halogen atoms, benzylz
cyanomethyl, ~Cl-C2-alkoxy)-carbonyl or the radical -CH2-NH-COO-
(C1-C2-alkyl);
Rl2 represents hydrogen, C1-C6-alkyl, phenyl substituted by 1 or
2 ~ubstltuents from the group consisting of halogen, C1-C2-alkyl,
C2-C4-alkenyl, C2-C3-alkynyl, Cl-C2-alkoxy and C2-C3 alkylthio, or
represents pyridyl, furan-2-yl, thien-2-yl or the radical
\ / \
; or
~-~ \O
Rl2 and RlI together represent one of the nroups tCH2~-,
-~CH2~- or -~CH
and
Rl3 and Rl4 each independently of the other represents hydrogen,
halogen or Cl-C3-alkyl; or
Rl 3 and Rl4 together represent the group -~CH2~-;
and
those compounds of formula I in which
Rl, Rz, R3, R4, Rs~ R6 and R7 represent hydrogen;
R8 represents fluorine~ chlorine or trifluoromethyl;
U represents the grouping -CH~;
X represents -0-;
~l3~L~
-- 8 --
Y represents -O-, -S-, -CH~- or -CO-;
n and m are the number O;
p i9 a number l or 2; and
7 represents one of the radicals
~0~ ~Rl I o ~Rl 3
- I 9 or -. I
~ 12 ~ R14
wherein
R11 represents hydrogen, C1-C6-alkyl, C3-C6-cycloalkyl, Cz-C4-
alkenyl, C2-CI,-alkynyl, alkoxyalkyl having a total of 2 to 4 carbon
atoms, C1-C4-alkyl substituted by 1 to 5 fluorine or chlorine atoms,
benzyl, cyanomethyl, ethoxycarbonyl or the radical -CM2-NH-COO~CH3;
R1z represents hydrogen, C1-C6-alkyl, phenyl subseituted by 1 or
2 substituents from the group consisting of fluorine, chlorine,
C1-Cz-alkyl and C1-Cz-alkoxy, or represents pyrid-4-yl, furan-2-yl
or thlen-2-yl, or the radical
~ \ /o\
! !i ; or
~.~ \0/
R12 and R11 together represent one of the groups tCH2~-,
-~CH2~- or tcH2~-;
4 s
and
R13 and R14 each independently of the other represents hydrogen,
halogen or Cl-C3-alkyl; or
Rl3 and R14 together represent the group tCH2~-.
Because of their biological activity, attention i~ drawn according
to the invention especially to the compounds of formula Ia
----O----CHZ_./ \ ~R11 (Ia)
\0/ ~l2
wherein
~c~
Rg represents fluorine, chlorlne or trlf:Luoromethyl;
p is a number 1 or 2; and
Rll and Rlz each independently of the other represents hydrogen,
cl-c4-alkyl~ C2-c4-methoxyalkyl~ Cl-C2-alkyl substituted by 1 to
3 halogen atoms, C2-C3-alkenyl or C2-C3-alkynyl, or R1l and Rlz
together represent one of the radicals -~CH2~- or
H2~.
Those compounds of formula I or Ia in which p ~ 1 and/or R12 repre-
sents hydrogen are also of particular importance.
The compounds of formula I and Ia can be prepared in a manner known
per se (see also US Patent Specification No. 4,007,280):
a) To that end, for example, a compound of formula II
~ \ _
(R8~ H (Il~
R7
can be reacted with a compound of formula III
Q~ ~ ~ ~ Z (III),
in which formulae II and III Rl to R~, U, X, Y, n, m, p and Z have
the meanings given above and Q represents a leaving group.
b) A compound of formula I in which Z represents the radical
\o/ ~ ~R
O Rl 2
can be obtained by condensing a compound of formula IV
:~3~5~
-- 10 --
\ _ y~ 3 ~ ~ OH (IV)
R7 2 " n 6 H~-OH
in the presence of Bn acidic catalyst with a compound of formula V
or Va
R160\ / ~11 /R11
/C\ (V) O~C~ ~Va),
Rl60 Kl2 Rl2
in which formulae IV1 V and Va Rl to Rl~, U, X, Y, n, m, p, Rll and
R12 have ths meanings given above and Rl 6 represents Cl-C4-alkyl,
preferably methyl or ethyl.
Process a) i~ preferably carried out in the presence of a solvent or
diluent that is lnert towards the reactants and in the presence of
at least one equlvalent of an acid acceptor or oE a basic substance
respectively. Suitable acid acceptors and bases are especially
tertiary amines, such as trialkylamines and pyridine, and also
hydrides, hydroxides, oxides, carbonates and bydrogen carbonates of
alkali metals and alkaline earth metals, and also alkali metal
alcoholates, such as, for example, potassium t-butoxide and sodium
methoxide etc~. Depending on the nature of the solvents used, the
reaction temperature is usually in the range of from -5C to
+140C, preferably from O to 60C. In the starting compound of
formula III, Q represents a usual leaving group, such as, for
example, a halogen atom, the mesyloxy group or the tosyloxy group.
Process b) is generally earried out in the presence of an inert
solvent or diluent and an acidic catalyst. As acidic catalysts there
are used, for example, sulphonic acids, such as methanesulphonic or
p-toluenesulphonic acid, including the acidic ion exch~nger resins
containing sulpho groups9 Lewis acids, such as boron trifluoride-
diethyl ethsr or boron trifluoride-dimethyl ether complexes, snd
also mineral acids, such~as sulphuric acid or phosphoric acid.
~3~ S~
~ 11 -
Dependlng on the nature of ~he solvents used, the reaction
temperature i8 in the range of approximately from 30 to 150C,
preferably from 60 to 120C.
The above-described processes are carrled out usually under normal
or elevated pressure and preferably in an inert solvent or diluent.
Suitable solvents and diluents for process a~ are, Eor example,
ethers and ethereal compounds, such as diethyl ether, dlisopropyl
ether, dioxan, 1,2-dimethoxyethane and tetrahydrofuran; amides, such
as N,N-dialkylated carboxylic acid amides; sulpholane and dimethyl
sulphoxide. Suitable solvents and diluents for process b) are, for
example, aliphatic, aromatic and halogenated hydrocarbons,
especially benzene, toluene, xylenes, chloroEcrm, dichlorGmethane,
chlorobenzene, hexane and cyclohexane.
The starting ~aterials of formulae II to Ya are known or, if novel,
they can be prepared by methods analogous to known ones and then
constitute also an obJect of the present invention.
For example, the diols of formula IV can be obtained (see US Patent
Specifications Nos. 4.590.282 and 4.097.581) by reacting a compound
of formula II with a hydroxymethylalkylene oxide of formula YII
R7~ - CN~OH (VII)
The compounds of formulae I and Ia may be ln the form of mixtures of
different enantiomeric forms, or they may be formed ln synthesls.
The mixtures of dlastereolsomeræ or the racemates of formulae I and
Ia can be resolved lnto the lndividual forms by known methods. A
compound of formula I is therefore to be understood as co~prislng
all the lndlvidual diastereoisomeric or enantiomeric forms and the
mixtures thereof.
~3~
Certain 4-(4-phenoxy)-phenoxymethyl-1,3-dloxolans are already known
as insecticide~ from US-PS 4.097.581. 1,3-dioxolan and l,~-benzo-
dioxin derivatives of similsr structure and their use as pesticides
are sub~ect matter of US-PS 4.590.282. The compounds o$ formula I
according to the inventlon differ from those known compounds essen-
tially by the presence of a phenyl group mandatorily sub~tituted by
1 to 3 R~ radicals.
Surprisingly, it has been found that the compounds of formula I of
the invention are excellently suitable for controlling a variety of
pests of animals and plants as well as soll pests. The compounds of
formula I can thus be used for controlling insects, for example of
the orders: Lepidoptera, Coleoptera, Homoptera, Heteroptera,
Diptera, Thysanoptera, Orthoptera, Anoplura, Siphonaptera,
Mallophaga, Thysanura, Isoptera, Psocoptera and Hymenoptera and
mltes and ticks of the order Acarlna.
The compounds of formulae I and Ia are sultable for controlling
plant-destructlve insects ln ornamentals and crops of useful plants,
in partlcular in cotton crops ~e.g. Spodoptera littoralis and
heliothis virescens). The compounds of formula I are also effectlve
against 90il insects (e.g. Aulacophora femoralis, Chortophila
brassicae, Diabrotica balteata, Pachnoda savignyi and Scotla
ypsllon), and especlally against sucking insects, such as aphlds
(e.g. Myzus persicae, Aphls craccivora and Aonldlella aurantli, and
also other representatives of the famlly Coccidae). The compounds of
thls lnvention are also actlve as ovlcldes ln plant protectlon,
especially for controlllng plant-destructlve insects, e.g. Cydia
pomonella, Lobesla botrana and Adoxophyes retlculana.
The compounds of ~o}mula I are also very effective against flies,
e.g. Musca domestica, and mosquito larvae, further agalnst stock
pests, e.g. Sitophilus orlzae and Rhizopertha dominica.
~3~
- 13
The compound~ of formulae I and Ia of the lnvention are particularly
effective against plant-destructive acarids (~plder mites e.g. of
the families Tetranychidae, Targonemidae, Eriophydae, Tyroglyphidae
and Glycyphagidae) and also against ectoparasLtic acarids (mites and
ticks e.g. of the families Ixodidae, Argasidae, Sarcoptidae and
Dermanyss~dae) that attack productive livestock. A number of the
compounds of this invention have good acaricidal-ovicidal activity
and leaf penetration properties. The compounda of the invention are
particularly suitable for controlling the following species of mites
which attack crops of fruit and vegetables: Tetranychus urticae,
Tetranychus cinnabarinus, Panonychus ulmi, Bryobia rubrioculus,
Panonychus citri, Eriophyes piri, Eriophyes ribis, Eriophyes vitis,
Tarsonemus pallidus, Phyllocoptes vitis and Phyllocoptruta oleivora.
The acaricidal or insecticidal activity can be substantially
broadened and adapted to prevailing circumstances by addition of
other insecticides and/or acaricides. Examples of suitable additives
include: organophosphorus compounds, nitrophenols and derivatives
thereof, formamidines, ureas, pyrethroids and also carbamates and
chlorinated hydrocarbons.
The good pesticidal activity o~ the compounds of formulae I and IR
of the invention corresponds to a mortality of at least 50-60 ~0 of
the above pests.
The compounds of formula I are used in unmodified form, or prefer-
ably together wlth the adjuvants conventionally employed iD the art
of formulation, and are therefore formulated in known manner to
emulslfiable concentrates, directly sprayabl~ or dilutable
solutions, dilute emulsions, wettable powder~, soluble powders,
dusts, granulates, and also encapsulations in e.g~ polymer
substances. As with the nature of the compositions, the methods of
application, such as spraying, atomising, dusting, scattering or
pouring, are chosen -ln accordance with the intended ob~ectives and
the prevailing circumstances.
~3~5~
- 14 -
The formulations, i.e. the compositions, preparations or mixtures
containing a~ least one compound (active lngredient) of ~ormula I
and, where appropriate, a solid or liquid ad~uvant, are prapared in
known manner, e.g. by homogeneously mixing and/or grinding the
active ingredients with extenders, e.g. solvents, solid carriers
and, in some cases, surface-active compounds (surfactants~.
Suitable solvents are: aromatic hydrocarbons, preferably the
fractions containing 8 to 12 carbon atoms, e.g. xylene mixtures or
substituted naphthalenes, phthalates such as dibutyl phthalate or
dioctyl phthalate, aliphatic hydrocarbons such as cyclohexane or
paraffins, alconols and glycols and their ethers and esters, such as
ethanol, ethylene glycol, ethylene glycol monomethyl or monoethyl
ether, ethyl acetate, propyl myristate or propyl palmitate, ketones
such as cyclohexanone, strongly polar solvents such as N-methyl-2-
pyrrolidone, dimethyl sulphoxide or dimethylformamide, as well as
vegetable oils or epoxidised vegetable oils such as epoxidlsed
coconut oil or soybean oil; silicone oils or water.
The solid carriers used e.g. for dusts and dispersible powders are
normally natural minsral fillers such as calcite, talcum, kaolin,
montmorillonite or attapulgite. In order to improve the physical
properties it is also possible to add highly dispersed silicic acld
or highly dispersed absorbent polymers. Suitable granulated
adsorptive carriers are porous types, for example pumice, broken
brick, sepiolite or bentonite; and suitable nonsorbent carriers are
materials such as calcite or sand. In addition, a great number of
pregranulated materials of inorganic or organlc nature can be used,
e.g. especlally dolomlte or pulverlsed plant residues.
Depending on the nature of the compound of formula I to be formu-
lated, suitable surface-active compounds are non-ionic, cationic
and/or anionic surfactants having good emulsifying, dispersing and
wetting propertle3. The term "surfactant" will also be understood as
comprising mlxtures of surfactants.
~3~ LS~
Suitable anionic surfactants can be both water-soluble soaps and
water-soluble synthetic surface-active compounds.
Suitable soaps are the alkali metal salts, alkaline earth metal
salts or unsubstituted or substituted ammonium salts of higher fatty
acids (Clo-C22), e.g. the sodium or potassium salts of oleic or
stearic acid, or of natural fatty acid mixtures which can be ob-
tained e.g. from coconut oil or tallow oil. Further suitable sur-
factants are also the fatty acid methyltaurin salts.
More frequently, however, so-called synthatic surfactants are used,
especially fatty sulphonates, fatty sulphates, sulphonatad benz-
imidazole derivatives or alkylarylsulphonates.
The fatty sulphonates or sulphates are usually in the for~l of alkali
metal salts, alkaline earth metal salts or unsubstituted or sub-
stituted ammonium salts and contaln a Cg-C22-alkyl radical which
also includes the alkyl moiety of acyl radicals, e.g. the sodium or
calcium salt of lignosulphonic acid, of dodecylsulphate, or of a
mixture of fatty alcohol sulphates obtained from natural fatty
acids. These compounds also compri~e the salts of sulphated and
sulphonated fatty alcohol/ethylene oxide adducts. The sulphonated
benzimidazole derivatives preferably contain 2 sulphonic acid groups
and one fatty acid radical containing 8 to 22 carbon atoms. Examples
of alkylarylsulphonates are the sodium, calcium or triethanolamine
salts of dodecylbenzenesulphonic acid, dibutylnaphthalenesulphonic
acid, or of a condensate of naphthalenesulphonic acid and formal-
dehyde.
Also suitable are corresponding phosphates, e.g. the salts of the
phosphoric acid ester of an adduct of p-nonylphenol with 4 to
14 moles of ethylene oxide, and phospholipids.
Non-ionic surfactants are preferably polyglycol ether derivatives of
aliphatic or cycloaliphatic alcohols, or saturated or unsaturated
fatty acids and alkylphenols, said derivatives containing 3 to
5~
- 16 -
30 glycol ether groups and 8 to 20 carbon atoms in the (allphatic)
hydrocarbon molety and 6 to 18 carbon atoms in the alkyl moiety of
the alkylphenols.
Further suitable non-ionic surfactants are the water-soluble adduct~
of polyethylene oxide with polypropylene glycol, ethylenediamino-
polypropylene glycol and alkylpolypropylene glycol containing 1 to
10 carbon atoms in the alkyl chain, which adducts contain 2~ to
250 ethylene glycol ether groups and 10 to 100 propylene glycol
ether groups. These compounds usually contain 1 to 5 ethylene glycol
units per propylene glycol unit.
Representative examples of non-ionic surfactants are nonylphenol-
polyethoxyethanols, castor oil polyglycol ethers, castor oil
thioxllate, polypropylene/polyethylene oxide adducts, tributyl-
phenoxypolyethoxyethanoll polyethylene glycol and octylphenoxypoly-
ethoxyethanol.
Fatty acld esters of polyoxyethylene sorbitan, e.g. polyoxysthylene
sorbitan trioleate, are also suitable non-ionic surfactants.
Cationic surfactants are preferably quaternary ammonium salts which
contain, as N substituent, at least one Cg-C22-alkyl radical and, as
further substituents, unsubstituted or halogenated lower alkyl,
benzyl or hydroxy-lower alkyl radicals. The salts are preferably in
the form of halides, methylsulphates or ethylsulphates, e.g.
stearyltrimethylammonium chloride or benzyldi-(2-chloroethyl)ethyl-
ammonium bromide.
The surfactants customarily employed in the art of formulation are
described e.g. in "McCutcheon's Detergents and Emulsifier~ Annual",
MC Pub~ishing Corp. ~idgewood, New Jersey, 1979; Dr. H~lmut Stache,
"Tensid Taschenbuch" (Handbook of Surfactants), Carl Hanser Verla~,
Munich/Vienna, 1981.
~3~5~L
- 17 -
The pesticidal compositlons usually contain 0.1 to 99 ~, preferably
0.1 to 95 %, of a compound of formula I, 1 to 99.9 % of a solid or
liquid adjuvant, and 0 to 25 %, especially 0.1 to 25 %, of a sur-
factant, percentages relating to weight.
Whereas commercial compositions are preferably formulated as concen-
trates, the end u3er will normally employ dilute formulations or
preparations containing e.g. 0.1 to 1000 ppm of the active compound.
The compositions may al~o contain further additives, such as
stabilisers, anti-foams, viscosity regulators, binders, tackiflers
and fertilisers or other active ingredients for achieving special
effect 9 .
Example _
a) Preparation of 1,2-dLhydroxy-3-[4-(4-fluorophenoxy)]- phenoxy-
propane (starting material):
0.7 g of tetr~methylammonium chloride i8 added to a solutlon of
71.4 g of 4-fluorophenoxyphenol in 70 ml of xylene, the whole i9
heated to 60C, and then 29.6 g of glycerol glycide are added
dropwise, within a period of approximately 30 minutes, while
stirring. The reaction mixture is then further stirred for approxi-
mately 16 hours at 90C. For working up, 200 ml of n-hexane are
slowly added to the reaction mixture at approximately 60C, the
whole is cooled to 20C while stirring, and the precipit~te which
has formed i8 filtered off. The crude product so obtained is
recrystallised from isopropanol and n hexane. In this manner, ths
title compound of formula
F~ O-cH2- ,CH--OhT
i8 obtained ln the form of colourless crystals with a melting point
of 86-87C.
31 3~
~ 18 -
The following compounds of formula IV are prepared ln analogous
manner, if appropriate ~lth puriflcation by chromatography (eluant.
methylene chloride/diethyl ether 1~
o-o~ \- m.p. 76-78C
~ O-CHz-lCH-OH
i il i il-O-CHz- ~ -OH m.p. 63-64C
Hz-OH
O-CH2- H-OH m.p. 52-54C
8~l 2 -OH
~ m.p. 64-65C
F ~o ~ ~. CH2-OH
F~ O-~ ~ m.p. 74-75C
--O--CHZ-ICH-- OH
~ -O-CH2- H-OH m.p. 109-110C
C~ . gH 2 -OH
O--~ m.p. 60-62~C
~CHZ-8~l--OH
Cl
O-CH2-CH--OH m.p. 94-9SC
.~ CH2-OH
CH2 i Il-O-CH2-CH- OH m.p~ 81-g2C
Hz-oH
~3~5~
19 -
o ~ o
,!~ ,! i~ /.-O-CH2-~H- OH m.p. 87-88C
b) Preparation of 4-[4-(4-fluorophenoxy)]-phenoxymethyl- 2-ethyl-
1,3-dioxolan
8 g of propionaldehyde diethyl acetal are added dropwlse, within a
period of 10 ~inutes, while stirring at reflux temperature, to a
solution of 13.9 g of the 1,2-dihydroxy-3-[4-(4-fluorophenoxy)]-
phenoxypropane prepared in accordance with a) and 40 mg of
p-toluenesulphonic acid in 75 ml of cyclohexane. After stirring for
one hour at that temperature, the ethanol which has formed is
distilled off continuously over a short Vigreux column within a
period of approximately 2 1/2 hours. The reaction mixtur2 is then
washed wlth 10 % sodium carbonate solution and then with water~ the
cyclohexane solution is dried over sodium sulphate, the cyclohexane
is distilled off under a water-~et vacuum, and the residue la
chromatographed on silica gel (eluant: n-hexane/diethyl ether 19:1).
The title compound (compound no. 1~ of formula
\. o
obtained in this manner has a melting point of 47-48C (mixture of
diastereoisomers~.
Example 2:
Preparation of 4-[4-(4-fluorophenoxy)]-phenoxymethyl-2-isopropyl-
1,3-dioxolan:
A solution of 13.9 g of 1,2-dihydroxy-3-[4-(4-fluorophenoxy)]-
phenoxypropane, 5.1 g of isobutyraldehyde and 50 mg of p-toluene-
sulphonic acid in 150 ml of benzene is heated for 5 hours while
stirring in a water separator. The reaction mixture is then washed
repeatedly first with 10 % sodium carbonate solution and then wi~h
water, and is then dried over sodium sulphate and filtered. The
rrade -~r~
13~S~
- 20 -
benzene is distilled off from the resulting filtrate in vacuo. The
crude product is chromatographed on sllica gel (eluant: ether/n-
hexane 1:20), the pure title compound having a melting point of
62-64C (compound no. 2) of formula
I il i ll-o-CH2~ CH~H3)Z
being obtained in the form of a mixture of diastereoisomers.
Example 3:
Preparation of 4-[4-~4~fluorophenoxy)]-phenoxymethy -2,2-dimethyl-
1,3-dioxolan:
I~ 3 i li-o-CH2-~/ \ /CH3
~0/ CH3
a) 6.4 g of 4-(4-fluorophenoxy)-phenol are reacted with 3.5 g of
potassium tert.-butoxide in anhydrous dimethyl sulphoxide, a
solution of the potassium salt of that phenol being obtained. A
solution of 9.1 g of R-(-~-2,2-dimethyl-1,3-dioxolan-4-methanol-
toluene-4-sulphonate in 20 ml of dimethyl sulphoxide ls added drop-
wise to the solution obtained above in a nitrogen atmosphere at
10C, while stirring, and the whole is then stirred for a further
20 hours at 20-22C.
For working up, the reaction mixture is poured into 300 ml of ice-
water and extracted four times with 100 ml of diethyl ethsr. The
combined ether phases are ~ashed repeatedly with water and dried
over sodium sulphate. Ater the ether has been distilled of, the
residue is puri~ied further by chromatography on 250 g of silica ~el
(elu~nt: diethyl ether/hexane 1.20), pure (S)-4-[4-(4-fluoro-
phenoxy)]-phenoxymethyl-2,2-dimethyl-1,3-dioxolaD being obtained:
nD = 1.5345, [~]D: +5.2 + 0.3 (CHCl3) (compound no. 3~.
~3~
b) In analogous manner there :Ls obtained from the potassium salt of
4-(4-fluorophenoxy)-phenol and S-(+)-2~2-dimethy~ 3-dioxolan-4-
methanol-toluene-4-sulphonate in dimethyl sulphoxide the enantio-
meric (R)-4-[4-(4-fluorophenoxy)]-phenoxymethyl-2,2-dimethyl-1,3-
dioxolan: nD _ 1.S350, [~]D20: -5.0 + 0.3 (CHCl3) (compound
no. 3a).
The optical purity of the two enantiomers characterised under a) and
b) above was checked by MPLC chromatography (medium pressure liquid
chromatography) on cellulose triacetate. It is ~99.8 % for the S
compound and ~99.4 æ for the R compound.
F.xample 4:
a) Preparation of 2-methanesulph _ lmethyl-6~methyl-1,4- dioxan_
(starting material):
13.7 ml of methanesulphonic acid chloride are added dropwise, over a
perlod of approximately 30 minutes, while stirrlng at 0 to SC, to a
solution of 21.1 g of 2-hydroxymethyl-6-methyl-1,4-dioxan, 15.2 g of
pyridine and 0.6 g of 4-di~ethylaminopyridine in 80 ml of dichloro-
methane. After stirring for a further 18 hours at room temperature,
the reaction mixture is extracted repeatedly with lN hydrochlorlc
acid and then washed with water until neutral. After the organlc
phase, which has been separated off, has been dried over sodium
sulphate and the solvent has been di~tllled off completely, the
tltle compound of formula
CH3S02-0-CH2-~ ~-CH3
\0/
ls obtained. The lH~proton resonance spectrum corresponds to this
structure; nD~ = 1.4607.
1 30 il~
- 22 -
b) _r ~aration of 2-[4-(3-flu~ropheno~ ~ h noxymethyl- 6 methyl-
1,4-dioxan:
A freshly prepared solution of 3.6 g of potasslum tert.-'outoxide in
20 ml of dimethyl sulphoxlte i8 added while cooling with ice to a
solution of 6.2 g of 4-(3-fluorophenoxy)-phenol in 20 ml of dimethyl
sulphoxide. To the resulting solution of the potassium salt of that
phenol there is then added dropwise at 10C, over a period of
approximately 30 minutes, a solution of 8 g of the 2-methanesul-
phonylmethyl-6-methyl-l,4-dioxan prepared as above in accordance
~ith a), in 20 ml of dimethyl sulpho~ide. The reaction ~ixture i3
then stirred for a further 16 hours at 22C. The mixture is then
poured onto lce-water and extracted repeatedly with diethyl ether/
hexane (1:1). The combined organlc phases are washed with 10 %
potassium hydroxide solution and then with water until neutral, and
dried over sodium sulphate. The solvents are then distilled off. The
title compound of formula
--o--CH2--7' `T--CH3
\O''
which has a refractive index n~2 - 1.5445 (compound no. 4~ i8
obtained by chromatography on silica gel (eluant: hexane~ethyl
acetate 6:1). The elementary analysis and the lH-NMR spectrum
correspond to the above structure.
The following compounds according to the invention of formula:
(Ra~~-+ 1~ o_cH2~
\0/ R~2
are also prepared analogously to the procedures described above, all
the compounds - except where indicated - being in the form of
mixtures of their diastereoisomers:
~S3~S~
- 23 -
. , ~
Comp. R3 P R11 Rlz Physical
o. ._
4F 1 -CH=CH2 H nD =1. 5477
6 4-F 1 -C-CH H m.p.74-76C
7 4-F 1 -CH3 -CH3 m.p .84-86C
8 3-F 1 -CHs~CH2 H nD -1. 5493
9 3~F 1 -CH3 -CH3 m.p.60-62C
3-F 1 -C2Hs H nD -1. 5379
11 2-F 1 -C2Hs H nD~1.5361
12 4-F 1 -(CH2)2-CH3 H nD~1.5301
13 3-F 1 -( CH2)2-CH3 H nD =1. 5321
14 4-F 1 -CH2-CH(CH3)2 H nD =1. 5257
3-F 1 -CH(CH3)2 Hm.p. approx. 32C
16 2-P 1 -(CH2)2-CH3 HrlD =1.5318
17 2-F 1 -CH(CH3)2 H nD ~1.5321
18 4-F 1--(CH2)s-- m.p.53-55C
19 3-F 1-CH=CH-CH3 H nD ~1. 5443
4-F 1OCH3 H nD ~1. 5281
21 4-F 1 -C2Hs -CN3 m.p.57-59C
22 2-F 1 -CH3 _ nD =1. 5410
*) not a diastereoisomeric mixture b~lt a racemate
l5~
. _
Comp. 8~ _ Rll Rl2 Physical
23 2-F 1 -CH3 H n~ ~1.5410
24 3-F 1 -CH2=CH~CH3 H nD 51. 5443
3-F 1 -CHzCH(OCH3)-CH3 H nD =1.5300
26 4-P 1 -CH3 H diaster~oi~o-
m.p.46-47C
26a 4-F 1 -CH3 H diasterealso-
nD =1 5428
27 4-F 1 -CH(C}13)-C2Hs H nD ~1.5369
28 2-F,4-F 2 -C2Hs H nD W1.5280
29 2-F,4-F 2 -CH(CH3)2 H nD =1.5225
4-F 1 ~ -Cl H m.p.89-91C
31 3-F 1 -~ Cl H m p.59-61C
32 4-F 1 ~ . H nD W1.5782
33 4-F I ~ --Cl H nD -1.5861
~ Cl 21
134¦3_F ~ H ~'{ L ¦~ ~nD ~1.5849
4-F 1 \ ./ -CN3
13~ 51~i4
-2S-
~ r . . n ~ ~ _ _ . . _ _ _ . _ _ . _ _ _ _ _ _ _
Comp. R8 P Rll Rlz Physical
No. data
. ......... . ... ~. _ ... .... _. _
36 4-F 1 ~CHz, s m.p. 53-55C)
37 4-F 1 ~ H m22. 73-74C
38 4-F 1 -C(CH3~3 H nD 5 1.5379
39 4-F 1 ~CHz~ m.p~72-73C
3-F 1 -CH2Cl ¦H nD-1.5504
41 3-F 1 ~CH2~ nD~1.5414 )
42 4-F 1 -CH2Br H nD=1.5769
43 4-F 1 -C0-OC2Hs H nD~1.5419
44 4-Cl 1 -CZHs H nD=1.5529
4S 4-Cl 1 -C3H7(n) H m.p.41-43C
46 4-F 1 -CHC12 H nD~1.5669
47 3-F,5-F 2 --CH=CH2 H m.p. 46-48C
48 3-F,5-F 2 --C2Hs H m p. 32-34C
49 3-F,5-F 2 -C3H7(n) H nD~1.5262
3-F 1 -C(CH3)3 H nD~1.5270
51 4-F 1 -C(CH3~3 H nD=1.5289
52 3-F 1 ~=N H nD=1.5390
53 3-P 1 --N H nD=1.5733
54 4-F 1 ~ m.p.60-63C
*) not a diastereoisomeric mixture but a racemate
- 26 -
Comp. R3 - Rll ~ Physical
No. ~ _ _ ~ data
SS 3-F 1 -NH-CO-OCH3 H m.p.65-66C
56 3-F 1 -CH=C(CH3) 2 H m.p.67~69C
57 3-F' 1 -C-CH H nD ~1.5539
58 3-F 1 -(CH2)s CH3 H nD =1.5245
59 3-F 1 -(CHz )4 CH3 H nD ~1~5260
3-F 1 -C~Hg(n) H nD =1.5290
61 3-F 1 ./j -CEI3 diastereoi~omer A
. (as racemate):
/ m.p. 69-70C
61a 3-F 1 _, ¦ -CH3 diastereolsom~r B
. (as racemate):
m.p. 44-46C
62 3-F 1 -CH-CH2 -CH3 diastereoisomer A
(as racemate):
nD ~1.5370
62a 3-F 1 -CH~CH2 -Cl13 diastereolosmer B
(a~ racemate):
nD -1.5350
63 3-Cl 1 -C2Hs H nD -1.5560
64 3-F 1 -CH2-C-N H m.p. 85-87C
3-F,5-F 2 -CH(CH3)2 H nD -1.5221
66 2-F,4-F 2 -CH=CH2 H nD ~1.5376
67 3-Cl 1 -C3H7(n) H nD ~1.5481
68 3-Cl 1 -CH.~_NH2 H nD ~1.5639
69 2-F 1 _,~ ~. H diastereoisomer A:
nD ~1,5~62
69a dlastereolsomer B:
nD ~1.5822
4-CF3 1 -C2Hs H m.p.62-63C
71 3-F 1 -CClz-CF3 H nD ~1.5695
72 3-F 1 -CHz-CH2Cl H nD =1.5341
73 3-F 1 --~CH2~ - Im.P 94-95C )
*) not a dia~tereoisomeric mixt~re but a racemate
~Q5~
- 27 -
_ .
Comp. R3 P Rll ¦ R~z Phy31cal
No ~ ~ ~ _ data
74 3-F 1 -CH2- H- - H- m.p. 36-38C
4-F 1 -.\ H / ¦H nD =1.5350
76 2-F 1 -CHzCl ¦~ nD ~1.5618
77 4-F l - (CHz)ll m.p.88-90C
78 4-F l -CHzCl H ~D a 1.5381
79 4-F l _.~ ~--CzHs H m.p.84-85C
=,/\,
4-F l ~ 0 H m.p.83-85C
.=./\.
81 3-F 1 _.~ ~ ~ H m.p.47-50C
82 4-F 1 H H m p.34-36C )
83 3-F 1 -CH3 H nD =1.5420
84 3-F 1 -C(CH3)~CHz H m.p.48-50C
3-P 1 (CH ) 4 - m.p.94-95C )
86 3-F 1 _1l il H nD -1.5620
87 3-F 1 _1l li H m.p.51-53C
88 3-F 1 ~C2Hs H m.p.84-86C
3 9 3-F ~ o H m.p.~30~C
*) not a diaYt~reoi~omeric mixturs but a racemate
~3~
- 28 -
Ihe following compounds of formula I are also prepared analogously
to the procedtlres described above:
Compound Physical
No. _ data
I `--I `- m.p. 57-58C
F 0
.~ \. O
91 !~ o-cH2-I~ 'i m.p. 60-62C
92 F- T i, T~ ~il - O-CH Z-i i m.p.65-67C
93 ! D i~ ,D~7 CN8~ -CH(C=3)Z
94 !~ `D-C~ .. p. s5-s7~c
~^~o ~ o
g5 !~ o-cH2-i \.-c3H7(n) m-p- 54-57C
96 !~ ll-0-CH2-If \ nD ~1,5479
\ / ~ / 2 1 / ff 2Hs nD ~1,530g
:~3~5~
-- 29 --
98F - i i1 i Il-O-CHz~ m.p.66-67C
~ -o-CHz-i \ C H ( ) P
100 P~ [~ ~i O-CHz--i ~ CH3 ~.p.~35C
The foll.owing compounds of formula I can be prepared ln a manner
analogous to that described above:
o_cH2~ C2Hs
\0/
~ '\ fH3
'! ! ~ -O-CH2~
,---C3H7 ( n)
\o/
I 11 ; Il_o-cH2-fi/ \--C3H7(i)
'~0/
CH3
~ .
: : :
13~51~4
- 30 -
Example 5:
Formulation Examples for liquid active ingredients of formula I
acco_i g to Examples 1 to 4 (throughout, percentages are b
w ght~
1. Emulsifiable concentrates a) b) c)
compound according to the
Preparatory Examples25 % 40 % 50 %
calci~m dodecylbenzenesulphonate 5 ~0 8 % 6 %
castor oil polyethylene glycol
ether (36 moles of ethylene oxide)5 %
tributylphenol polyethylene glycol
ether (30 moles of ethylene oxide) - 12 % 4 %
cyclohexanone - 15 % 20 %
xylene mixture 6S % 25 % 20 %
Emulsions of any desired concentr~tion can be produced from such
concentrates by dllution with water.
2. Solutions a ) b) c) d)
compound sccording to the
Preparatory Examples 80 % 10 % 5 % 95
ethylene glycol moDomethyl
ether 20 % - - -
polyethylene glycol
(mol. wt. 400) - 70 % - -
N-methyl-2-pyrrolidone - 20 %
epoxidised coconut oll - - 1 % 5 %
ligroln (boiling range
160-190C) _ _ 94 %
These solutions are sui-table for application in the form of micro-
drops .
~3C~
- 31 -
3. Granulates a) b)
compound according to the
Preparatory Examples 5 %10 %
kaolin 94 %
highly dispersed æilicic acid 1 %
attapulgite - 90 %
The active ingredient is di~solved in methylene chloride, the
solutio~ is sprayed onto the carrier, and the solvent is subse~
quently evaporated off in vacuo.
4. DustY a) b)
compound according to the
Preparatory Examples 2 % 5 %
highly dispersed slliclc acid 1 % 5 %
talcum 97 %
kaolin - 90 %
Ready for use dusts are obtained by intimately mixing the carriers
with the active ingredient.
Formulation Examples for solid active in _edients of formula I
according to Examples I to 4 ~throughout, percentages are by
weight)
5. Wettable powders a) b) c~
compound according to the
Preparatory Examples 20 % S0 % 75 %
sodium lignosulphonate 5 % 5 %
sodium laurylsulphate 3 % - 5 %
sodiu~ diisobutylnaphthalene-
sulphonate - 6 %10 %
octylphenol polyethylene glycol
ether (7-8 moles of ethylena oxide~ - 2 %
highly dispersed silicic acid 5 % 10 % 10 %
kaolin 67 % 27 %
~3~
- 32 -
The actlve ingredient is mixed with the adjuvants and the mixture is
thoroughly ground in a suitable mill, affording wettable powders
which can be diluted with watar to give suspen.sions of the desired
concentration.
6. Emulsifiable concentrates a) b)
compound according to the
Preparatory Examples 10 % 10 %
octylphenol polyethylene glycol
ether (4-5 moles of ethylene oxide) 3 %
calcium dodecylbenzenesulphonate 3 %
castor oil polyglycol ether
(36 moles of ethylene oxide) 4 %
castor oil thioxilate - 25 %
cyclohexanone 30 %
butanol - 15 %
xylene mixture 50 %
ethyl acetate - 50 %
Emulsions of any required concentration can be obtained from this
concentrate by dilution with water.
7. Duets a~ b)
compound according to the
Preparatory Examples 5 % 8 %
talcum 95 %
kaolin - 92 %
Ready for use dusts are obtaiDed by mixing the active ingredient
with the carrier and grinding the mixture in a suitable mill.
:L3~5~
- 33 -
8. Extruder granulate
compound according to the
Preparatory Examples 10 %
sodium lignosulphonate 2 %
carboxymethylcellulose 1 %
kaolin 87 %
The activa ingredient i5 mixed and ground with the adjuvants, and
the mixture is subsequently moistened with water. The mixture is
extruded and then dried in a stream of air.
9. Coated granulate
compound according to the
Preparatory Examples 3 %
polyethylene glycol (mol. wt. 200) 3 %
kaolin 94 %
The finely ground active ingredient is uniformly applied, in a
mixer, to the kaolin moistened with polyethylene glycol. Non-dusty
coated grant11ates are obtained in thls manner.
10. Suspension concentrate
compound according to the
Preparatory Examples 40 %
ethylene glycol 10 %
nonylphenol polyethylene glycol
ether ~15 moles of ethylene oxide) 6 %
sodium lignosulphonate 10 %
carboxymethylcellulose 1 %
37 % aqueous formaldehyde 301ution 0.2 %
silicona oil in the form of a 75 %
aqueous emulsion 0.8 %
watsr 32 %
:~3~
- 34 -
The finely ground active ingrsdient i8 intimately mixed with the
ad~uvants, giving a suspension concentrate from which suspensions of
any desired concentration can be obtained by dilution with water.
Example_6- Action against Lucilia sericata
I ml of an aqueous solution contsining 0.5 % of test compound i9
added at 50C to 9 ml of a culture medium. Then about 30 freshly
hatched Lucilia sericata larvae are added to the culture medium, and
the insecticidal action i8 determined after 48 and 96 hours by
evaluating the mortality rate.
In this test, compounds of the formula I according to Examples 1
to 4 show good activity against Lucilia sericata.
Example 7: Action against Aedes aegypti
A concentration of 100 ppm is obtained by pipetting a speciEic
amount of a 0.1 % solution of the test compound in acetone onto the
surface of 150 ml of water in a beaker. After the acetone has
evaporated, 30 to 40 two-day-old larvae of Aedes aegypti are put
into the beaker containing the test compound. Mortality counts are
made after 2 and 7 days.
In this test, compounds of formula I according to Examples 1 to 4
show good activity against Aedes aegypti.
Example 8: Action against Aonidiella aurantii
a) Immersion test - potato tubers
Small potato tubers are infested with crawlers of Aonidiella
aurantii (California red scale) by keeping the potatoes for about
24 hours in direct contact with largæ pumpkins which are heavily
infested with Aonidiella~ so that ultimately a population of 200 to
300 crawlsrs is present on each potato. When the crawler populatio~
on the potatoes has attsined the 2nd nymphal stage, i.e. after about
14 days, the potatoes are immersed, u~ing pincers, for about 2 to
3 minutes in an aqueous emulsion formulation which contains the
respective test compound in a concentration of 50 ppm. The treated
- 35 -
potatoes are dried and then kept for 10 to 12 weeks in plastic
containers which are ringed at the top with glue to trap the winged
males. EvallJation i8 subsequently made by comparing the state of the
treated Aonidiella population wlth that of untreated control
populations. This is done by counting the number of males and
assessing the development of females ~formation of scales) and the
production of crawlers of the first generation of progeny.
b) Spray ~reatment - citrus plants
_ _ _ _ _ . _ _
Citrus trifoliata cuttings are brought into close contact wlth a
pumpkin which is heavily infested with Aonidiella (q.v. test a)
above~, so that each cutting i~ populated with about 150 to
200 crawlers which have migrated from the pumpkin. When the crawler
population has attained the 2nd nymphal stage, i.a. after about
14 days, the infested cuttings are sprayed to drip point with an
aqueous emulslon formulation containing the respectiv0 test compound
in a concentration of 50 ppm. After about 10 to 12 weeks, fln
evaluation is made of the development of the population (percentage
of male and female scales) and of the production of crawlers
compared with untreated controls.
Compounds 1-20, 25, 27-35, 37-41 75~ 77-83 and 90-92 of formula I
according to Examples 1 to 4 were 100 % effective in this test.
Example 9: Insecticidal 3tomach poison action
Cotton plants about 25 cm high, in pots, are sprayed with an aqueous
emulsion containing the respective test compound ln a concentration
of 400 ppm.
After the spray coating has dried, the treated cotton plants are put
into metal pots (3 plants per pot) and populated with 50 Spodoptera
larvae in the L1 stage. Each pot is then covered with a glass plate.
The test is carried out at 2gC and about 60 % relative humidity.
The percentage mortality of the test insects compared with untreated
controls is determined aft0r 96 hours.
:~L3~
- 36 -
Compounds of formula I according to Examples 1 to 4 exhibit good
activity in this test.
Example 10: Actio~ _st ticks
Adult females of the cattle tick, Boophilus microplus~ which are
replete with blood are used as test organi3ms. The test is carried
out with 10 tickg each of an OP resistant strain (e.g. Biarra
strain) and o~ a normal sensitive strain (e.g. Yeerongpilly strain).
The ticks are affixed in the dorsal position to plates to which
double-sided adhesive tape has been applied and then contacted for
1 hour with a cotton wool swab which is impregnated wlth a solution
or aqueous em~lsion containing the test compound in a concentration
of 400 ppm. After removal of the cGtton wool swab, the ticks are
dried overnight at 24C and then kept in a controlled environment
chamber under constant conditions (28C, 80 % relative humLdity) for
4 weeks until ov1position has taken place and the larvae have
started to hatch. Evaluation is made by making a mortality count and
determining the percentage inhibition of fertile egg depos:Lts
(inhibition of embryogenesis and hatching~ compared with untreated
controls.
Compounds 1-11, 13-21, 25, 27, 28, 39, 77, 78, 83, 90 and 100 of
formula I according to Exsmples 1 to 4 were 100 % effective in this
test.
xample 11: Action against ticks: killing action in various develop-
ment stages
About 50 larvae, about 25 nymphs or about 10 imagines of each of the
tick species ~hipicephalus bursa, Amblyomma hebraeum and Boophilu~
microplus are used as test organisms. The test organisms are
immersed for a short time in aqueous emulsions containing the
respactiva test compound in a concentration of 800 ppm. The emul-
sions, which are contained in test tube~ are then absorbed by
cotton wool, and the wetted test organisms are left in the test
~3[3S~
- 37 -
tubes which have thus been contaminated. Evaluation of the percent-
age mortAlity i8 made 3 days later in the case of the larvae and 14
days later in the case of the nymphs and imagines.
Compounds of formula I according to Examples l to 4 exhibit good
activity in this test.
xample 12: Ovlcidal action against Cydia pomo _lla~ Adoxophyes
reticulana and Lobesia botrana
Egg deposits not more than 24 hours old of the above fruit pests are
i~mersed three times for a few seconds in an aqueous acetone
solution of the respective te~t compound having a concentration of
400 ppm. After the test solution has dried, the eggs are placed in
petri dishes (diameter: 5 cm) and kept at a temperature of 26C and
55 % relativae humidity. The treated egg deposits of Cydia pomonella
(codling moth) are placed between two round paper filters in the
petri dish. The egg deposits of Adoxophyes reticulana (summer fruit
tortrix moth) and Lobesia botrana (vine moth) are placed between two
round cloth filter~ beneath the cover of the petri dlsh, into the
bottom part of which a normal Lepidopter feed has been po~red.
Evaluation is made 6 days later by determining the percentage of
larvae which have hatched from the treated eggs, using untreated
control~ for comparison purposes.
Compounds of formula I of Examples l to 4 exhibit good activity in
this test.
Example 13: Chemosterilisation action against Nilaparvata lugens
The test is carried out with growing plants. To thls end, 4 rice
plants (thicknesq of stem ca. 5 cm, height ca. 20 cm) are planted in
pots (diameter: 8 cm).
The plants are sprayed on a rotary -table with 5 ml of an aqueous
emulsion formulation containing the tegt compound used in a concen-
tration of 400 ppm. After the spray coating has dried, each plant i~
populated with 4 adult females and 2 males. To revent the insects
~3~5~
- 3~ -
from escaplng, a transparent plastic cylinder is slipped over each
infested pot and sealed with a gau~e cover~ The insects remain for
6 days on the treated plants for oviposition. Ihe surviving lnsects
are oounted and then removed.
The rice plants wlth the egg deposits are incubated for 14 days at
20C and 60 % relative humidity. ~ count is made of the young
cicadas which have hatched out during this time. The precentage
reduction of progeny (chemosterillsatlon effect) is determined by
comparin~ the number of larvae whlch have hatched out on the t~eated
plants with that of the larvae whlch have hatched out on untreated
control plants.
Compounds of formula I according to Examples 1 to 4 exhibit good
activlty ln this test.
Example 14: Action Against Bemisia tabaci
a) Apllcation of the test compound before lnfestatlon
Cotton plants in the cotyledon stsge, in pots, are sprayed to drip
point with an aqueous emulsion formulation of the respective test
compound in a concentration of 400 ppm. After the spray coating has
dried, 40 adult~ of Bemlsia tabacl (white fly) are kept on each
plant in plastic cylinders. A flrst evaluatlon is made by determi-
ning the percentage mortality of the adults present on the plants
3 days after infestatioan. The surviving adults are removed. A
second evaluation is made 24 hours after infestatioan by determining
the percentage mortality of the nymphs, pupae and adults of the
first generatioan of progeny. The test is carried out in a con-
trolled environment chamber at 25C and a relative humidity of ca.
50-60 %.
b) Application_oE the test compound after infestation
Cotton plants ~untreated) in the cotyledon stage, in pots, are
populated as described in a) with Bemisia tabaci (white fly) such
that 40 unsexed adults are present on each plant. After oviposition
over 3 days, all adults are removed. Ten days after lnfestation,
~3(;~L5~
- 39 -
i.e. at a tlme when about two-thirds of the nymphs are in the 1st
nymphal stage and one third are in the 2nd nymphal stage, the
infested plants are sprayed to drip point with an aqueous emulslon-
formulation of the test compound (concentration: 400 ppm~. A count
of dead and living nymphs, pupae and adults is made 24 days after
infestation. The test i8 carried out in a controlled environment
chamber at 25C and at a relative humidity of about 50-60%.
Compounds of formula I of Examples l to 4 show good effectiveness in
this test.
Example 15: Action against storage pests - Sitoph lus orizae and
Rhizopertha dominica
lO0 g of wheat grains are thoroughly mixed in a lO0 ml plastic
beaker with 1 ml of an aqueous emulslon formulation of the respect-
ive test compound, said aqueous emulsion formulation containing ~he
test compound in a concentration such that, based on the weight oE
the wheat grains, the final concentration is lO0 ppm. Then 25 un-
sexed adults of Sitophilus orizae (rice weevil) and Rhizopertha
dominica ~les~er grain borer~ are put into each beaker (filled with
lO0 g of treated wheat grains). After infestation, the beakers are
kept in the dark at 26-28C and 60-65 % relative humidity. The
percentage mortality of the adults is determined one week after
infestation and the percentage reduction of the first progeny is
determined 8 weeks after infestation.
The compounds of formula I according to Examples l to 4 were very
effective in this test.