Note: Descriptions are shown in the official language in which they were submitted.
~3~
l 27979-5
PRO~ESS FOR PREPARING BISPHENOL A
BACKGROUND OF THE INVENTION
The present invention relates to a process for preparing
high-purity 2,2-bis(4-hydroxyphenyl)propane (referred to as
bisphenol A hereinafter).
Bisphenol A i& used as a raw material for polycarbonate
resins and epoxy resins and also for engineering plastics.
Colorless and high-purity bisphenol A is xequired for these
uses.
Bisphenol A is prepared by the reaction of acetone with
excess phenol in the presence of an acid catalyst such as
hydrochloric acid. The product mixture contains bisphenol A
and also the catalyst, unreacted acetone, unreacted:phenol,
water, and by-products such as coloring substances.
There are many known processes for obtaining high-purity
bisphenol A from the product mixture. For example, in the
case where hydrochloric acid has been used as the catalyst
in the reaction, the product mixture is heated at 90 to 120C
:
under reduced pressure, thereby~remo~ing hydrochloric acid,
unreacted acetone, water, and a small amount of phenol and
thereaEter bisphenol A in the form o an adduct with phenol
is separated by cooling. ~nother process includes distilla-
tion to separate bisphenol~A from other substances having
a higher and lower boiling point than that of bisphenol A.
- ~ 30~?7
The thus-obtained bisphenol A may be further purified by
extracting with a solvent or recrystallizing from a solution.
In the case where hydrochloric acid has been used as the
catalyst, the product mixture which has been distilled to
remove hydrochloric acid, acetone, and water still contains
a trace amount of hydrochloric acid which causes some troubles
in the subsequent purification steps.
One of the tIoubles is the corrosion of equipment due
to the acid. The corrosion yields metal salts which contami-
nate bisphenol A, and the removal of the metal salts requires
a complicated purification procedure. A possible countermeasure
is to use equipment made of an acid-resistant material; however,
this is not economical because such equipment will be expensive.
Another trouble is that bisphenol A is decomposed due to
the acidic substance during distillation, as described in
USP 3,073,868 and Japanese Patent Publication No. 4875/1963.
A crystallization process of the~adduct of bisphenol A
with phenol is disclosed in Japanese Patent Laid-open
No. 135832/1983. In the process, water is added to the product
mixture and the product mixture is cooled by evaporating a
water-phenol mixture, thereby performing crystallization.
In the evaporation, a trace amount of hydrochloric acid distills
together with water and phenol. If the distillate is discarded,
hydrochlorlc acld does not accumulates, but discardin~ the dis-
tillate is economically undesirable because useful phenol is
lost. If the distillate is recycled and reused, hydrochloric
5~
acid accumulates and the accumulated hydrochloric acid causes
some corrosion of equipment and some decomposition of bisphenol
A in the subsequent steps.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a
process for producing high-purity bisphenol A without causing
the above-mentioned troubles (i.e., the loss of useful phenol
and the accumulation of hydrochloric acid) in the step of
crystallizing out the adduct of bisphenol A with phenol.
To achieve the aforesaid object, we carried out a series
of researches, which led to the finding that high-purity
bisphenol A can be obtained by improving the step of crystal-
lizlng out the adduct of bisphenol A with phenol by evaporating
a water-phenol mixture which follows the addition o* water.
According to the improved~step, a mixture of water and phenol
is recovered from the product mixture under reduced pressure,
the recovered mixture is treated with a specific ion-exchange
resin, and the treated mixture is recycled and reused as the
water to be added to the step. The present invention was
completed on the basis oi this finding.
In accordance with the present invention, there is provided
: ~
a process for producing high-purity bisphenol A by reacting
phenol with acetone in the presence of hydrochloric acid as
the catalyst to obtain a~product mixture, removing the hydro-
chloric acid from the product mixture, thereby yielding a liquid
:
.
- 4 _ 27979-5
mixture, adding water to the liquid mixture, evaporating a water-
phenol rnixture from the liquid mixture under reduced pressure,
thereby cooling the liquid mixture and crystallizing out the
adduct of bisphenol A with phenol, and finally recovering bis-
phenol A from the adduct, wherein an improvement comprises treating
the water-phenol mixture with a weakly basic ion~exchange resin
and recycling and reusing the treated mixture as the water to be
added to the liquid mixture.
RIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawing is a flowsheet showing an
embodiment of the process of the present invention for producing
bisphenol A.
DETAILED DESCRIPTION OF THE INVENTION
The reaction of phenol with acetone is well known in
the art. Usually an excessive amount, namely, more than the
stoichiometrically required (i.e., 2 moles) amount of phenol is
reacted with acetone. More practically the molar ratio of
phenol to acetone in the starting mixture is from 4:1 to 12:1
and usually the reaction temperature is 40 to 70C.
The reaction yields a product mixture containing
bisphenol A, and also unreacted phenol, unreacted acetone, hydro-
chloric acid, water, and by-products. The product mixture is
distilled under reduced pressure to remove water, acetone, hydro-
chloric acid, and a small amount of phenol. The vacuum distil
lation is also well known in the art and is performed preferably
at a pressure of 20 to 200 mmHg and a temperature of 90 to 120 C.
Thus there is obtained a phenol solution of crude bisphenol A,
~ ~3~i5~
- 5 - 27979-5
which also contains a contaminant amount of hydrochloric acid.
The thus-obtained phenol solution of crude bisphenol A
is cooled according to a known manner, usually to 35 to 70C in a
crystallizer so that the adduct of bisphenol A with phenol cry-
stallizes out. The crystallization is accomplished by adding
water to the phenol solution o~ crude bisphenol A in the crystal-
lizer and evaporating water and phenol under reduced pressure
(preferably 20 to 100 mm~g), thereby removing heat. The
evaporation yields a distillate composed mainly of a mixture of
water and a small amount of phenol. This mixture is condensated
and then is treated with a weakly basic ion-exchange resin, and
the treated mixture is recycled and reused as the water to be
added to the phenol solution of crude bisphenol A in the crystal-
lizer.
The amount of the water-phenol mixture to be recycled
should be sufficient to cool the phenol solution of crude bisphenol
A and also to remove the heat of crystallization of the adduct by
its evaporation. This amount is often 2 to 20 wt% of the phenol
solution.
The weakly basic ion-exchange resins used in the process
of the present invention should preferably be substantially in-
soluble in the water-phenol mixture. They include ion~exchange
resins which contain secondary or tertiary amine as the exchange
group, for example, "LEWATIT MP-62*" (Bayer AG) and "WA-20*"
; (Mitsubishi Chemical Industries, Ltd.). The treatment with the
ion-exchange resin should preferably be carried out continuously
at 20 to 70C. The amount of the water-phenol mixture
*Trademark
~5~
to be added to the ion-exchange resin should preferably be
0.5 -to 10 kg/hr for 1 kg of the ion-exchange resin.
According to the process of the present invention, the
crystals of the adduct of bisphenol A with phenol are separated
by any known method, and the bisphenol A is freed of phenol.
An embodiment of the present invention will be explained
with reference to the accompanying drawing.
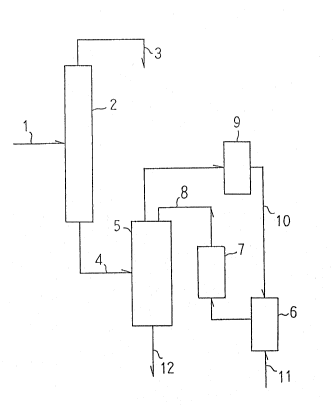
At first, phenol and acetone are reacted in the presence
of hydrochloric acid tas a catalyst). The resulting product
mixture 1 is fed to a dehydrochlorination column 2 for vacuum
distillation. From the top of the column 2 is removed the
mixture 3 containing water, hydrochloric acid, and a small
amount of phenol. From the bottom of the column 2 is obtained
the mixture 4 containing bisphenol A, phenol, and by-products.
The mixture 4 enters a crystallizer 5 in which water and
phenol are evaporated under reduced pressure. The evaporation
cools the mixture 4, crystallizing the adduct of bisphenol A
with phenol. During the crystallization, the water-phenol
mixture 8 is fed to the crystallizer 5 through a column 7 of
a weakly basic-ion-exchange resin from a phenol-water receiver
6, for the replenishment of water and phenol which evaporate
in the crystallizer 5.
The water and phenol which have evaporated in the crystal-
lizer 5 enter a condenser 9. The condensate 1~ returns to
the phenol-water receiver 6. In this manner, the water and
phenol are recycled.
~3~ 7
The adduct in the form of slurry 12 yielded in the
crystallizer 5 is transferred to the subsequent steps. As
much water as lost along with the slurry 12 is replenished by
the feedwater ll to the phenol-water receiver 6.
Examples
The invention will be described in more detail with
reference to the following examplesj in which "~" means "wt%",
unless otherwise indicated.
Example 1
Condensation of phenol and acetone was carried out in
the presence of hydrochloric acid as the catalyst. There was
obtained a product mixture composed of 54~ of phenol, 35% of
bisphenol A, 2% of by-products, 4% of water, and 5% of hydro-
chloric acid. The product mixture was fed to a dehydrochlori-
nation column in which water, hydrochloric acid, and a small
amount of phenol were removed at a pressure of 70 mmHg and
a temperature of 120C (at the column bottom). The bottom
liquid contained 10 ppm of hydrochloric acid.
The bottom liquid was continuously fed at a flow rate of
400 kg/hr to a crystallizer kept at a pressure of 50 mmHg
and a temperature of 45C. Simultaneously, the water-phenol
mixture was fed at a flow rate of 40 kg/hr to the crystalli~er
through a column filled w1th 20 kg of "LEWATIT MP-62" (made
by Bayer AG). The water-phenol mixture was kept at 40C.
The water-phenol mixture which had evaporated was
~L 3~. S ~
condensed by a condenser for recycling.
The slurry of the adduct of bisphenol A with phenol which
was discharged from the crystallizer contained 2 ppm of hydro-
chloric acid. After washing with an equal amount of phenol,
a part of the adduct was dissolved in ethanol to give a 50
solution. The solution had a Hazen color of 10 APHA.
Comparative Example 1
The same procedure as in Example 1 was repeated to produce
bisphenol A, except that the weakly basic ion-exchange resin
was not used. The slurry of the adduct discharged from the
crystallizer contained 10 ppm of hydrochloric acid. After
washing with an e~ual amount of phenol, a part of the adduct
was dissolved in ethanol to give a 50% solution. The solution
had a Haæen color of 20 APHA.
EFFECTS OF THE INVENTION
According to the process of the present invention, the
product mixture is freed of hydrochloric acid effectively.
It is possible to prevent bisphenol A from being contaminated
by any corrosion of eguipment. Thus it is possible to produce
colorless and high-purity bisphenol A.