Note: Descriptions are shown in the official language in which they were submitted.
:1 3C~ 7
S P E: C I F I C A T I O N
TITLE OF T~IE INVENTION
-
3-Propenylcephem derivative
BACKGROiJND OF THE INVENTION
Field of the invention:
The present inven,tion rela-tes to novel cephem
derivative useful for anti-bacterial agents. More
particularly, the presen-t invention relates to 3-
propenylcephem derivative. The present inven-tion
also provides process for the preparation of the 3-
propenylcephem derivative, anti-bacterial agents,
intermediate for the 3-propenylcephem derivative, and
process for -the preparation of the intermediate.
Descrip-tion of the prior art:
;
- Cephem deriva-tives having ammonio group have
been conventionally known Erom Japanese Patent Appli-
cation Laid-open Nos. 174,387/83; 198,490/83; 130,295/84;
172,~93/84; 219,292/84; 97,983/85; 197,693/85, 5,084/86,
etc.
ParticuIarly, cephem derivative having an ammonio-
propenyl group at the 3~position thereof, similar to
the compound of the presen-t invention, have been
disclosed in Japanese Patent Application Laid-open
~;~ Nos. 17Z,493/84 and 5,084/86.
,
'
: : ~
`.
;~ 305'~
S~MMARY OF TIIE INVF,NTION
The pr~sent inventors have found -that cephem
derivative having an ammoniopropenyl group at the
3-position thereof and a Eluoro-substituted lower
alkoxyimino group or a cyano-substituted lower
alkoxyimino group in a side chain at the 7-position
thereof have excellent anti-bac-terial activities,
leading to comple-tion of the present invenl:ion.
An object of the present invention is therefore
to provide novel cephem compounds useful as anti-
bac-terial agents; a process for -the preparation
thereof; pharmaceutical composition containing the same;
intermediates for -the derivatives; and a process for
the prepara-tion of the intermediates.
DETAILED DESCRIPTION OF THE INVENTION
AND PREFERED EMBODIMENTS
The present invention rela-tes to 3-propenylcephem
derivative of the following formula (I):
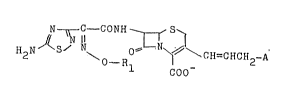
: ~ C ~ CON~ ,S
~: H2N~S~ ~CH=CHCH2-A (I)
O-R, COO-
:
:~ wherein Rl represents a fluoro-substituted lower alkyl
group or a cyano-substituted lower alkyl group, and A
represents a cyclic or acycllc ammonio group, and a
: pharmaceutically acceptable salt thereof.
. . , , ~ . , . ~ , .
~l3VS~'7
As illustrative examples of the Eluoro-
substituted lower al.kyl group represented by Rl
in the formula (1), may be mentioned fluoromethyl,
difluoromethyl, 2-fluoroethyl, 2,2-difluoroethyl,
2,2,2-trifluoroethyl, l-fluoroethyl, 2-fluoropropyl,
l-(fluoromethyl)-2-fluoroethyl, 3-Eluoropropyl and the
like with fluoromethyl being particularly p~eferred.
Regarding the cyano-subs-titu-ted lower alkyl
group represented by R~ in the formula (I), there
may be enumerated cyanomethyl, 2-cyanoe-thyl, 3-
cyanopropyl, and the like.
As illustrative examples of the acycli.c ammonio
group represen-ted by A in the formula (I) may be
mentioned a group of the following formula:
R2
N - R
1 4
R3
:~ in which R2, R3 and R4 are the same or
: different and mean individually a group
~ selected from the group consisting of lower
:: :
, ~::
: ~ 3
'~:
: ~ :
; : .
alkyl, hydroxyl-substituted lower alkyl, carbamoyl-
substituted lower alkyl, cyano-substituted lower alkyl,
amino, (lower alkyl)carbonylamino-substituted lower alkyl,
aminosulfonylaminocarbonyl-substituted lower alkyl,--(lower
alkyl)sulfonylaminocarbonyl-substituted lower alkyl, (lower
alkyl)aminocarbonyl-substituted lower alkyl, hydroxyl- and
carbamoyl-substituted lower alkyl, hydroxyl- and
hydroxy(lower alkyl)aminocarbonyl-substituted lower alkyl,
(lower alkyloxy)aminocarbonyl-substituted lower alkyl,
hydroxyaminocarbonyl-substituted lower alkyl, carbamoy.l(lower
alkyl)aminocarbonyl~substituted lower alkyl, hydroxy(lower
alkyl)aminocarbonyl-substituted lower alkyl, (lower
alkyl3amino-substituted lower alkyl, carboxylate(lower
alkyl)di(lower alkyl)-ammonio-substituted lower
alkyl,di(lower alkyl)amino-substituted lower alkyl, di(lower
alkyl)amino- and hydroxyl-substituted lower alkyl, ureido,
hydroxyl, carboxyl-substituted lower alkyl, hydroxyl- and
carbamoyl-substituted lower alkyl, lower alkyloxy-substituted
lower alkyl, di(lower alkyl)amino-
'
;
- .
~ ~ 25
~a~4~!~
carbo~yl-substituted lower alkyl,
dicarbamoyl-substituted lower alkyl,
: bis[hyd~oxy(lower alkyl)]aminocarbonyl-
substituted lower alkyl, dihydroxyl-
substituted lower alkyl, trihydroxyl-
substituted lower alkyl, bis[hydroxy(lower
alkyl)amino-substitu-ted lower alkyl,
amino-substituted lower alkyl, oxo-
substituted lower alkyl, di-lower alkyl-
substituted lower alkyl, 5-membered
he-terocycle-substituted lower al.kyl
wherein said heterocycle stands for
pyrazolyl, imidazolyl, oxadiazolyl or
te-trazolyl.
Fur-ther, illustrative of a cyclic ammonio group
represented by A in the Eormula (I) may include, for
example, a group of the following formulae:
. .
:
S
~: :
..,. : : :
13~5~87
R~ -~N N
~ N~ ~N ~ -\ ~ ~ N~N~
+ ` ~? ~ ~ ~, ~o
,~
Rs ~ / 5
+ ~ N~
; ~ in which R5 means a group selec-ted from
:~ lower aIkyl, carbamoyl-substituted lower
; :
: :
~;
~; :: ::
~ . ,
~L~3Q54~7
alkyl, amino-substituted lower alkyl, hydroxyl-
substituted lower alkyl, carboxyl-substituted lower
alkyl, cyano-substituted lower alkyl, dihydroxyl-
substituted lower alkyl and ureido-substituted-lower
alkyl groups,
said cyclic ammonio group optionally containing on the ring
thereof one or more substituents selected from hydroxyl-
substituted lower alkyl, hydroxyl, formyl~ sulfonic,
carboxyl-substituted lower alkyl, carbamoyl, sulfamoyl,
carboxyl, hydroxyimino-substituted lower alkyl, imino-
substituted lower alkyl, bis[hydroxy(lower
alkyl)]aminocarboxyl, hydroxy(lower alkyl)aminocarboxyl,
amino, morpholinocarbonyl, carboxy(lower alkyloxy)-
substituted lower alkyl, carboxy lower alkylthio and lower
alkyl groups.
Illustrative of the lower alkyl group in the definition
for A (R2 - R5) in the formula (I)
: :
: ''
~:
~. .
~3~)54~37
may include alkyl groups having 1 - ~ carbon atoms,
such as methyl, ethyl, n-propyl, i-propyl, n-butyl,
i-butyl, sec~butyl and t-butyl.
As non-toxic salts of the compounds of the
~o;-mula (I) may be mentioned their
pharmaceutically acceptable salts, for example, alkali
metal salts such as sodium salts and potassium salts;
ammonium salts; quaternary ammonium such as tetraethyl-
ammonium salts and betaine salts; alkaline earth metal
salts such as calcium salts and magnesium salts;
inorganic acid salts such as hydrochlorides, hydro-
bromides, hydroiodides, sulfates, carbonates and
bicarbonates; organic carboxylates such as acetates,
maleates, lactates and tartrates; organic sulfonates
such as methanesulfonates, hydroxymethanesulfonates,
hydroxyethanesulfonates, taurine salts, benzene-
sulfonates and toluenesulfonates; amino acid salts such
as arginine salts, lysine salts, serine salts, aspar-
tates and glutamates; amine salts such as trimethyl-
amine salts, triethylamine salts, pyridine salts,
procaine salts, picoline salts, dicyclohexylamine
salts, N,N'-dibenzylethylenediamine salts, N-methyl-
glucamine salts, diethanolamine salts, triethanolamine
salts, tris(hydroxymethylamino)methane salts and
phenethylbenzylamine salts; etc.
. 8
.~
3~5~1~37
Each of the compounds of the Eormula
(I), which pertain to the present invention, has its
syn-isomer (2) and anti-isomer (E) with respect to its
stereoscopic conEiguration at the following moiety:
-C- ..
N
O -R
~lthough both isomers are included in the present
invention, the syn-isomers are desired owing to their
antibacterial activitiesO
The compounds of this invention can be produced
by the following process.
Namely, the compounds of the formula (I) and
their pharmaceutically acceptable salts can individually
be obta1ned by reacting a compound, which is represented
by the formula (II):
N~rC-CONH~S~
H 1~S~ N~CH-CHCH2~ (II)
2 ' O--R, COOH
; wherein Rl means a fluoro-substituted lower alkyl group
or a cyano-substitu-ted lo~wer alkyl group,
and X denotes a halogen atom, a compound wherein the amino
and/or carboxyl groups are protected with protecting
group(s), or a salt thereof wi-th a compound represented
: by the formula (III):
A' (III)
g
- . ' ' :
:
~3~S~L15~7
wherein A~ mea~ls an amlne corresponding to A a
compound wherein the functional group(s) are protec-ted
with protec-ting group(s) or a salt thereof; followed
by optionally removing the protecting group(s).
As halogen atoms represented by X in the above
Eormula (II) may be mentioned iodine a tom bromine
atom and chlorine ~tom.
The above reaction may be carried out at a
reaction temperature of -10C - 60C,preferably, 0C
- 40C. ~s a reaction solvent, an anhydrous organic
solvent is desired. As usable organic solvents, may be
mentioned lower alkylnitriles such as acetonitrile and
propionitrile; halogenated lower alkanes such as
chlorornethane, dichioromethane and chloroform; ethers
such as tetrahydro~uran, dioxane and ethyl ether;
amides such as dimethylformamide; esters such as ethyl
acetate; ketones such as acetone; hydrocarhons such as
benzene; alcohols such as methanol and ethanol; and
sulfoxides such as dimethylsulfoxide; as well as mixed
solvents thereof.
The removal of the protecting group(s) may be
carried out by a method known per _ in the art in
accordance with the kind(s) o~ the protecting grou~?(s)
used, such as hydrolysis or reduction.
As the salts of the compounds of the
formulae (II) and (III) and the protecting groups for
, 10
54~
the compounds, those employed routinely may also be
used SQ long as they do not impair the above reaction.
Exemplary protecting groups for the amino group
may include formyl group, acetyl group, chloroacetyI
group, dich]oroacetyl group, phenylacetyl group,
thienylacetyl group, t-butoxycarbonyl group, benzyloxy-
carbonyl group, trityl group, p-methoxybenzyl group,
diphenylmethyl group, benzylidene group, p-nitro-
benzylidene group and m-ch]orobenzylidene group. As
illustrative protecting groups for the carboxyl group,
may be mentioned p-methoxybenzyl group, p-nitrobenzyl
group, t-butyl group, methyl group, 2,2,2-trichloro-
ethyl group, diphenylmethyl group and pivaloyloxymethyl
group. Here, use of a silylating agent such as N,O-
bis(trimethylsilyl)acetamide, N-methyl-N-(trimethyl-
silyl)acetamide, N-methyl-N-(trimethylsilyl)trifluoro-
acetamide or N-(trimethylsilyl)acetamide is convenient
because such a silylating agent can protect botil amino
and carboxyl groups at the same time.
As salts of the compounds of the
formulae (II) and (III), suitable selection may be made
from their salts such as alkali metal salts such as
sodium salts and potassium salts; alkaline earth metal
salts such as calcium salts and magnesium salts;
ammonium salts; quaternary ammonium salts such as
triethylammonium salts and betaine salts; inorganic
11
- '
13(15~37
acid salts such as hydrochlorides, hydrobromides,
sulfates, carbonates, hydroiodides and bicarbonates;
organic carboxylates such as acetates, trifluoro-
acetates, maleates, lactates and tartrates; organic
sulEonates such as rnethanesulEonates, hydroxymethan.e-
sulEonates, hydroxyethanesulfollates, taurine salts,
benzenesulfonates and toluenesulfonates; amine salts
such as trimethylamine salts, triethylamine salts,
pyridine salts, procaine salts, picoline sa].ts,
di.cyclohexylamine salts, N,N'-dibenzylethylenediamine
salts, N-methylglucamine salts, diethanolamine salts,
trlethanolamine salts, tris(hydroxymethylamino)methane
salts and phenethylbenzylamine salts; amino acid salts
such as arginine salts, aspartates, lysine salts,
glutamates, serine salts and glycine salts; etc.
The compounds of this invention show strong
antibacterial activities against both gram-positive and
gram-negative bacteria and are hence useEul as anti-
bacteria1 agents. The compounds are used ~or treating
a disease caused by bac-teria.
When using the compounds of this invention as
injections, they may be administered generally at a
daily dose o~ 100 mg - 10 g in 1 - 4 portions either
intravenously or intramuscularly. Meedless to say, the
dose may be increased or decreased depending on the age
and conditions of disease.
Their injections may be produced by a method
12
:13~5~L~3'7
known ~ se ln the art. For example, each compound of
this inventioll may be formulated into an injection by
dissolving same in distilled water, if necessary, in
the presence oE an isotonic agent, solubilizer and/or
the like. They may each be filled as powder in a vial
or the like, thereby providing injections which require
dissolution before use. These injections are hence
dissolved in distilled water for injection, physio-
logical saline, glucose injection, amino acid infusion
or the like upon administration.
A compound of the above-mentioned formula (II)
i.e. an lntermedia-te; a compound wherein the amino
and/or carboxyl group(s) are protected with a protective
group; or salts of these compounds are all novel compounds.
These compounds can be prepared by the following process.
That is -to say, the compounds can be prepared by reacting
a compound oE -th~ formula:
N \\ C - COOH
J ~ / N (IV)
H2N S ~O - Rl
wherein Rl has the same meanings as mentioned above,
a reac-tive acid derivative thereof, a compound wherein
an amino group is protected wi-th a protective group or
; a salt of the compound with a compound of the formula:
~ 13
:
13~5~8'7
H2N
(V)
O ~-- ~ \
C~l = C~IC~l2X
COOH
wherein X has the same meanings as defined above, a
compound wherein a carboxyl group is protected with
a protective group, or a sal-t thereof, followed by
optionally removing the pro-tective group, and/or
convertin~ a halogen a-tom represented by -the symbol X
into the o-ther halogen atom.
The above-mentioned reaction can be conducted in
accordance with a conventional reaction condition for
an N-acylation. ~or example, the reaction can ke
carried out at a temperature from -50C to 50C in
an inert solvent such for example as tetrahydrofuran,
ethyl acetate, acetone, N,N-dimethyl formamide,
acetnitrile, dioxane or a mixed solvent thereof.
As for a reactive acid derivative of -the compound
of the formula (IV), there may be exemplified an acid
halide such as acid chloride, acid bromide, etc.
symmetrlcal acid anhydride, a mixed acid anhydride,
~ ~ an active ester, an active acid amide, and the like.
: When a free carboxylic acid of -the formula ~IV)
or a salt thereoe is used in the reaction, it is
preferable to conduct the reaction in -the presence of
:a conventional condensing agent such as N,N'-
:~ ~
~ 14
,
.
~3~ '7
dicyclohe,Yylcarbodiimide, p-tuluene sulfonic acid, and
the like, for example.
The conversion of a halogen atom represented by
X into the other halogen atom is carried out in a
conventional manner. For example, -there can be
prepared a compound of the formula (II) wherein X
represents iodine atom, when a compound of the formula
(II) wherein X represents chlorine atom is reacted with
an alkali metal iodide.
Further, the intermediates of -the under-men-tioned
formula (VI) are also novel compounds, such as a
compound of the formula:
N \\ C -- 6
~ N N ( VI )
H2N S OCH2F
wherein R6 represents carboxyl group, a halogenocarbonyl
~roup, carbamoyl group, or cyano group, a compound
wherein amino and/or carboxyl group(s) are pro-tected
with a pro-tective group, or a salt thereof.
Illus-trative oE protec-tive grloups for -the amino
qroup and the carboxyl group may include a similar
group as exemplified in the compound of -the formula (II).
The above-mentioned compounds can be prepared by
~ the following exemplary process.
:
: :
,
:
.
3(~5~t7
NC - C - CONH 2
OH (VII )
H 2 N S ~ ~ ( X I V )
NC - C - CONH 2 .
N
~ OCH E~ ~ VIII )
NC - C - CN - N ~CI --COX
N ( IX ) H2N ~OCH2F
~ OCH2F ,~ ( X: halogen atom )
/ (XV)
`~ . ~ ~ /
N ~ COOH
H 2 N \ C C C N H 2 N ~S / \ ( X I I I )
; HN N (X) . OCH2F
\ OCH2F / ~
: .
f ~\S / N\
~ C CN /~7 ( X I I )
H2N S" N
: \OCH F
; : ( XI )
:::
1 6
:~ :
o :
' ~ `
.
13~ t~
The compound of the formula (VIII) can be prepared
by reacting a compound of the formula (VII) with a
halogeno fluoromethane in an inert solvent.
As the halogeno fluoromethane, there may be
exempliied bromo fluoromethane, iodo E]uoromethane, and
the like.
The reaction is 'carried out a-t a tempera-ture ranging
rom -30C to 100C.
The compound of the formula (IX) can be prepared
by reacting a compound of the formula (VIII) with a
dehydrating agent in an inert solvent. As the reaction
tempera-ture, it is preferable to use room tempera-ture or
above. A dehydrating agent may include oxyphosphorus
chloride, thionyl chloride, etc.
The compound of the formula (X) can be prepared
by reacting -the compound of the formula (IX) with
ammon1a and/or ammonium salt in an inert solvent such
as wa-ter, a lower alcohol, acetone, chloroform, etc.
Suitable reaction temperature may range from -20C to
room temperature. Ammonium salt may include ammonium
chloride, ammonium aceta-te, ammonium sul.fa-te, and the
like.
:
The compound of the formula (XI) can be prepared
by reacting tbe compound of the formul2 (Xj with a
halogenatina agent such as gaseous bromine, gaseous
chlorine, etc. to effect t~e halogenation, follo.~ed
~ 7
;~ .
.
'. :: ' : ~- :
' ' , ' - ~ :
.
~3~5~ 7
by reacting it with an allcali metal thlocyanate,
pre-ferably in the presence of a base. Suitable
reaction temperature may range from -20C to room
temperature. As an alkali metal thiocyanate, -there
may be used potassium thiocyanate, sodium thiocyanate,
and the like.
A compound of thé formula (XII), a compound wherein
amino group is protected with a protective group, or a
salt -thereof can be prepared by hydrolyzing a compound
of the formula (XI), a compound wherein amino group
is protected with a protective group, or a salt thereof
in -the presence of an oxydizing agent and a base,
followed by optionally removing the protective group.
The reac-tion can be carried out at a reaction
temperature of from 0C to 70C in water, a buffer
solu-tion or a mixed solvent of the former with a
lower alcohol.
There may be used hydrogen peroxide, oxygen, etc.
; as an oxydizing agent; and sodiurn hydroxide, po-tassium
hydroxide, etc. as base.
A compound of the formula (XIII), a compound wherein
amlno group is pro~-tected with a protective group, or a
salt thereof can be prepared by hydrolyzing a compound
of the formula (XII), a compound wherein amino group is
~; protected with a pro-tective group, or a salt thereof
; in -the presence of a base, followed by op-tionally
removing the protective group.
18
~: .
,
.. ~
,
13~'S4~'7
Tlle types of the base, solvents, the reaction
tempera-tures, etc. may be the same as those described
in -the reaction sequence from the compound of the
formula (XI) to that of the formula (XII).
Fur-thermore, a compound of the formula (XIII),
a compound wherein amino group is protected with
a protec-tive group, or a salt thereof can be also
prepared by reacting a compound of the formula
(XIV) wherein amino and/or carboxyl group(s) are
protected by a pro-tected group, with a halogeno
fluorome-thane, followed by optionally removing
the protective group.
The halogeno fluoromethane may indlude bromo
fluoromethane, iodo fluorome-thane, chloro fluoro-
methane.
The reaction can be carried out in an inert
solvent at a reaction temperature ranging from
-30C to 100C.
Illustrative examples of the inert solvent to
be used may include sulfoxides such as dimethyl
sulfoxlde, etc., amides such as N,N-dimethylacet-
amide, for~amide, hexamethylphosphoryl triamide,
etc., ke-tones such as ace-tone, etc., or a mixed
solvent thereof.
; :: :
. :
,
1 9
~: :
~, ' ~ ~ ' ' : - '
. :
' .
,
~3~4~
A compound of the Eormula (XV)~ a compound
wherein arriino group i~s protected with a protec-tive
group, or a salt thereof can be prepared by reacting
a compound of the formul.a (XIII), a compound wherein
amino group is protected with a protective group,
or a salt thereof with a halogena-ting agent.
Illustra-tive examples of the halogenating agent
may include phosphorus pentaoxide, thionyl chloride,
-thionyl bro~ide, oxyphosphorus chloride, and the
li!ce
The above reaction can be carried out in an inert
solven-t such for example as dichloromethane, te-tra-
hydrofuran, ethyl acetate, chloroform or a mixed
solvent thereof at a reaction temperature of from
-50C to 50C.
The present invention will next be descri.bed
: in fur-ther de-tail by the followina Experiments and
~xamples.
~ '
:: :
~ .
; 20
~ .
~ .
~ :L3~ '7
Experiment l (Synthesis of` the raw material compound)
Ethyl 2-(5-tritylamino-1,2,4-thiadiazol-3~yl)-(Z)-
2-fluoromethoxyi~ino acetate
@~- CHN~ COOC2Hs
\OCH2 F
Ethyl 2-(5-tritylamino-1,2,4-thiadiazol-3-yl)-(z)-2-
hydroxyimino acetate (60.4 g) was dissolved in dimethyl sulfoxide
(210 ml), and then potassium carbonate (96.1~8g) was added thereto
under ice-cooling. The solution was stirred for lO minutes.
Thereafter, bromofluoromethane (19 g) was added thereto, and
the solution was stirred for 3 hours at room temperature.
Ethyl acetate (1 liter) was added to the reaction solution and
the solution was washed with water and then with a saturated
brine, followed by drying with addition of anhydrous magnesium
sulfate. The solvent was distilled off, and ethanol (120 ml)
was added to the residue. Crystals deposited were collected
by filtration, and washed with ethanol to obtain the object
~; product (58.2 gj.
Experiment 2 (Synthesis of the,raw material compound)
2-(5-Tritylamino-lJ2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyimi~no acetic acid
2 1
: ~ ~
:
;: :
~HN~ 5 ~ ~
~ `OCH2F
Into a mixed solution contai.ning sodium hydroxide (2.04 g),
ethanol (146 ml) and water (29 ml) was added the compound
(17.87 g) prepared in Experiment 1, and the solution was stirred
for 20 minutes under reflux. After the soluticn was concentrated
under reduced pressure, ethyl acetate (.200 ml) and lN hydro-
chloric acid (77 ml) were added thereto. The ethyl acetate
layer was separated to collect and washed with a saturated
brine, followed by drying with addition of anhydrous magnesium
sulfate. The solvent was distilled off to obtain crystals. The
crystals were crushed with addition of petroleum ether, and then
recovçredby filtration to obtain the object product (16.55 g).
Experiment 3 (Synthesis of the raw material compound)
p-Methoxybenzyl 7~-[2-(5-tritylamino-1,2,LI-thiadiazol-
3-yl)-(Z)-2-fluoromethoxyiminoacetamido~-3-~(Z)-3-chloro-
l-propen-l-yl]-3-cephem-4-carboxylate
C-CONH~S,~Cl
bCH2F -IOOCH2~0CH3
- :
, .
22
.
~3~
Dime-thyl~ormamide (348 ~1) and tetrahydrofuran (4.1 ml)
were cooled to -10C, and phosphorus oxychloride (418~1) was
added thereto, and stirred for 90 minutes under ice-cooling.
To this solution was added a solution of the compound (1.73 g)
prepared in Experiment 2 in tetrahydrofuran (5.5 ml) with
cooling to -10C, and the resulting solution was stirred for
90 minutes under ice-cooling. The reaction solution was
cooled to -20 C,and a mixed solution containing p-methoxyben
7~-amino-3-[(Z)-3-chloro-1-propen-1-yl]-3-cephem-4-carboxylate
hydrochloride (1.78 g), N-(trimethylsilyl)acetamide (2.95 g),
ethyl acetate (18 ml) and tetrahydrofuran (5.5 ml) was added
thereto, and the resulting solution was stirred for one hour at
-10C. To the reaction solution was added ethyl acetate
(100 ml), and the resulting solution was washed succesively
with water, a saturated aqueous sodium hydrogencarbonate and a
saturated brine, followed by drying with addition of anhydrous
magnesium sulfate. The solvent was distilled off, and the
residue was purified by silica gel column chromatography to
obtain the object product (2.65 g).
::
Experiment 4 (Synthesis of the raw material compound)
p-Methoxybenzyl 7~-[2-(5-tritylamino-1,2,4-thiadiazol-
3-yl)-(Z)-2-fluoromethoxyiminoacetamido~-3- t(E)-3-iodo-
l-propen-l-yl~-3-cephem~4-carboxylate
,
~; ~ 23
::
:
.
L3(?~L8
C - CONH S
~N'~N ~F~, I
~D ~OCHzF (~OOCH2~0CH3
The compound (10.11 g) prepared in Experiment 3 was dissolved
in acetone (212 ml), and sodium iodide (9.03 g) was added thereto
under ice-cooling. The resulting solution was stirred for
15 minutes under ice-cooling and for additional 90 minutes at
a room temperature. The solvent was distilled off, and the
residue was extracted with ethyl acetate (500 ml). The extract
was washed with a saturated aqueous sodium thiosulfate solution
and with a saturated brine, followed by drying with addition
of anhydrous magnesium sulfate. The dried extract was concentrated
under reduced pressure and n-hexane was added thereto. The
resulting precipitates were collected by filtration to obtain
the object product (10.92 g).
.
Experiment 5 (Synthesis of the raw material compound)
p-Methoxybenzyl 7~-[2-(5-tritylamino-1,2,4-thiadiazol-
3-yl)-(Z)-difluoromethoxyiminoacetamido)-3-~(Z)-3-chloro-
l-propen-l-yl]-3-cephem-4-carboxylate
N~C-CONH~CI
'OGHF2 OOCH2 ~OCH3
24
S~'7
In the sarne marlller as described in Experiment 3, 2-(5-
tritylamino-1,2,~l_thiacliazol-3-yl)-(Z)-2-difluoromethoxyimino-
acetic acid (2.00 g) was reacted with p-methoxybenzyl 7~-arnino-3-
[(Z)-3-chloro-1-propen-1-yl]-3-cephein-4-carboxylate hydrochloride
(1.795 g) to obtain the object product (3.17 g).
Experiment 6 (Synthesis of the raw material compound)
p-Methoxybenzyl 7~~[2-(5-tritylamino-1,2,4-thiadiazol-
3-yl)-(Z)-2-difluoromethoxyiminoacetamido)-3-~(E)-3-
iodo-l-propen-l-yl]-3-cephem-4-carboxylate
~) C-CON~I S
~(~HN~S~
\OCHFz COOCHz~OCH~
In the same manner as described in Experiment 4, the
compound (3.00 g) preapred in Experiment 5 was reacted with
sodium iodide (2.62 g) to obtain the object product (2.92 g).
Example 1
7~G'-[2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoacetamidoJ 3 t(E)-3-(carba~oylmethyl-
ethylmethylammonio)_l-propen_l_yl]-3-cephem-4-carboxylate
~ 25
:~
~ ~3~1S~
CH2CH3
~C-CONH ~ S `, I
H2N~S' N ~ N~ I~CONH2
\O-CH2F COO- CH3
The compound (550 mg) prepared in Experiment 4 was
dissolved in a mixed solution containing ethyl acetate (20
ml) and ethyl ether (lO ml), and then ethylmethylamino-
acetamide (117 mg) was added thereto. The resulting solution
was stirred at room temperature for 4 hours and 30 minutes.
Isopropyl ether was added to the reaction solution, and the
resulting precipitates were collected by filtration and dried
to obtain the yellowish brown powder (400 mg).
The powder was stirred in a mixed solution of trifluoro-
acetic acid (4.5 ml) - anisole (4 ml) for one hour under ice-
cooling, and ethyl ether was added thereto. The resulting
precipitates were collected by filtration and washed with
ethyl ether. The precipitates were suspended in water (5
ml). The suspension was adjusted to pH 5.5 - 6.5 with sodium
acetate. Insolubles were removed by filtration, and the
filtrate was purified by reversed phase chromatography to
obtain the object product (49 mg).
~xample 2
7~-[2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoacetamido]-3-[(E)-3-[1-(2-
hydroxyethyl)-4-carbamoyl~l-piperidinio]-1-propen-
1-yl]-3-cephem-4-carboxylate
.
26
, ~ .
-
3~3~S~7
0~1
,,~C-CONH ~ S
HzN S N ~G~/N~CO~Hz
\O-C~T~F COO-
The compound (700 mg) prepared in the Experiment 4 was
dissolved in dimethylformamide (3 ml), and a solution of 1-
(2-hydroxyethyl)isonipecotamide (194 mg) in dimethylformamide
(0.5 ml) was added thereto. The solution was stirred overnight.
The reaction solution was added to ethyl ether (120 ml), and
the resulting precipitates were collected by filtration to
obtain yellow powder (680 mg).
To this powder was added anisole (4.5 ml), and trifluoro-
acetjc acid (5.3 ml) was added drop by drop over 30 minutes with
stirring under ice-cooling. After the dropping was completed,
the mixture was stirred for additional one hour and 30 minutes.
To the reaction solution was added isopropyl ether (50 ml), and
; ~ the resulting precipitates were collected by filtration.
The precipitates were suspended in water (30 ml), and the
suspension was adjusted to pH 7.0 with sodium acetate. The
result:ing insolubles were removed by filtration, and the
filtrate was purified by reversed phase silica gel column
chromatography to obtain the following two types of isomers
of the subject compound: -
~; Isomer (2-1) 21 mg
Isomer (2-2) 20 mg
~ 1 Mixture of the two isomers 50 mg
: ~: 27
:
~3~ '7
Example 3
7~-[2-(5-Amirlo-1,2,4-thiad.iazol-3-yl)-(Z)-2-
fluorom~thoxyiminoacetamido3-3-~(E)-3-~lS-carbam~ylethyl~
dimethylammonio]-1--PrPen-l-yl~-3-cephem_~_carboxylate
(3-1)
rC-CONH ~S CH3CH~
H~N ~ ll ~ N ~J ~'`.'N ~ CONH2
\OCH2F COO~ dH3
7~-[2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoromethoximino-
acetamido]-3- L( E)-3-[(lR-carbamoylethyl)dimethylammonio]-
l-propen-l-yl~-3-cephem-4-carboxylate (3-2)
C-CONH ~ 9~ OH
\OCH2F COO- dH3
~ '
The compound (600 mg) prepared in Experiment 4 was dissolved
i.n a mixed solution con~aining ethyl acetate (20 ml) and ethyl
ether (10 ml), and then 2-dimethylaminopropylamide (150 mg)
was added thereto. The resulting solution was stirred for 3
hours at room temperature. Isopropyl ether was added to the
reaction solution, and the resulting precipitates were collected
by filtration, followed by drying to obtain yellowish brown
;
~ powder (50 mg).
. ~
~ ~ 2~
,~ ., : .
:~3~ 7
This powder was stirred in a mi,ced solution containing
trifluoroacetic ~cid (5.5 n~l) and anisole (5 rnl) for one hour
under ice-cooling and, thereafter, ethyl ether was added thereto.
The resulting precipitates were collected by filtration and
washed with ethyl ether. The precipitates were suspended in
water (5 ml), and the suspension was adjusted to pli 5.5 ~ 6.5
with sodium acetate. The insolubles were removed by filtration,
and the filtrate was purif~ied by reversed phase silica ~el column
chromatography to obtain the object products of 8 mg of the
(3-1), 7 mg of the (3-2), and Ll mg of the mixture (1:1) of the
(3-1) and the (3~2).
Example 4
7~-[2-(5-A~ino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoacetamidO~-3-~(E)-3- ~(2-hydroxypropyl)
dimethylammonio]-l--propen-l-yl]-3-cephem-4-carboxylate
CH3
N ~\ C--CONH~ ~S~ ~ I OH
H2N'4S~ 11 ~N~N~
\OCH2F COO- CH3 CH3
The compound (550 mg) prepared in Experiment Ll was dissolved
I in a mlxed solution containin~ ethyl acetate (20 ml) and ethyl
ether (10 ml), and then 3-dimethylamino-2-propanol (0.124 ml)
.
was added thereto. The resulting solution was stirred at
room temperature ~or one hour and 30 minutes. Isopropanol was
2~
~3~f~7
added to the reactiotl solution. The resulting preclpitates wer-e
collected by filtration and dried to obtain y~llowish brown powder
(530 mg).
This powder was stirred in a mixed solution containing
trifluoroaceticacid (5.5 ml) and anisole (5 ml) for one hour
under ice-cooling. Thereafter, ethyl ether was added thereto.
The resulting precipitates were collected by filtration and
washed with ethyl ether. ~The precipitates were suspended in
water (5 ml). The suspension was adjusted to pH 5.5 - 6.5
with sodium acetate. Insolubles were removed by filtration
and the filtrate was purified by reversed phase silica gel
column chromatography to obtain the object product (70 mg).
Example 5
7~-C2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoacetamidO~-3- ~(E)-3- ~(lR-carbamoyl-
2-hydroxyethyl)dimethylammonio]-1-propen-1-yl]-3-cephem-
4-carboxylate
~C-CONH S I H3 OH
H2 ~S ' N ~_,~ N ~H
\OCH2 F ~OO- bH CONH2
The compound (1.00 g~ prepared in Experiment ll was dissolved
::
~: :
: :~
~ ~ ~ 3
~ ~:
~ ~ '.' . '
.
~3~ 37
in dimetllylformaide (2 ml). A solution of ~ dimethyl-D-
serinamide was prepared by dissolving N,N-dimethyl-D-serinamide
trifluoroacét~te (590 mg) in methanol (5 ml), adding lN-aqueous
sodium hydroxide solution (2.4 ml) thereto, followed by distilling
off the solvent under reduced pressure,~and extractir;g the
residue with acetonitrile (2 ml). The N~N-dimethyl-D-serinamide
solution was added to the solution of the compound prepared in
Experiment 4 in dimethylformamide under ice-cooling. Th2 resulting
solution was stirred for 30 minutes. The reaction solution
was added to ethyl ether, and the resulting precipitates were
collected by filtration to obtain yello~ powder (1.1 g).
To this powder was added anisole (8 ml), and trifluoroacetic
acid (9 ml) was dropped over 30 minutes with stirring under
ice-cooling, followed by stirring for additional one hour and
30 minutes. Ethyl ether was added to the reaction solution,
and the resulting precipitates were collected by filtration.
The precipitates were suspended in water (10 ml). The
suspension was then adJusted to pH 7 with sodium acetate.
The isolubles were removed by filtration, and the filtrate
was purified by reversed phase silica gel column chromatography
to obtain the object product (30 mg).
Example 6
7~-[2-(5-Amino-1,2,4-thi,adiazol-3-yl)-(Z)-2-
fluoromethoxyiminoace~amido~-3-~(E)-3-(4R-hydroxy-2R-
hydroxymethyl-l-methyl-l-pyrrolidinio)-l-propen-l-yl]-
3-cephem-il-carbOxylate
~: :
;: ::
31
~: :
.
'7
N\\ C-CONH ~ \ r
H2N~Sy 11 ~ N ~ ,
OCI-12F (~00- ,
CH~OH
The compound (700 mg) prepared in E~periment 4 was dissolved
in acetone (4 rnl),and a solution of N-methyl-cis-LI-hydroxy-
D-prolinol (89 mg) in acetone (2 ml) was added thereto. The
resulting solution was stirred overnight. The reaction solution
was added to ethyl ether (100 ml), and the resulting precipitates
were collected by filtration to obtain yellow powder (700 mg).
To this powder was added anisole (4.5 ml), and trifluoro-
acetic acid (5.3 ml) was dropped over 30 minutes with stirring
under ice-cooling, and stirred for additional one hour and 30
minutes. To the reaction solution was added isopropyl ether
(50 ml). The resulting precipitates were collected by filtration.
The precipitates were suspended in water (30 ml), and the
suspension was adjusted to pH 7 with sodium acetate. The
insolubles were removed by filtration, and the filtrate was
purified by reversed phase silica gel column chromatography to
obtain the following two types of isomers (relating to a
nitrogen atom of pyrrolldine):
Isomer (6-1) 27 mg
Isomer (6~2) 100 mg
~; :
~ ~ 32
.:;; :
~.
`-~13~5~
Example 7
7~-[2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoacetamido]-3-[(E)-3[4R-hydroxy-1-(2-
hydroxyethyl)-2S-hydroxymethyl-1-pyrrolidinio]-1-propen-
1-yl]-3-cephem-4-carboxylate
OH
C-CONH ~ S ~ OH
H2 N~ 11 ~ N ~ N~J
\ OCH2F COO- ~
C~ OH
The compound (2.0 g) prepared in Experiment 4 was added
to a solution containing (R)-4-hydroxy-1-(2-hydroxyethyl)-
(S)-2-hydroxymethylpyrrolidine (450 mg) in dimethylformamide
(5 ml). The resulting solution was stirred overnight. The
reaction solution was added to ethyl acetate. The resulting
precipitates were collected by filtration to obtain yellow
powder (1.65 g).
, :
~ To the powder was added anisole (10 ml~, and then
; trifluoroacetic acid (11.7 ml) was dropped over 30 minutes
with stirring under ice-cooling, and further stirred for
~ additional one hour and 30 minutes. Isopropyl ether was
added to the reaction solution, and the resulting
~; ~ precipitates were collected by filtration. The precipitates
were suspended in water (4.5 ml), and the suspension was
adjusted to pH 7 with sodium acetate. ~he insolubles were
removèd by filtration, and the filtrate was purified by
reversed phase silica gel column chromatography to
:
~ ~ 33
:
~: ~
ol)tain two l;ypes of the isorners (relating to a nitrogen atom
of pyrrolidine) of` the subject compound as follows:
:[somer (7-1) 96 mg
Isomer (7-2) 207 mg
Example 8
7~-[2~(5-l\mino-1,2,LI-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoa~etamido~-3-t(E)-3-(4R-hydroxy-2s-hydr
methyl-l-methyl-l-pyrrolidinio)-l-propen-l-yl]-3-cephem-
4-carboxylate
CH3 ,~OH
H2N S N ~ ~ ~
\OC~ F COO~ CH2OH
The compound (700 mg) prepared in Experiment 4 was dissolved
in acetone (4 ml), and a solution of N-methyl-trans-4-hydroxy-
L-prolinol (89 mg) in acetone (2 ml) wa`s added thereto, and the
resulting solution was stirred overnight. The reaction solution
was added to ethyl ether (100 ml), ancl the re~sulting precipitates
were collected by f~iltration to obtain yellow powder (700 mg).
To the powder was added anisole (4.5 ml), and trifluoro-
acetic acid (5.3 ml) was dropped with stirring under ice-cooling
over 30 minutes, and then the mixture was stirred for additional
one hour and 30 minutes. To the reaction solution was added
isopropyl ether (50 ml), and the resulting precipitates were
collected by filtration. The precipitates were then suspended
~:
34
::
' . '
' .
-:
in water (30 rnl). The s~lspension was acljusted to p~l 7 with
sodium acetate. The insolubles were removed by filtration, and
the filtrate was purified by reversed phase silica gel column
chromatography to obtain the object compound (92 mg).
E~ample 9
7~-[2-(5-Arnino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoacetamido~-3-~(E)-3-(1-carbamoylmethyl-3-
hydroxy-l-pyrrolidinio)-l-propen-l-yl]-3-cephem-4-
carboxylate
CONH2
~N ~ C CON ~ ~ ~ ~ OH
'OCE~2~ COO
The compound (1.0 g) prepared in Experiment 4 was dissolved
in dimethylformamide (4 ml), and a solution of N-carbam
methyl-3-hydroxypyrrolidine (186 mg) in dimethylformamide (2 ml~
~was added thereto. The resulting solution was stirred overnight.
The reaction solution was addedto ethyl ether (200 ml). The
resulting precipitateg were collected by filtration to obtain
yellow powder (970 mg).
To the powder was added anisole (9.0 ml), and then
trifulooroacetic acid (10.6 ml) was dropped over 30 minutes with
stirring under ice-cooling. The mixture was further stirred
for additlonal one hour and 30 minutes. To the reaction solution
:: : :
:: :
:
~",..,:
was added isopropy~. ether (80 ml), and the resulting precipitates
were collectdd by filtration. The precipitates were suspended in
water (50 ml). The suspension was adjusted to pH 7 with sodium
acetate. The i.nsolubles were removed by filtration1 and the
filtrate was purified by reversed phase silica gel column
chromatography to obtain the respective types of isomers
(relati.ng to a nitrogen atom, and carbon atom on the 3-position
of pyrrolidine, and there are four types on high pressure liquid
chromatography) of the subject compound as follows:
Isomer (9-1) 71 mg (mixture of two types)
Isorner (9-2) 70 mg (sihgle material)
Isomer (9-3) 54 mg (single material)
Example 10
7~-~2-(5~Amino-1,2,4-thiadiazol-3 yl)-(%)-2-
fluoromethoxyiminoac2tamido~-3-~(E)-3-tl-m~thyl-4
sulfo-l-piperazinio)-l-propen-l-yl]-3-cephem-4-
carboxylate
CH3
~ \~ C-CONH ~ S~ ~r-~
H2N ~ S~ N ~ N ~ ~_~N~_~N-SO3H
\OCH2F 00-
:
The compound (2.0 g) prepared in Experiment 4 was added
to a mixed solution containing 4-methylpiperazinosulfonic acid
I sulfate (718 mg), N-methyl-N-(trimethylsilyl) trifluoroacetamide
(2 ml) and dimethylformamide (6 ml), and the rFsulting solution
'
36
...~,:
~ ~3~
was stirred overnight. To the react;ion solution was added
methanol (2 ml), and the insolubles were removed by filtration.
The filtrate was added to a mixed solution containing ethyl
acetate (50 ml) and ethyl ether (50 ml), and the resulting
precipitates were collected by filtration to obtain yellow
powder (1.79 g).
To this powder was added anisole (10.9 ml), and trifluoro-
acetic acid (12.7 ml) was dropped over 30 minutes with stirring
under ice-cooling. The resulting solution was further stirred
for additional one hour and 30 minutes. To the reaction solution
was added isopropyl ether (100 ml), and the resulting precipitates
were collected by filtration. The precipitates were suspended in
water (4.5 ml), and the suspension was adjusted to pH 7 with
sodium acetate. Insolubles were removed by filtration and the
filtrate was purified by reversed phase silica gel column
chromatography to obtain the object product (50 mg).
~ Example 11
;~ 7~-[Z-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-
~fluoromethoxyiminoacetamido~-3--~(E)-3-(1-carbamoylmethyl-
4-hydroxy-1-piperidinio)-1-propen-1-yl~-3-cephem-4-
carboxylate
:
: : 'CON~2
,~Cj-CONH~S~ N/~OH
Hz~l~ S N : d-
\ OCH2F COO-
:
~ ` 37
: : :
~3~
The compound (1.0 g) prepared in Experiment 4 was dissolved
in acetone (9 ml), and N-carbamoylmethyl-4-hydroxypiperidine (206 mg)
was added thereto. The solution was stirred overnihgt. The
reaction solution was added to a mixedsolution (lO0 ml) containing
ethyl ether and isopropyl ether (2:1), and the resulting precipitates
were collectecl by filtration to obtain yellow powder (l.0 g).
To this powder was added anisole (9.0 ml), and trifluoro-
acetic acid (10.6 ml) was~dropped over 30 minutes with stirring
under ice-cooling, and the resulting solution was further stirred
for additional one hour and 30 minutes. To the reaction solution
was added isopropyl ether (80 ml), and the resulting precipitates
were collected by filtration. The precipitates were suspended
in water (5 ml)~ and the suspension was adjusted to pH 7.0
with sodium acetate. Insolubles were removed by filtration,
and the filtrate was purified by reversed phase silica gel
column chromatography to obtain the object product (166 mg).
Example 12
7~-[2-(5-Amino-l,2,4-thiadiazol~3-yl)-(Z)-2-
fluoromethoXYiminoacetamido~-3-~E)-3-(S~aza-l-methyl-
2,8-dioxabicycloC3.3.1~nona-5-io)-1-prlopen-1-yl)-3-
cephem-4-carboxylate
N C-CONH , ~ S ~ --o
H~N~ S~ N ~L N~ ~CH3
OCH2F COO- ~'
3~
: ~ :
.
The compound prepared in Experirnent 11 was dissolved in
d~methylformamide (10 ml), 5-aza-]-methyl-2,8-dioxabicyclo
[3.3.1]nonane (800 mg) was added theretc at room temperature.
The resulting solution was stirred for 20 minutes. ~he reaction
solution was diluted with ethyl acetate (25 ml) and the solution
was added to ethyl ether to obtain brown precipitates (3.85 g).
The precipitates were dissolved in anisole (23 ml), and
tr:ifluoroacetic acid (26 ml) was added thereto uncler ice-cooling.
The solution was stirred for 30 minutes at the same temperature.
Ethyl ether was added to the reaction-solution, and resulting
precipitates were collected by filtration. The precipitates
were suspended in water (40 ml), and the suspension was adjusted
to pH 7.0 with sodium acetate. Insolubles were removed by
filtration, and the filtrate was purified by reversed phase
silica gel column chromatography to obtain the object product
( L108 mg).
In the same manner as described in the Examples 1 - 12,
the following compounds in Examples 13 - 113 were preapred:
~ C-CONH S
HN ~ S~ N ~ ~ I
OCH2F COOCH2 ~ OCH3
(compound in Experiment 4)
: ~ A' (amine corresponding to A)
~ . :
~ H2N ~ N ~ ~ A
\OCH2F COO--
: :
:
39
:~3C~
~ 'lur~ Lsomers may be formed due to the portion of ammonio
group on A. When these isomers were separated~ the respective
yields were shown for the respective isomers individually..
The following abbreviations were used:
Boc: t-butoxy carbonyl group
t~u: t-butyl group
Bh: benzhydryl group .
Tr: trityl group
~: : : : :
: :
__ _ _ ~3l~5~
.. ~ _ _
C~ C~
rl c~ ~ o 13 Il~ Ir
>~ 3 E o X o cO E E~
E ..
~ ~1~ _
.
E C E ~ E~ Ch t1o
s~ O O O O
~: E ~L co O
~ r _
~ ~0
~: E ~ ~ 1~ ~
o . ~ ~ ci) r-
E ~ 00 O Cl:) cO
_ __ _~_ .
: ; ~
~ ~ ~ ~ /-7j ~ Z]
; E ~ _ ~
41
:
: :
:: :
::
:~3~5~7
-~' ~
,~ I C~ C~ U~ o ~
! co C~l co a> ~D
~ _. _
.E 'a~E crO ~ ~ el,
a E t~ ~ o O O
h ~ . i~ . . _
,C ~ ~ .
~0 ~ E~ ~ ~ ~ ~
C C~ c~ C-- ~D
a F C`~
a) ¢ .
11 ~ ~ O ~
~ ¢ C ~ ~ ~'\<~ ~ ~ ~
` :
; ~ ..... ~... _
x t_ aa o __
~ :
~ .
~ ___ __ r ~ __ ___
U ~ CY~C~
~ h ~ , f~
0~ ~ ,
:~ . 1~ E E ~ ~ ..
.a ~r ~ c~ ~ c~
~ ~ 1
~ ~ . . ~
O C ~r Z E E E E
C E m h
~ ~ a ¢ ~ . ~:-
~: _ _ ._ .___ ~ X
; ~ ~: h~ ~ ~
¢ ~ zl? ~ ?
: : : : ~ : ~
E - . ~ . .... _ .... ...
E ~ _ ~ . . . .. __ ..
:: :
43
~3(~t'~4~7
~ _ _ __ .... _ . _ ___ _ .. _~ _ . _ . _____
~O~ E~ ~ O -'O
. ~ ~ _ ~
o ~ a o c~O ~ ~ ~
,~:' ~'~
W .
C C 00 O O N O
D ¦ ¢
~ -_ ~ . . _ ...._
~ O ~ N N N
,f ~ O O O ~
~: ~ I ~z~ ¦~o ~l~ lo 'I? ~ ~rl?¦ ~'1?
~ ~ ~ E d ~ ~ .
~ ~ ~ ~ ~`,
'
5'~B~
_~.._ ~
-~v~ .,.
h .
O N O O LO N
ra ~x ~ ~ __
E O ~1 ._ l-- O .
C E Q~
Ll _ . ~
~ ~ m o
~ C ~ E~ ~ ~ ~ ~
C E O ~Z; 0 ~7 O O
~ O ~ ~ ~ Cl~ O
: ~ : E . N N l J ~ N C`l .
: ~ ~ ~ 3 :` : 1~ ~
__ ~ m _
; ~¢ ~ m I ~ o o
~
~ zz ~z~ m l?~ ~Z~ m~? Zm ~?
~ ~ I . I I I
h ... _
: ~ E ~ C~ c~) d~ Is~ ~D
X~ ~ . .... ___
: :
'7
;_ ~ _
U ,,
'o~ ,
rl
rl ,1~ t t tt tt t tt t t
; ~ ~
It .-~ _ .
t I
r c E ~ E E N E
C E
~ `
': ~ ~ ¢ .
_--_ _~ ,__
;~ ~ :
: ~ ~`t
~ ~ ~ t rt
~ ~ ~ ~ ~ ~z~ ~t ~~ . O~'
~ ~ ~ ~I~+ ~ ~ Z,+ ~ , " I
r-l _ _ ~
E d t-- ~ao t~t O ._
~ ~ 'X tXt _
ll ~t
:
- ' , : ,
: ` : , `
a),,~ ., .
`o~ .
~ ~ ~ E~ ~ E3 ..
'- ¦ ~ ul --~ 1 D~' ~
o o ~ o . ~ o o
_ J ~ O _ A
r , ~ , ~
~ ~ ,. ~ :~ q - c
E : ~ ~ ~ _
~ !~7
~ ' ~
~3~5~37
;_.. ,.~. . ,.- _ _ ....... _
rrl ~
~ ~ ~o ~ ~ ..
~ . ~D ~ Ln C" CY~
o c~ ~ ~r c~
. 1~ 'o~ ~-~ ...... .
E O ~ ~ C~ t
~ ED'Q
.. _. .
r I ~ ~ o ~ ~ ~ r
o . o ~ z; c, o~ ~ o o
E ~ C~ O ~) ra NO
:~ ¢ _
~ _ _ .
~ :
: ~ N N ~
,~ c~ " ~ J ~S ¦ "l $-5
~ ~ ~ a ~ _ ~ _ --- --- I - ~-- -~--
~: ~ w _ _ _ ~ ~
.
.
t'~
~ ~ ~ ~ - ~
,~,,u ' ~ r~ ~ ~ " E~
r~ C~ ;~1 _~_ O
~l r I --~ ~
~ _ .
~ ~. ~ ~ ~ ~ E~
j~ C\~ U~ 0
__ : ~
~ ¢ ~ n O O ~ O
;~ ~ ~} ~ 0~
L~- 1'~
o
~ ` : ' ' '
~ ~ ~ -- -- ~ --- --~
s ~ ,,
a~
a ~ E~ ~ ~ ~;
a co c~ c~l ~ u7
. ~o o~ , _
~ ~ ~ a o o ~ u~ ~ ~
W ~ ~ ~ _ .
t~
:~ _ - ~ : ._
\/ 3 ~ z
~ ~ ¦ ~ ~ Z C~ C~ Z~ Z ( ~
: ~ __ . . _ .. ~ _ .~_
~ ~o ~ o
:~ : 50
:
: : - ` - ' `- .:
.
,
:-. ' " ~
~3~4~
~ h ~ ~ ~
'OQ' ,
~ . r--~ ~ ~o ~ ~ C~o
co ~t~ , ~ o r-
_ _ ___
. :Q 'U ~o Cto .
e c E Cto ~o ~ i~ E~
U O ~ N CO O . O O
C E Q . N O O Lo
h t.) t~
nl ~ ._ ,
C ~ ~ ~ ' ~ ~
C C ~' C~ O 00 O
E ` .
~ ¢ .
_ __ . ... ___
: ~: : ~ ~
~; : ~ Z ~ ~ ~ ~ ~ ~
¢ I ~ O ~ o~ I ~ V ~ V
~/ \j/ \1~/ 0~ ~ 0
~ I ~ v~ I ~q ~ ~ c~ / ~ ~ ~ r~
: ~ ~ I ~ ~ I ~ ~ ~ ~ ~ ~ ~ \
C~ V Z;~ ~ V~ C) ~ V ~ V
~: . _ _ . _
I QE' d ~ C`J CY~ ~' ~S)
IIJ :G . . .. __ ~V _.__ .
5:~
: ~ :
l ;~r~ 7
~ _ __ ._ ____ __ ____ __ ~ --___ ___ r ~
c'~ ~ ~
~aJ ~
V ~ ~ ~ ~ .. ~
. ~ C~ C~ ~D __ LO
~a ~a a
E E O ~ ~ . ~o
h E ~ Ln .
~ ..
.s:: ~ ~ .~ ~ .
v a o o o ~r
a ¢ .
: __ ~ ... .
¢ ~ D:~ W ~ W
~ l ~Z_~C W ~_W^ W'_z_W W'~ ' W~_z_W
~ . . ~ __ .
~; E d c0 ~ Ot:) cn O
h~ ~ __ .
~ . 52
-
:
~? ~
.,~. ~ ~ .. .. ____
~,~v ~ ~ ~ ~ - C~o
~D U~ , ~ ~ 00
~C `'c .......... ~ :
Eo' ~e ~ ~ ~ ~
U U ~ o o o o
a ,~ ~ .
`o C ~ ~ O ~ ~
~ ,~ ~ O / U~ ~ ~D
~ a E / \ ~ ~ t\~
O ¢ ~
_ _ .... _ .... ~ ._
~ ~ P~ ~ ~ O ~ ~
¢ g O g O O
~ P~ < ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
C~ ~Z-c) V ~ C) C) ~ V ~-0 V ~ I-V
_ _ . . _
E ~ ~ N C~:) . ~ Ln
..
- 53
.
.
~3~ 4tB ~J
~ _____ ____ _ ___ .____ _ .____
U , ,
'O~Q~ ,
(U'U ~ ~ ~ ~ ~
,ra~ CD Oo CO ~ ..~
__ 'I~ ,~C
E ~: E3 O O O O O
O ::~ ,1 ~ O O O O
O h t-- CO o0 L~ U~
h t,) W
r .
~O ~ '~ .
O C ~ ~ ~ U~ O
: a ¢ Z u~ c~ ~ c-
~: ~ .__ _ _
;: ~
¦ E ~ tD r-- ~ O
W ~1 _
` 54
:
~ 3 ~ 7
,~,u~ ~ ~ ~ ~ ..
n . C10 o r- u~ C`J
~a a C _
E C E E~ ~ ~ ~ ~
a CElP O O O O O
~r 1~ -- r r~T
O C ~ ~ ~ ~ O
~ID ¢ .
_ _ . , ~ ._ .__ .. __
¢ ~ O O
; ~ ~ z~ C~ ,~7~
~ _~ ._ .. . . ~ .._ _ ..__
: ~ ~ oo . ..._ ~__ . .. I . _ .
CC
`
~C~4~'~
' ~-- -~ ~
~ o _ Cf~ , co , ~, o~
E C ~ C~, ~ ~o Cb
C~ C~ Ll Ln ~ Ln U~
, c'3~
~ . .
: ~ ,~ o
~ `c) a~ \
~ ~ ~ ~ \ ~ ~
~: E O ~D ~' C,~ O ~D
:~ ra O c~ \ C~ 1~ lr~
E ~ c~ IZ~ ~ ~ c.
~_~1 ~ ,
:
¢: ~ ~ ~ ~ ~ R
~ ~`,z; ~
~ ~ ~ .. ~ ~........... . ... ~ _
E ~ CD C-- c~ 0~ O
w : :_ a oo
:
~ . -
.
'7
, ~ ...... ~ ~ _ _ ...... _ .
U .,
, n. .
'~ .~ E~ ~ ~ c~ ,. E~
~ ~D ~ ~ LO O
O Lf~ CD cn c~ In
a o _
Eo C ~ t:~ c~ t~ E~
U: o,)Q, ~ C~\~ ~ O c~,
~ " 0_ _ ~ ~ ; .
~ C C ~ ~0 LO crO~ CO
~ ¢ . .
:~ ~ __ _
~ I ~ ~Z ~ ~ ~
~1 w' <~o ~ z~ z_~c
n _ . _ _ _ _ . . _
` : X _ ._
: 7
~3(~ 4~'7
~ i : ~ '
~ ~o c- t- ~ ~r
_
~U .
e ~ E Clo E~ 1~ e10 C~o
. t~ O Ll C~ O oo ~ C~
,J E ~ ~
1,~, _
C , ~ ~ ~ ~ , ~
4~ rU O O O C~ O
E C~ C~ (:n cr) ~
e, ~,, .
U) ¢ .
_ _ ~' : ~ ~ _
:~ : : ~ ~
~ ~: d Z \ / ~ ~ O O
: ( O r~ $ m O ~ ¦ ~
: .
~; ~ ~: ~ ~,Z- C) ~Z--V 0 ~ ) C) ~ C)
r-l ~ :
E ~ ~ ` ~.D ~ CO C~) O
~ 1.1 _
~3~?5~ 7
. _~ _._ _ _~ __ _
s~L, .,
~.. ci~ ~ o c~ ..
r - ~~~~- __
C ~H
.' ~ ~ ~ ~ ~ ~b
E C E I _ O O _O
v E X~ ls~ IS~ Is~
nJ ~ _
.
' O
:~ E E~ ~ ~ ~r~
o . ~ o o oO
E ; ~ c~ ~ o
(1) ¢' . _
~ 1 L
E ~ o o O O o
~ ~ : ~ Z _
59
: ~ ::
'
~3~5~t7
V l l
~ a
'~r~ ~ ~ ~ E~
~ n _ __ Lr~ . . ~
_ r h 00
0 l ~ .. _ _ - __
: a E .
~: E I i~ i~ ~ i~ E~
d . O N O O ~ .
~_ ~_. ~1 ~` \
~ ~ ~S ~ J ~ J
. _ _....... ___ ._
h~ ~ _ _ __ ...... _ _ O O
-..
~3~ '7
~ :1 ~
,~ .
.
r , o~ o ` o
v E t- ~. ~ ~ 00
: 1!~ ¢ ~ ~J 5
-18
~
~ [~
:
61
` ~3~54~'7
ple 114
7~-~2-(5-~mino-1,2,4-thi?~-liazol-3-yl)-(Z)-2-di~luoromethoxy-
iminoacetamido~-3,~(E)-3-(dirnetllylcarbamoylmethylammonio)-1-
propen-l-y]~-3-cep~em-4-carboxylate
N ~ C - CONH ~ ~S ~7H3
2 ~ C~I3
The compound (500 mg) Or Experiment 6 WaB dissolved in a mixed
solution Or ethyl acetate (10 ml~ and ethyl ether (10 ml). To the
solution was added dimethylglycine amide (160 mg), followed by
stlrring for 30 minutes at a room temperatureO To the reaction
solution was added iso-propyl ether. The resulting precipitate
was recovered by filtration, and dried to obtain yellowish brown
powder (400 mg)0
This powder was stirred under an lce-cooling in a mixed ~olution
Or trlrluoroacetic acid (5 ml) and anisole (4.5 Dll) for one hour. To
the reaction solu-tion was added ethyl ether, and the resultlng
precipitate was recovered by riltration, rollowed by washing with
ethyl e~ther, This precipitate was suspended in water (5 ml),
followed by adJusting its pH to 505 - 605 with sodium acetate, The
insolubles were removed by filtration, and the riltrate was puriried
through a reversed pha~e silica gel chromatography to obtain the
obJec~t product (55 mg).
I~ the same manner as described in Example 114, there were
produced the compounds of' the f`ollowing Examples 115 - 117.
~ . .
62
~: :
:
~::
~3~:~54~'7
~3 N C {~"NH S
~- - CHN~N o~Nf,~ / I
OCH:F ~
Z COOCH2~=\~> OCH3
. .
(~ompound of E:xperiment 6)
A' (The corresponding amine to A)
NC--CON~ S
H2N~N O ~/A
\ocHF2 boo~
:
: ~ :
:: :
: : 63
o
iu l ~ I ~
¦ r; O I ~
~a ~ ; ¦ o I ,
a~ 1~, a d ~
~ ~ ¦ C ~ ~ ~ ~ O
a a o~o o o
I ~
_ ~ _~
e~ _
; ~ 64
~3~
. ..... ,_ ~
t_. ~ N _ _ N I I~ X
O ~r~ N
~o ~ OCON ~: ~ ~ O
~ m m ~ - O '' m ' ~ m ~
V ~ t-- tf~_~ N O
C~ ~ Nr~~ ~_ N ~ _ 5~
Y N ~~ ~5~ ~ ~d ~ t
~ ~ 1000C~ CO ~ t- ~
Z 11 . ll ~ ~ ~ ~ t~D ~
~ u3~1 ~ N N,~ ~ ~ ~ ~ N N
~ ~ ~ 3:~ N U~ - X U~ ~ t--
C~ -- C~ _ -- __ _ ~ ~
. ~ t-- ~ `~
_ O 1~ C~O CO~ GO N CO tD
~ 0 ~ ~ ~ 1
~ ~ ~__~ ____
: o u~ o b ~ ~ x ~ ~ ~
V~ ~, U~ U~ , C~
~ o ~ ~ a ~ ~ ~ O
a~ ~ ~ ~ c~ _ o
_ ~ ~ a) ~ L(~ ~D t~
. . . '
C _
o In tt~ o L~ O
: ~ ~ 0~ C~ _ _ _
v n. -- u~ u~ t- ~D t- ~D
' ~ _ _ ._
O U~ O- O Lf~
U E ~ N ~D CO t-- CO
~ ~ _- t_ t- t- ~D t- ~7
r ~ M ~ ~ . _
~C'~ _ _ ~
::
:
~ 65
~3~?5~
. . .
,..~_ ___ __.,.....
N ~ ~
3~ ~ W
C`~ ~1 C~
. ~ ~ o
N ~ D ~ ~
D 1~ ~ CD," 1~ ~ W
CO ~ C~ W C`~ 1
Co ¦ '_ ¦¦ ~ ¦ _ 11 ~ _ ~ _ .,
. _ `- ~ O. ~ _ ~ c~J Xo C~ C,~
E ~C~,, ~ _ ~ ~
Ll ~ ~ ~ '~ ~ .
V r~ ~o~'~ ~
~ ~ O _ ~_ ~ ~ ~ ~ ~ ~ .
~ U-) Wc~~c, tD ~ ~
Z; a~ ~r _ ~ X
Nt-- I ~ --' N U~
U'7 _ ~COID ^ O ~ C ~_
N~ N ~ C
C-~ C~i o N3 ~ U~ o ~, CO ~D
c'~ 11 Ir~ 11~ cO ct~c~ 11 CO
~ I ~
X ~ ~ ~ ~r~
r) ~ _~1 _ Nr~ N _ ._ ~
~ ~ _ ~, , ~ ~
Q Lo o c~ n ~ co cn co o
C) ~ ~ ~C~ C`~ V CJ~ cO, ~ o
. ~ C~ tC) 1~ 'J C'~ D t--
.
~ C _ ~
O U~ O O O
'.'1 C~ ~ C~
~ ~: ~ ~ ~ ~D
O O _ ~ _
:1 U~ O O O
'~1 E ~ t_ C~ 00 C~
I,~L-~ ~, ~D ~ ~
H U~
.. _ __._ ....
4 d .
X 11 .. .__. .
~ _
: ~ 66
.
13~S~7
. _ .. , _ . .. . _ . . . _ . . .
, ~ ~ '' X E~
E ~ Ln ¦ N ~ N CJ) X ~ Ln
0 t'~Lt~ C~ t.~ _ t~O ~ ~
U '~ ~~) ~ ~N t~ ~ ~ N
1~ N ,~0 ~ t.~; Lt~ N Ei ,~
N - ~ - 5
~i t-- ~ V~, _t,~ X t~
~ o 1V ~r t
X t~ o ~ t~l ~r Ln N N ~ Ln N
. ~ , ~ D t~ ^
~o N_, D ~ ~X ~ ~ ~ ,
O t7~ ~ N ~1 tr~ ~ ~ o
Q ~'? ~ '" Q . ~ ~ ~ Ln a~ cn
~ ~ Ln t~ ~tt7 Ln ~- ~ ~ Ln Lt;
;~ C ~ ._ _ .
o Ln o o o o o
t~ Ln N L~ t.`J
_l CD Lt~ U~ Lt~ tl~ Ln
:; : O _ _ O O O O
6 _ CD o~ Ln cn Lr~ a~
~a U ~, ~ L~ -- _~ ~- Ln
:~ h ~
~ .~ .,._ . .__ ,_ _~__.~
: ~ _~ ._ . ._
:
~ 3~5~87
. . __, ~ , .a~
Ll~ ~ _ ~
,~ ~ O ~ ~
_ X N ~ E~--~ _ _ .
--` X ~ ' LO~ [ N U~ . C~ ..
U~ r~ ~~~L~7 ~n 1 I L
~ ~ ¦ I~ E3¦ '~ '~ 1I N
.~ . O ~ O_ N ~~ ,~, ~ .
u~ ~ C_ u~ `-- N '
1~ _ - ~ ~_ _c~ ~ - N ~
L/~ N ~L(~ ~O
L.~Lf~ O--I ~r O --I O -- N -
u~c~ ) c-~ 1111 ~~ o E~
~: '~ '~ '~
N X~ ~:1 X ~ N _ll C`~ ~n
O IT~ O h ~ ;~ N ~ Ll~
Z t:--~ ~ ~r ~~1 X`-- o ~ N
¦¦ `_Lf~ C~ ~ ) i11 ~ LO
)~ LO ~ ~ 11 ~ '11 ~
~D LO ~ ~7 ID t~ . I N
_ L~ ~ ~ ~C~ _ _ c~~IL~
. ~ ~N ~ ~~ N~ ~N ~ N
0~ CLl~ ` U~ N~ ~ ~_~ , ' O
_ _L ~ ~_ OLt~ o ~ U~
~ ). ~
. ~ - - ~~ __ L~ ~ ~ hC~) _ _
~ ~ X $ ~ ~ - ~ $ ~
~1 ~I N r-l ~1 ~ N .--1 C`l
~ CO tD ~~ ~ C`J C~
1~ ~ ~? ~ ~`~ ~ ~ t- ~D p N tD '? '~
__ ~_~ Cr~ U U~ _' C-~ U~ U~ CO ~ C i C~) U~ ~
~: : __ _ ~
C O- U~ O O O- ~
,~ ~ _ t- C~ ~
h --~ ~D LO C.D Lf) U~ U~
0 0 _ _ ~1 ~ ~ _
O O O O L~~ O
~ ~ tl E -- ~-- c~ Lt~ ~D O
; ~ ~ ~ ~_____.. : ~
~; ~ ~ __~C~ I . .
~: :
:~ :
: 68
': . , .
~3~4~3
. .. = . ~ .. ~
_ ~ U) ~
N --~ W ~ t~ N . tC ^ ~
,_ ~ ? û~ ~r ,1 ~ o ~ ~;
. ~ D - ~C -- ~ ,;
E ~ ~ .
':~' ~ , _ N O ~ ~c~ _ ~
U W~ ~ 00 . . ~ t~
D~ ~ . C~ ~ I C~
~ ~ ~ ~ _~ ~ - ~ ~ i~ .~ ~ ~
cr~ ~~ X N il ~ ~ ~ ~ CO .
. ~ CO ~ ^ a7 ~ ~ '
~ ~ ~ ~X ~~Cli ~ - ' --
~i 11 3 ~ ~ ~ ~ ~ ~ o ~ ~ ~ o
. ,, ~ ~ ~ ~ ~
, N ~
O.~ 1 N-- O I I ~ C`J O I I _ N
ir7 ~ ~r o ~D N i ~
CO ~ ~ CO
._ . . I
C' O- O O O O O
CL ~ ~ ~) ~D IS'~ tD If~
: 0 0 _ : _1 _ _
:: a z u~ ô ô o' o o
~:1 E tC~ o U7 C~ 1~ C~
~,_ t- ~D _ _ t~
~C~
~U ... ,,,__~. ._ _____ .___. .. __
7 ~ _ _ 9 ~__
~ : .
: ~ ~ . 69
.
`~ ~3~ '7
__ E
. ~D ~ ~ ~ W
E3 ~ , ~ ,_ ~ N N I ~--
~ b~ 5 ~ .r, ~
~ ~ ~ ~ X `~ L~
~J ~ ~ C~
C~ ~ ~ N N O
C`J';P N N .~ ~, 5 E3 ~ ~3 ~
N E3 ~ Il~ ~ ~ ~~ C0
. ~ ~CO ~ ~ O ~ NC0 ~) .
-- ~~C X ~ --'$ N N ~
~ . U~,~, 00~ o O U: ~ U~ ~ CO --'
N~ ~ 11~ N U~ 1~ C0
~ E~ J _~ . h _ ~ ~
O ~~ I X ~ ~q I ~ N
~ ~` O _ N -- O~1 1 ~ N
L ~ D, ~ ~:i'CO _ ~Cl) O tDN
~ ~ ~ CO
.. _ ~_. .. _. .. __
C ._ .
O ~ U~ O O O
. CC~ N ~ U~ ~
~ ~ tD , U~ ~ CD 1
O O _ _ ~ _ _
.q r~ O- 0 O- O O O
~1 E t~ 0 co 0~ IS~
^ t- ~ t- IS) ~ Ir~
0 ~ ~_ _ ~ ~ _ ` _
_ --- --__ ._ .. _ ._.... .~_
~: ~1 d _ : N
~ ~' 1~ ~ ._~- ~.. ___ .
~ ~ 70
~ 3~ 37
. . ~ ~ , ,,
~ ~ m ~ ~ ~r e I D~ .,
. _ _ ~ N ~ ~1,,, ~ ~
Ll ,~ e N ~ U~ N --~ N ~ CEi -
U ~ ~ c~ a~ ~_, 1
C~ ~ ~ ~ ~
- ~ N
' - ~ ' OU ~ N ~ ~ ~ .
o~ a~ m ^co,~ ~n r- ,~
.I ~~D o c- E ~, ~ ~u~ E ~ ~
~ I~ ~ 11 11 c~ m ~ ~ _
: IC,O _ _ ~ _ --'~ ~ W ~ ~ ~' W
~ o ~ o 0 ~ c~ ~ ~OD o ~- ~N
: `~Jcou~ ~ où ~C~u~ In ~ n
;~ ~ e ~- ~ .. ----
o o o o o o o
o o _ _ U~ In tD ~n
L U t~ _ _1 : _ _
: . H ~
~; : ~ E .. _ _.~ _ __
:::: :: ~ . __ . a~ a~
:: ~ ~ ..
~: :
: ~ : 71
: :
.'
~3~ 37
., ............. ... ... .. ; .~ _ .
~ o
~J ~ ~ _ ~ X ~ N --'
. 31 ê` 1' 11 "' - ~ 1I t- O ~ ..
X ~-- ~ ~/ ~ --- ~ .
. ~~ ~ ~ ~, a, ~ _, ~ . , -d
E C~ ~: I ~ ~: _ I ~I .~
Z _ ~ _
~ CO ~D ,a - ~ ~ u~ C~- ~ X ~
N N ~C N~1 ~ N ~ _~ ~ t CO -
u~ a~ co ~r~~ co ~N o
: c~ r- O ~ U~ C~ tD U~
. : ~ 11 ll ll ~ E~ co
~d -O ~ 1 ~:1 ^ ~ U~
~ ~ : O ~t _ O N U~~ O C`~ N ¦ C-- CO ~
: : ~ ~ P ~ U
~ ~ , ~ U~ , ~U~ ~ C.
__ .~. _ .. . . .
C: ~ ~ . .~ ~
O O U) O O O O O O
t~ u~ c~) ~O a~ t- C~
4 --C~ U.~ tD 1~) U~ U~ ~
O :0 ~ _., . _ _~ ~ :_, _ _ _
U,)^ L~ O O O O O O
'~l E ~ tD a~ ~ L~ ~1 ~ cn
~ _ _ ~ _ _ ~ r-- ~D I_ . U)
:~ ~ cQ~-; , :
: ~ ~ : O : ._ ~ ___. .. .__ . .
~ ~ lâ: d O _ N .
~_ ' . C~
_ ,~ ._ . _ ___
72
` ~3~S~il7
.. _ _ _ ~
._
_~ ~ ~ ~
o E ~ ~D ~ ^ o a~ o o~
r-- N N ~ ` ~ ) ` 11
~r 0, ~r ~ ~ ~ ~ ,
-~ ~ 11 11 5~ C'~ N ~ ~ N
~o ~ ~ ~ N D ~ ~ 1 ~ C 1l - 'O
E ~ ~ ~ ~ ~ O ~ ~ ~ ~ - ~ _
~/~ O ~ ~ m -~ ~ N _ ~ ¦ - c~ O
C~ N ~1 11 1I X ~ N . C~
:~ ~ E ~. ~ ~ ~ ,C^ ~n $
t, X m ~ u~ ~ ~ m m ~ N m m ~ `~'
~ ~ _ c~ ~ I ~ ~1:1 ~ '--' ~ N N
'~ ~ o ~ ) oo _ ~i 11 In
_ N_ ~ C~ , t~
l~ ~ ~ r~~~ _ r ~ ~ _
O C oo - ~ ~ U~ O I _ _
~ I m o c.~~ c~ cn ~ ~ r~
Ci u~ c~ ~ c~ ~ ~ o Q c~ ~ o
_ . ~ ~ tD -' C~ - ~
c ~
o ô ~ ~ o o c~ o o
.,~ C~ C~ ' ~D C,`3 U~ _ U~
h _I U~ CD iD ~ ~
u) o -- ~ _ _ _ ._ ~ ~
.4 Z I~ O _ _ O O O O
E ~ O tD a~ 15~ a) U~
V ~ ~ t- tD t- ~ ~ ~ e- In
L~ _ 1_ : _ _ ~ _ ~ ._
U~, ~
__ ~ . ~ C~
E _ _ l _ . _
73
~3~ 7
.. _ ~ __. ~
~ ê ~ ~, ê ~c ê ~
C" ~ 00 ~,CO1~) ~ , t`~ ~ C~ .
r~ N ~_~ _ I CO .
~o ~ oU~ ~1 ~ .~ CO I~
~ ¦ ~ ~ ~ F
~ ~ E~ ~ er ^ C~ ~ ~ O
U~ , ~ _ ':~' - ~ N ~ U~
N N ~ ~3~ I--
. I ~ c~ ~ 1 X 1~ C~ ) U~
CO ~ ~ ~CO C~ Ncn o COL~ O ~ ~ ~
.-U~U') 00 ~~ u~ ~ ~ C~
ll ll ll llll . ~ 11 ~
:~ ~ ~ ~ ~ D ~ ~ ~ _O ~ ) ~ U~
~ ~ I ~ ~ ~ I ~ ~ ~ I ~
: O ~C~ ~ O ~~ C~ O ~ _ ; I O
t/l ~ U~ U~ U~
--CJ) O ~ ~CO
, ~ ~ t- t`J ~ CO ~ ~ ~1 ~ u~
cO ~ ~ ~ I
_
~:: : _
O O 0~ O O
.,~ ~ t~ U~
^ ~D U~ U~
O o _
~ Z ô o o o o o
: ~co u~ cn ~ o
~tl E _ c~ ~_ 10 ~ tD
~,,U ~ _l ~ ~ _
~ , ._.................. . .
~. d ~D t_ co a~
~' ~ _ _ ~ ,: _
'
74
:: ~ :
'7
. ._. _ . ~ _ ... _,_
_ ~t~ ~ N E
~ ~I CO CO ~ ~ ~ CO _
Otl- I X ~ 9 . ,
N ~ N C ~ ~ ~ 5
E ~ ~ X CC! u~ N ~CO E~ ' -- CO
_ ~ O ~~ _ ~ CO~ ~o CO ~ I _
u~ X E~ co 11 ~~r -- 1I co ~
d~ N ~ _ ~ _ ,_~ I~ C~ _ ¦¦
:Z; c~ _ E3 X ~ u~ E3 ~
~ 5~ w ,,~ C 5~ ,
. ~~ ~ X I ~ ~ ~ N~-- ~ ~ ~ ~ '
o~O o 1~ o 1:- ~0 ~. c~ co E3 -'' In --
N_ ~ CO _ ,~ C`~ ~ C~ ~ CO
CO
.~
I ~ CO 1:: 5 ~o I
O~ ~ O ,~ L~ ~ I O _ N
~ OCO O ~ ,~ ~) O ~ CO cr~ _
~ O O. t_ ~ ~ ~ ~O ~ n ~ O ~ O ~ ~
~ ~ ~ ~ Is~ ~O ' In
~- O
O
~_¦n. ~ . ; - -- ..... ___._ _.___._.. . _
~ 1~ :~ , .. __ ........... . ~
.,
. .
~ - r~
~ _ N ~i N CO
~ ~ , ~ ..
CO ~ X ~ o -d ~ C~
. . ~ ~1 ~10 ", ~ L~
C/~ ~ ~ ~ ¦ ~" N~1
E E :~ E ~ v~ o ,"
U C`~ m ~~ oo
u t~ ~ t~
. I Ct~ ~ 11 W _
;~i ~ N ~ X Cl~ ~ ~ E35~ 0
:Z; C~i ~ _ ~ ~ ~ _ ~ ~
ct~ ~ ~ ~~O ~O N N ~ N
, ~ ~ C I X N 1~ N
C`O,.~ ' t~ oO 11~ ~o
Ei ~.u~ o - ~o u~
N ~ ~\r ~ ~ N
E~ El 11 11 1111 11
-
~C '
O I _N C )~ ~ C~
~ OO ~ ~
Cl)~D, N~IJ~~O C`J
00 ~
~ . _
:
C .. _
: O O OU~ C~ O
JJ U) Ir) N Ir~ CO
Q~ ~ CD ~ ~
O O ~ ~ ~ _~ ~
: ~ O O U~U~ O O
e u~ oo tD~ Lt~ N
~D ~ ït-- ID t-- 1~)
,,.U ~ ~ ~ _
~u c~ . . ____ . _
_l N
~' d l ~ 1
W C'~ .
. _ ~ . _ .
76
~ 5~7
. . _~.. ___ ~_ __ . ~_ __ ,
. ~ . ~C ~ ,_ ~ ~
_ N ~ ~~ _ N t~ ~ ~ C E3
0U~ 1I N ~ ~_IN ~ 1-- ~ N U~
~o .~ E ~ ~ ~3 ~ ^ ~ ~ c~ co ~ .
. ~ 3: ~ '' I ~ E ,, ~ c~ ~ E
~1 ~ ~ ~ _ U~ ~ ~ ~ 'J ~ N~i - ~D
E ~ tD ~ C ~ U7 ~ , ~D '~ a>I~
J~ ~ U~ ~ ~ N '~ t-- N t--
U U~ ~ X ~ C`l ~- ' C" ~.D - ~1
X cn C~ ,~ ~ a:) ~ c~ c~ ,_,
C2::~ _If) 0 ~ NC~ N ~C~
~ .,'' c~ 5~ E~ o ~,
~^ - , ~~1^ a~ ~ ~ u,, ~ ,
~`_ _ ~) ~ tD~t~CO _ oN ~D
COU~ ~o~11') t~
~ ^ ~ ~ , , ~ ~ ~ ~ ~ o, ~ '~
" ~ N ~~ N~t`l:l ~ ~~ ~ N ~r N N
COC~) ~Cl~ ~qCO~ ¦ ~ ~l ~ ) I
N~ ~ ~ ~ oo C~ ~ U~
C~J ~ 1C~ ~ ~ ~rcr~ _ ~\ 1
~ ~11 11~ ~ 11 1111 11 11
~ , ~ . ~ _ ~ ~'~
'~ i _ _ ~ ~ ~ ~ ,
I _ CO X I ,, ~ ~ ~ I . _ ~ 3~
O~ ~ Oc~ l~ N _ O ~_ ~ C~ ~
Cl~ ~ ~ O ~ ~ U~ ~ ~ O ~
~ D N ~ a ~ tn ~ co co a ~. tD
~ ~ ~ ~ U~ ~, _~ ~1 ~ ~ U~ ~ ~ 10 ~
_ _
: C O O O O O
r~ ~ ~ CD ~' U~ ~
~ ~ ~ U~ ~D (n ~o . ~D
O O ~ _ ~ _ _. _
Z ~ O- O- O- O- O O O
E ~ o~ ~ C~ Lt~ ID 00
_ ,t- Ir) ~ U~ ~ ~ U~
L/t) ~ _ _ ~ ~ ~ _ _ _
qC~-' ,
Q ._ _ _ _ _
R, d tD t-- CO CS~ O
~e _ , .. ___ c~ c~ .
77
~ . :
' ' ~'
~3~5~87
.. ~ ê ê
~ ~ _ N ~ ..
Cl') ~ ~ N r~
~C O ~ _ ~ . `
. ~ o ~r ~ ~
E ~ ~ ~ ~ ~ . . ~ c.
:~N ~ O ~~ U lo X O ~r
O. C~CO~ ~ ~C~ C- C~
~ ~~I~ C~11 Cl)~ _ C~ h
Z; ~ ~ ~ Ei c~ N
~ , a~ ~ P co c~ o N
: ' ~ ~ r _ N _ _ C`~ _ 10 0
~ 1 '~ 1 ~ ~ ~ ~
~: O `~ ~ ~ ~ O ~ ~ O _ N ~1
~_q 8 6 q ,
;: : : O : : O O O O
O 0 ~D . ~r CD U~ ~
~ b: _ _ _ ô O O O O O
. __ , . . . ~ _ . -- _ , _ . _
~' ~ ~ _ ~ ~ ~
~:
~: 78
.
`~ ~L3~4~'~
_ __ _~_ . _. .~ ~ ~_ _
N C' ) f3 Ei ~ C~l
N - ~ b ~ b ...... w
c~ ~ ~ . ~ ~i c~
3~ ~ C`l CO ~ U~. Il cr~ O ~, ,
~ U~ '~ ~ ~ ~ ~ ~ ~ .
_ ~ '~ r~ ~ ~ ~ ~ ~ _
~ ,. I _ b~~ ' b '`
~Z; ~ E3 ~ c~ E3 ~ , ~ r~ ~ c~
~ `' `-- ~i ~ O N W U~ W O ~ b~ ~ ~ b m
.- . w w E ~~ . w w E w .~ 7 o
~ ~ l~ l~ 11 ~ 11 ~ ~:C E O
~ _ N tq ~ _ ~ ' ~ _ lo o C~ a
O ~ I ~ O ~C ~ O ~ I O ~ I O I u~ ~
~ O L~ ~;r ~ _ c.~ ~ ~ o ~ ~ ~ Q c o ~D _
c~ ~ u~ ~ ~ ~ _, cr~
o ô o o o ... _ . . . _
~ n Z w ~o n n ~ I I
V Il~ N t:-- ~D
~ _~ _;l
__ __ ,._. .__ .. _
~ ~ tcO ,~_ 00 CO~
_ .~ .__ - .__ '__ _ .. _
: 79
.
.. -
~S~7
. _.. . ~ _. ..._ ~
~ . . N
e ~ t ~ ~ ~O
_ ,~ _ _ $ ,_ ~,
3 1 ~ ~t~ C- N Ln ..
~ ~ ~ ~ 3 ~ ~
, ~rco ~ ~ N 11~r . Il
. ~ ~ _ 5~ ~ ~ 1~ ~
,_,~r ~ ~ - ~r '' ~
E e ~, ~ ~ ~ m ~ ~tn ~
h O ~o _ tC ~ ~, _ U~
D: Lt~ ~ tD ~ Ot ¦ ~ ~ w
Z. ~ . N . N N L - ~
0~ ~ N ~ ~ t~
~_ N ~ ~ 00 5~ N ~ O O
$ LO ~O c~ ~ 00 , tD ~
l ~ N CD ~ ~N C`J ~ ~ ~ X
LD ^ ~ ~ tD ~ tn tD ~ O
_ tn co _ _. Il~ C`J --~ Ln In ~
X~ ~ ~ ~ ~ ~ I~
~ ~ _ ~ ~d _ ô ~ ~ ~ tO
I ~ ~ I ~ 1 ~
~N ~1 O ~ _ _ O ~ _ C~
U~ U~ U l
Q $ L_ ~ ~ tD tD _ 2 ta' ~ L_ ~
~ a) ~ tn C~ `~ C~ LD tD CO
~ 0 0 O O
r1 tD C~l tO LD
O O ~tD LD tD tD
U~ r~ .
r~ Z O O OO O O
E tD ~ tn :) tf) 00
m~ I ^ C- Lt~ ~ Lr~ ~ tt~
4 ~ ~: _1 _. ~~1 ~ : _~
~ ~:L
~: 'CJ ~- _ ,~ _ ...... _ .. , .
~ :~ O _~ C~
: :: ~_ _ ___ .__._____~
~ : ~ ~ :
:' :
s'~
--- - - - --~
_ , ~, ~ ~
. ~ ~ r~ ..
r ~ L~ ~ N
_ ~ ~ I I ~~ L _ ~
~~ C~l ~ _ ~) ~C`i ~ 5C r-l
~O ~ o cS.~ a~ O ~
r ~ ~ ~ ~ ,_~
E - ~ -- X - ~ N ~_ ~ ~ N ~
Ll E~ 00 `~ _ C~ ~ ~ ~-1C" I ~ r,
~J ~ C ~r ~ a) tD ~ r- ~ r- ~_
v~ d' ~ _r- ~ ~C ~ 11 ~;~ ~
~~ N ~ ~ _ /~ U~ Ei
N ~C~ ~ _ _ ~
~I ~ o - $ ~~ ~ ~ _
Z; . C~ ~ co û~ ~ r- r~ J r- _ co
~ _, ~ _ 11 ~ 11
~ - t 1 C~l CO ~ I
t~ _ ~- ~ CO .. ~
' r-~ O ~ ~ ~ N~ ~ ~ _
: ~~ ~ C~ U) r-
11 ~ r- r r 1~ 1~ 1~1~ 1
O
I ~ c I ~ r
O _ ~ O r-~ _ _ ~ _~
t~ O CO r-~ C ~ U~ CD r~~1 r . ~_ c~
c~ ~ cY~ u) t-- ~ c~ ~ ~f~
::
::: ~ :
~ ~ ô c~ O O
V Cl ~ C~ 1CNI CD C~J
L~ --~ CDIr~ . C~ IS)
O Or-~ _ ~1 ~ _
ZC) O O O O O
E Ll~ ~ tD O CD O
~a ~ t-- Ln r- c~ t- CD
10 ~ CEI r-l _ ~ _ r-l
L~
~ l . ~ .
~ Z ~ ~r .. ~r
.... _~.. _.__ ., _ _ . .
.
~ ,, ~ " ' '
'' ' '
"~ ~3~s~3t7
~~
_
CO ,, N M _ d'
N NC~ ~ ~ ~ N _ .~
N ._C~ _ ~ CO ~ N
t~ El ~ _~ '~ ~ ~ ~ , $ , ..
'~ U l '~ _~CO '~ _ ~ ~ N -- ~ . o~
~ ~ ~ 11 ~a m ~ ~
_ _ CO - $ N ~ t-- _ ` _
~ ~ `~,~ o~ N N _ ,~ `J -
ut t--~r~ ~ ~ 11 ~ ~e-- ~ ' 3
~0 ~ , O ,~
~ ~ ~ ~ ~ u~ ~ ~ ~ ~ .
1 ~ n
O C~ -~O $ ~ ~ ~ ~ ~
~ ~~ 00 o C~ r ~ ~ er ~
~ - ~ - cl~ ~r ~ ~) n ~
~ ~ - ~ - ~ ~ - - ~ -
.t~ ~ ~ 0 ~ - E~ ~ u~ ~3 t
: I ~~ ~ I~ ~ I $ $ X
~Ln-- -- -- ~t' ~~' d'
cn ~_ ~ u~~~, u~ , , u~
: ~ C~ ~ COc-:~ ~ ) d'~1~`d' O tD N
: : ~ tD tD tD ~ ~ O ~ NC-- ~ ~ CO tD O Ln
~ --~ mtD`~ tl ~`~ n t~
_
: C ~ .
o o o o o oLn o o
v tD C~ n _ CD N t-- _
CL -- tD IntD ntD Ln tD Lf~
O O _ _~ ~. ~ _ _
: : 0 ~ ¦ o o o O Ln Ln L~ Ln
I n a~ Ln O tc~ ~ t~ a~
E t~ t--tDt-- Ln ~ t~
h ~ ~ ~ _ _ _ ~ _ ~ , _,
~C'~
~ .
~ ~ ~ ~ ~ . ~ _
82
:~
.
.
'7
. . ~ .. .__ -
E ~ ~ ~
- ~ N E~ Il~--
N N N CO ~ ~ _
. l `- ~ t" L~ _~ _ tl:~ O ~
U~ U~ ~ ~ 11 -- _ 1 11 _ ~J
ct~ ~r '~ ~ ~r . ( '~ tD
N ~ _
F ~ ~~ ~ ~ ~ qtD r-- O
V _ ~~ ~ ~ O ~ _ ~C'~ ~ ~
U~ t~ _11 ~ ~_~ ~U~ C~ .~ .
N~ ~ ~ ~ N ~ ~ N
_ co1~) ~ E~a~c~i O _ . u~ ~ ~, CO ,
- COIf~ ~ ¦ ~ -COt~
o~ ~ _ _ a~ ~~ ~ ~ ~ ~ ,
~r N, t_ ~ ~11~--.1C~ ~D ' ~ U~ t--
: O ~ ~ 1 ~ ~ - ~E3 o E~ u~
~ ~ ~; ~ ~ I ~ $
~ I ~ r-~ ~ C~J ~ U~ ~ ~ O c~3 N
~ ~ ~ o ~ ~ ~ ~ ~_ tD ~. ~ o L~ $ N
~ ~ ~ u~ ~ ~ ~ ~~
C ...._. _ ..
.~ U~CO O O O O
: D :~ .--~ ~ l 1~ L~ t~ 11~
: ~ Z O O O o O O
~ ~ ~ . `1~ lo r- a~
': . ~ ..... ~....... ......
~: i~ O __ ~__ N a~ _~
' .
~ ' ' ` ' `" ` ` ' :
`` ~3QS~
. ~' ~C ~
~ o~
C -- l ~ N
. 03 ~ N U~
_tD ^ ` ~N ~ Ir~ t~
. 4 ~ ~ 'r ~~ o ~ ~ C_ ~r
C~:1 11 ll COt/~11 d ~ 11 ll
,~ 11 ~ ~
~ ~d ~ ~ ~ , , ~ _~ ~ ~ ~
~ ~ oo ~ ~ ~r ~ , a~ ~ ~
Z ~ , ~ ~i~Nt--
¦¦ ~ _ N~U~ ~ t~ '
~ ~ ? XtD C1~7
~ ~ ~ - ~
'`-- ~ N i:C NC'~N ~ ~-- ~ ~ ~ ~
N ~ d' U~ ~ a ~a~ X ^ _ _ U-) --
~ ~ '~ ~a .~ ~ 1~ 1~
~ ~ ~ ~ ~^
V X ~ ~ ~C I ~ ~C CO ~ ~ ~
~ ~ N O ~,,, ,~ ~I N ~1
: U~ U~ I
~ ~ ~ V
,~ ~ O ~ _ ~ ~D O ~ Q c~ ~ o
; _, ~ ~ ~ a~ ~ ~ ~
_ .......... ... .. ~ . _
~: ~ ~
.: ô o o :o o o
v 1~ Cl~ L~ N C-- N
1.1 ,_1 CD Ir) ~ u~
:: O O _l _ _ _ _ _~
~: ~ O 0~ 0 0- 0 _ _
'1:1 E ~ ~ N CO O tD O
~ ~ t-~D : t- ~D
C D. ~ ~ _~ ~ _
~ ~ _~ . ._.___
E Z Lt~ U:~ .. t_
._ . ~ .
_ ._
~: .
~t
~ ' ~ - ', .
,
' '
.
\
4~'7
_ _ .__
m ~ '' ~ ~ ~ e
~ N N ~ N ~ t_ Lr) Ln
co ~ 1 ~ 11 _ N ~ _ 11 _
. ~ _ ~ ~ ~ Ln _~ ~ $
E ~ ~Ln _ I _ CO
U ~r Ln ~ Ll~ L~ L ~ N CL~ o 11
D~ ~ N¦¦Lt) ~:~ ~ ~ ~ ~~,~ _ ~ _ _
~ ~D o ~ . O o ,_~ ~ _O ~ ~ _ N
~ 11 ~ ,, '~ 11 . ~ `~ ^ O `~ ~ a~
_ ~ , ~ , X C-- ~ ~ â
. ~ _ LX)_ _ _ _ N
O ~ ~ ~ Ln ~Ln C~ ~~DL~ Ln
~ ~ ~~` ~ 11 111111 CO
~; ~ _ ~ Lq ~ Lq N ~ ~ ~ _ ~ _ ~
~ I 1~ 0~ I ~Ln ~:~ I X
O ~ Ll~ ~ O C~~ p N ~ 0~1 _ N tN
CO Ln I ~ ~ :~;LOCO a~
Lna~ ~ P Lno> ~ ~Lnu~ o~
::~ ~ _ _ _
O Ln Ln O Ln OLn
v ~ N ~ N t--._
L1 - tD Ln ~D Ln CD Ln
0 0 ~ _~ ~ ~1 ~
Ln ô Ln ô ô Ln
: ~ E _ U~ O ~D O 1~ Ln
~.u E~ I ~ ~ .
C,"_ _
~ : . _
~ ~ _ _. _
8~
:~ .
.
.
.
3~5~7
~ __ _
. .
_
~D w ~ e
~ ~ ô ~ 'o oi .
--~ W ~: ~ ~ 11 :~ ~
~ ,~, E3 ,~ w .
. ~ ~
~ . ~ I 1I w w 1l X m ~ w
c~~ E3 - ' ~ ~ W
N ~ _ _ ~ _ ~ ' ~ X
- O ~ tD X ^u~ u~
~ -- ~~ _ - 0~ ~ ~Cr~' I ~ O
I ~ ~ û~ _ _ CO _ ~ Lt~
C~ N ~N 3C~ N N N'i~ ~C~N ~ ~ _
. ' ~ ~X N ~ ~ ~ ~ N ~ C N N N
1~ ~ C t- o ~ ~~ ~C
t- tD ~tD ~`J ~ ~ ~ ~Elco "~ O
c~ C~ --11 ~ ~ _ ~ Ll~ a~
11 11 11 CO 11 ~ ~1111 11 ~ 11 11 11
. ~ ~~ _ ' ~ _ _'~ ~ ~C~ '~ ~ '~
'I ~ ~ '~ ~ ~ ~ ~ ~ 'd ~ -' ~ ~ ~
O ~ ~ ~ I ~~ ~ ~ ~ I ~ 3 ~ ~
_ _ ~ _ O ~~ ~ ,-,~ O ~ ~P ~ C`l _
::U~ U~ U~
~ ~D ~ ~ CO ~ ~o CO t~ Cl~ ~0 ~ t_ O ~
~ ~ u~,u-) t-- ~ Cl) ~
: . __
: C
~; O U~ U~, O- U~
. t-- N 00 C`l
~ ~ tl~ t~
: O ,0 ~ _
~ ~ ,~ :1 _ _ _ .
11 E t~ ~
U1 ~- t- _ t~
H U)
n~ _ .. _ ..... ____
: : E ~ . . tO C`l
2i
._ ... __ , .... ~ _ . ... _.. _._.__ . ......... . . _
86
: ~
w m ~ m. W
_ I ~ m ~ "
E ~ N . N ~ N ~, 1:-- - N ~
m ~ ~ m O ~ ,0 ~ ~" O "
~C~ ~D -- -- ~ N ~ ~ _
~ I u~ ~ ~ rl ~
C~ O ~~ , ~ ~ ~.
N X N~C ~ 5 t_ 5
c~ U') CO - ~ 3 - 11 1I E3 c~
~ ~ ~ ~ '~ ~cî
~ D ~ ~s>D D ~_, ~
~ 1~ I~ $
O ~( _ N -- C:~N C~ ~ O N ~ U~
~ U) ~ U~
: :~3 'rO ~ ~ ~ Q N O '~
~ ~ ~`~~ d' U) a~
--I _
: _ _
O O U~ O ~ .
.,1 . CD N CD c~
~ ~- ~ tDI~ ~ , ~
~ r- ~D o ~ o
~ ~ V ~ _I _
:: : __ _
~ ~ ~ ~_
:
~7
' -': :
' ' '
````~ ~3~S4~'7
_ _
...._. ~ __...
C~ ~
N C~ r-~
,_ ~ N N ~ ~ U') ..
_ , . . 3 ~ _ N U~
In ~~
o~ a~ t-- N
. N ,;~ N~ 5
O~ ~ ` ~ U~
E ~ CD "~ -- ~ W ~ $ ~ ~ N ~
~ ~_ ~ ~ ~ ~
J N ~ m ~c O~ . ~
~ ~ ~ Nt~O ~ U~ W ~:i
Z; 'D ~ ~ ~ ~ a:J N $
11 ~ ~ ~ ~ D C~
~_ . X - N
. ~ ~~1!- Nt~~r ~ NtD ~ N O N
~ ~ N E~ ' ~ ~ ' ~~ C~l ~~ 11 '
: r ~ N~J ~I$ ~~ . ~ ~ ~ D
~ ~ _ ~1
: ~ O _ O N ~ O _ . ~ N
u~ I `~ cn ~ ~ ~ ~ u~
3 1 ~ I ~o ~ I I co ~o ~ ~ .D ~ o
m cn ~ ~ cn
~: C o o o U~ o o
.,~ : ~ ~ ~ ~ N ~S) N
: ~ ~ ~ tD ~
R ~ ~1 ~ _~ _ _ ._
0 Z U~ O 11~ 0 O Lt~
~D O U~ O
~1 E~ _ _ ~t-- ~D
a ~ ~ ---- -
: ~ : ~ d t-- : ~n
~: : ~ Z ~7 ~D
:, ~ ~ .. . _
: ~
`:
~3~S48~
_. _ ", _ __~
., ~ ~ .
C~ ~
N ~ N N I
~ U~ ~ ~ N
N~ , ~ ~ ~o~
1~ N ~ r--l ,t-- ~:J N u7
.~ N t~ ~ ~ ~
il ~ ~ ~ C~, 1~
~c~ ~ u~ ~ ~ ~;
. ~ , ~ 1- X ~
_ _ ~ cr~ ~ ~
E O~~ q Ei ~ ~D ~ t-- N ~ N
C~ ~ ~ ~
~ ~ o ~ ~ _~ o ~ ~
V~ U~ O C~; U~ ~ U~ 11 11
~ _ - ~D - ~ N ~
~ ~ ~ C ~ ~, D
_ ~ ~0 ~ ~ C~ X CO ~ ~
^ ~ ~ ~ ~ O~r
. . û~ ' î.~ ~ _ ~â .
~ ~D N _r~ N
C~ N ~ OOtD X Lt~
~~ . ~ ~ o~ ~r~ r
~ ~_ 11_I tY~ ~ r,r~ )
,~ .
~ ~ ê ~ ~ ê ~ ~
I ~ ~ ~ ~ ~ I X ~ X ~
O _ ~ _ ~ ~~ ~ O ~C~l N
C~ O ~ ~ rq ~ ,
~ t~ ~ CS~ r~ a~ O ~
tD ~D O Ll ~ Cl~ ~ ~D~ ~ 1
u7 r- ~ ~
~: : :
::~ :
O C~U~ O O
. t-C~l ~D tD
_~ ~ ~ ~D
O O _~ _
Z O U~ O O O r~
E ~CJ) Lf~rJ~ Ir~ C0
: ~:: ~ r~ ~ u~ ~ ~, ~ u~
~ ~ V ~ _ ~ _ _ _
~: . ..... _ __
: :~ :
~: . -___ ~ .
39
:: :
:~:
. , . .,: . . .
., ~.
` ~3~54~3~7
,. ~ _ ~ ___
, .1 _ N
_ X
~ m ~ ~
~ ~ _ N ll
C`~ ._ ~ ~ _ ,
U~ ê ~ ~ ,
e O ~ O~ ~ ~ E --
,~ ~ O ~D o U~ N
. X 3 ~ ~ N N ~ C~ 0 1 X i-~
~ ~ ~ , ~ ~ ~
L~ ~N N ~11 ~C~tD ~ Ei~ ~
~~ ~ ~,e ~", o - cn~ ~
. . u~ ~C" u~ ~rtD._ ^d
tL ,~ - ~ d~ ^ ~
u~ o~Il11 ~ ~ ~ 11 o11
c~:: , ~ m E~ ~ ~ ~ N
~ u~ ~ ~ x ~ 1 ,~ ~ ~ ~ ~
7 .c~ _ . .~J . r-~ .
) ~u~`~ t~
' ~__ ~D ) ~ ~ N
o ~ ^o ~ ~
. U~ tD~ U~ ~O ~ ~ .
mN,~ N ~ ~N ~ N ~'. ~ ~ _
' ~q X ~ ~ ~ 5 _~ ~ ~ $ ._
.
u~ u~ c~ QO m .
.
~ ~ ~ . ~
u~ ~ ~ ~ ~ ~0 ~ ~ ~cn
I ~ X ~ $ I I ~ ~ ~ ~
O .-~ ~1 N C`J O ~ --~ C`J ~ _ N ~ N
: ~U~O ~-- N ~ r- o r-1~~CO m ~ N
~: ~ cO ~ ~
C .
: O O O O O O
'V U') I~ U~ l:`J
~ ,~ ~D ~ u~ tD ~n
O ~ O ~ : _~ ~ ~ ~
:: rOZ OO OOO OO
~: E U~ tD ~ C`~ u~ 0
~ ~ ~ r~ t~ ~ u~
~: V ~ _~ _~ _ _ _ _
:: ~:
~ -- . ~ ..
~ ~ ~ E ~?i t_ t-- C--
~-_ .. ..... _
:
::
~3~
_ N _ ~ X
1 X ~ ,, ^ E ~ X
_~ ~ , Cl~ ~ _ N CO ~ c_
X C~ ~ C ~ ~ ~ c ~
. C" Ci). _D C~ ~ _ _ t_ ~ _ C~ ,
C~ ~ C`~~ - ~
Ll ~ , ~C`it-- 5~~1 .~ U~ ~ -
u ~ lâ'~CO O~ ~ _ ~ ~ N
v~CO ~1 ~ X _ Ei _ N ~ X
I:Y Cf l ~ _ U~ ~ X n~
~ I ~ . ~ ~ ~ ~ ~ o ,~ o
Z; ~r ~ Lr~ ~ c~ o ~
cq ~ ~ _ c~ C~J cn ~ .
^ N X N L~ A ~n lo ~ ~ o
o~ CO ~ ~ ~ 10 U~ .
t~ ~ $ ~ ~ N ~,$ N ~ 'a _ _.~ _
. ~ ~ ~ Lo co O Lr~ cn I ~ ? ~
~ I A I-- \ ~ ~ C O _--I C_
~ ~' ~ ~0 In C ~î ~ ~ C L E3 cn
o ~ _ b cr~ N N --I I ~ C C ~ C
? ~, ~, u~ u~
2 ~ ~; _ cq a) N ~ ~ N t-- O
p, ~D tD, _ ~~1 O t-- t- ~ C~ O c-- .
~ ~ cq ~ LO cn ~c~ Cq Lf~ U~ cn
~ o o O
V 1~0 ._ ~1:7 ~D
O. ^ ~D LO ~D ~D
L~ _, _ _ _ _
~ ~ O O-. O O O O
E Lf~ c~ LO clO 10 c~
~:' r- ~ ' ~ ~ ~ .~,
L, L~ ~ _ _ _ _ ~
~ _ __ ___.... ,
.~ ~ ~ . ___
~ d tD 1 _ Ct7
. ---~ ._._.. ' _.
'' ~
91
: ` ` , . .
c~
~-- ~ ~ ~
_ N ~ ,~ N
,_ a~ ,,, . ~ ~ ~ I ~ ~
co 11 ~ ^ ~ ~ ~ c~ ~ ~r ~
'Ci ~ t_ tC ~ - I Ei N
~` ~ ~ ~ ~'> _ o ~~ .
~ ~ CO N ~ ~ N N r~ 5~
Z _, _tq ~, 11 .-- N t N _ ," _ O
: ~ co ~ t~ ~ ~
. _ N ~C~ - ~ c~ _ ~ N T--~ ~ N N
. ~ _ ~ ~I N~-- ~ N X ~
o ,. ~ ~a~ ~ ~.~ a~
~ tD CO~ ~ ~~_ _
~ t~ ~ 11' ~ ~ N ~ U) r-l
'
I ~ ~ ~ I U)
~ ~ C~ ~ ô ~ ~ ¦ 0 7~ N ~ ~
~ N ~ ~ ~ I ~ ~ O
~ ~r tD, N ~ c~ _ OO ~ D ~ N t~ _ c~
: ~ ~ ~ ~ ` ~ tl~ ~ CO
~ . ~
~ ~ C ~ ~ ' _
'V ~ 0 0 O O
O ~ ~ ~
: : ~ ~ O- O- O- O
c1 E _ _ t-- lo
: h U _
:~ : ~ _
~,
d r_O _ _ ____ _
~ ~ 92
; .
.
~ ~3 ~7
_. . _ j,.... -- , ._ . _.
_. N ~:C ~
~ - ~ .. _ ~ ~ O .
~ .~ ~
~ '~~ --' ~ ,~ N
c~ ~, ~ ~r u) ~--
c~u~ cO ~ r
t-- _~C~, ~ ll N ~ N
~_ U~ _ a~ ~ O ~ _ "
~O m ~ ~ ~. m . ~ m m U~
E .~ ~ _ O r~
v, ~ ~ ~ t~ ~n _ ,~ _ 11 ~ o
~ ~ ~ ~" ~ ~ m m ~, o m w
. X ~ ~C 11 X ~J ~' ~ a~
~ ~ ~ m m~ m I m ~ m ~~, m m ~
r~ ~ ~ ~ ~ ~ ~c~
~ ~ ~ o ~
: ~ ~ ~ .
~ In ~ ~ ~ cn ~ ~ ~ co
~ :
c: :o o ô o: t~ o
. t-- C`;l tD C~ tD C~
h . I --I ~ U) O ~ ~_
tO c~ . tD O tD a~
L~ v ~ t-- ¢~ ~ tD t-- U~
~ ~ èl ~I _ _~ j _ I .
~ ~ ~ .~ ~o
': ~
93
: : :
...
_ _,, _ ~...... _ _ _ ~ ____
~ ~. X ~ .
E3 ll ~ '
~ ~ N ~--
~ 1I m N ~ . O ,~ _ ..
m ~ 1I w E m ' m '. ~ ~ m
O _ ~- ~ ~ --
0`7 ~ t_ ~ ~ ` ~C) ~ . ,~
~ ~ 3: X C~ ~ ~
Z --~ N '-- --~~ C~ ~ ~ N ~
X, o ~) U~
. u~ U~ t_ - ~
~ m m ~O m ~... 9 ~ :
E3 ~ ~ ~ ~ ~ ~ ~
~d _ _ _ _ _~1 o U~ d
Oc~ ~ ~ I'd
U ~ ~` I C/:l ' ( --' '
: ~ , ~ V o00
` ~ L~ ~D 00 ` U~ ~D ` ~ Ll~
_
C O U~ O
~r~ tDU~ l ~)
'~:1 E _ U~ cn Ir~ a)
~:U ~ _ _ _ ._
` ~ ~ ~ 11,
.~ . _
~ ~ d ; tD CO
2~i _ . ~ _
:
94
:
,
~3~5~
.. ._. ._ . _ ~ .. ~
3:~ , ,_ . N
~ ~ ' ~, ,~,
_ U~ Lr~ ~ ' CO
~ N ~ .
a~ , : ~ , co
m ~ :C ~ E ~ C C`~ ,
C~ X tD ~ _ ~ C~ X '~ ~ ~
E O Lf~ c~ _ E~ ~ ? ~p
:~ . L 11 ~ ~Lt~ N ~ ~ L~
~.~ C~ ~ ~ D ~ U~ _ ~ _
L-L U~ ~ _~ , L~_o Ln N N ~ N
n ~ ~ N _ C~
; ~ Z N _ ~ O O ~ ,
: ~ 11 E3 ~ l ~, ~ ~ ~ ~ ,~
~ ~ 00 , 3 ~ _ _ ' ~' ~ .
. L~ ~ ~,,, O ~> o ~ O
~ ~ 11 tD ~ ~D Ln ~ ~4 -- ~
. Il 11 ~ ~ ~ Ln ~ N
'~ ) ~ C~
~ . ~ ~ ~ ~ ~ I a c c ~C c
O ~ _ C~ O _ ~ ~ _ O (d "~
u~ ~ ~ , u~ cq ~,
~n ~ ~ o ~ ~ ~ ~;r ~ a~
O, u~ ~ ~ u~ o, r- ~ ~ o~ tD O tD
"' : ~~U?CO ~~)Ln Lt) O~ --' N ~ In U~ ~
:~ ._. _ .
~ : :C
~ O L; L~ O U~ O O
V Ll-~ N It~ N I~') ~
P~ ~U::~ Ll~ Cl:~ Ll~ CD L~
O O , _ _ _~ _ ~
: ,a 2 Ll~ O Ll~ O O O
e Lr) cn ~ ~ CD a~
~:: : ~" r^
:~ 4V ~ ._1 _ _ _~ ~ _
H U) ~
-- _ ._. ~ .__
~` _ C~
~: ~ : ~ ~ . _ _ . _ _ . . _
~'. ':;
: ~ 95
:::
::
~3~3~
_.. _ ~ ~ .. _ ~
~ ~ ~ . ~
_ CN ^ --~ W X
'~ ._ ~ X ~ ~
~:)CCI , ~ _ ~ N tC~ 1~ CO ..
_ ~ E~ ~r ~r ' 1I N t--
(D ~ 11 ~ C~ ~ ~, N
`-- N ~ ) ~ C`~
. ~ CO N ~i' ~1 ~D ~1
o _ ~ ff _ - ~
0 ~~ X o,~ ~/ COC~ ~
~ ~co ~ l ~ 11 ` "~
~ ~ o _ ~ ~, E ~ I ~
.'N ~ ~ ~ ~ U~ _C~
~' 1-)a) ~ t-.-1 o, ~ ~ _,
o ~ ~ ~D ~D CO CO ~
~ C~ 11 X ~ ~ 11 11 11 C~
,~ ~ ~ ,~ ~ râ ~ ~â to
O ~ ~ 1; I ~ ~ ~ I ~ 3~
cq N _ N O _ ~ _ ~ ~ C~ C`l
_ D C~ ~ ~D O I O ~ ~ O 1-- N
~ CO ~ ; ~ ~ ~
_.. . . .. _ . .____
: C O O 10 0 O
V U') t~ t~ C~ tC~
C\. _ ~ U~ CD U~ U~
o: o ~ ~ ~ -I ~-1
Itl Z O O O O O IS:~
CI E tD a~ tC~ a7 ~D
~J ~ ' ^ t- Lt) t- U~ t~
L,:U ~ ~ ~ ~ _ ~ _
: ~ ~
~: ~ : '_~ ,_ .~._ ~ ,.. __. .....
~: E _ _ _ . . _.. _ _ .
~ ~ 9~
: .
:
. .
~ 3 ~
_ . .
, _ _ m m m m
_ r~ ~ ~ ~ , _ _ ~
^U~ ~ '` ~ . ~ ~ ~ 11
CO C`~ - Xil ~ l
~3 ~ N1~ ~ t~ ) co ol ~ ,~
~O ~ ~ ~ ~ l ~
_, O ~ _ m m ~ m ~ ~ m m E
~~ E ~ ~1~ ' - m ~ t~ 11 o m
U~ _ _ U~ _ ~ ~ ~ ~ --' _
c~ ~r o, ~ ~ ~ o _ . ~_ ,, ~ ,
~ m u, ,, ~O ~ m. ~, ~ ~ 'D 1l m m ~ ~
N ~ N ~ 1~ N LO N ~D ~ N ~ N ~D 5 N
`-- M ~ M ~ ~ 3 ~ `-- N ~ C~
o~ c~ ~ oO ~ ~'U~ ~~ ~ r
O ~ ~ E; C`J a~ U~ IJ ct~ U~
11 11 ll 11 ~ 11 ~r 11 a> c~
,~~4 ~ ~ .
,~ ~ ^ D~ n , ~ ,~ ~
x ~ x
~ ~ ~ C~ O r-1 1 ~ O ~ t~ O C~ N
~ o ~ ~; o ~~1 ~
O1-- r-- ~~D ~D ~ ~ N ~ cn ~ ~
C~ ~ t-- ` c~ 1~ CO ` N ~ t--
. _ ... _ .. _ .. _
C ~
0 O O O O U~ O O
JJ <S N C~ N ~D C~1 ~
U~ CC: U~ CD m ~D
o o _ _ _ _ _ _ _
o- o o~ o- ~ ô o
~ E CC a) tD 0~ ~ ~D O
:~ Q~ ï t_ U~ r ~ C~ ~ ~D
~,,t) ~ _ ~ ~ _~ ~ _ ~ _.
_ .. _ .. __
. .
~ ~ ~................. .
::~
:
:
97
::
?5~
--_.._ ~ _........ _.. _ __
~ ' . N
~D t_
ct~ _ --~ N _~
'-- X ~ ~ ~ ~ 00
~i I , ~ W ~ ..
_~ ~ 11 ~ ~ 1I C~ N
_, a~ ~ _ ~ ~ In
. ~ _ N WC~ , _ _
ECl) ~ ~ ~ ~C`~ U:l N ~q N ~ O
~ _ - _ _~' _ _ O~ N _~
V) ~ 11 û~
R: ~ ~ , ~ U~~ ~ N ^
. _ CO _ N ~ ~ C`J
C`~ ~ ~ 11
u~ ~ '~ ~ ~ ( co
tn ~ u~
X - ~ ~ ~ ~ o ~ U~
. . c~~ ~ ~ , ~ ~ ^~: N~ ~ u~ N
CO N ~ XCJ> N~C ~ ~ O O~ O --' ..
C`J _~ ~ CO N ~ U~U~ ~
1111 11 11 .Il 111111 11 ' 11
'~ '~ ~
~ ~ ~ ~ ~~5 ~ U~ ~q
O ~ $ ~ $ I ~ I ~
C ~ _ O _ ~I C`J _ O ,_ N --I
~ , , u~ , cn
:Q ~r O ~ t ~ ~ ~ ~
_~..... .. . .. ._
'
o o ô u, ô o
V tl:~ ~D N t-- N
~ a z ô L~ o ô
E t~ O ~D ~ ~
aJ ~ _^ ~ - ~ L~ ~ Lr)
h 1~ 1~ ~ ~ . _ _ ~ ~
~Ha' :
_ ._ _ ,
~ ~ C~ ~ oo
~' 1~ CJ~ C~ . _, .
_-' .. ,_ . _____ ._ ......... . ~
.
98
:
~3~J54~'7
. ___ ~ - ._.. _.__, _ ___._ ___
r~N , ~ X m E
m m~ N , 'O ~ ~_ ,
,0 mN m ~ ~ ~ m m ", ~D 1' m m
. .~ ~ ~ L~l ~ _ 11 ~ '-
E ~ ~~:1 L~ ~ Lt~ 1I Ll~ ~ _ ~ Lo
~ Lt~ ~C 11 ~ ~ C ~ ~ g ~ ~ N ~
~ ~~ 0,_ ' m~ ,~
) ~n ~1 ,_ ~ ~ Lt~ ~ N t'' ) U) ~ Ln
( ~1 ~ - Lf~ ~ _
t~ L~ ~ C~ N ~ E~ - _
. r I O ô~ O r~ C`J O _ r ~ o 3
~l~i _ I,i tD _ ~ , ~D ,
. . ~ N ~q N --~X ~ ~ C ~ ~ ~ --~ ~ N
C~ LO ) 00 C~ t~ Ln ~ 1I N Ei ~ t_ CO
~ ~ ~ ~ ~ ~ ~ ~ !~ ~
: ~ ~ ~ ~ ~ ~ r~ n
1 ~ ~ ~ X ~C ~ I C ~ - ~ $^
_ ~ ~ l ô ~
o t ~ Q _ co ~r ~ ~ t~ o ~' ~ ~r 1
~ L~ L~ ~ ~ Lt~ ~ ~ ~ ~ L~ U~
~ . .. __ ........ . .
o o o ô o o
Ln a~ ~D ~
~ u~l ~V Lr~ ~D ~D ~
: ~ R :1 0 0 O O O O
'U E _ C~ t~ tD a~ Ln C`~
- h h ~ t-- ~D t--Ll~ t--
~ ` ~C~ .
~ ~ ~ _ __ . ,___ .~., _ __ _._
~ o N O O
~ ll~i_ . _ I
: : . _ . _ .. . .. ____
99
.
.. , :
48~
1 ~ ,_ .,. ..~ .~ ,
, _.1 ~ N N N
- .~1 ~N , 1:_ r-- LO W
L -
.~ _ ~~
. X~ o ~11~q ~cn ~ ~ ~ C`J
EN -d' - ~ ~N _Ln O Ot) O
U~ ~~ ¦¦ ~~ U~
o, _I . ~~ ~ _ ~ ~ _" ~r w
~ cr~, _ ~ ~ ~ 'r ~ U~
Z ~ _ ~ X ~L,i E3- ~i ~ ~
C~ ~ ~ ~ . _ ~; ~1
U~ û~ ~ o ~ ~ ~ ~ w ~ ~ ~o
, ~ N ~ ~ ~ _ ~ ~ ~ LO ~ N ~ N N
0~ N N ~ CD ~ ~ I ~ ~ ~_ LO ~ ~ ~
_, . o ~) c~ t- t- c~ '' E3 tD
cr~ _ LO ~ ~ ~~ a) ~ ~ ~ c~
~ ~ ~ .'~ ~ 1I E .
_ _ _ _ ~ ~~1 ~ h _ _~ -
, ~ ~ ~ ~ ~ ~ ~ ~ I ~
; ~ _ C`J ~ O ~r-l ~D C`~ C~3 ~
~ ~. ~0 ~0 L~ ~ ~ O ~ ~o C~
_, ~ LO t-- ~ ~ LO ~ ~ L~ ~o --~ ~ Ln ~ o~
_ ~ ... ___.
: : C . O LO
CD ~0 ~ Ln
~ O O O O _ _
~ E ~ ~ ~ tD a~
U ~ _ ._ _
~: ~_ _.__ . .. _ ... __ .... __. ..... _._
:~ : ~ ~ O _ t-, . O
: : 100
~ .
'
.. .
,,,,~ ~ 3~?S4~7
~ ~ .~ ~
~0 u~ ," , ~ ~ ~ O ~ ~
. ~ ~ ~ ~ o C~ ~ N
N 00 tD ~,r~ O
~ U ~ ~ U: o~ ô ~ ~ ` ~
~ ~ ~ ~ ~ ~ N 11 ~ '~ O C~
Z o ~ ~ ~ ~ X ~ ~ ~ ~
~r ~ ~ 11 N ~ll ~ ~ M
,. ~ - ~ N a~, ~ ~ ~ ~q O .. ~ _
N ~ I C --~ ~NC`~ ~ N ,~ ~ ~ ~
C'~ ~ ~ ~ U~ CO ~ _ 1~ ¦¦ tD N ~ _
11 11 11 11_11 ~1 11 11
, ~ ~ ~ '~
I ~ J
~ X O ~ ~ ~ X 1~ q ~
~N _ _ N _ _ O ~I N _ N O _ _
o~ ~_ U~ U~ Cl~
~ O ~ ~ 1 ~ o ~
~ ` Il~ a) ~ co ~
--- . .~ .
~ rl - ô o u~ o
t- tD C~ U~
t~. - tD U~ tD U~ U~
o o o o o - - - -
Q~ h 7 tD _ N t-- CD ~D
: . ,,, ~ _ _ .~ _, _ _ _~
ql~ ~
.__ .~__ ._ .___ ~
O ~ t~ C`l
. ~ . . _
-~ --- _ . __ ___ - _
~ . 101
. ~ ~ ,,
, ~ ~ -- ~
- ~ N a1 ~ -- 11 - . ~ ~ tD ~ ~ O
aa 3 1 `~ ~ , ~i N 3 ~ _ ~ ~
. ~ ~ D _ ~ N E~ ~ X
E3 ~ X o CO N ~ ~ ô ~ N N ~ , N
,, ~ m '' ~ ~ m ~I m m I m m
~ cn D1I N ~ ~ ~ 1 ~ N
Z; m ~ ~ . m ~ ~. m ,~ ~
. `-- N C-- ~ ~ ~ ~ _ _ N N N C~ .
c~ 3 ~ ~ ~ ~ cr~ ~, O N .
i ~ 11 ~ li 11 11 3
. ~ ~ ~ ~ ~ ~ ~ _ ~~ ~
O ~ N _ O ~, ~ ~ X O _ I N
~ ~ N N ~ ~D ~ O~ I ~ a~ $ ~ N O O
~ ~ c~ ~ ~ ~ ~ Ir~ 02)
C ._._ ... ___ ..
: .,1 ~ .. t- O
: ~, _ tD ~D ~D U~ ~
0: 0 _ _ _~ _ ._
: ~ Z O ~ O O O O
~I E _t-- U~ t-- c~ t-- ~D
~'~ ~cu'a,~ ~ ~ ~ _ _, _; ...... _ .____ ._ ......... ___ - ... __ ..
~:~ ~ ~ . - ~ _ .
~ . 102
': ' . ' .. ~. ~
. .
;~3~ 87
_, ___ N
, ,_~ O
00
` N
U~ ~ ~;
~ O ~
~ ~ ';
' ~ $ ~
11 ~ CO
`~
U~ ~
O O m ~c
~; O ~ ,_ ~
~ O ~ t
. _ ~ .
a
ô lS~
: 1:1 E ~_ ~ o
H _.
~ ~ ~ :
:
. ' ~
'
:~3~:~S~8'7
Experiment 7 (Synthesis of Raw Material Compound):
p-methoxybenzyl jB- ~2~(5-tritylami.no-102,4-thiadiazol-
3-yl)-(Z),2-[2,2,2-trifluoroethyl)oxyiminoacetamido3-
3- L(Z)-3-chloro-1-~ropen-1-yl~3-cephem-4-carboxyla-te:
~ N \~-C--CON~ S e
~S~
OCH2CF3 ~2~0CEI;
A mix-ture solution comprising dimethylformamide
(0.247 ml) and -tetrahydrofuran (3 ml) was cooled to -10C,
and phosphorus oxychloride (0.297 ml) was added thereto and
stirred for 40 m.inutes wi.th ice-cooling. To the resulting
solution was added the tetrahydrofuran solution (4 ml) of
2-(5-tritylamino-1,2,4-thiadiazol-3-yl)-(Z)-2-(2,2,2-
trifluoroethyl)oxyiminoacetic acid (1.36 g), followed by
stirring for fur-ther one hour at the said temperature.
The resulting reaction solu-tion was added to a mix-ture
solution comprising p-methoxybenzyl 7B-amino-3- t(Z)-3-chloro-
l-propen-l-yl~-3-cephem-4-carboxylate hydrochloride (1.145 g),
N-(tri.methylsilyl)acetamide (2.09 g) and ethyl acetate
(10 ml), with cooling at -20C, followed by e]evating the
temperature up to 0C with s-tirring for one hour. After ethyl
acetate was added to -the reaction solu-tion, this was washed
: ~ :
~ with water and dried with anhydrous sodium sulfate~
~ : 10~
: ~
.
'7
The solvent ;~as evaporated out and the residue was purified
by silicagel column chromatography to obtain the desired
product (1.43 g).
Example 118
7B-~2-(5-~nino-1,2,4-thiadia~ol-3-yl)--(Z)-2-fluoro-
methoxyimlnoacetamido~-3- t(E)-3-(carbamoylme-thyldimethyl-
: ammonio)-l-propen-l-yl]-3-eephem-4-carboxylate:
N~-C-CONH ~/S CH,
boo
The compound (500 mg) obtained in Experiment'
4 was dissolved in a mixture solu-tion comprising
methanol (3 ml) and di.emthylformamide (l ml), and N,N-
dimethylglyeinamide (71.3 mg) was added thereto wi-th ice-
cooling and then stirred overnight at room temperature.
The resulting reaction solution was added to a mixture
solution comprising ethyl aeetate (5~ ml) and ethyl ether
~. ~
(50 ml) and the preeipitate formed was eollected by filtration
: and dried to obtain an yellow powder (382 mg).
This powder: was added to a mixture solution
: comprising trifluoroace-tic acid (2.7 ml) and anisole (2.3 mll,
~: ~: followed by stirring for 2 hours with iee~cooling. The
::
.
: :
105
:; : :
.
3~3~ii4L~'7
reaclion solution was added to a mixture solution comprising
ethyl ether (25 ml) and isopropyl ether (25 ml), and the
precipitate Eormed was collected by filtration and washed
with ethyl ether. The thus--obtained precipitate was suspended
in water (4.5 ml), followed by adjus-ting the p~l of the
resulting suspension to the range of Erom 5.5 to 6.5 with
sodium aceta~e, and -the insoluble subs-tances were removed
by Eiltration. The filtrate was purified by reversed phase-
silicagel column chromatography to ob-tain the desired product
(94 mg).
Example 119
7~-~2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyiminoacetamido3-3- r(E)-3-(1-methyl-4-sulfamoyl-
l-piperazinio)-l-propen-l-yl~-3-cephem-4-carboxylate:
N ~ -
H2~S N`oC~ F ~LN~ , ,N ~N SO2N~
COO-
The compound (500 mg) obtained in Experiment
4 was dissolved in a mixture solution comprising
methanol i3 ml) and dime-thylformamide (1 ml), and N-
sulfamoyl-N'-methylpiperazine (116 mg) was added thereto
with ice-cooling and then stirred overnight a-t room tempera-ture.
: ~:
106
: ::
~3~59L87
The reaction solutlon was adcled to a mixture solution
comprising ethyl acetate (50 ml) and ethyl etller (50 ml),
and the precipitate formed was collected by filtration and
dried to obtain an yellow powder (402 mg).
The powder was added to a mixture solution
comprising trifluoroacetic acid (2.8 m]) and anisole (2.5 ml)
and stirred for 2 hours with ice-cooling. The reaction
solution was added to a mixture solution comprising ethyl
ether (25 ml) and isopropyl ether (25 ml), and the precipitate
formed was co]lec-ted by filtration and washed with ethyl
ether. The resulting precipitate was suspended in water (4.5
ml), followed by adjusting the pH of -the resulting suspension
to -the range of from 5.5 to 6.5 with sodium acetate, and the
insoluhle substances were removed by filtration. The
Eiltrate was purified by reversed phase-silicagel column
chromatography to obtain the desired produc-t (58 mg).
Example 120
73- ~-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyimi~oacetamido]-3- ~E)-3- ~(1,3,4-oxadiazol-2-
yl)methyldimethylammonio3-1_prOpen-l-yl~-3-cephem
4-carboxylate:
, ~ ~q CONH~ ~ CH,
OO CH3
107
~3~5~'7
The compoulld ~50!~ mg~ obtained in Experiment
4 was dissolve~ in a mixture solution comprising
methanol (1 ml) and ethyl ace-tate (4.8 ml), and the ethyl
aceta-te solution (1 ml) of 2-dlmethylaminomethyl-1,3,4-
oxadlazole (88.8 mg) was added thereto wi-th ice-cooling and
then stirred overnight at room temperature. The resulting
reaction solution was addéd to a mix-ture solution comprising
ethyl acetate (~0 ml) and ethyl ether (50 ml), and the
precipltate formed was collec-ted by filtra-tion and dried to
obtain an yellow pow~der (443 mg).
The powder was added to a mixture solution
comprising trifluoroacetic acid (3.1 ml) and anisole (2.7 ml)
and stirred for 2 hours with ice-cooling. The resulting
reaction solu-tion was added to a mixture solution comprising
ethyl ether (25 ml) and isopropyl ether (25 ml), and the
precipita-te formed was collected by filtration and washed
,
; ~ with ethyl ether. The thus-obtained precipita-te was suspended
n water (4.5 ml), followed by adjusting the pH of the
resul-ting suspension to -the range of from 5.5 to 6.5 with
sodium aceta-te, and the insoluble substances were removed by
filtration. The filtrate was purified by reversed phase-
sllicagel column chromatography to obtain the desired product
(92 mg).
:
xample 121
7B-~2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z~-2-fluoro-
~:
~ :
108
::
.
~ 3~t3~
methoxyiminoacetamidoJ-3- ~E)-3-(1-methyl-9-carbamoyl-
l-piperazinlo)-l-propen-l-yl~-3-cephem-~-carboxyla-te:
N ~ N ~l CONHz
COO
The compound (500 mg) ob-talned in Experim'ent
4 was dissolved in a mix-ture solution comprising
methanol (3 ml) and d;methylformamide (1 ml), and N-methyl-
N'-carbamoylpiperazine (100 mg) was added thereto with
ice-cooling and then stirred overnight a-t room temperature.
The reaction solution was added -to a mixture solution comprising
ethyl aceta-te (50 ml) and ethyl ether (30 ml), and the
precipitate formed was collected by filtration and dried to
obtain an yellow powder (425 mg).
The powder was added to a mixture solution comprising
trifluoroace-tic acid (3.0 ml) and anisole (2.6 ml) and stirred
for 2 hours with ice-cooling. The reaction solution was
added to a mixture solution comprising ethyl ether (25 ml) and
isopropyl ether (25 ml), and the precipitate formed was collec~ed
bs~ filtration and washed with ethyl ether. The precipitate
was suspended in water (4.5 ml), followed by adjusting the
pH of the resulting suspension -to the range of from 5.5 to
6.5 with sodium acetate, and the insoluble subs-tances were
:
:
109
~ .
13~
removed by filtra-tion. The filtrate was purified by reversed
phase-silicagel column chromatography to obtain the desired
product (107 rng)~
~xamp]e 122
7B- ~2-(5-~nino-1,2,4-thiadiazol-3-yl)-(Z~-2-fluoromethoxy
iminoacetamido~-3-((E)-3-(1,2-dimethyl-1-pyrazolidinio)-
l-propen-l-ylJ-3-cephem-4-carboxylate (Isomers: A and B)-
M~ CONH~S CH,
H2~S `0(~I2F c~ N~\6~/+~ N
C00 CH~
The compound (783 mg) obtained in Experiment4 was dissol-~ed i.n a mix-ture solution comprising
; me-thanol (1.5 mll and e-thyl acetate (7.1 ml), and -the ethyl
acetate solution (1.5 ml) o~ N,N'-dimethylpyrazolidine
(103 mg) was added thereto with ice-cooling and then stirred
overnigh-t at room temperature. The reaction solution was
added to a mixture solution comprising ethyl ace-ta-te (50 ml)
and ethyl ether (S0 ml), and the pre~ipitate formed was
~ ~ collected by filtration and dried to obtain an yellow powder
- (631 mg).
The powder was added to a mixture solution comprising
trifluoroacetic acid (4.5 ml) and anisole (3.8 ml) and s~irred
~ ~ for 2 hours wi-th ice-cooling. The reaction solution was
::: : ~ :
:: :
110
: ::: ::
'
'
:
~3~ S ~L~ 7
added to a mixture solutiotl comprising ethyl ether (2S ml)
and isopropyl ether (25 ml), and -the precipitate formed was
collected by filtration and washed with ethy] ether. The
precipitate was suspended in water (4.5 ml~, followed by
adjus-ting the pll of the resul-ting suspension to the range
of from 5.5 to 6.5 with sodium acetate, and the insol~ble
substances were removed by filtration. The filtrate was
purified by reversed phase-silicagel column chromat;ography
to obtain the desired isomer A (37 mg) and isomer ~ (27 mg).
Example 123
7~-t2-(5-~nino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyiminoacetamido~-3- ~(E)-3-(1-me-thyl-4-formimidoyl-
l-piperazinio)-l-propen-l-yl~ -3-cephem-4-carboxylate
hydrochloride:
N ~C- CONH~ S ~ (~3
H,N `OCH~F (r~ N ~I-C~ H
The compound ~750 mg) obtained in Experiment
4 was dissolved in a mixture solution comprising
methanol (4.5 ml) and dimethylformamide (1.5 ml), and N-
methyl-N'-formimidoylpiperazine hydrochloride (l5a mg) was
added thereto with ice-cooling and then stirred overnight at
.
,,
: 111
~ .
room temperature. The reac-~ion solution was added to a
mixture soluti,o~ comprising ethyl acetate (50 ml) and ethyl
ether (3n ml), and the precipi-ta-te formed was collected by
filtration and dried to obtain an yello~ powder (485 mg).
The powder was added to a mixture solution
comprising trifluoroacetic acid (3.4 ml) and anisole (3 0 ml)
and stirred for 2 hours wi-th ice-cooling. The reaction
solu-tion was added -to a mixture solution comprising ethyl
ether (25 ml) and isopropyl ether (25 ml), and -the precipitate
~ormed was collected by filtra-tion and washed with ethyl ether.
The precipitate was suspended in water (4.0 ml), and the
insoluble substances were removed by filtration. The filtrate
was purified by reversed phase-silicagel column chromatoyraphy
to obtain the desired product (58 mg).
In the same manner as Examples 118 to 123, the
cornpnunds of the following Examples 124 -to 140 were synthesized.
Example 124
7BL2-(5-Amino-1,2,4-thiadiazol~3-yl)-(Z)-2-fluoromethoxy-
iminoacetamido~-3-[(E)-3-(l~methyl-1-piperazinio)-1-
propen-l-yl~-3-cephem-4-carboxylate:
N--C-CONH
H~N S ~ F ~
COO-
.
~ ~ 112
The compound (750 mg) obtained in Experimen-t
4 was reac-ted with N-methylpiperazine (116 ~1),
ollowed by removing the protective group, to obtain the
desired product (16 mg).
Example 125
7B-~2-(5-~mino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyiminoacetamido~-3- [(E)-3-(4-carboxypyridinio)-
l-propen-l-y~ -3-cephem-4-carboxylate: .
H2N S `OCH2F ~ N~COOH
COO
The compound (750 mg) obtained in Experiment:
4 was reacted with isonicotinic acid tl98 mg), followed
by removing the protective group, to obtain the desired
product (143 mg).
Example 125
7~-~2-(5-Amino-1,2,4-thiadiazol-3 yl)-(Z)-2-fluoro-
methoxyiminoace-tamido~-3-~E)-3-(1-methylpyrrolidinio)-1-
propen-l-y~ -3-cephem-4-carboxylate:
H2NJ~ S ' N~
boo-
:,
~;
113
:: :
3..3~
The compound (500 mg) obtained in Exp~riment
4 was reacted with N-methylpyrrolidine (55.8 ~1),
followed by removing -the protective group, to obtain the
desired produc-t (21 mg).
Example 127
7B- t2-(5--Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyiminoacetamid~ -3- [(E)-3-(4-carbamoylpyridinio)-
l-propen-l-yl~-3-cephem-4-carboxylate:
M- ~ C-CONH r S
HzN S N~ocH F~rN ~ vN~ CO~
COO
The compound ~500 mg) obtained in Experiment
4 w~s reacted with 4-carbamoylpyridine (131 mg),
followed by removing the protective group, to ob-tain the
desired product (42 mg).
Example 128
7B-~2-(5-A~nino-1,2,4-thiadiazol-3-yl)-~Z)-2-fluoro-
methoxyiminoacetamido3-3- t(E)-3-(trime-thylammonio)-
l-propen-l-yl~-3-cephem-4-carboxylate:
N~C-CONH _,S CHJ
/~ S' ~ CH~
H2N 1 `O~I F 0~ N~\~/CHJ
::
:: :
~ 114
:
~ - ~
~ . .
~3~ 4~ ~
The compound (500 mg) obtained in Experiment
4 was reacted with trimethylamine (whcareupon tri-
methylamine hydrochloride (57 mg), as neutralized, was used),
Eollowed by removing the protec-tive group, to ob-tain the
desired product (79 mg).
Example 129
7B- ~2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-~luoro-
methoxyiminoacetamido~-3- ~(E)-3-(1,4-diazabicyclo-
~2,2,2J octan-l-io)-l-propen-l-yl~-3-cephem-4-carboxyla-te:
HtN S ~`OC~ F O~N~\~/N'/\--
00
The compound (500 mg) obtained in Experimen-t
4 was reac-ted with 1~4-diazabi~yclo~2~2~2~octane
~72 mg), followed by removing the protective group, to
ob-ta:in the desired product (61 mg).
Example 130
7B- ~2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoromethoxy-
iminoacetamido3-3- ~(E)-3-(1,5-diazabicyclo ~3t3~0~ octan-
l-io)-1-propen-l-yl~-3-cephem-4-carboxylate:
N~C- CONH~ S
HZ1 S `OCHtF ~ N `~
115
'7
The compound (500 mg) obtairled in Experiment
4 w~s reacted with 1,5-diazabicyclo ~3,3,0~octane
(120mg~, followed by removing the protective group, to obtain
the desired product (32 mg).
, ~ .
Exarnple 131
7B-[2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyim;.noacetamido~-3- [(Ej-3-(4-methylthiomorpholine-
1,1-dioxid-4-io)-1-propen-1-yl~-3-cephem-4-carboxyalte:
~C-CONH~r~s~ CH,
~2N S `OC~IIF ~ N` ~,~y~, ~ o
00
The compound (250 mg~ obtained in Experiment.
4 was reacted with 4-me-thylthiomorpholine-1,1-dioxide
(52 mg), followed by removing the pro-tective group, -to obtain
the desired produc-t (17 mg).
Example 132
7B-t 2-(5-~mino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyiminoacetamido~-3- [IE)-3-(1,4-dimethyl-l-piperazinio)-
l-propen-l-yl~-3-cephem-4-carboxylate:
H..7`1 S `O~I~F ~
coo
:
:
The compourld (500 mg) obtained in Experi.Ment.
4 was reacted ~ th 1,4-dimethylpiperazi.ne l94 lul),
followed by removing the protective group, to obtain the
desired product (35 mg).
Example 133
7B-r2-(5-Z~mino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyiminoacetamido~-3- ~(E)-3- ~(2-aminoethyl)dime-thyl-
ammonio3-1-propen~l-yl~-3-cephem-4-carboxylate;:
N ~C-CONH _~ S~ CH3
H2N S `OCH~F ~ b - I--NH2
The compound (750 mg1 ob-tained in Experiment~
a was reacted with N,N-dimethylethylenediamine
(115 lul), followed by removing.the protec-tive group, to
obtain the desired product (15 mg).
Example 134
7B-~2-(5-Amino-1,2,4-thiadiazol-3-yl)-~Z)-2-~luoro-
methoxyimlnoacetamido~-3- [(E)-3- t(tetrazol-5-yl)methyl-
dimethylammonio3-1-propen-1-yl~-3-cephem-4-carboxylate:
: N~C-CONH~S~ C~ N--N
H2N ` ~OCH2F o~ ~ N
:
:: :
: 117
:
gL&i'7
rh compoulld (soo mg) obtained in E,cperimant
d ~as reacted with 5-dime-thylaminomethyltetrazole
(150 mg1, followed by removing the protective group, to obtain
-the desired product (37 mg).
~xample 135
71~-~2-(5-~mino-1,2,4-thiadiazo].-3-yl)-(Z)-2-fluoro-
metho~yiminoace-tamido~-3- ~E)-3-(3-sulEopyridinio)-
l-propen-l-yl~-3-cephem-4-carboxyla-te:
N -~-C-CONH ~ ~S ~ ~ ~H
H2N S N`oc~ F ~N~\~/
COO
: The compoun~ (750 mg) obtained in Experiment
4 was reacted with 3-pyridinesulfonic acid (384 mg),
followed by removing the pro-tective group, to obtain the
desired produc-t (68 mg).
: Example 136
7B- ~2-(5-Amino-1,2,4-thiadiazol-3-yll-(Z)-2-fluoro-
methoxyiminoacetamido~-3- [(E)-3- ~(2-dimethylaminoe-thyl)-
~: dimethylammonio~ propen-l-yl~-3-cephem-4-carboxylate:
:~ N ~ ll_CONH ~,S~ CH3
H,.'\~ S N`oc~; F ~N`~'\~/ 1 N
:: :
~ 118
:::
:
.`
. . ,
.
. ~ . . .
~.3~,~S9~7
The compound (500 mg) obtalned in Experiment
4 was reac~ed wi.h N,N,N',N'-tetramethylethylene-
diamine ~105 pl), Eollowed hy removing the protective group,
-to obtain the desired produc-t (22 mg).
Example 137
7B-[2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyiminoacetamido~-3- ~(E)-3- ~(2-oxopropyl)dimethyl-
ammonio~ propen-l-y.l~-3-cephem-4-carboxylate:
N ~ C-
COO CH3
The compound (500 mg) obtained in Experiment
4 was reac-ted with (dime-thylamirlo)acetone (80 lul),
~ollowed by removing the pro-tective group, to obtain the
desired product (60 mg).
Example 138
7B-~2-(5~Amino-1,2,4~thiadiazol-3-yl)-(ZJ-2-fluoro-
methoxyiminoacetamido]-3- [(E)-3-(4-carbamoylquinuclidinio)-
l-propen-l-yl~ -3-cephem-4-carboxylate:
~: N ~- C- CONH;~ ~N;~CONH2
00
119
:.
The compound (500 mg) obtai.ned ln E:~periment
4 was reacted ~ith 4-carbamoylquinucli.dine (107.6 mg),
followed by removing the protective group, to obtain the
desi.red product (77 mg).
Examp]e 139
713-t2-(5-~mino-1,2,4-th,iadiazol-3-yl)-(Z)-2-Eluoro-
methoxyiminoace~amido~-3- t(E)-3- ~1-methyl-2-(2-hydroxy-
ethyl)pyrrolidinio~-l-propen-l-yl~-3-cephem-4-carboxylate
(Isomers: A and B):
'~ S N N~ V/~,~
CHZC~20H
The compound (500 mg) obtained in Experim~nt
4 was r~acted with 1-methyl-2-(2-hydroxyethyl)-
pyrrolidine (83.3 mg), followed by removing the protective
. .
group, to obtain the desired isomer A (24 mg) and isomer
B (26 mg).
Example 140
7B-L2-(5-~mino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyiminoacetamido3-3- r(E) -3-(4-carboxyme-thylpyridinio)-
l-propen-l-yl~ -3-cephem-4-carboxylate:
C-CONH ~S
N Z,LN ~3CH2COOH
HZN S ~CH2F O
~ ~ coo
:; ~
120
~,
. . : . , :
`
~3~5487
The compound ~500 mg~ obtained in Experiment.
4 was reacted with 4-pyridyl. acetic acid hydrochloride
(280 mg), followed by removing the protective group, to obtain
the desired product 15 mg).
Example 141
7B- t2-(5-~mino-1,2~4-thiadiazol-3-yl)-(Z)-2-fluoro-
methoxyiminoace-tamido~-3- L(E)-3-(1,4,4-trimethyl-1-.
piperazinio)-l-propen-l-yl~-3-cephem-4-carhoxylate iodide:
~N ~ 1 CONH ~ r ~ ~ CH~
00
The compound (1.0 g) obtained in Bxperiment
4 was suspended in ethyl ether (100 ml), and the
ethyl aceta-te solution (40 ml) of 1,4-dimethylpiperazine
(189 jul) was dropwise added there-to and s-tirred overnight.
The precipitate formed was collec-ted by filtra-tion, and this
was fur-ther re-prec;.pitated in tetrahydrofuran/ethyl acetate
and then washed wi-th ethyl acetate to obtain an yellow powder
(992 mg). This was dissolved in dichloromethane (2 ml), and
:~ me-thyl iodide (4 ml) was added thereto with ice-cooling and
then stirred overnight at the same temperature. The reaction
solution was pu-t into e-thyl acetate, and the precipitate formed
:
':
~3~ 37
WdS co~.lected by filtration. to obtain an yellowlsh brown
powder (190 mg).
This powder was added to a mixture solution
comprising triE~uoroacetic acid (1.35 ml) and anisole ~1.16 ml)
and stirred for 2 hours with ice-stirring. The reaction
solution was added to a mixture soluti.on comprising ethyl
ether (25 ml) and isopropyl ether (25 ml), and -the precipitate
formed was collected by filtra-tion and washed witll ethyl
ether. The resulting precipitate was suspended-in water
(4.5 ml), and the insoluble substances was removed by
filtration. The filtrate was purified by reversed phase-
silicagel column chromatography to obtain the desired product
(23 mg).
Example 142
7B-~2-(S-Amino-1,2,4-thiadiazol-3-yl)-~Z)-2-(2,2,2-
trifluoroethyl)oxyiminoacetamido~-3- [(E)--3-(carbamoyl-
methyldimethylammonio)-l-propen-l-yl~ -3-cephem-4-
carboxylate:
CH~
N C--CONEI~ S~
T ~ CO~DH2
~2N OCH CF ~
2 3 C00
~ ~ The compound (1.43 g) obtained in Experiment
'~ ~
:
12,~
.
,
~,
~;~q~5~h~7
7 was dissolved in acetone (20 ml), and sodium
iodide (0.927 ~) was added thereto with ice-cooling and stirred
Eor 10 minutes at the same temperature and successively for
1 hour and 30 minutes at room temperature. The solvent was
evaporated out, and after ethyl acetate was added to the
resulting residue, this was washed with a dilute~sodium
thiosulfate solution and saturated brine and then
dried with sodium sulfa-te as added. The brine was evaporated
out, and the residue was dissolved in ethyl acetate (~0 ml).
~fterwards, dimethylgycinamide (237 mg) was added to the
resulting solution and stirred for one hour at room -temperature.
To the resulting solu-tion was added isopropyl ether, and the
precipi-ta-te formed was collected by filtrat;on, to obtain an
yellowish brown powder (1.07 g).
The powder was added to a mixture solution comprising
-trifluoroacetic acid (8 ml) and anisole (6 ml) and stirred for
one hour with i.ce-cooling. Ethyl e-ther was added to the
resulting reaction solution, and the precipita-te Eormed was
collected by filtration. This precipitate was suspended in
water (10 ml), followed by adjusting -the p~ of the resulting
suspension to the range of Erom 5.5 to 6.5 with sodium
acetate, and the insoluble substances were removed by filtration.
The filtrate was purified by reversed phase-silicagel column
chromatography to obtain the desired product ~268 mg).
: :
.
'
123
~-3~ ~a~ ~B'~'
ê 1l ~
~ ''7
. ~
O~ ~ ..
.
C ..
O 0 .
U ll i
.
24
~: : :
;
::;:: ::
. .
:
.
~3~S~ 7
.. _ _ __ _ ............... _ ~ ~
~ I_ ~ _ _l ~ A ~
CD ~ O~ _ ~ ~ ~ 11 ~ ~
r~ _ ~ ~ ~ 1 tq~ ~ ~ ~ ~ O
~O ~ 1~ ~ ~ ~ ~ t~ m U~ ~ ~ ~ -
_~ ~q o ID ~ C.~ , ..
e ~q ~ _ C,~ ~ li ~ ~ ~ _ O = _ "
Z; C~ ~ N _ ~,~ C~ Ct~ _ ~ , ~ ~_ ,
~ ^ ~:C ~ ll ~ 1 E~
~ ~~ ~ ~~ U~ ~ ~ ~ ~î C~ ~ 3 ~n ~ _
. ~ C~ _~ o ~ cr~ c~ ~ 3 lo - _,
~1 ~D O 0 ~ -- O ~ _ ~ cr~ C~ 3 .
. . u~ cn ~ ~li ~ ~ Cl U~ 0 E3 ~ ~
:` ~ ~ ^ ~ ~ t~ ~ ~ ~ ~ $ ^ ~ ."
I ~ o I ~ ~n I
. o ~ o ~q ~ o 3~ o co $ $ o
u~ u~ c~ ~ _ cq ~ ~ _ c~
~ ~ ~ _ _ ~ , ~
Li ~ ~ c~ q ~ ~ ~ l o _ ~ ~ o o ~ .~, ~ co
:: ~ ~ ~ 10 ~_ ~ ~~ o 5~ ~~ C.7 ~r ~_ .
C .. ~ ~ . ~ ~
o o o ~ o o
vt_ . . tD IS~ tD
O O _~ tD tD CD tD
~ ~: ~ Z _ ~1 ~ .-1 _~
~1 E _ O O Ir~ O O 11'~ ~0 O 10 ~
h 1.~ rtD D~ ! tD O tD C~) ~ ~ tD ~)
t-- ~ t_ tD ~-- 1~ t-- U~ t~
~, ~ D, ~1 _ _ ~ ~ ~1 _ ~1 _
: ~ ~ ~ . . ~ . .. ,.. _
E :~ _ ... O . N
:
: ` 125
.
.
~3~3~
.~ .,_~ _ ~ .. .. . .___._ .. _ ...
~ ~ .
-- N . ~ _ _ N >-~
_ ~E! ~ ~-- C
_, ~ n
R . ~ 7
I W 0 ID W l ~W ~ W ~ w~
. e~ $ _ N e'~ ~", ~3 ' N ~o N ~ --
'~ ~ 1l 11 1 ~11 1l 11 ~ 11 11 CO
. i ~ ~ I 10 ~ ~ I ~ î ~ I ~ --
~ ~ _ o ~ ~ O ~ E3
O U:~ ~ ~ O ~ t_ ~ ~ O o _
~; ~ ~ `~ c~ _~ ~0 to CO ~
C ~ .
~: ~ V~ ~ ~ t_ C~ O
4 _~ ~D ~ U~ U~ C::~
3 z _ ~ _ _ _
e o o o o ~ u~ u~ ~ o
4 ~ 1~ ~ C~ tD a~ ~ D O U) a)
c o, r- m ~ _, _ ~ _ ~ _ _
.... __
~ ~ ~ ~ _ . ~1 o
: ~ . 126
~ .
54B~7
~V'~` ~ ~
~ ~_ ~ ~ a _ ,~ ~-- ~
, '-.
_
N ~ t~ ~ 10 N ~ `' ~C N
~ . ~ o r~ 0 U~ ~
,~ a G G ~ G ~ ~ G ~ ~ ~ G C`/
C G G . _ _ _ . _ . . _ _ 0
: ~ 0~0 U~ ~ U~ O~ ~
~ G ~ _ 0 . . ._
;~ ~
127
:
?S~7
.~ .. ~ ~_
~o t~ ~ 'i 5 5 ~ ~ 5
_ ~ ! ~o
~; C`J ~ _~ ~ C~ '~:1 , ~ N ~ ~ 1~ . ~ W .~
~0 W _ .~ Ul ~ t '1:1 W C~ ~ U~ _ _
_ ~ ~ 1 ~_ ~ O ~ _ .-- ~ h _ ~
~ ~ . ~ ~ 5 co , _ ~ N N ~ _
o 11 11 ~ 11 ~ ~
I ~^ ~ 0 j I ~î ~î I ~ I ~ ~ ~î I ~ ~ w
O ~ 0 ~q ~ ~ ~ ~ ~
1 ~ ~ Q "' cr~~ P~ ~ co ~ '~ 7
`~ ~0 ~ 0
C -_ j _ ~_........... ~
o 1 m o o o o
V t- t_ tD ~ ~ ~r ~_
~ _/ ~D ~ ~ ~ ~ tD
U) r~ .~ ¦ _ _ ~ ~ _~ _
~a E _ Lt~ Lt~ O O ID U~ O O It) If~ O
h::~ r ~ ~~ ~D O tD a> u, c~7 ~ ~ ~ O
~ ~ W ~ ~ e~ 7 r- tD
H U~ _ _ ~ _~ _ ~ _ _
~ - -r-- - -
~:; E~ ~ ~ ~ .~ ~ U~
_,
12
,
~3
,. "
1l ~ ~ ~ _ ~ ,~
~ ~ ~O ~ oq c~ _ I_
.' e -~ ¦ ~
~ 1 ~! o ~ ~ ~
Z; O , _ _~J ~ ro ' ~ N " ~ ~ -- -
tl~ ~ 1 ~ ~ m ", ~ U~ ~
~ -- N C`~ 1 ~ ~ ~ ~r U~ , _ 0
. ~ _ ~ N -- Lr~ 3 --
,~ 1, 1l ~ ~ ~ 1l'
I ~ ~ r~ I I ~ El ¦ ~ 1 E3 I r
3 ~ O x ~ ~q ~ ~ ~ ~ ~
Q ~ ,~:, 0 ~ o ~ ~ '3 ~ ~ ~ o
_ c~ ~ ~ ~ U~ ~ ~ ID ~ ~n 0
_
O _ L~ O 0
D~ - ~O ~ U~
o o ~o ~o ~ ~
:,~ ', ~ _ _,
~I E o U~ O O O O O O
~D ~ r ~D ~ ~ ~ ~D ~
~ ~ ~_ t-~ t-~ ~U~ ~U~
__ _ . . _
W _~ CO O~ o
__
129
~,3r.,1s~3~
~o ~
~, ~-7~
z; -- 3 co cn
~ e ~ It~O ~ ~
. c~ ~ ~r
~; ~ .
. 1 ~ ~ I x ~q
O ~ ~
.~ ~' c~ ~n '' ~ co
~: __ '
O O O
: v
n Z _ ~
~ ~ ~ I o n c~ o
: U ~ ~ U~ _ ~
,~ a~ _
~I d , ~ _ _
':
::
130
i
: ~ .
Example 14~
7~-[2-(5-Amino-1,2,4-thiadiazol-3-yl~-(Z)-2
fluoromethoxyiminoacetamido~-3- ~(E)-3-¦tris(2-
hydroxyethyl)ammonio]-l-propen-l-yl] 3-cephem-4-
carboxylate
\~ C--CoNH \ pH
'O--CH:.FC~fJoo \ ~
OH .
400 Mg of the compound prepared in the Experiment 4
was dissolved in 4 ml of ethyl acetate, and 96 mg of
triethanolamine solution in 4 mQ of ethyl acetate was added
thereto. The resulting solution was stirred for 6 hours
at the room temperature. To the reaction solution was added
24 mQ of diisopropyl ether, and the resulting precipitates
were collected by filtration. This precipitates were added
to 4.5 m~ of a mixed solution containing trifluoro acetic
acid and anisole (1:1) under ice-cooling. The mixture
was stirred for one hour and 30 minutes ~t the room
temperature. To the reaction solution was added 18 mQ of
dilsopropyl ether. The resulting precipitates were collected
by filtration, and washed with diisopropyl ether. This
precipitates were suspended,in 3 mQ of water, and sodium
acetate was added thereto to adjust the resulting solution
to pH 6. Insolubles were removed by filtration, and the
filtrate was purified by a reversed-phase silica gel column
.
~ 131
~3~S4~7
chromatography to obtain 17 mg of the objective product.
Example 144
7~-[2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoacetamido]-3-[(E)-3-[bis(2-
hydroxyethyl)methylammonio]-1-propen-1-yl]-3-
cephem-4-carboxylate
/OH
N-~ C--CONH ~,~ ~ ~
l~SyN o~N~WN--C~3
O--CH2F C013- --\OH
In the same manner as the Example 143, 500 mg of the
compound prepared in the Exeriment 4 was reacted with 150 mg
of N-methyldiethanolamine, and the protecting group was
eliminated to obtain 15 mg of the objective product.
.,
~ ~ :
,~ :: : : :
:~
:~ ~o
, ~
132
,
, ~ :
:
~::~ :::
: .
.:
::
~3~:~5~7
_ ,~ _
N "~
^ C~ _
~ ~ ~^
0 ~, '11 ~ ~1
~ a~ ~ 11 ~ .,
E 1' m ~- ~ m o'
7 ~
~ ~ ~^ ~
C`l CO ~ ~
.--~ ~D ~ ~ ~, ~T1 N t--
C~ ~ N ~ ~D 5
c~
U~
. o D _ ~ ~ ~ _-1
~
~ Q C~
~ ~ ~ C ~
a. ^ ; ~ o o
~ ~ : ~ E l ~D O
~ ~ laU ~
:~ ~ ; .___ . :
.~. _ .
` ~
133
~:
'
: .
:~3~4~
E~periment 8 (Synthesis of the raw material compound)
2-(5-Tritylamjno-l,2,4-thiadiazol-3-y])-(Z)-2-cyano-
me~hoxyiminoacetic acid
C - ~00~
~3CHN~3~N
O-CH2CN
~ ..
8.00 g of N-cyanomethoxyphthalimide was suspended in 50
ml of ethanol and 1.93 ml oE hydrazine monohydride was added
thereto at roorn temperature. The resulting mixture was
stirred for 1 hour and 45 minutes. Then a saturated
brine and ethyl ether were added thereto. The mixture
was made basic with conc. aqueous ammonia and then extracted
with ethyl ether. The ethyl ether layer was washed with a
saturated brine solution and dried over anhydrous sodium
sulfate. Subsequent]y the solvent was distilled off.
To the residue, 350 ml oE methanol and 6,50 g of
2~(5-tritylamino-1,2,4-thidiazol-3-yl)glyoxylic acid were
aclded and the resulting mixture was stirred at room
temperature Eor one hour. After clistilling off the solvent,
the residue was dissolved in ethyl ace~tate and then washed
with 0.1 N hydrochloric acid~followed by with a saturated
brine solution~ After drying the organic layer over
;anhydrous sodium sulfate, the solvent was distilled off.
Thus 7.64 g of the ob~ective compound was ohtained.
Experiment 9 (Synthesis~of the raw materia.l compound)
p-Methoxybenzyl 7~-[2-(5-tritylamino-1,2,~-thiadiazol-3-
8'~
yl)-(Z)-2-cyanometlloxyiminoacetamido]-3-[~Z)-3-chloro-1-
propen-1-y1~-3-cepllem-4-carboxylate
~r N~7-CON~sl rC
6~H~ S ~ N~O
O-C~H2CN
~D COOCH~OCH~
.::
1.51 ml oE dimethylEormamide and 18 ml oE tetrahydro-
furan were cooled to -10 C and 1.82 ml oE phosphoryl
chloride was added thereto. The resulting mixture was
stirred for 40 minutes under ice-cooling. Then a solution
of 7.64 g of the compound of Experiment 8 in 24 ml of
tetrahydrofuran was added thereto and the resulting mixture
was stirred at the same temperature for additional one hour.
A solution comprising 6.00 g of p-methoxybenzyl 7~7-
amino-3-[(Z)-3-chloro-1-propen-1-yl]-3-cephem-4-
carboxylate hydrochloride, 12.8 g of N-trimethylsilyl-
acetamide and 60 ml of ethyl acetate ~las cooled to -25 C.
Then the above-~,entioned reaction mixture was added thereto
and the resulting mixture was stirred for 40 minutes while
raising the temperature to 0C. This reaction mixture was
extracLed with ethyl acetate and the organic layer was
washed with a saturated brine solution and dried over
anhydrous sodium sulfate. After distilling off the solvent,
~ ::
the residue was purified with silica gel column
135
chromatographyO Thus 7.80 g of the objective compound was
obtained.
Experim~nt 10 ~Synthesis of the raw ma-te~ial compound)
p-Methoxybenzyl 7~-~2-(5-tritylamino-1,2,4-thiacliazol-3-
yl~--(Z)-2-cyanomethoxyiminoacetamido]-3-[(E)-3-iodo-1-
propen-1-yl~-3-cephem-4-carboxylate
. ~D ~,~C-~N~S
~ S N c~N~
~(~
~D C~H2 ~
To a solution of 7.80 g of the compound of Experiment 9
in 120 ml of acetone, 6.9 g of sodium iodide was added under
ice-cooling. The resulting mixture was stirred Eor 10
minutes and then for additional 1 hour and 30 minutes at
room temperature. The solvent was distilled off and the
residue was extracted with ethyl acetate. The extract was
washed with a dilute aqueous soltuion of sodium thiosulfate
followed by with a saturated brine solution and then dried
over anhydous sodium sulfate. The solution was concentrated
and added dropwise to a mixture of isopropyl ether and ethyl
ether. The precipitate thus formed was filterd out to
thereby give 6.50 g of the objective compound.
Example l45
7~-[2-(5-Amino-1l2,4-thiadiazol-3-yl)-(Z)-2-cyanomethoxy-
: ~
~ ~ ~ 136
~ :
` ' :
~3~59L~
iminoacetamido]-3-t(E)-3-[~15-carbamoyl-2-hydroxyethyl)-
dimethylammonio]-l-propen-l-yl]-3-cephem-4-carboxylate
~C-CONH~S CH~ H
H~N S ~OGH CN~LN~NH2
00- . '
1.0 g of the compound of Experimentlo was dissolved in
2 ml of dimethylformamide and 210 mg oE (IS-carbamoyl-2-
hydroxyethyl)dimethylamine was added thereto at room
temperature. The resulting mixture was stirred for one hour
and then diluted by adding 10 ml of ethyl acetate thereto.
The resulting solution was added dropwise to 100 ml of ethyl
ether to thereby give 710 mg of a brown precipitate.
This precipitate was stirred in a mixture of 6 ml of
anisole and 6.5 ml of trifluoroacetic acid under ice-cooing
for one hour. Then ethyl ether was added to the reaction
mixture to thereby give 430 mg of a brown precipitate. This
precipitate was suspended in 10 ml of water and the pH value
of the obtained suspension was adjusted to 7.0 with sodium
acetate. After filtering off the insoluble matters, the
filtrate was purified with reverse phase silica gel column
chromatography to thereby give 50 mg of the objective
compound.
Example 146
7~-[2-l5-~mino-1,2,4-thiadiazol-3-yll-(Z)-2-cyanomethoxy-
13l
~ . . ` '''' ' "
:
~*~
iminoacetamido]-3-[(E)-3-(1 methyl-2R-hydroxymethyl-4R-
hydroxy-1-pyrrolidinio)-l-propen-1-yl]-3-cephem-4-
carboxylate
bCH~
CH20H
1.0 g of the compound of Experimentlo was dissolved in
a mixture of 10 ml of ethyl acetate and 8 ml of ethyl ether~
Then 167 mg of N-methyl-4R-hydroxy-D-prolinol was added
thereto and the resulting mixture was stirred overnight.
The reaction mixture was added to 100 ml of ethyl ether and
the precipitate thus Eormed was filtered out to thereby give
840 mg of a yellow powder.
To this powder, 6 ml of anisole was added and 8 ml of
trifluoroacetic acid was added dropwise to the ~esulting
mixture under ice-cooling for 30 minutes. Then the mixture
was stirred for additional 1 hour and 30 minutes. 100 ml of
ethyl ether was added to the reaction mixture and the
preclpitate -thus formed was filtered out and suspended in 4
ml of water. The pH value of -the resulting suspension was
adjusted to 7.0 with sodium acetate. After filtering off
the insoluble matters, the filtrate was purified with
:
reverse phase silica gel column chromatography to thereby
give 24 mg of the objective co~pound.
~ ~ 130
;::
3~ 7
The following comp-unds of Examples 147 - 154 were
obtained in the same manner as those described in Examples 145
and 146,
ONH~
~N
O~L2C~ I
(compound of Experimentlo~
.~
j' (amine corresponding to A)
,~ CONH ~S~
H2N S ~ Ct--N`~
oo~
: In a case where plural isomers were formed depending on
: the ammonio group of A, the yield of each isomer, if
~ isolated, was given.
.~ :
~: ~:: :
:: 13
.: '' '
:
3~
~,, ~ ~
.` . ~
~: _~ . . _ i
~ ~ ~W ~'
~d
~: : ~ __ L , ._
: ~: _
~ 140
4~7
~ ~ ____. ._ ._
. o . . _ . _
CIH ~;
E ~ O ~ ~ ~b
O E R, _ O O O
. ~1
~, .. _ _ .
,~ ~ ~ .
~H O ,
~ C ~ V _~ ~ ~
E J u ~ 1~
E ` O \~ E O t-- C`l
: . ~ ,_~ ~ O
: -'. ~ ~ . .
__ _
:
: ~ ~ ~z;: ~ r-
¢ 1 ~ ~g ~o~
~ ~ -~ ~
. .
:~ ~ - --~-----=
E ~ u~ - ,~ ~ ~I u~ u;
__ _ . . __ ~
_ ,.,,_
~ ~ :
" '
~3r~ 7
z ~, ,~ m ~o ~n ~m, 4,
. ,~0 ~, ~ ,
,~
: ~ ~ ~
C) U g N u~ C~
~ . _ ~ . Co ~
R ~ ~ ~
~ l ~- ~D'
.. ~._ .~. _ _ ~
~;. ~'c Cl ._. ..... __ .. _____
~: : 142
~:
1 t3~?S4~7
. ~ . ~ . ~ ~ ~ o o~
~ ~ ~ ~. ~ ~ m ` 5~ 5 ~ - tq.
. >~ ~ $ _~ Ul q~ ~ 10 e`J ~
~ ~ _ ~ ~ r r ~ _ W' c" _
! ~ $~ ~ oJ ~ ~- ~ , ,.
I ~ ~ ~ ~. ~ ~ ~ ~ ~ ~ 1, ~ 1l ~ ~
!l ~ ,~ ~ ~:: m ~ ~ u~ ;~. ~ ,~
O ~ O 0, ~ ~3 i ~ O ~
~ Q ~ o C-~ ~ 1 u~ ~ o .
I '' c~ ~ ~ r '' ~
a l . __
o ~ o o o o o o
~ - 0~ ~ . ~ ~ t_
4 _1 ~ ~D 11) ~D ~ ~0
111 Z r--I r~l r--l ~ ~
~,~ r ~ ~ ~ O 3 0
o u ~ cn ~ ~ a~
t- ~ ~ U~ t- CD t- U:
~ ~,q ~ _~ ~ ~ ~ ~ _, ~
~ ~ ~ _ _
143
I
c~ i ~r O ~ u~ ~ CD ~
^ C~. 'd _~ c-~ ~ ~ oo N ~ ,_ d
~o i0 ~ 0 ~0 0 11 _ 0 ~ ~ ~ o~ 0
~1 - o W 0 0 -- ~ ~ N 0 N ~ ' ~ N
i,, " a
U~ ^ O
:~ . If _ W~ ~ ~ ~ _ _ W W ~
o ~ ~ ~ m ~ ~ ~ ^ 0
: ~ ~ ~ ~ ~ ~ ~ ~ ~ 3 a o ", ~ ~
:~ ~_ Co ~ 10 ~_ Co 10 CO Y C.~ ~
~ ~ ~ ~ ,........ ._, . _
o C) o U~ o ô o ô ô o
Q. _ t- ~ C- ~ . C~ ~ ~D O
O O ~D ~ ~ tD U~
~ ~ ~ ~:Z~ ^ ^: ~ _~ ~ ~ ~
:: ~ E _iS~ o , Lt~ 1~7 In U~ 11~ O O
~ ~ O ~ 0tD 0 U~
L~,U ~ C_ CD t- 10 t- 10 t~
0. ~ _ ~ ~ ~ _ . ~ _~ _
, ~ - ._ _ . ,
' ~ Cl~ _ __ . ~ ~
. ~_
44
::
:
~!.3t~S9L~7
~ ~ r ~
~0 11
.~ 1'
C'~ U~ ~ ~q O
., , a , ~, ~ .
~ ~~ ~ ^ U~
:Z; O~ O ~ _
11 ~ ~
D
.
~C~
:
$^ ~q ~ ^
~: ~ ~ 1 ~ ~ o ~
V C~V ~ 10 0
._ .
C U~ o ô o
: -~ ~D C`l ~O ~
O O ~O m ~ In
; ~ ~:
: ~ Z
:~ ~,, I O O O O
V ~ ~ U~
: C~
._ . ~ ... ._
: Q' d t~
X . I U~
~ 1:~1
~_ ~_ ~.
::
; .
.
J1~3~.~5
~xamPle 155
7~-[2 (5-~mino-1,2,LI-thiadiazol-3-yl)-(Z)-2-cyano-
methoxyim:Lnoacetoamido]_3_[(E)_3-carbamoylmethyldimethyl-
ammonio)-l-proPen-l-yl~-3-cephem-4-carboxylate
IN~ I ~ONH r~ S ~ CHa
H2N S N, ~g N~ ~N~coNH2
Dimethylglycinamide (64 mg) was added to a solution of
the compound (390 mg) of Experiment loin ethyl acetate (15 m~) 9
and this mixture was stirred at a room temperature for 1 hour.
Ethyl ether was added to the reaction solution and the produced
precipitate was filtered and dried, thus providing a yellowish
brown powder (260 mg).
A mixture of trifuloroacetic acid (2 m~) and anisole
(1.5 m2) was added to this powder and stirred for 1 hour under
an ice-cooling condition. Ethyl ether was added to the resulting
solution, and the produced precipitate was filtered and washed
with ethyl ether. This precipitate was suspended into water
(5 m~) and pH of the suspension was adjusted to 5.5 to 6.5,
following which the insoluble material was filtered off. The
filtrate~was refined in a reversed phase chromatography, thus
providing the desired material (37 mg).
:
' ~ '
146
'7
E.Yample 156
7~-[2-(5-Arnino-l,2,4-thiadiazol-3-yl)--(Z)-2-cyano-
methoxyiminoacetoamido]-3-[(E)-3-(l methyl-4-sulfamoyl-
l-piperazinio)-l-propen-l-yl)-3-cephem-4-carboxylate
SO2NH2
The compound (500 mg) of ExperimentlO was dissolved into
a mixture of dichloromethane (5 mQ) and methanol (l m~, and
N-sulfamoyl-N'-methylpiperazine (145 mg) was added thereto,
and the whole was stirred at a room temperature for 4 hours.
The resulting solution was concentrated, and ethyl ether was
added thereto. The produced precipitate was filtered and
dried, thus providing a yellowish brown powder (450 mg).
A mixture of tr1fluoroacetic acid (3.5 m~) and anisole
(3 m~) was added to this powder, and the whole was stirred
for l hour. Ethyl ether was added to the resulting solution,
and the prod~uced precipitate was filtered and washed with ethyl
ether. The preoipitate was suspended into water (5 rn~) and
the pH of the suspension was adjusted to 5.5 to 6.5, following
~; which the insolubIe material ,was fiItered off. The filtrate
was refined in a reversed phase chromatography, thus providing
the~ desired material (39 mg).
~: ::: :
: , :
~: :
47
~ , .
. ~
`S~W~r
Example 157
7~-[2-(5-Amino-1,2,ll-thiadiazol-3-yl)-(Z)-2-cyano-
methoxyiminoacetoamido]-3-[(E)-3-(1,4-diazabicyclo[2,2,2]
octane-l-io)-l-propen-l-yl~-3-cephem-~-carboxylate
N-~C--CONH-~_~S
H~N `O-~2CN ~3LN i
co(r
The compound (500 mg) of Experiment lOwas dissolved into
a mixture of ethylacetate (6 m~) and methanol (0.5 mR),
and 1,4-diazabicyclo[2,2,2]octane (90 mg) was added thereto
and stirred at a room temeprature for 20 minutes. Ethyl ether
was added to the resulting solution, and the produced
precipitate was filtered and dried, thus providing a yellowish
brown powder (330 mg).
A mixture of trifluoroacetic acid (3 mQ) and anisole (2.5 m~)
was added to this powder, and the whole was stirred for 1 hour
under a ice-cooling condition. Ethyl ether was added to the
resulting solution, and the produced precipitate was filtered
iand washed with ethyl ether. The precipitate was suspended
:~ into water (5 m~) and the pH of the suspension was adjusted
to 5;.5 to 6.5, following which the insoluble material was
filttered off. The filtrate was refined in a reversed phase
chromatography, thus providing the desired material (48 mg).
Compounds of the following Examples 158 to 161 were provided
in the same manner as in Examples 155 to 157.
14 8
:: : :
Example 158
7~-t2-(5-Amino-1,2,LI-thiadiazol-3-yl)-(Z)-2-cyano-
methoxyiminoacètoamido¦-3-[(E)-3-(4-carbamoylpyridinio)-
l-propen-l.-yl~-3-cephem-4-carboxylate
N~C-CONH S +
HZN S N ~ N J~/N~CONH2
coo- .
The compound (600 mg) of Experimentlowas reacted with
4-carbamoylpyridine (235 mg), and the protective group was
removed to provide the desired material (37 mg).
Example 159
7~ [2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-cyano-
methoxyiminoacetoamido~-3_C(E)-3-(1,3,4-oxadiazol-2-
yl)methyldimethylammonio]-l-propen-l-yl3-3-cephem-4-
carboxylate
N~rC-CONH r~S~ C~3 N~
H~N S N ~J\~\/~\J~ oJ
O CHzCN I oo- CH~
; The compound (600 mg) of ExperimentlO was reacted with : 2-dlmethylaminomethy~ 3~4-oxadiazol (163 mg), and the
protective group was removed 'to provide the dasired material
; (54 mg).
~: :
~: :
149
:
'' ~" '
;.
'7
Example 160
7~-[2-(5-Amino~ L~-tili~di~zol-3-yl)-(z)-2
methoxyiminoacetoamido]-3-[(E)-3-(1,2-dimethyl-1-
piperazinio)-l-propen-l-yl~-3-cephem-4-carboxylate
(i~omers: A and B)
~ I--CONH ~/S~ ~Hl "
H2N `0-CH2CN ~
oo CHl
The compound (510 mg) of ExperimentlO wàs reacted with 1,2-
dimethylpyrazolidine (0.4 mQ), and the protective group was
removed to provide the desired isomer A (20 m~) and the
desired isomer B (20 mg).
Example 161
~ 7~-[2-(5-Amino-1,214-thiadiazol-3-yl)-(Z)-2-cyano-
; ; methoxyiminoacetoamid]-3-[(E)-3-(1,5-diazabicyclo
~ ~ [3.3.0]octane-1-io)-1-propen-1-yl~-3-cephem-4-carboxylate
:
CI--CONH~f S~ ~
H2N S No ~ N~J\~/~N
COO
The compound (600 mg) of ExperimentlO was reacted with
1,5_dlazablcyclo[3,3,0]octane (220 mg), and the protective
group was removed to provide the desired material (39 mg).
.
~3~S~
- .. _ _ ~ _, _
m
~a '' m ~ ~ s:i à~
.8 I _ _ . i ~ m ~
W ~ I ~
C~ .3 ~ , _
1 t~ t- . 4 ~ m ê ~
--~ O ~ r~ o ~ .~ O ~ ._~ oO .
_ U~ _- ~ r1 ~~ ICO
O ~ ~ N ~ V V
~o ~ _4 _ ~ ~ ~ ~ ~ _ _
~ ~ O ~ ~ O ~ ~ O ~ , 0 ~
o ~ Q . a~N ~ D .
C ~ __ . ~ . __ . _ _ _ .
~ : ~ O _ O . .
E_l O 00 ~ 1
; ~ 1- D-- O _ _ _
~1 ._ ._ ._ _ _ _ . ____
:~ ~1~i Z ~ _ ~
51
' ' ,
.
_~ ~ . _ N
.4`-
. U~ ~ ~ ~ 11
â ~ ^ O
¦ 0 ~ ~ 1~ ~o O ~ e ~ ` ~
~ c~ 5 ~ ~ ~ ." N ~ ~ CO
S~. ~ ~ ~ 1., 2~ ~ ~^
~ r: . GO ~ cr~
z 1l 0 ~n ê ê lî' cn, ê 1l 1. ~
~ O s ~ o ' ,0 m o ~ m ~, " "
00 ~ O 1~ GO _ ~ O C~
--'~ GO ~ `~ ' It~ 0:)
a _ ~ _
'o a~ . l
'~I E ` LO G~ C~ O
~ Z_l ~ _ .-1 ~.
,a ~ E ~
C Q O C` O W
~1 __ _ _ ~ , _ .
I ~ ~ ~D ~ .-~
;: ~ --- -- - --- ----- -- --- - ----
~ . 152
' '
.: .
Experiment 11 (Synthesis of the raw material compound)
2-(5-Tritylamino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoacetyl chloride hydrochloride
~1J N ~ C--COCl
~CHN ~ ~N N HCl
S ` OCH2F
Phosphorous pentachloride (395 mg) was dissolved in
dichloromethane (2.9 mQ) and cooled to -5C. To the solution
was added the compound (627 mg) of Experiment 2, and agitated
for 2 and half hours at the same temperature as described
above. The reaction solution was added to a mixture of n-
hexane (9.4 m~) and n-octane (9.4 m~). The resulting
crystalline substance was collected by filtration, and washed
with n-octane to obtain the objective product (325 mg).
Melting point: 139 - 140C (decomposition)
Mass spectrum (m/e): M+....... 480 (35CQ), 482 (37C~)
In~rared absorption spectrum (cm 1, Nujol): 1795, 17~0,
1740, 1630
NMR spectrum (~, DMSO-d6): 5.79(2H, d, J=54Hz), 7~31 (15H,
s), lO.O9(1H, s)
~: :
153
,~
~.
~ .
::
, . . .
, ,
-` ~3~
Experiment 12 (Syn-thesis of the raw material compound)
2-(5~Amino-1,2.4-thiadiazol-3-yl) (Z)-2-fluorome-thoxy
iminoacetic acid ~thYl ester
N \~ C - COOC2H6
~z ~ S' N ~ oCE2F
The compound ( 2.00 g) of Experiment 1 was agitated .in tri-
fluoroacetic acid at room temperature for 30 millutes. The
solvent was distilled off, and the residue was purified bY
silica gel column chromatography to obtain the objective product
(405 mg).
~lel-tincJ Point: 172 - 173C
Infrared absorPtion spectrum (cm 1, Nujol): 1730. 1615
NMR sPectrunl (~. DMSO-d6): 1.28t3H. t. J=7.0Hz). 4.34(2H. q,
J-7.0Hz). 5.83(2H, d. J=54.5Hz). 8.27t2H. brs
~i ~
54
:` ::
:
.
~ll3~;~S~
ExFeriment 13 (Synl:hesis oE the raw material comPUnd)
2-(5-~mino-l~2~4-thiadiazol-3-yl)-(z)-2-fluorometh
iminoace-tic acid
N ~ C--COOH
H2N~S~N N~OCHF
The comPound (200 mg) of Experimen-t 12 was suspended in
mixture oE ethanol (6 mQ) and water (2 mQ). IN aqueous sodlum
hydroxide solution 1l.75 mQ) was added thereto, and s-tirred at
60C for I hour. Ethanol was distilled off from the reac-tion
solution. and the solution was adjusted to PH 2 with the use of
IN hYdrochlorlc acid. The resul-tin~ solution was Purified bY
means of "Dia-lon SP207" (trade mark for nonionic adsorPtion
resin manufactured bY Mitsubishi Chemical Industries, Ltd.) to
obtain the objective product (30 mg).
Infrared absorPtion sPectrum (cm l, Nujol): l720, 1620
NMR sPectrum (~, DMSO-d6): 5.74~2H, d, J=55 Hz), 8.24(2H, br)
:
~ ~ 155
,
'
Experiment 14 (Synthesis of the raw material compaund)
P-Methoxybenzyl 7~-[2-(5-tritylamino-1,2,~-thiadiazol-3-
yl)-(Z)-2-fluoromethoxyiminoacetamido]-3-[(Z)-3-chloro-1-
propen-1-yl]-3-cephem-4-carboxylate
~3 N~ C-CONH ~S rCl
(~HN S `OCXzF oJ N ~
@l COOC~I~ ~OCEs
To a mixture of ethyl acetate (37 mQ), tetrahydrofuran
(5 mQ), and dichloromethane (15.7 mQ) were added N-
(trimethylsilyl)acetamide (8.17 g) and p-methoxybenzyl 7~-
amino-3-[(Z)-3-chloro-1-propen-1-yl]-3-cephem-4-carboxylate
hydrochloride (3.33 g) to dissolve the latter materials. The
solution was cooled to -20C, then the compound of Experiment
11(3.80 g) was added thereto, and agitated at lO~C for 1
hour. After the addition of ethyl acetate (500 m~) to the
reaction solution, the mixture was washed successively with
water, a saturated a~ueous sodium bicarbonate solution, lN
hydrochloric acid, and a saturated brine solution, and then
anhydrous magnesium sulfate was added thereto to dry the
same. The solvent was distilled off, and the residue was
purified by silica gel column chromatography to obtain the
objective product (4.33 g~.
The infrared absorption spectrum and the NMR spectrum of
the resuItant product coincided with those of Experiment 3.
, ~
~ 156
~ . ~
' .
.?~4~7
\
periment :L5 (Syntllesis of the raw material cornpound):
2-Cyano-2-E:Luoromethoxyiminoacetamide
NC- C - CONHz
N
\ OCH2F
2-Cyano-2-hydroxyiminoacetamide (22.6 g) was dissovled in
dirnethyl sulfoxide (100 ml), and then potassium carbonate (55.2 g)
was added thereto with stirring at room temperatureJ and the
solution was Eurther stirred for additional 20 minutes
Fluorobromomethane (27 g) dissolved in dimethylformamide (20 ml)
was then added to -the solutionr and the solution was stirred
for 20 hours at room temperature and -then allowed to cool. The
reac-tion solution was added to iced water (1 liter)~ and extracted
-twice with ethyl acetate (150 ml)c The organic layer was washed
twice with a satura-ted brine, and dried with addition of anhydrous
magnesiurn sulfa-te, followed by distilling off the solvent, The
residue was washed with e-thyl ether, and dried -to obtain the
objective product (14,4 g)~
Melting point: 124 - 125C,
: : ; Infrared absoprtion spect~um (cm , Nujol):
: 3410, 3290, 3150J 1690~ 1590
NMR spctrum ( ~, DMSO-d6):
5.94 (2H, d, J=54.0 Hz), 7.85 - 9.40 (2H, b)
57
:: ~
~: ~
.:
,
Experiment 16 (Synthesis oE the raw material compound)
2-Fluoromethoxyiminopropanedinitrile
NC-- C--C~N
N
\ OCH2F
A mixture con-taining the compound (14.0 g) prepared in
Experiment 15, acetonitrile (15 ml), sodium chloride (lS g) and
phosphoryl chloride (14 ml) was reacted under reflux for 2 hours.
To the mixture was added phosphorylchloride (5 ml), and the whole
was reacted for 2 hours. The reaction solution was, after cooling,
added to lced water ~200 ml) and stirred at room temperature for
one hour. The solution was extracted twice with methylene chloride
(50 ml). The extract was washed with 5% aqueous solution of
sodium bicarbona-te, and with a sa-turated brine, and then dried
wi-th addition of anhydrous magnesium sulfate. The solvent was
distilled off. The resulting oily product was subjected to
distilla-tion under reduced pressure to obtain a colorlessoily
objective product (9.1 g).
~oiling point: 69 - 70C/25 mmHg
NMR spectrum (~, CDC13): 5.85 (2H, d, J=52.0 Hz)
`
:::
:: :
: , :
Experirnent 17 (Synthesis of the raw material compound)
2-Cyano-2-fluoromethoxyiminoacetamidine
~2N ~
C- C - CN
HN D l¦
OCH2F
A mixed solution con-taining 28% aqueous ammonia (50 ml),
amrnonium chloride (8 g) and ethanol (50 ml) was cooled to -5C.
and the compound (9.1 g) prepared in Experiment 16 was added
thereto with stirring, and -then Eurther stirred at the same
tempera-ture for additional 3 hours. Water ~100 ml) was added
-to the reaction solution. The solution was extracted thrice
with methylene chloride t50 ml). After drying the extract with
addi-tion of anhydrous magnesium sulfate, the solvent was distilled
off. The residue was washed with ethyl ether and dried to obtain
-the obejctive product (3.4 g).
A portion of the product was dissolved in ethanol, and
glacial acetic acid was dropped thereto_with stirring. The
resultant precipitates were recovered by fil-tration and washed
with ethanol, followed by drying to obtain an acetate of the
subject compound. The following data of physical properties
are those of the acetate.
Melting poln~t: 125 - 127C
Infrared absorp-t1on spectrum (cm 1, Nujol):
3200, 1670, 1570
NMR spectrum ( ~, DMSO-d6)
1.90 (3H, s), 5.95 (2H, d, J=54.0 Hz), 7.40 (3H, b)
59
:
~: : :
r~ 3 ~
~xperlment 18 (SynLhesis of the raw material cornpound)
2-(5-Amino-1,2,~1-thiadiazol~3-yl)-(E)-2-fluorometoxyimino-
acetonitrlle,
N- C CN
N N
H2N ~ S'/
OCH2F "
The compound (3.0 g) prepared in Experiment 17 was dissolved
in methanol (50 ml), and triethylamine (4.2 g) was added thereto,
After cooling the solution to -5C, bromine (3.5 g) was dropped
to the solution. A solution of potassium thiocyanate (2.1 g)
in methanol was -then dropped thereto at a -temperature from -3C
to -5C, and the solution was stirred at the same temperature
for 2 hours. The resulting precipitates were recovered by
filtration and washed with wa-ter and with methanol. The
precipitates were then recrystallized from acetone to obtain -the
objectlve product (3.4 g).
Melting point: 236 - 238c.
Infrared absorption spectrum (cm 1, Nujol)
3450, 3250, 3075, 1610, 1520
NMR spectrum (~`, DMSO-d6):
6.02 (2H, d, J=54.0 Hz), 8.32 (2H,b)
::: :
~ '
:
160
:
.
..xperiment 19 (Synthesis of the raw materi.al compound)
2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoxyiminoacetam..ide
N ll C CONH2
N N
H2N \ / OCH2F
To a solution of sodium hydroxide (0.23 g) in water (18 ml)
was added 35% aqueous hydrogen peroxide (7.4 ml). And;~ the compound
~2.0 g) prepared in Experiment 18 was added -thereto with stirring
at room temPerature. The solut.ion was further stirred at a
temperature from 2SC to 30C for additional 8 hours. The
precipitates deposi-ted were recovered by filtration, and washed
with water and with acetone, followed by drying to obtain the
objective produc-t (1.3 g).
Mel-ting point: 210 - 211C.
In~rared absorp-tion spectrum (cm 1, Nujol):
3450, 3260, 3180,1690, 1610
:~ MNR spectrum (~ , DMSO-d6):
5.73 (2H, d, J=55.0 Hz), 7.69 (2H, br),
7.98 (lH, br), 8.10 (lH, br)
;~
~ 161
)'s~
E.Yperirnent 20
2-(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-2-
fluoromethoY~yiminoacetic acid
N , C - ---------COOI]
~ N N
/ \S / ~OCH2F'
~l2N
A mixture containing the compound (1.1 g) prepared in
Experiment 19 and 2N aqueous sodium hydroxide solution (10 ml)
was stirred at 50C for 5 hours. The react~on mixture was cooled,
and adjusted to pH l.0 with concentrated hydrochloric acid,
followed by extraction thrice with ethyl acetate (20 ml). After
addition of anhydrous magnesium sulfate to the extract, followed
by dryingJthe solvent was ditilled off. The residue was washed
with isopropyl ether to obtain a crude product (0.8 g). The
crude produc-t was purified by reversed phase silica gel colurnn
chromatography to ohtain the objec-tive product (0.4 g).
The infrared absorption spec-trum and NMR spectrum were
identical with t~hose of Experiment 13.
:
16~
, ,
t7
xample 162 (Preparation of an injection)
The compound (10 g) prepared in Example 1 was dissolved in
distilled water (50 ml). The solution was divided and infused,
so that the respective vial rnay contain 5 ml o-f the solution.
This solution was lyopllilized to give an injection.
Example 163 (Preparation of an injection~
The compound tlO g) prepared in Example 151 was dissolvbd in
distilled water (50 ml). The solution was divided and;infused,
so as -to contain 5 ml per one vial. This solution was lyophili~ed
to give an injection.
The acu-te toxici-ty and the anti-bac-terial activity of the
compounds according to this invention were determined as follows:
(1) Acute toxici-ty in mouse:
The compoundsaccording to this inven-tion dissolved in a
physiological saline solution were intravenously dosed to five
ICR male 6 weeks-old mouse. As the result, the values of acute
toxicity o~ the compounds prepared in the following Examples were
all ln excess of 2g/kg.
Example Numbers:
1, 2-1, 2-2, 3-1, 3-2, 5, 6-1, 6-2, 7-~, 7-2,
9-1, 9-2, 9-3, 10, 11, 12, 13-1, 13-2, 96, 121,
137, 145J 146, 150, 151, 159 and 155
(2) Anti-bacterial activity (MIC):
MIC (~Ug/rnl) were determined by an agar dilution method
[Chemo~therapy (Japan), 29, 76 - 79, 1981~. Overnight cultures
of the bacterial strains in Mueller-Hinton broth were diluted -to
163
. ~
::
5''~
final concentratioll of about 106 CFU/ml, and 5~1 of each bacterial
suspension was spotted onto Mueller-Hinton agar plates that
contained twofold serial dilutions of antibio-tics. MICs were
measured after incubation for 18 hours at 37C.
As the controls, CAZ (Cefatazidime) and CTM (Cefotlam) were
selected.
:
: ~
~; :
:: ~
~:
:: ~
, ~ ~
, .~ ¦ , 31.13i.,~
'n ¦
~u c o ~ w
~ .,, l cx~ In In oo Ln oo C10
5n ~ O ~ ~-- O ~--5 0 0
OJ .
__
~a :''
D C r O O O O O ~ O O
h E ~ O O O O O O O
. ~
~ ~, _ _ . _. ; _ _
~'5 u)
\ c u~ ~ L) o5 In ~, ~ N
~ ~'lu u~ o o o o o o o
a ~ \/11 ~/11
E
----_ . ._ . .. _ ~ ._ .~ _
.; t_) O ~ o ~ ~o N 15~ N C~-5 -5 C~-5 C`J
I_ C tL~ O O O O O O O
51 \/~ \/51~
~ .~ .. __ __
:~ '5
h 1~ C~J ~ o o o o o
: ~ C H O O O O O O O
. u z \/11 V,l \AI \/11 \/11
:;, . .
, ~: ~n
a D ni
,U C O C`~ -5 C~.-5
~ .a ~ o o' o o o o o
c : v)
cs - . . . ...... ,
O Ih)/ C~ 1 ~ c~ ,_,
~n a ~ ' ~ E E ~
~ ~/ _ ~ x c~
_ _ . _ _ . _ .. __
/G ~
~3(~
o ~1
C o 00 00 CO 00 GO CO CO 00
~' ~" [LIJ1
n, ~, "
a :,~
C C r , , O O O 0
O L~ O O O O O O O 0
~:
~ _ _ . _ _ __
~ U)
\ C N N C~ N C`J C~J N C~3
U~ O O O O O O O O
a) o l o o o o o o o o
~ u~ ~ ~ \AI \/11 ~/11 \./11 \111 \AI \/11 Vll
E
._ ,__ ._
~ ~
~ C U~
a) O ~D C'~ C~ N C~ ~ C~ N N
E X o O , . o o O
. ~ \/11 \1~1 ~11ll \/11 \~1 \~1 \AI \1
..- ___
~1
O ~ L~
U 1~ N C~
~: ~ 1~ O o ~o o . O o
~u z \/11 \/11 \/11 \/11 \111 \/11 \AI
. L~
.,
: ~ C~.
. a~ C~
: D~ o O O o O O O O O
V~
1 ~ _ .
IJ U /~ ~ ~ C~J ~ C`J ~ C`l ~
U L~ _
1 6~
~3j~ t~
~ ~i o ~ ~ ~
~ . ,
. .. .. _ .. _
r~ rl C~ ~S U~
a) c ~ o o o o o o o o
C ~ O O O O o o o O
O h \/11 \/11 \AI \/11 \/11 \/11
~ ~ ._... __ ' .._
~ Ul ., ~
\ h I u~ ~ O 0 ~1 0 0 0 0 0
~ ~lu u~ o o., o o o o o o
~,~ E t-l \/~
.: : _ _ . _ .-- - -- ~--- - --- ---- - - - :
1~) Il) IS~ If) U~ Lr~ Sl~l, Lr~ Lf)
(_) Q C C Co~ Co~ Co Co`J ~o o o
~1 E u:) O O O O O O O O
~'~ ~ ~, ~CU [Ll \/11 \/11 \~11 \/11 \~1 \,/11 \~11 \/11
s)~ . .
~: ~ .. __ _ . . _ . ~
: ~
.' UO
i: S-~ . ~ O O O O O O O O
~: C H O O O O O O O O
-~ ~ u 2;
; ~ . ~
~ _ - .~. _ ._ . __ __
~ ~ ~S
: I ~ ~ ~ ' O 0~ O' O 0
~:~:: '1 O
: u~ : '
~ ~ a ~
E ~,1 C~l I I ~ ~ C`~
~u,~ ~ 8 ,x~
:: -:
.
: ' , ,
. .
,
-_ ~ O r~ .
~ rI O rx~r~jO r,~o r~ IS) '.~1 I
rl~ ~ I)J O O O
J.
~1 .~
C C ~r Lr) u ) ~ u~
r U~ rl, O O Co O O O O
O
O E ~1 O O O O O Ci O O
. ~ \/11, \AI \AI \AI ~/11 \AI
~ C . .____
\ a) c~l c~ c~ c~
, U ~n O O O O ~ C~J O O
~v' 'v ~ o o o o o o o o
h r~l ~Al\AI \111 \/11
ra ~
(~ ~ -C U~ n
V O ~o C`lC`J t~ C`J N Ll ) C~ C`J
.~. E Y O O o O 0. Oj Oj o,
; ~ Q. ~/11'/11~Al ~Al ~111 \/11 \/11
~ ~ _ _
U ~:: O o o o O
:' C H \AI \AI~/11 \/11 \AI \/11
. ~ ~ . __ . _ __ _ _ _ _ ___ . . _
r~a Q.
~a o O O O O Ci O O O
, :: u~
~ : ;l --~ r , ___
~ ~ j - E X C~J N
S
- ~a
~o o
.... _ _, . . ... _ ._ ' _.,_
. ~Ic~ . o o o o o o o o
L~ l o l [-1 \111 ~1 "
, ~_ . - - --- .- . . ._ ._ . .. _
n O ~ ~ ~ ~ ~ o
, ,,,_ .. -- . . . ._ ._ ._.._ . ~ _ . __
O ~'C ~ ~ g O 0~ 0 ~ O
,_1 E ~D O O O O O O O O
~ ~ r~ ~ ~Al \/11 '\/11 ~/11 ~/11
~ _- _ ._ _ ._ ... _ ... . ._
,~
L~ ~ C`J U~ U ~)
: C H O O O O O O O O
: u z \AI \/11 `\
: ~ , .
~ ~ ~ _ . .. _ . ._ ,_ ._ .__
h ~ C~ C~ ~ ~ ~ ~ ~ C~
~: r o O O o O o O O O
; ~ ~ Q
: ~ _ ,~ ..... -
~ .~~ ~ ~D ~ 00 O~ O ~ C~
~E E3 ~ C~ C~ J C~
~: ~ ~ E~ U ~ .. _ ... _ ~._ .. , ... . _
~ . ._
, C o
U~ CO
o ~ o ~ o
Q~ ,,
_ . _
a) 31 ~ ~ o o ~
o~
~ ~ o o o o o o - o o
h h , \/~
_~ ~ . _
E~ v~ L~
\h (n r-- ~, ~ ,--1 C~ ~1
t~otl7 U 1~ O O O O O O O O
: E hl \
. .
O ~ ~ , o O O C\l C~
~1 E ~o O o O o O O O O
~ ,_ ~ ~,, ~/11 \/11 ~Al ~/11 \/11 \/11
:~ ~ - - _ - -- - --- --- - - _
~; : ,(
~ ~ h 1~ O O O --I 0 0 0 0
: ~ ~ : O O O O O' O O O
; ~ ~ h , \~ ¦
~; :~ ~ ~ ' ~- '-'--- - - ~-- ' -- _ __
~ ~ l d'
~; ~,~a ~ ~
r .
~ I h
~ ~ '
~3~J~i~
~'1,, o
~ I ~` rl~ ~ o ~i ~, o o ' ~ o
~ .
. ... ~ ........... . ... . _
m ¦ O O O O O O O O
., C ~ O O O O O O O O
h E \/~
., _. . _.. . .. __ .. . ._ . ; . . _ _
~ C
\ ~JIaU) u~ ~ l O ~ ~0 ~ C`l
~iV~ I U V~ O O OO O O O O
E W \AI
. ._ _ ,, , ..... _ ,_ . _ ._
V ~''C O ~ O 0~ 0 ~ O O
~ E ~D O O O O O O O O
~ ~ :1 ~: \/11 ~1 \/11
I ~ . -.
~ ~ - ~ . .__ ._ ___ _
r l H o O O OO O O O
~ : Ul Z
I ;
_ _ .. ~
3 : :
~ ~ ~ ~ o ~ o~ o
~ ~a N
1 U)
=
~: ~
6~l'
. :
.
- l ~ 3~
~:) C o ~ D ~D
~Vj'~ [-~ ~ O, O) O
t~ h ~
__ ,
r C I ~r O O O ~ O o O O
C 1_1 O O O O O O O ~O
h L~ W \/11 \AI \/11 \AI \/11 \111
\ 1 U ~n O O O O O O ~ O
rv I a) u~ O O O O O O O O'
E IL1 \/11 \111 \/11 \/11
.. . . . _ , _ . ,, __ . .__ _ ._ .. __
~_) Q C LoO Co~ eO~ o U~ o
V ~0 ~o O O O O O O O O
~ - .~ x \/11 \J~
~ ~ ~ ~ - . _ , _ ..... _. _ .. _ _ . .. .___ _
¦ ~ U T ~ O . o O O
~ ~ . t3~ Z \~/11 \JII \/11 \/11 \/11 \/11 Vll
~ - ... - ,
: ~ n~ c~
::: : : ~ -l o o o o~ o o o o
~ : r~
;; :
-- ---- ----
l 7 ~
.3
. o ,~
'1 ) ri O ) CO 00 Ot l 0~
a ~S o o o o o o
a) ll
~.
......
~-1 ,~ N N N C~ C~J N
aJ c ~r O O O O ~=) O
' C ~ ~-1 O O O O O O O O
h L~ ~ . \1~ '
O O ~ ,,
,
._ ~
~ .,
~ C
\ a) C~
\ , 1~ U~ O O O O ~ O O ~i'
C~h a) I~ O O O O O O O O
v~ U v~ \~1 `\AI \/11 \111
E
.. _. . _ . . .
a) Lr~
~ C~
(_) .R r O O O O O O O O
a) o tD O O O O O O O O
I_ ~ r-l E ~C \AI \AI \AI
-. .
.
:~ ~ rl
O 1~ U') lS~ l~ LO Ll~
U C~ C~l ~J N N C~ U~
. O O O C~) O O 0 ~1
a~ . ~ o o o o o o o o
S H \~1 \/11 \/11 \/11 \/11 \/11
: ~
. . __
: : _ . ~ ~
: a)
h ~t~ ~I C~ C~3 ~ ~ ~ 00
D~ O ~ O O O O O O
:,~
:::
~ ~ u~
E
~:: rh /~ a
a) / j ~D ~ 00 C~ O C~
r~dc ~ ~ e-
....__ ~.
/~ 3
.
.. ~ ~?~
. O ~ CD C~r.~ tD ~D r. ~
ra r _ Ln r-~ ~ Ln l.n ~ Ln Ln
r~ Ll n, r~l C~' ) C~ r--l r--l ~ r--~ r--J
'a . . . _
.. ~ Lr) Ln Ln Ln
~-1 .~ Ln N C~ Ln N
~1 'C ~1~ O ~C~l O O O O O
' C rr 1_1 O O O O O O O O
, ~ O [L~ , ~A , \/11
. ~
~ rn Ln Ln
\ L; I rn 1~ N O r--I r--l O
~ '`"I '
~ E .
-_ ,
I ~ ~ O O O O O O O
,_ c ~ \/11 ~/11 \/11 \/11 \/11
. _ _,
h H N C~ o C~oJ L L
hl \/11 \,/11 ~/11
: ~ : 1~ _ ___ _ ._ _ ,. . _ ._ _
LJ ~4 O O O O O O O O
Q r~l
V
'~ ~ _- - .. _ -. _._ __ .
rlJ,~ E u~ ~ ~ ~ ~ c~ ~ Ln
U ~L7 00 00 a:~
- ------ - ~
~ 7y
l - - ---
' 1 ~ ~ ~
c~ h7 o o, CoO o LO CO 0~ 00
~J .,
. . _ _ ..... ... ._ ._
I ~ ~ c r O O O N o N N
h h n. \111 \/11 \/11 ~./11 \/11 \/11 \111
O E
. ... _ ._ ._._ _ '
V7 .,
~ Ln
\Q~ C\l C`J ~ N C~ N C~ N
L; V7 ~-- O O O O O O O O
V~J7 U l O O O O O O O O
hl \AI \/
: ~ .. _ _ _ _ _ _
~ L~
(~ . ,~ N N C~ C~l C~ C`3 C~ N
.: ~1 E ~ O o o . O o
~ X ~ LX7 \/11 \/11 \/11 \~1 \/11 \/11 ~Al \/11
' . .
~ ~ _ . . ._ _ ._ .
U ~ ¦ N N In N N N
u z \/11 \AI \/11 \/11 \Jll \/11 .
~' ~ ' .
~: __ - . __ _ ._ ._
~7
~: : ~ a~ C~, C\l N N N N N C~
: r o - O O O O O O O O
V
~ .... ~ .. __ ,,
/~, ~ ~ I O ~ N C
3~ ~ 8
~: . -
. ~
I1~ LB'7
ta ~1
C~ o
,~ ~ ,~ o o o .
~v .,
. .. _ . .. . ~
U C I ,, O O O O O O O O
' c ta ~1 O o o o o o ti O
O E c~ \A~
____ _ ._ . . ._ .... . _
\ Lt I U~ t` O O O O O O O O
~ rU I (U t I O O O O' O' O O' O
~ E ~3 \AI \~ 1 \AI
. .._ ~
t_)R I C O O O O o C~ N
(U E ~o O O ~ O O O O O
: ~,Y C ~ \/11 \~1 \/il \/11 ~/11 \/11 \~11
G
~ ._ _ - _ ._ . __ - .. .. _ .
~1
O lr~ C~ C~ l.Q N N 11~ N
O O O O O O O O
(U '~ O C) O O' O O O O
rnU Z ~/11 ~AI \/11 \/11 \/11
~ .
. _. . ... ~
: ~ ~ :~
) t tl. ~ C~J e~1 C`l t.~ N C~
': ttJ rl O O O O O O O O' .
tJ~
~ ~ ~ _ ___ .
JJ / 0~ E L~ C~7 ~ t t 5~ ~ C~l
~,~ tn o ra t~ Cl) c~ 0~ 0 0
~ _ . _ _ .__~
1 7~ .
~,
~ C ~ o --1 r~l U~ r--I (~
~ U~ U~
~ :
;~ ~
~ c ~ o o ~o o ~o
~o o o
c ~( o o o o~ o
o o o
~ ~ w ~1 ~1 ~Al ~1 , \AI ~1
O E
,- , _ _ .- ~.- - . ._ .. _ . ._._ _
~ C U~ - U~
\ ~ C~ U~ C~
h !n ~n O ~ O ~ O O O C~
u~ U O O O O O O O O
E
.. _ ._ . _ ..
a C`J u~ J N C~l ~ u~
C),q c o o o o o o o o
,_1 E ~o O O O O O O O O
~ I ~ :~ w , \111 \/11 \Jll \AI \~1
~ _ ... _ . _ _ ..
~1
h ~ O O O O O O O O
-C H O O O O O O O O
: u~ z ~1 \AI ~1 ~Al
. .
: ~ ~ . . - , .. - .
: ~ ~ ~ ~ ~ C~l ~ C~3 GO
~: : ~ o o o o o o o o o
`:: : u~ :
~ i ~___ __ _ __ _ _ _n___ . _. _ _ _
U ~ . & E O O `O ~1 ~1
U1 ~ _~ ~ E 111 ~ ~1 ~
:~ ~ 8 ~
_. _
- ~77
: :: .
__ _ . ..._ .... . . .. .. . _
~33
~ O O ~ c~) ~ tx ) L~
w O O O O O O _ O _ O O c~ O
1~ h l
_ .. _
ta :,~1
C ~ U~ Ln Ln Ln Ln U~ Ln
C _l CJ N N N N C~J Lt~ u~ N C~ C~ Ln C~
~ ~ 1~ O O O O C~ O O O O O O O O
s~ o ~ ~ o o o o d o o o o o o o
~ ._ _ ~
~ .,
\ 41 ~ L~ In ~ Ln ~ Lf)
~o ~ ~ l ~ o o ~ o o c~ ~ o o o ~ o
[~ o o c~ o ~ o o o c) o o o o
~ E \~11 . \AI
.. - _._ . . _ .... _ .. . . .. _
a)
~> . ~ L~ L~ L~ Lr)
R C u:~ N N C~ N C~ C`~ L~
r~ O o o O o O o O O O O O Ci
~ ~! \/1 \A! \~1! \/11 \AI \/11 Vll ~/1! `vli \11!
_ . ,.. _ . . .. _ ._ .... .... . . I
o ~ Lr~ Lr)U C~l N N C~ C~lC~3 L~ Lr~ N N ~ Lt~ N
h ~ O O O o o O O O O O O O O
~J H O O O O O O o O O O O O O
w z
. .,
: ~ I --_ ._ . . ._ _ .. .. ... .
: h C~ C~ ~1 N N `~ l ~ ~1 ~ N C~
O C~ O O O O C~ O O O O O O'
O
U~
: ~ _ , . ._ .~
~ Cl~ I N N N 1` N N N N N ~ ~ l
'`~
1~
:,
.
,
~ ~ ~.~3~
,~j' o~ ~, ~,
,
~ o o
,~ . _
~ ~ a ¦ ~
_
' ~I)
o o
: ~, . ___
~ 1 ~
`\
. O ~1 ~D tDtD CD tD CD tO tD CO
~a c O Ll~ ~ tr~ tn m u~ Lr~ ~ Ln oo
.~ P~ ~ o
. _ .__. . . .
C! ~r ~ O O ~ O ~ O O _
C ~, ~ O O O O O O O o C~ G
O E Pl
,_ ~
,~ ,
\ C u~ ~ O C~ O ~ ~ O O ~ ~
~ ~ O O O O O O O O O I
._ _ . ~ . . _.__ . _
.~ ~
O ~ C . U~
~ O ~D ~ O O O C~ ~ O O ~ ~
X E X O O O O O O O O O O
Q.
__-_ .......... _ ... _ . .. - ._ ._
U.
U ~ OOO'C~OOOOOO
~ :~ ~
~ _ . _ .. _ ._ _ . . .___
~: :~1
:~
~U N O O O O O O O O O O
= Ul _
~: ~ /~ ~
~ ~ U) ~ E E ~ ,,1 ~ Ul N ~ ~,
~ ~: ~ _ _~ U _
: .___ ~_
1 ~0
~ '
~.3~ B~
__ ._ I_ __ _ .. . .. ..
~u,
~ O o U~
~ CLI U~ CO U~ .~, U~ oo o~
U ~ ~ o ~ o o
c :~ Lr) ~n
~ ~ Q. _ ~ ~ _ ~ O ' O ~
h O ~3 O O O O O O O O
__ . - .,
E~ cl
\ - L~ U In ~ ~ C~
~ ~ ~ v~ o o o o o o o o
E W
~ .
t ) ~ I L ~ C~ ~ C~
E ~D O _ _ O O O O O
~ ~ o o o o o o o o
,_, c ~11 ~1 .
;~ . ., ~ -- --- -
r~ , C~
: o ~ _ ~ o o o o o
~; 1~ o o o o o o . o o
~s~ z ~1 ~1
. .
a~ ~
~ l o o o o o o o o
~ o
u~
1 ~1
'; ~ ~ , :
U~C~ ~ '7
~ ~ ~ o
a. ~a il.1 o
___~ ~
~ ~ I~~
h h a, o o
1 \ ~
~ E
._. _ ~ . .
c~~ I c ~ ~ ~
' ~ ~ o o
. . _ .. _ . _ ___.____.. ~
~0~ ~ ~
U I Z o' o
1 ~ 1 ~ ~
~iY, , / ~
;''` '
: :
~ ~ '
~3~S~
3. Antl-bacterial activity (MrC):
Comparison tests oE the anti-bacterial activities were
conducted between the compounds of the following ~ormula according
to the present invention and the corresponding control compounds
wherein Rl represents methyl group. Measurements of MIC are the
same as described in the above item 2. The results are shown in
the following table.
N , --- C CONH~ S
H2NJ~ ~ O--Rl ~--CH=CHCH2-A
COO
~: :
a3
:
~3~-~ t~
__.__ .. ,_
~0 ~ o n
C~ ~ r-l ~D O ~I Ul ~I O
_ . . , /\,_
U~
_ (~1 ~ 111 0 U) ~O U~
E L, O r~ O o u ) o ,~ (~1 m ,-1 N
~ ~Y I~ ~ ~ ~
~'U O o O O o ~ O O
~ ... _ . .___ . __
~D . .
,~ ~ r-l ~ U~ In ~ U~
V~ 111 W r~ ~D ~ ~D ~D ~1 ~1 ~D
: ~D ... .. _ _ ,_
a~ ,~ ~ ,~ ~ ,~ ~ ,~ ?
,_~ ~4 E h CL~ E Ccl 6 ~? ~ E ~?
~ ~: ~ t, 3 ~. . ~ ~ ? 3 ~ n? ~ C v~ ,
~: t ,~ - ~
, ~ ~
` ~ ;'
, . . . . .
.
.
.
, , ,