Note: Descriptions are shown in the official language in which they were submitted.
1 3 0 7 7 6 2 FP-1555
Method for evaPoration treatment of PhotoqraPhic
processing waste solution and device therefor
BACKGPCOUND OF THE INVENTION
This invention relates to a method for evaporation
treatment which comprises carrying out evaporation
treatment of a waste solution generated with developing
processing of a light-sensitive photographic material by
means of an automatic processing machine for photography
Sherein abbreviated as photographic processing waste
solution or waste solution) and a device therefor,
particularly, to a method for evaporation treatment of a
photographic processing waste solution and a device
therefor which is suitable for treatment by arrangement
within an automatic processing machine or in the vicinity
of an automatic processing machine.
Generally speaking, photographic processing of a
light-sensitive silvér halide photographic materiai has
been performed by a combination of the steps each ~
employing a processing solution having one or two or more
functions of developing, fixing, water washing, etc., in
the case of a monochromatic light-sensitive material or
color developing, bleach-fixing Sor bleaching and
fixing), water washing, a stabilizing, etc., in the case
of a color light sensitive material.
1 307762
-- 2 --
And, in photographic processing which processes a
large amount of light-sensitive materials, there has been
employed a means to maintain the performance of a
processing solution constant by supplementing the
components consumed by processing on one hand, while
removing the components which are thickened by dissolving
out into the processing solution or evaporation by
processing (such as bromide ions in a developing
solution, silver complexes in a fixing solution) on the
other, thereby maintaining constantly the processing
solution components. For the above replenishment, a
replenisher is supplemented to the processing solution,
and a part of the processing solution is discarded for
removal of the thickened components in the above
photographic processing.
In recent years, the replenisher, including
washing water which is the replenisher for water washing,
is changing to a system in which the supplemented amount
is reduced to a great extent for the reason in pollution
or in economy, but the photographic processing waste
solution is led from the processing tank of an automatic
processing machine through a waste solution pipe and
diluted with wacte solution of washing water or cooling
water of the automatic processing machine, etc., before
discarded into sewage, etc.
However, on account of tightened pollutative
regulation in recent years, although washing water or
cooling water can be discarded into sewage or rivers, it
has become substantially impossible to discard other
photographic processing solutions other than these [for
example, developing solution, fixing solution, color
developing solution, bleach-fixing solution ~or bleaching
solution, fixing solution), stabilizing solution, etc.].
For this reason, the respective photographic processing
dealers are asking professional waste solution disposal
dealers for recovery of the waste solutions with payment
1 307762
of recovery fees or providing installations for
prevention of environmental pollution. However,
comission to waste solution disposal dealers requires a
considerable space for storage of waste solutions and is
very expensive in cost, and further the installations for
prevention of environmental pollution have the drawbacks
that initial cost is extremely great, and that a
considerably vast space is required for installation.
More specifically, as the pollution treatment method
which can reduce the pollution load of a photographic
processing waste solution, there have been known the
active sludge method (e.g. Japanese Patent Publications
Nos. 12943/1976 and 7952/1976, etc.), the evaporation
method (Japanese Unexamined Patent Publications Nos.
89437/1974 and 33996/1981, etc.), the electrolytic
oxidation method (Japanese Unexamined Patent Publications
Nos. 84462/1973, 119458/1974, Japanese Patent
Publications No. 43478/1978, Japanese Unexamined Patent
Publication No. 119457/1974, etc.), the ion exchange
method (Japanese Patent Publication No. 37704/1976,
Japanese Unexamined Patent Publication No~ 333/1978,
Japanese Patent Publication No. 43271/1978, etc.), the
reverse osmosis method (Japanese Unexamined Patent
PUblication No. 22463/1975, etc.), the chemical treatment
method (Japanese Unexamined Patent Publication No.
64257/1974, Japanese Patent Publication No. 37396/1982,
Japanese Unexamined Patent Publications Nos. 12152/1978,
58833/1974, 63763/1978, Japanese Patent Publication No.
37395/1982, etc.), etc., which have not yet proved to be0 satisfactory.
on the other hand, for the reasons such as
restriction in water resources, elevation in water
feeding and discharing costs, simplicity in automatic
processing machine installation, working environment
around automatic processing machine, etc., photographic
processing by means of an automatic processing machine
1 3377~2
requiring no piping for feeding and discharging of water
washing outside of the automatic processing machine by
use of stabilizing processing substituting for water
washing (the so-called no water washing automatic
processing machine) is prevailing in recent years.
According to such a processing, it has been desired to
omit also the cooling water for temperature control of
the processing solution. Such photographic processing
using substantially no washing water or cooling water has
features that its pollution load is extremely greater as
compared with the case when water washing processing is
applied, because the photographic processing waste
solution from the automatic processing machine is not
diluted with water, while on the other hand the waste
solution amount is small. Thus, due to the small waste
solution amount, the piping outside of the machine for
feeding the waste solution can be omitted, whereby an
extremely great advantage can be exhibited that
compaction and simplification of the machine to the
extent available as an office machine can be
accomplished, with cancellation of all the drawbacks
which have been considered the drawbacks of the automatic
processing machine of the prior art, such that the
machine can be moved with difficulty after provision of
~5 the piping, that the space around foot is narrow, that
enormous cost is required for pipeline work during
installation, that energy cost is required for feeding
hot water, etc.
However, on the other hand, its waste solution has
an extremely high pollution load, and therefore it cannot
be discarded at all not only into rivers but also into
sewage in view of the pollutative regulation. Further,
although the amount of waste solution from such a
photographic processing (processing performing washing
with large amount of water) may be small, even by a
relatively small scale processing, its amount can be
1 307762
about 10 liters per day in processing of, for example,
X-ray light-sensitive materials, about 30 liters per day
in processing of light-sensitive materials for printing
plates, and about 50 liters per day in processing of
S color light-sensitive materials. Thus, processinq of the
waste solution is becoming an increasingly great problem
in recent years.
For the purpose of carrying out easily processing
of a photographic processing waste solution, a device for
evaporating water to dryness by heating a photographic
processing waste solution is disclosed in Japanese
Unexamined Utility Model Publication No. 70841/1985.
Even in such a device, since a vapor generated by
subjecting the photographic processing waste solution to
lS evaporation concentration or drying is discharged into
atmosphere, is poses a problem in view of prevention of
environmental pollution and a problem of worsened working
environment due to generation of objectionable odor.
Also, as an embodiment, the device is provided with a
means having an activated charcoal for adsorbing and
removing injurious substances in vapor. However, the
adsorption of injurious substances is not sifficiently
performed only by use of the activated charcoal, and the
activated charcoal has a problem of absorption of vapor.
Therefore, an exchanging frequency of the activated
charcoal is large and the cost concerning them is raised.
Moreover, since this device discharges hot vapor into
atmosphere, it requires a means for discharging vapor to
outside of room when this device is employed in room.
Also, this device treats the photographic processing
waste solution to dryness, and therefore had the
drawbacks such that a part of the photographic processing
waste solution components was converted to a tar which
was attached on a heating source or an evaporation kettle
wall to cause lowering in heat efficiency, that due to
the presence of a surfactant added into the photographic
1 307762
processing solution or dissolved out and accumulated from
the light-sensitive material, foaming may sometimes occur
to cause bumping, that objectionable odor is conspicuous
and excessive decomposition may sometimes occur, and that
breaking of the evaporation kettle may sometimes occur,
etc.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 and 10 is a schematic illustration showing
an evaporation treatment device for a photographic
processing waste solution of the present invention;
Fig. 2 is a flow chart showing another example of
the present invention;
lS Fig. 3 through Fig. 9 are each schematic
illustration showing the cooling treatment means of the
present invention.
SUMMARY OF THE INVENTION
The pricipal object of the present invention is to
further develop the thought of the evaporation treatment
device for photographic processing waste solution as
disclosed in Japanese Unexamined Utility Model
Publication No. 70841/1985 and at the same time clarify
various improvements for practical application of the
device, particularly to clarify a treatment method and an
evaporation and cooling treatment device of a
photographic processing waste solution which is optimum
for treatment of a photographic processing waste solution
in a photographic processing installation where a single
or a small number of automatic processing machines are
arranged. Further, another object is to provide a
treatment method and an evaporation and cooling treatment
device of a photographic processing waste solut on which
is free from the drawbacks or inconveniences, etc., as
1 307762
mentioned above and can process easily the vapor
generated by treatment of a photographic processing waste
solution to obtain distilled solution.
The method for treatment of a photographic
processing waste solution according to the present
invention accomplishing the above objects is a method
which comprises evaporating a photographic processing
waste solution by heating and cooling a vapor generated
by the heating by use of a cooling means to obtain a
distilled solution.
More preferably, the method of the present
invention further comprises absorbing a conentrate of the
photographic processing waste solution concentrated by
heating onto a carrier to perform solidification
treatment.
The evaporation and cooling treatment device of
the photographic processing waste solution according to
the present invention accomplishing the above objects
comprises a heating means for heating photographic
processing waste solution and a cooling treatment means
for cooling vapor generated by said heating means, the
device has a constitution in which said cooling treatment
means receives the vapor directly or indirectly from said
heating means.
~5 In the present specification, distilled solution
means a distilled liquid, including the case containing
components other than water.
A preferable embodiment of the method of the
present invention comprises (1) performing at least one
3U cooling treatment selected from (A) to (~) shown below,
that is (A) the treatment by means of a baffle or a
radiating plate, (B) the treatment in which the waste
water before evaporation concentration or drying
treatment is used as the cooling heat medium, (C) the
treatment which is carried out in a tank which stocks the
waste solution, ~D) the above treatment ~C) in which
1 307762
there are a plural number of stock tanks, (E) the
treatment in which the waste solution before evaporation
concentration or drying treatment is introduced into the
cooling treatment section to be utilized for cooling, (F)
the treatment in which a fan for air cooling is utilized,
(G) the treatment which is carried out through heat
exchange with the processing tank, the replenisher tank
or the dissolving water tank of the automatic processing
machine; (2) the photographic processing waste solution
containing a waste solution of the stabilizing solution
substituting for water washing; or (3) gas adsorption
treatment being performed before cooling treatment.
Preferable embodiments of the solidification
treatment of the concentrate according to the present
invention is (A) wherein the solidification treatment is
a liquid absorbing treatment onto a liquid absorbable
resin, (B) wherein the solidification treatment is the
treatment with addition of a solidifying agent or a
drying agent, (C) wherein the solidification treatment is
~ carried out by use of a pack for waste solution housing
at least one of the absorbable resin, the solidifying
agent and the drying agent, the concentrated soluiton of
the photographic processing waste solution being
solidified by absorption into said pack and the solid
being housed into said pack, or (D) wherein the
solidification treatment is carried out by throwing at
least one of the liquid absorbable resin, the solidifying
agent and the drying agent into an evaporation
concentration kettle.
Alternatively, according to the most preferred
embodiment of the method of the present invention, a
photographic processing waste solution overflowed from
the photographic processing tank`of an automatic
processing machine is stored in a stock tank, and
following the photographic processing waste solution
information obtained by detecting its amount and/or the
1 307762
photographic processing waste solution amount in the
treatment means, the photographic processing waste
solution is fed from the stock tank to the treatment
means, wherein it is evaporated by a heating means to
evaporate the photographic processing waste solution, and
its vapor is cooled to distilled solution according to
the energy-saving and efficient heat exchange method.
On the other hand, the treatment device of the
photographic processing waste solution according to the
present invention accomplishing the above objects
comprises a treatment tank for performing heating
treatment of a photographic processing waste solution, a
means for discharging from said treatment tank the heated
and concentrated solution of the photographic processing
waste solution, a receiving tank for receiving the
discharged solution, a means for performing cooling
treatment of vapor generated by said heating treatment, a
recovering tank for recovering a distilled solution
generated by said cooling treatment, and a control means
for controlling heating treatment according to the
progress of treatment of the waste solution.
Still another preferable embodiment of the
evaporation treatment device according to the present
invention is one wherein the solidification treatment
means is a waste solution pac~ housing at least one of a
liquid absorbable resin, a solidifying agent and a drying
agent for one treatment, having a constitution such that
said waste solution pack is thrown into the concentrate
of the photographic processing waste solution or that the
concentrate of the photographic processing waste solution
is introduced into said waste solution pack.
Alternatively, according to the most preferred
embodiment of the evaporation treatment device of the
present invention, the photographic processing waste
solution is stored in a stock tank, and following the
photographic processing waste solution information
1 307762
-- 10 --
.
obtained by detecting its amount and/or the photographic
processing waste solution amount in the treatment means,
the photographic processing waste solution is fed from
the stock tank into (evaporating concentration) treating
means, wherein the photographic processing waste solution
is concentrated by evaporation with a heating means, and
the concentrate is solidified within said treatment means
or by flowing out of said means, simultaneously with
cooling of the vapor to distilled solution to be
lU reutilized in the photographic processing steps.
In the present invention, it is preferred to
recover silver according to a means such as the
electrolytic method, the precipitation method, the metal
substitution method, the reduction method, etc., before
heating treatment.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Hereinafter, the present invention will be
described in detail.
First, the cooling treatment means 60 in the
present invention shown in Fig. 1 i5 described.
For coolinq of the vapor generated by evaporation
of a photographic processing waste solution, all kinds of
heat exchange means can be employed, including any of the
constitutions shown below:
(1) the shell and tube type ~multi-tubular type,
muffled tube type);
(2) the double tubular type;
(3) the coil type;
(4) the helical type;
(5) the plate type;
(6) the fin tube type;
(7) the trombone type; and
(8) the air cooling type.
The heat exchange type reboiler technique can be
1 307762
also used, for example:
~1) the vertical thermosiphon type;
(2) the horizontal thermosiphon type;
(3) the overflow tubular type (the kettle type);
(4) the forced circulation type;
(5) the insertion type, etc.
Further, the condenser type heat exchange
technique may be employed, and any of the following
systems may be employed:
~1) the direct condenser system;
(2) the tower built-in system;
~3) the tower top setting system;
(4) the separation system, etc.
It is also possible to use a cooler, and any
desired type of cooler may be used.
It is also advantageous to employ an air cooling
system heat exchanger and either one of
(1) the forced drafting system and
(2) the blowing drafing system may be employed.
In the following, preferable examples of the
cooling treatment means 60 are described.
~1) As shown in Fig. 3, a constitution wherein the
vapor Q generated by evaporation treatment of the
photographic processing waste solution P is passed
through a radiation plate device 62 having a large number
of fins 61 for air cooling.
(2) As shown in Fig. 4, a constitution wherein the
vapor Q generated by evaporation treatment of the
photographic processing waste solution P is passed
through the cooling section 66 such as a coil 63, etc.,
where heat exchange is effected with the photographic
processing waste solution P in the stock tank 30.
(3) As shown in Fig. 5, a constitution wherein the
vapor Q generated by evaporation treatment of the
photographic processing waste solution P is passed
through the cooling section 66 such as a multi-stage coil
1 3077~2
- 12 -
64, etc., where heat exchange is effected with
photographic processing waste solution P in a plural
number of stock tanks 30 and 31.
(4) As shown in Fig. 6, a constitution wherein the
vapor Q generated by evaporation treatment of the
photographic processing waste solution P is passed
through the cooling section 66 such as a coil 63 (or 64)
which sprays the photographic processing waste solution P
from a spraying member 65.
(5) As shown in Fig. 7, a constitution wherein the
vapor Q generated by evaporation treatment of the
photographic processing waste solution P is passed
through the cooling section 66 such as a coil 63 (or 64),
etc., against which wind is blown by means of an air
delivering means 67 such as a fan, etc.
(6) As shown in Fig. 8, a constitution wherein the
vapor Q generated by evaporation treatment of the
photographic processing waste solution P is passed
through the cooling section 66 such as a coil 63, etc.,
where heat exchange is effected with the photographic
processing solution used in at least one tank of the
color developing tank SD, the bleach-fixing tank BF, the
stabilizing tank substituting for water washing Sb or at
least one solution in the replenisher tank S.
(7~ As shown in Fig. 9, a constitution wherein the
vapor Q generated by evaporation treatment of the
photographic processing waste solution P is passed
through a heat exchanger 69 having heat exchange
particles 68 comprising a large number of glass beads,
etc., built therein.
~8) Otherwise, as to the cooling means by use of a
baffle, for example, a constitution wherein the vapor Q
generated by evaporation treatment of the photographic
processing waste solution P is passed between the baffles
as disclosed in Japanese Unexamined Patent Publioation
- No. 125600/1978.
1 307762
- 13 -
In the present invention, it is preferable to use
one of a combination of two or more of the heat exchange
cooling treatment means of these (1) to (8) and others.
When the gas adsorption treatment to be used in
the present invention is desulfurization treatment, said
desulfurization treatment may be either dry system
desulfurization or wet system desulfurization. As the
former dry system desulfurization, there can be used
various desulfurization methods such as the iron oxide
method or the soda-iron method and contact adsorption by
use of activated charcoal, silica gel, molecular sieve,
etc., or the oxidation desulfurization method, etc. On
the other hand, as the wet system desulfurization methad
of the latter case, either non-regeneration method or
regeneration method may be employed. For example, there
may be employed any of the method in which sulfur is
absorbed into an alkaline solution, the method in which
reducing property of hydrogen sulfide is utilized or the
mehtod in which a compound of hydrogen sulfide is formed,
etc~, and, as to the operational method, either one of
the methods such as the seaboard method, the cyclox
method, the vacuum carbonate method and the garbottle
method, etc.
The gas adsorption treatment to be used in the
present invention is not limited to the above
desulfurization treatment, but the zeolite adsorption
treatment, the activated charcoal treatment, etc., may be
also practiced for the purpose of removing ammonia gas or
sulfur gas.
In the present invention, the preferable
embodiment of the gas adsorption treatment further
comprises a means for reutilizing distilled solution R
including a gas adsorption means 50 such as a filer, an
adsorbent and a cooling means for vapor 60.
In the drawings, the gas adsorption means 50
separates and recovers injurious gas such as hydrogen
1 307762
sulfide, oxygen sulfide or ammonia gas (H2O, SO2, NH3,
etc.) contained in the vapor of the photographic
processing waste solution by use of various
desulfurization and adsorption techniques utilizing, for
example, zeolite adsobent, activated charcoal as
mentioned above.
According to a preferred embodiment of the present
invention, (1) at least one secondary treatment selected
from the following (A) to (I) is carried out to the
distilled solution, namely (A) activated charcoal
treatment, (B) UV-ray irradition treatment, (C) reverse
osmosis treatment, (D) oxidation agent treatment, (E)
electrolytic oxidation treatment, (F) aeration treatment,
(G) electrodialysis treatment, (H) redistillation
treatment, (I) ion exchange resin treatment is carried
out; (2) the distilled solution is used as the dissolving
water for photographic processing solution; (3) gas
adsorption treatment is conducted together with
distillation, preferably one or two or more of
desulfurization treatment, zeolite adsorption treatment,
activated charcoal treatment is conducted in combination.
Also, according to the most preferred embodiment
of the present invention, the photographic processing
waste solution overflowed from the photographic
processing tank of an automatic processing machine is
stored in a stock tank, and following the photographic
processing waste solution information obtained by
detecting its amount and/or the amount of the
photographic processing waste solution in the treatment
means, the photographic processing waste solution is fed
from the stock tank to the treatment means, where the
photographic processing waste solution is evaporated by a
heating means to be concentrated or dried, and the
resulting vapor is cooled and reutilized as distilled
solution in the photographic processing steps.
The activated charcoal to be used in the present
1 307762
- 15 -
invention may be any activated charcoal having adsorbing
capacity for at least one materials of benzyl alcohol,
ammonium compounds and sulfur compounds.
In the present invention, regardless of the
starting material and the method for activation, either
powdery or granular activated charcoal can be used,
preferably granular activated charcoal, particularly
preferably coconut shell activated charcoal and activated
charcoal having molecular sieve ability. The activated
charcoal having molecular sieve ability as herein
mentioned has slit-like fine pores, and the size of the
fine pores should desirably be 6 A or more, with the
width being 15 A or less. As to the activated charcoal
having such molecular sieving ability, reference can be
made to the description in Japanese Unexamined Patent
Publication No. 14831/1983.
As the adsorbable substance to be used in the
adsorption treatment, etc., in the present invention, the
following substances other than activated charcoal can be
also used.
(1) Clay substance
~2) Polyamide type polymeric compound
~3) Polyurethane type polymeric compound
~4) Phenol resin
25 (5) Epoxy resin
(6) Polymeric compound having hydrazide group
(7) Polymeric compound containing polytetrafluoro-
ethylene
(8) Monovalent or polyvalent alcohol methacrylic acid
monoester-polyvalent alcohol methacrylic acid
~9) Polyester copolymer
As to details about these substances tl) to ~9),
reference can be made to the description in JapaneSe
Unexamined Patent Publication No. 124639/1984
~particularly pages 62 to 66).
The UV-ray to be used in the present invention can
- 1 307762
- 16 -
be generally obtained by a commercially available UV-ray
lamp or UV-ray irradiation device or a halogen lamp,
etc., and is not particularly limited. The output of the
UV-ray lamp, etc., has been known to be 5 W to 1 KW, but
it is not limited thereto. Also, in the present
invention, electromagnetic wave and light with wavelength
outside the range of 190 nm to 400 nm is generated from
the UV-ray lamp, and it may be irradiated on the
distilled solution obtained from the photographic
processing waste solution. Also, Infrared-ray, etc., may
be used in combination.
~ he UV-ray lamp, etc., to be used in the present
invention can be also made a double tube.
In the present invention, by UV-ray irradiation is
lS meant W-ray irradiation of the distilled solution
obtained from a photographic processing waste solution by
use of a UV-ray lamp, etc., and these UV-ray irradiations
may be effected on said distilled solution continuously
or, if desired, intermittently.
In the reverse osmosis treatment to be used in the
present invention, various kinds of reverse osmosis
membranes, desalting concentration methods and devices by
use of reverse osmosis membranes can be utilzed without
any limitation.
The reverse osmosis device should preferably be
run under a pressure of 40 kg/cm2 to 55 kg/cm2 with
respect to separation performance and treatment capacity.
The oxidizing agent to be used in the oxidizing
agent treatment of the present invention is inclusive of
3~ metal, non-metal oxides, oxyacids and salts thereof,
peroxides, organic oxygen containing compounds, etc.
Examples of oxides may include nitrogen peroxide NOx,
anhydrous chromic acid CrO3, selenium dioxide SeO2,
manganese dioxide MnO2, lead dioxide pbO2~ osmium
tetraoxide OsO4, silver oxide Ag2O, copper oxide CuO,
mercury oxide HgO, etc. Oxyacids may be exemplified by
1 337762
hot sulfuric acid H2S04, nitrous acid HN02, nitric acid
HN03, etc. As the salt, there may be employed sodium
hypochlorite NaOCl, bleaching powder CaOC12~ potassium
e K2Cr207, potassium chromate K2Cr204, potassium
permanganate KMnO4, potassium chlorate KC103, potassium
perchlorate KC104, etc. Typical examples of peroxides
are hydrogen peroxide H202, sodium peroxide Na202,
benzoyl peroxide (C6H5coo)2l etc. There may be also
included substances which can take 2 or more kinds of
1~ valences, such as trivalent iron ion Fe3+, divalent
copper ion Cu2+, lead tetraacetate Pb(CH3Co2)4~ etc.
Otherwise, Fentone reagent (Fe++ + H202), dehydrogenation
catalysts (Pt, Se, zn), etc., can be also used as the
oxidizing agent.
The electrolytic oxidation treatment to be used in
the present invention is a method for oxidizing a
substance on the anode by electrolysis, and it may be any
system by way of increase of positive charges of cations,
decrease of negative charges of anions, polymerization of
anions, increase of oxygen atoms and decrease of hydrogen
atoms in atomic groups. The excellent point of such
electrolytic oxidation as compared with oxidation with an
oxidizing agent is that very strong oxidation can be
effected and also that there is little by-product.
The aeration treatment in the present invention is
to promote oxidation by air delivery into the distilled
solution of the photographic processing waste solution,
and it is preferable to make finer the air bubbles by use
of a distributor, etc., whereby the bubbling effect can
be improved to enhance removal efficiency of organic
solvents, etc.
The electrodialysis treatment of the present
invention is to charge the distilled solution of the
photographic processing waste solution into the chambers
each partitioned by a diaphragm between the cathode and
the anode of an electrodialytic cell and pass direct
1 307762
- 18 -
current therethrough.
Preferably, the diaphragms are ion exchange
membranes. More preferably, the space between the
cathode and the anode should be partitioned with anion
exchange membranes and cation exchange membranes to
provide a cathode chamber, a plural number of
concentration chambers (the chamber partitioned with an
anion exchange membrane on the cathode side and a cation
exchange membrane on the anode side), a plural number
desalting chambers (the chamber partitioned with a cation
exchange membrane on the cathode side and with an anion
exchange membrane on the anode side) and an anode
chamber. The distilled solution of the photographic
processing waste solution is preferably charged into the
desalting chambers, but it is also preferable to charge
it into the concentration chamber. The electrolyte
solution to be charged into the concentration chambers
and the anode chamber is not particularly limited, but
0.1 to 2 N solutions of, for example, sodium sulfite,
sodium sulfate, sodium chloride, potassium sulfate,
sodium thiosulfate, etc., can be preferably used. In
this case, a processing solution having fixing ability
(bleach-fixing solution or fixing solution) or its waste
solution can be very preferably used as the electrolyte
solution to be charged into the concentration chambers
and the anode chamber, because no electrolyte solution is
particularly required.
The redistillation treatment to be used in the
present invention is to carry out distillation treatment
of the concentrate obtained from the photographic
processing waste solution, which is one of the so-called
rectifying operation. It may be either batchwise
distillation (including simple distillation, batchwise
rectification) or continuous distillation, and it is also
possible to employ the continuous equilibration
distillation method for continuous rectification. To
1 307762
-- 19 --
obtain pure water (containing remarkably little
distillate other than water) by the redistillation
treatment will make it possible to feed effectively water
to the photographic processing solutions. Also, an
5 appropriate separating agent may be advantageously used
in azeotropic distillation and extraction distillation.
In the present invention, the secondary treatment effect
can be obtained also by the so-called steam distillation.
Also, as for the operational pressure, it may be either
of high pressure distillation, normal pressure
distillation, vacuum distillation and molecular
distillation.
The ion exchange resin treatment in the present
invention can be carried out by the contact betweent
various kinds of ion exchange resins and the photographic
processing waste solution, and the ion exchange resins
may comprise three-dimensionally polycondensed polymer
substrates having functional groups bonded thereto,
including cation exchange resin, anion exchange resin,
chelating resin, absorptive resin, etc.
As to the chemical structure examples of the ion
exchange resins preferably used in the present invention
and the methods for using them, reference can be made to
the description in Japanese Unexamined Patent Publication
No. 124639/1984 (particularly pages 54-57).
The carrier to be used in the present invention is
capable of absorbing the concentrate of the photographic
processing waste solution, preferably one which is free
from liquid dripping even when the liquid absorbable
carrier having absorbed a liquid is held by hands, and
the so-called high liquid absorbable resin is preferably
used.
As the high liquid absorbable resin, there may be
used, for example, seed polysaccharides, algae
polysaccharides, resin polysaccharides/ fruits
saccharides and route stock polysaccharides. There may
1 307762
- 20 -
be further included zansane gum, zanflow, guardrane,
succinoglucane, sizophirane, pullulan, gelatine, casein,
albumin, shellac, starch derivatives, derivatives of guar
gum, locust bean gum, and cellulose derivatives, alginic
5 acid derivatives, vinyl type compound, acrylic compound,
polyethylene oxide, etc.
Next, preferable examples of the high liquid
absorbable resin to be used in the present invention are
shown below.
(A) Grafted starch type
(A-l) Starch-acrylonitrile grafted polymer
(A-2) Starch-acrylic acid grafted polymer
The above ~A-l) can be prepared according to the
methods disclosed in Japanese Unexamined Patent
Publication No. 43395/1974 and U.S. Patent No. 4,134,863,
and the above (A-2) can be prepared according to the
method disclosed in Japanese Patent Publication No.
46199/1978. (B) Acrylic acid type:
(B-l) Sodium polyacrylate type
(B-2) Vinyl alcohol-acrylic acid copolymer type
The above (B-2) can be also used repeatedly by
natural drying and/or forced drying.
~C) Polymers having recurring units having the
structural formula represented by (I) or (II) shown
below, more preferably having 10 to 70 % by weight of (I)
and/or (II) copolymerized with another ethylenic
unsaturated monomer
(I) B
~C~2--C~ R2
3u (COZ) R~ ~ ~ B~ X
(II)
R
~CH2--C3-
C~)ZRI--SOa lt
1 307762
- 21 -
In the above formulae, R is a hydrogen atom, a
methyl group or a halogen atom; Z is an oxy group or an
imino group; n is O or l; Rl is an alkylene group having
1 to 6 carbon atoms (including also substituted alkylene
groups), a cycloalkylene group having 5 to 6 carbon atoms
or an arylene group, and arylenealkylene group or an
arylenebisalkylene group, wherein said alkylene moiety
has 1 to 6 carbon atoms and said arylene moiety (which
may be also substituted) has 6 to 10 carbon atoms, and
includes an arylene substituted with a hydrophilic polar
O NR5
group such as -NHCR5, -OH, -C-N, -~=0 or -C-O-M~ (wherein
R5 is an alkyl group having 1 to 4 carbon atoms); R2, R3
15 and R4 are each hydrogen atom or an alkyl group having 1
to 6 carbon atoms, or forms together with N a
heterocyclic group which can also contain optionally
sulfur or oxygen atom; M is a hydrogen atom, a soluble
cation or an ammonium group including quaternary ammonium
20 cations having alkyl groups having not more than 6 carbon
atoms; and X is an acid anion.
The halogen substituent on R can be bromine or
chlorine, the alkylene group having 1 to 6 carbon atoms
of Rl may be also substituted with hydroxyl group, the
arylenealkylene group of Rl includes phenylenemethylene
group, phenyleneethylene group, phenylenepropylene group
and phenylenebutylene group, and the arylenebisalkyl
group of Rl includes phenylenedimethylene group.
Examples of the soluble cation of M are sodium and
potassium.
Examples of the heterocyclic group formed by R2,
R3 and R4, and the ~ atom to which these are bonded may
include pyridinium, imidazolium, oxazolium, thiazolium
and morpholium.
The acid anion of X may include chloride, bromide,
acetate, p-toluene sulfonate, methane sulfonate, ethane
1 307762
- 22 -
sulfonate, methylsulfate, ethylsulfate and perchlorate.
The ethylenic unsaturated monomer to be
copolymerized with the monomer of the above formula (I)
and/or the monomer of the above formula (II) comprises
preferably one or more monomer having a crosslinkable
group such as 2-hydroxyethyl methacrylate, 2-hydroxyethyl
acrylate, and a monomer containing active methylene
group. The copolymerizable ethylenic unsaturated monomer
polymerized of this type is disclosed in, for example,
U.S. Patents Nos. 3,459,790, 3,488,708, 3,554,987,
3,658,878, 3,929,482 and 3,939,130.
The preferable polymer to be used in the above
description has 10 to 70 ~ by weight of recurring units
derived from at least one monomer enumerated below:
2-aminoethyl methacrylate hydrochloride;
N-(2-methacryloyloxyethyl)-N,N,N-trimethylammonium
chloride;
N-(2-methacryloyloxyethyl)-N,N,N-trimethylammonium
methosulfate;
Sodium 2-methacryloyloxyethyl-1-sulfonate; and
2-(N,N-dimethylamino)ethylmethacrylate
hydrochloride.
The acid addition salt coinciding with the above
structural formula ~I) can be converted to a free amine
when neutralized with a base.
The above polymer can be prepared according to a
conventional method by carrying out polymerization
reaction of a suitable monomer in an aqueous solution.
The monomer of the above structural formula (I)
can be prepared according to the methods described in
"functional monomers" edited by R.H. Yocum and E.B.
Nyquist, Marcel Dekker, Inc., New York (1974) and U.S.
Patent No. 2,780,604. The monomer of the above
structural formula (II) can be prepared according to the
methods described in U.S. Patents Nos. 3,024,221 and
3,506,707.
1 3 0 7 7 6 2
- 23 -
In some cases, the polymer can be prepared
by (a) quaternarizing a polymer having amine groups
with an alkylating agent, or alternatlvely ~b) by
reacting an amine with a polymer having a group
reactive with the amine, for example, an active
halogenic group. Such techniques are known in this
field of art and described in U.S. Patents
No. 3,488,706 and 3,709,690 and Canadian Patent
No. 601,958.
The resins as mentioned above are also
available as commercial products. Examples of such
commercial products may include Sumikagel N-100,
Sumikagel SP-520, Sumikagel S-50, Sumikagel NP-1020,
Sumikagel F-03, Sumikagel F-51, Sumikagel F-75,
Sumikagel R-30 (all are trade marks, produced by
Sumitomo Kagaku Kogyo Co.), Sunwet IM-300, Sunwet
IM-1000 (all are trade marks, produced by Sanyo Kasei
Co.), Aquakeep IOSH-P (trade mark, produced by
Seitetsu Kagaku Co.), Ranjiel F (trade mark, produced
by Nippon Exran Co.), etc.
The high liquid adsorbable resin preferably
used in the present invention shoud preferably have a
shape which can easily absorb liquid, and those
shaped in powder or granules with diameters of about
0.01 to 3 mm can be advantageously used in handling.
The solidifying agent to be used in the
present invention may be any one capable of
solidifying the concentrate of the photographic
processing waste solution, and the chemical reaction
may be either accompanied or not during
solidification. As the solidifying agent of the
present invention, for example, CaO, Ca(OH)2, CaCO3,
silica gel, calcium chloride, aluminum oxide, calcium
sulfate, magnesium oxide, barium oxide, granulate
soda lime, diphosphorous pentaoxide, etc.
1 307762
- 23a -
In the present invention, it is preferred
that the processing chamber 42 in Fig. 1 should be
constituted so as to store the photographic
processing waste solution to-----------------------
,~
1 307762
- 24 -
be treated or the concentrate which has been already
treated, an inner kettle or inner liner 44 formed of a
metal or a porcelain or a synthetic resin, etc., and the
treated concentrate solidified according to the present
invention as shown below in Examples, by use of a
separating means such as bag filter or pack, etc., and
taken out together with the inner kettle or inner liner
44 or together with the bag filter or the pack to be
discarded or disposed. Other than utilizing the above
mentioned inner kettle or inner liner 44, the discharging
means 43 can be designed variously. For example, the
concentrate of the photographic processing waste solution
is permitted to fall naturally into a vessel containing
one or two or more liquid absorbable resin, solidifying
agent and drying agent of the present invention through
the valve from the bottom of a known discharging device
utilizing rotary screw pump or the treatment chamber 42
to effect solidification according to the present
invention therein.
The inner liner 44 for pack may be preferably made
of a heat-resistant and chemical resistant material, such
as carbon fiber, aramide fiber, Teflon resin fiber, hemp,
glass fiber, polyethylene foam, polypropylene foam, etc.
Next, typical examples of the photographic
processing waste solutions which can be treated according
to the present invention are described in detail. In the
following description, photographic processing solutions
in the case of photographic materials for color to be
processed are primarily described, but the photographic
processing waste solutions are mostly overflowed
solutions discharged during processing of silver halide
color photographic materials by use of these photographic
processing solutions.
The color developing solution is a processing
solution to be used in the color developing processing
step (which is the step for formation of a color image,
1 3077~2
- 25 -
the step for forming a color image through the coupling
reaction of the oxidized product of a color developing
agent with a color coupler), and therefore a color
developing agent is required to be contained generally in
the color developing solution in the color developing
processing step, but the case of having a color
developing agent built in the color photographic material
and processing it with a color developing solution or
alkali solution (activator solution) containing a color
developing agent is also included. The color developing
agent contained in the color developing solution is an
aromatic primary amine color developing agent, including
amino phenol type and p-phenylenediamine type
derivatives.
Examples of the above aminophenol type developing
agent may include o-aminophenol, p-aminophenol, 5-amino-
2-oxy-toluene, 2-amino-3-oxy-toluene, 2-oxy-3-amino-1,4-
dimethyl-benzene.
The color developing solution may sometimes
contain an alkali agent conventionally used in a
developing solution, and further sometimes contain
various additives such as benzyl alcohol, alkali metal
halides or development controllers, preservatives.
Further, various kinds of defoaming agents or
surfactants, or organic solvents such as methanol,
dimethylformamide or dimethyl sulfoxide may be sometimes
conveniently contained.
Also, antioxidants may be contained in the color
developing solution, if desired. Further, in the color
3U developing solution, various chelating agents may be used
as the sequestering agent in combination.
The bleach-fixing solution is a processing
solution to be used in the bleach-fixing step ~which is
the step in which the metal silver formed by development
is oxidized to be converted to silver halide, and then
water-soluble complex is formed simultaneously with color
1 307762
- 26 -
formation of the uncolored portion of the color forming
agent), and the kind of the bleaching agent used in the
bleach-fixing solution is not particularly limited.
The bleach-fixing solution may sometimes contain
various pH buffering agents either singly or as a
combination of two or more kinds. Further, various kinds
of fluorescent brighteners, defoaming agents or
surfactants may be contained. Also, preservatives such
as bisulfite adducts, etc., organic chelating agents such
as aminopolycarboxylic acids, etc., stabilizers such as
nitro alcohol, nitrates, etc., or organic solvents, etc.,
may be sometimes conveniently contained. Further, the
bleach-fixing solution may sometimes contain various
bleaching promotors as disclosed in Japanese Unexamined
Patent Publication No. 280/1971, Japanese Patent
Publications Nos. 8506/1970 and 556/1971, Belgian Patent
No. 770,910, Japanese Patent Publications Nos. 8836/1970
and 9854S1978, Japanese Unexamined Patent PublicationS
Nos. 71634/1979 and, 42349/1974 added therein.
The stabilizing processing by use of a stabilizing
solution substituting for water washing according to the
present invention is ~ot the conventional processing in
which a large amount of water is employed for washing and
removing a processing solution of the pre-step adhered to
or permeated in the light-sensitive photographic
material. This is a processing carried out by
replenishing the stabilizing solution in an amount of as
small as 30 ml/m2 to 9,000 ml/m2, preferably 60 ml/m2 to
3,000 ml/m2 to the light-sentsitive photographic material
into the stabilizing tank to secure the effects similar
as or more than the above conventional processing, and is
specifically disclosed as an image stabilizing processing
in Japanese Unexamined Patent Publication No.
134636/1983, etc. ~ 6 /;
Accordingly, by use of the otab-lizji~g solution
substituting for water washing according to the present
1 307762
- 27 -
invention, there is no requirement to provide pipelines
for supplying from and discharging to the outside of the
automatic processing machine for water washing as in the
concventional process.
The evaporation treatment of the present invention
is preferably employed in conmbination with the
processing by use of the stabilizing solution
substituting for water washing, because an amount of
processed waste solution is small and hence a large
effect of heat exchange is obtained.
There may be a kind of stabilizing solution which
has a function for stabilizing the color image and a
function of draining bath for preventing the
contamination such as uneven washing. The stabilizing
solution may further contain coloring controlling
solution and antistatic solution containing antistatic
agent. When bleach-fixing components are brought from
the pre-bath into the stabilizing solution, there may be
provided any provisions to the stabilizing solution for
neutralizing, desalting and inactivating the components
to prevent a deterioration of dye.
As the component contained in such a stabilizing
solution, there may be included chelating agents having a
chelate stability constant with iron ions of 6 or higher
(particularly preferably 8 or higher). Such chelating
agents may include organic carbonic acid chelating
agents, organic phosphoric acid chelating agents,
polyhydroxy compounds, inorganic phosphoric acid
chelating agents, etc. Particularly preferably for the
3~ effect of the present invention, diethylenetriaminepenta-
acetic acid, l-hydroxyethylidene-l,l-diphosphonic acid or
salts thereof may be employed. These compounds may be
used generally at concentrations of about 0.1 g to 10 g
per liter of the stabilizing solution, more preferably at
concentrations of about 0.5 g to 5 g per liter of the
stabilizing solution.
1 307762
- 28 -
As the compound to be added in the stabilizing
solution, ammonium compound may be employed, these are
supplied from ammonium salts of various inorganic
compounds. As the ammonium compounds, there may be
specifically included ammonium hydroxide, ammonium
bromide, ammonium carbonate, ammonium chloride, ammonium
hypophosphite, ammonium phosphate, ammonium phosphite,
ammonium fluoride, ammonium acidic fluoride, ammonium
fluoroborate, ammonium arsenate, ammonium hydrogen
carbonate, ammonium hydrogen fluoride, ammonium hydrogen
sulfate, ammonium sulfate, ammonium iodide, ammonium
nitrate, ammonium pentaborate, ammonium acetate, ammonium
azide, ammonium-lauryltricarbonate, ammonium benzoate,
ammonium carbamate, ammonium citrate, ammonium
diethyldithiocarbamate, ammonium formate, ammonium
hydrogen malate, ammonium hydrogen oxalate, ammonium
hydrogen futarate, ammonium hydrogen tartarate, ammonium
lactate, ammonium malate, ammonium maleate, ammonium
oxalate, ammonium futarate, ammonium picrate, ammonium
pyrolidine dithiocarbamate, ammonium salicylate, ammonium
succinate, ammonium sulfanate, ammonium tartarate,
ammonium thioglycolate, 2,4,6-trinitrophenol ammonium,
etc. Amount of the ammounium compound added may be
within the range of from 0.05 to 100 g, preferably from
0.1 to 20 g per liter of the stabilizing solution.
As the compound to be added in the stabilizing
solution, there may be included pH controllers,
antifungal agents as disclosed in Japanese Unexamined
Patent Publication No. 43741/1986 pages 26 to 30, such as
5-chloro-2-methyl-4-isothiazoline-3-one, 2-octyl-4-
isothiazoline-3-one, l,2-benzisothiazoline-3-one and
others, preservatives such as water-soluble metal salts,
etc., dispersing agents such as ethylene glycol,
polyethylene glycol, polyvinyl pyrrolidone (PVP ~-15*
Luviscol ~-17, etc.), etc., film hardening agents such as
formalin, etc., fluorescent brighteners and so on.
e ~ '~
1 307762
- 29 -
Above all, in the present invention, a stabilizing
solution substituting for water washing containing an
antifungal agent as mentioned above may be particularly
preferably used, because generation of tar in the
evaporation treatment device is little.
When the light-sensitive material to be processed
is for negative, an aldehyde derivative may be sometimes
added in said stabilizing solution for negative for
improvement of storability of the photographic image.
1~ In the above stabilizing solution for negative,
there may be sometimes added various additives, if
necessary, including, for example, water droplet
irregularity preventives, pH controllers, film hardeners,
orgnaic solvents, humectants, otherwise additives for
improving and expanding the processing effects such as
tone modifiers, etc.
Also, a stilbene type fluorescent brightener may
be sometimes used in a color developing solution or
stabilizng solution for color paper.
The components contained in the waste solution of
the above color developing solution are various
components or additives as described above and the
components dissolved out and accumulated from the
~ photographic material to be processed.
The components contained in the waste solutions of
the above bleach-fixing solution and the stabilizing
solution are various components or additives as described
above and the components dissolved out and accumulated
from the photographic material, etc.
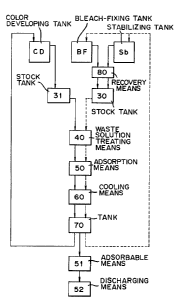
Next, referring to the drawings, described is an
example of the evaporation treatment device of the
photographic processing waste solution which is the most
preferred embodiment in practicing the present invention.
A).... Automatic processing machine
The automatic processing machine for which the
present invention is applied is indicated by the symbol
1 397762
- 30 -
10 in Fig. 1, and the machine shown in the Figure is of a
system in which a roll-shaped light-sensitive
photographic material F is guided continuously into a
color developing tank CD, a bleach-fixing tank BF and a
stabilizing tank substituting for water washing Sb to be
photographically processed therein, and after drying D,
wound up (although not shown in the drawing, the
automatic processing machine is inclusive of various
kinds such as the embodiment having a color developing
tank CD for permitting a short leader to guide the
light-sensitive photographic material, a bleaching tank
BL, a fixing tank FIX, a stabilizing tank substituting
for water washing Sb and a second stabilizing tank, but a
typical example is shown in the Figure).
11 is a replenisher tank, and the replenisher is
replenished to the respective processing tanks according
to the control device 20 by detecting the photographic
processing amount of the light-sensitive photographic
material F with a sensor 21 as described in detail below
and following the detected information.
The system of photographic processing, the
constitution of the photographic processing tanks, the
replenishing method of the replenisher are not limited to
those as described above, but the present invention is
applicable for other systems and constitutions, including
the so-called no water washing system as disclosed in
Japanese Unexamined Patent Publications Nos. 14834/1983,
34448/1983, 132146/1982, 18631/1983, 263941/1985 and
2153/1986.
Incidentally, though the most preferred embodiment
of the present invention is that equipped in the
automatic processing machine, when a large amount of the
photographic processing waste solution is processed
collectively, the processing is preferably carried out by
use of the treatment device of the photographic
processing waste solution as shown in Figure 10.
1 307762
- 31 -
Regarding to the device of Figure 10 is essentially the
same as the automatic processing machine in the present
specification and hence described simulataneously
hereinunder.
B).... Recovery of photographic processing waste solution
and silver recovery
When the replenisher is replenished to the
respective processing tanks, overflowed waste solutions
are discharged from the processing tanks and collected in
the stock tank 30. During this operation, the waste
solution in the color developing tank CD is charged into
the stock tank 30 without passing through a silver
recovery means 80 according to the silver recovery
treatment technique of the present invention, but the
waste solutions of the bleach-fixing tank BF and the
stabilizing tank substituting for water washing Sb are
subjected to the silver recovery treatment by the silver
recovery means 80 according to the silver recovery
treatment technique of the present invention as described
above before they are charged into the stock tank 30. In
the automatic processing machine shown in Figure 1, the
solutions overflowed from the upper portions of the
processing tanks by replenishment of the replenisher are
to be processed as the photographic processing waste
solution.
Provision of a plural number of silver recovery
means 80 or stock tanks 30, and provision of a plural
number of treatment means 40, utilizing one or two or
more the stock tank (for example, using them alternately
as the stock tank and the treatment means), etc., are
also included in the present invention. If a certain
amount is treated at a time by use of the stock tank 30,
the concentrated or dried photographic processing waste
solution can be uniformized, and the stock tank 30 is
useful as the buffer from the photographic processing
tanks to the treatment means.
1 307762
- 32 -
As a means for transferring the overflowed
photographic processing waste solution to the silver
recovery means or the stock tank 30, a simple method is
to permit it to fall naturally through a guide pipe.
S However, there may be also provided a means for
collecting the heat energy possessed by the photographic
processing waste solution by arrangement of heat exchange
means in the course of transfer, or alternatively a means
for preheating or evaporating the solution of the
photographic processing waste solution before collected
into the silver recovery means 80 or the stock tank 30 by
- - utilizing the heat energy of the automatic processing
machine or the evaporation treatment device as described
below. Also, it can be sometimes transferred forcibly by
means of a pump 23, etc.
Also, since the components in the photographic
processing waste solutions in the respective photographic
processing tanks CD, BF and Sb differ from one another,
the case of treating separately by preparation of stock
tank 30 for each photographic processing tank or for the
waste solutions of the processing tanks divided into 2 or
3 or more groups without treating all the photographic
processing waste solutions at a time is also included.
Particularly, with respect to recovery of silver, it is
advantageous to treat the waste solution of the color
developing tank CD separately from the waste solutions of
the bleach-fixing tank BF and the stabilizing tank
substituting for water washing Sb.
Also, by connecting a pipe to the already existing
waste solution tank in the automatic processing machine,
etc., the waste solution may be transferred forcibly into
the stock tank by means of a pump. Further, the waste
solution tank of the automatic processing machine itself
can be also utilized as the stock tank. In this case, it
is preferable to transfer forcibly the waste solution
through the pipeline by actuating the pump with detection
1 307762
- 33 -
of the weight of said stock tank. It is also preferable
to have a float floated in the waste solution tank and
actuate the pump by detection of the liquid surface at a
certain level or higher, because it can be easily set in
an existing automatic processing machine.
C).... Treating means
The treating means 40 includes a heating means 41,
a treating chamber 42 containing a means for storing the
photographic processing waste solution (concentrate)
during treatment or after treatment, a means for
discharging the photographic processing waste solution
which has been treated (concentrate) from the treating
chamber 42.
Also, it may be also made an example added with a
lS gas absorbing means 50 and 51 such as a filter,
adsorbent, etc., and a means for reutilization of
distilled solution 60 including a cooling means of vapor.
As the heating means 41, the heat source and the heating
(evaporation) means are important, but there exists no
reason to limit these to specific ones in application of
the present invention, but there may be included the
embodiments in which the photographic processing waste
solution is heated by utilizing one or two or more
combination of effective heat sources, such as
electricity, gas, solar heat, etc., to vaporize and
concentrate said photographic processing waste solution.
Also, various constitutions are possible from one in
which the photographic processing waste solution is
stored in the treating chamber 42 and heated as a whole
to, for example, one in which the photographic processing
waste solution is fallen or thrown (including spraying)
onto a heated body such as overheated metal plate, etc.,
to effect evaporation and one in which it is fed
~uantitatively to the heat source to be processed
continuously. Further, the photographic processing waste
solution may be evaporated by atomizing photographic
1 307762
processing waste solution in mist within the treating
chamber 42 and applying heated air thereto. Also, the
heated air may be thrown into the photographic processing
waste solution.
The means for atomization should preferably be one
which carries the photographic processing waste solution
on a heated whirling gas stream, preferably a spray
drying device.
The heating means 41 may be positioned above or
internally of the stored photographic processing waste
solution or outside of the treating chamber 42, etc., as
- desired.
Preheating of the waste solution may be performed
on the way from the stock tank 30 to the treating chamber
40 by utilizing heat exchange between the waste solution
and the vapor generated.
Other than the constitution utilizing the inner
kettle as mentioned above, as the discharging means 43,
various designs are possible such that the concentrate of
the photographic processing waste solution is permitted
to be discharged by use of the cischarging means
utilizing rotary screw pump or to fall naturally from the
bottom of the treating chamber 42 through the valve and
recovered by recovering container 45, followed by
addition of the carrier to effect solidification
treatment.
Also, the concentrate may be added with the
absorvable resin or solidifying agent to conduct
solidification and the solidified product is discharged.
Further, discharging may be carried ont through the
separating means such as bag filter.
In the evaporation method of the photographic
processing waste solution of the present invention,
particularly when the heating means 41 contacts directly
the photographic processing waste solution such as the
heat source built-in quartz tube or electrical heating
1 307762
- 35 -
plate of nichrome wire, etc., it has been found that the
photographic processing waste solution is secured onto
the surface of the heating means under the scorched state
to lower remarkably heat efficiency in the process of
concentrating or drying the photographic processing waste
solution. Also, the photographic processing waste
solution is liable to be secured on the thermally
conductive member which conducts heat from the heat
source to the photographic processing waste solution, and
further it has been found that corrosion is liable to
occur when the waste solution or its vapor contacts
directly the portion not related to heating.
Accordingly, the present inventors have conducted
various experiments to obtain the following preferable
example.
An example was attempted to apply, for example,
Teflon working (coating of fluorine resin) onto the heat
source in the case when the photographic processing waste
solution or its vapor contacts directly the heat source,
or to the surface of the thermally conductive portion in
the case when the heat source does not directly contact
the photographic processing waste solution as shown in
Fig. 1. ln this example, two 750 W nichrome wire
built-in quartz tubes were used, a metal kettle was
provided above the quartz tubes and the photographic
processing waste solution was placed therein. When the
experiment of treating the photographic processing waste
solution was conducted by arranging the heating means
applied with Teflon working (coating of a fluorine resin)
as the means for preventing securing of photographic
processing waste solution 44 on the surface of the metal
directly into the photographic processing waste solution
to be treated, 2 liters of waste solution could be
concentrated to 0.5 liter within 1 hour and no securing
phenomenon of tar material of the concentrated waste
solution was seen over a prolonged use. On the other
1 307762
- 36 -
hand, on the metal without any working treatment, a tar
material was found to be secured to take a long time for
concentration of the photographic waste solution for the
second time or thereafter, whereby not only heat
efficiency was remarkably lowered, but also progress of
corrosion was seen at the metal portion.
When the experiments were conducted with Teflon
working means other than coating of a fluorine resin,
such as binder type, plating type, oil mixing type,
heating treatment type, normal temperature wet cloth
type, respectively, good results could be obtained.
Also, when the experiment was conducted in the
same manner by use of inorgnaic fibers as described in
"Collective Composite Material Technology" (published by
Sangyo Gijutsu Center, P.213-219, 1976), "New Material
1984" (published by Toray Research Center, P.287-315,
1984) and "Composite Material" (published by Todai
Shuppankai, 1984), the same results as described above
were obtained.
As shown in Fig. 1, in the case of utilizing an
inner kettle, a means for preventing securing or the
photographic processing waste solution as described above
$s applied on the inner side thereof, particularly at the
portion where heat of the heating means 41 is
transmitted, but it is preferable to apply the means for
preventing securing of the photographic processing waste
solution on the whole instrument within the treating
chamber for prevention of damages of the instruments by
corrosion, etc.
Next, the experiment was carried out for an
example in which a nichrome wire built-in quartz tube was
arranged (not shown) as the heat source at the bottom of
the treating chamber 42 in Fig. 1, a liguid layer 44 of,
for example, silicone oil was formed as the securing
preventing means to slightly the upper portion thereof
and the photographic processing waste solution was stored
1 307762
- 37 -
thereon to carry out heating treatment. As the result,
no securing phenomenon of waste solution onto the heat
source could be seen to obtain good result similarly as
with Teflon working.
The constitution of the treating chamber 42 may be
determined corresponding to the heating means 41 as
described above, but it is preferably brought to an
atmosphere under reduced pressure to lower the boiling
point for promoting evaporation of water.
It is preferable that the treating chamber 42
should be constituted so as to store the photographic
processing waste solution or the concentrate which has
been treated, an inner kettle or inner liner 44 formed of
a metal, porcelain or synthetic resin and, by use of a
separating means such as bag filter or pack, the treated
concentrate should be solidified and taken out together
with the inner liner 47 or the bag filter or pack to be
discarded or disposed. Other than the constitution
utilizing the inner kettle or inner liner 44 as described
above, as the discharging means 43, various designs are
possible such that the concentrate of the photographic
processing waste solution is permitted to fall naturally
through a valve from the known discharging device
utilizing a rotary screw pump or the bottom of the
treating chamber 42 into a vessel containing 1 or 2 or
more of the liquid absorbable resin, solidifying agent
and drying agent.
The inner liner 44 or pack should preferably be
made of a heat resistant and chemical resistant material
such as carbon fiber, aramide fiber, Teflon resin fiber,
hemp, glass fiber, polyethylene form, polypropylene form,
etc.
Also, the concentrate may be added with a liquid
absorbable resin, a solidifying agent or a drying agent
such as lime, etc., before discharging. Further, it is
also preferable to effect discharging through a
1 307762 `
- 38 -
separating means such as bag filter, etc.
The gas adsorbing means 50 separates and recovers
harmful gases such as hydrogen sulfide, sulfur oxide, or
ammonia gas (H2s~ SO2, NH3, etc.) contained in the
evaporated photographic processing waste solution by
utilizing various desulfurization and adsorption
technique such as zeolite adsorbent, activated charcoal,
etc.
The cooling means 60 performs secondary treatment
of the distilled solution subjected to evaporation
treatment by the waste solution treating means 40 with
activated charcoal, reverse osmosis membrane, UV-ray
irradiation, oxidizing agent, etc., to obtain distilled
solution, which is utilized in photographic processing
solution in the automatic processing machine 10. That
is, for example, it is used when utilized as the
dissolving solution for the replenisher or the
stabilizing solution.
D).... .......Control
The control in the evaporation treatment device of
the photographic processing waste solution of the present
invention is important primarily for the respective items
of:
~1) discharging of photographic processing waste
~5 solution to the silver recovery means 80 or the stock
tank 30 and 31;
(2) feeding of the photographic processing waste
solution from the stock tank 30 to the treating means 40;
and
(3) actuation of the treating means 40, and these
items are described in this order.
(1) Discharing of the photographic processing
waste solution to the silver recovery means 80 or the
stock tank 30:
The amount and the temperature of the photographic
processing waste solution in the silver recovery means 80
1 307762
or the stock tank 30 are detected by the sensor 24, and
their informations are successively memoried at the
memory portion of the control device 20. Accordingly,
when the photographic processing waste solution in the
silver recovery means 80 or the stock tank 30 is detected
to be under the full state, replenishment of the
replenisher is prohibited so that no photographic
processing waste solution may not newly discharged, or
emergent discharging by means of a pump is effected from
the silver recovery means 80 or the stock tank 30 to the
treating means 40 following the supplement indicating
information of the replenisher. For prevention of
erroneous actuation, it is preferable to give the silver
recovery means 80 or the stock tank 30 surplus of volume
or arrange a plural number of silver recovery means 80,
stock tank 30 or preliminary tanks. On the other hand,
in the system of treating separately following the kinds
of the photographic processing waste solutions without
treating all of the photographic processing waste
solutions at a time, detection of liquid amounts and
temperature, etc., is performed for respective silver
recovery means 80 and stock tanks 30.
The temperature detection of the photographic
processing waste solution in the stock tank 30 is
important as the information of the photographic
processing waste solution in the actuation control of the
treating means 40 described later, particularly for
control of the heating temperature.
~2) Feeding of the photographic processing waste
solution from the stock tanks 30 and 31 to the treating
means 40:
For feeding control of the photographic processing
waste solution from the stock tanks 30 and 31 to the
waste solution treating means 40, there are the case when
the number of the waste solution treating means 40 is
single and the case when it is plural. In the latter
1 307762
- 40 -
case, further plural number of waste solution treating
means 40 are prepared, and 1 or 2 or more of them may
sometimes function also as the stock tanks. In such a
case, the photographic processing waste solution is
discharged as divided into the waste solution treating
means 40 prepared in a plural number for each waste
solution of the photographic processing tanks similarly
as in the case of discharging into the stock tanks as
described above, and as a general rule subjected to
evaporation treatment by the waste solution treating
means 40 thrown.
When the waste solution treating means 40 is
single, so that the photographic processing waste
solutions stocked separately in the stock tank 30 and 31
may not be mixed with each other, another photographic
processing waste solution is fed after completion of the
treatment of the preceeding solution.
Feeding of the photographic processing waste
solution from the stock tank 30 to the treating means 40
may be performed according to a system in which a
constant amount (the amount which can be stored at a time
within the waste solution treating means 40) is fed at a
time and a system in which it is fed continuously in
equal amounts or variable amounts. In the former case,
feeding of the photographic processing waste solution
from the stock tank 30 to the treating means 40 is
controlled following the detection informations of the
reduced quantity of the photographic processing waste
solution in the stock tank 30 by the sensor 22 and/or the
photographic processing waste solution quantity in the
treating means 40 by the sensor 24. In this case,
feeding may be also controlled following the detected
information by a flow meter provided in the photographic
processing waste solution feeding pipe from the stock
tank 30 to the treating means 40.
In the case of the system of feeding continuously
1 307762
- 41 -
in equal amounts or variable amounts, the amount of the
photographic processing waste solution is controlled
according to the temperature of the photographic
processing waste solution fed, the temperature of the
heating means 41 of the treating means 40 or the treating
chamber 42. Alternatively, with the amount of the
photographic processing waste solution fed being made
always constant, the amount of the photographic
processing waste solution within the treating means 40
may be detected by the sensor 24 and the heating
temperature of the heating means 41 such as heater may be
controlled to be elevated or lowered depending on its
amount, or the heating time may be controlled to be
increased or decreased.
(3) Actuation of the heat-ng means 40:
Control of actuation of the treating means 40, as
also described in the previous item, may be done
according to the difference in amount of the photographic
processing waste solution fed and the photographic
processing waste solution treated, or the amount of the
remaining photographic processing waste solution or the
amounts of the concentrated photographic processing waste
solution.
In the sy~tem of feeding the photographic
processing waste solution in a constant amount at a time,
actuation of the treating means 40 can be controlled by
controlling the heating time if the temperature of the
photographic processing waste solution fed and the
temperature of the heating means 41 or the treating
chamber 42 are detected.
Peeding of the photograpnic processing waste
solution to be treated and actuation of the treating
means ~control of the heating means, discharging of the
treated waste solution) can be designed variously such as
3S actuation stopping during discharging, low energy
actuation of the heating means during discharging,
1 307762
- 42 -
treatment simultaneous with discharging on feeding, etc.
In the above operation, the degree of treatment
progress of the photographic processing waste solution by
the treating means is controlled by the treating time, or
other~ise by detection of the viscosity of the
photographic processing waste solution, the lower limit
level of the photographic processing waste solution
within the processing chamber 42, the vapor temperature,
pressure, weight, electroconductivity, turbidity,
transmission or temperature outside the device, etc., and
it is preferable to change the actuation of the
photographic processing waste solution treating means 40
to stopping or low energy running at the stage when the
photographic processing waste solution has been
concentrated to a certain level.
As described above, feeding, treatment
(evaporation, concentration), discharging of the
photographic processing waste solution are controlled by
a variety of items, and various sensors 24, etc., are
used for detection of time, viscosity, temperature,
pressure, liquid surface level, concentration, electric
resistance, weight, etc., corresponding thereto, and the
sensors 24, etc., can be mounted at various positions.
In the case of time control, it differs depending
on the batch treatment and the continuous throwing
treatment, and the time is also different depending on
the temperature of the photographic processing waste
solution fed.
For measuring the treatment progress by detection
of the viscosity of the photographic processing waste
solution, various viscometers, such as capillary
viscometer, etc., may be employed, or otherwise, for
example, a load imposed on a propeller or rod for
stirring is detected, or the viscosity is detected by the
load imposed on the driving motor when using a bar screw
as the discharging means. For detection of viscosity by
1 30776~
- 43 -
utilizing such a rotary screw or a propeller, the
treatment completion signal is generated by a certain
elevation in rotational loading or reduction in
rotational number according to the elevation in
viscosity.
For detection of concentration, for example, a
light emitter, a reflective plate, a light receiver,
etc., is arranged at a predetermined height in the
treating chamber and a measuring instrument for measuring
transmittance (attenuation degree) of light or refractive
index is used. It can be also detected by the change in
electrical resistance accompanied with the change in
concentration.
For detection of the vapor temperature in the
treating chamber 42, although not shown, for example, the
device may be constituted such that the liquid surface of
the waste solution will be lowered with the evaporation
treatment until the heated portion is exposed on the
liquid surface at a predetermined level or lower. With
such a constitution, the vapor temperature within the
treating chamber will be abruptly elevated by the
overheating phenomenon from the stage when the heating
portion has been exposed, whereby the progress of the
waste solution processing can be detected.
Also, by mounting a sensor causing change in
electrical resistance depending on presence or absence of
the liquid at a predetermined height on the inner wall of
the treating chamber 42, the liquid surface level can be
also detected. The liquid surface level can be also
detected by a mechanical mean by use a float, etc.
For measurement of weight, for example, it can be
detected by arranging an electrical or mechanical
gravimeter below the inner kettle arranged in the
treating chamber 42.
The amount of the vapor generated can be detected
by arranging a flow meter in the stage preceding to the
1 307762
- 44 -
gas adsorbing means, and it can also be detected by
measuring the amount of the stored distilled solution
(weight, liquid surface height) when the cooling means 60
is provided.
In the latter case, actuation of the waste
solution treating means 40 may be changed to stopping or
low energy running by detection of the vapor temperature,
weight or the temperature outside the device.
In the present invention, "concentration" means
reduction of the waste solution volume to one half or
less of the volume when exiting from the photographic
processing tank, preferably one fourth or less with
respect to discarding, more preferably one fifth or less,
optimully one tenth or less. By concentration,
generation of precipitates or tar, etc., may occur. The
liquid as a whole is required to be fluid, and presence
of precipitates or sludge may be permissible.
lExamples]
The present invention is described in detail by
referring to the following examples, by which the
embodiments of the present invention are not limited at
all.
f~ The Sakura~Color SR paper (produced by Konishiroku
Photo industry Co.) was picture printed and then
subjected to continuous processing by use of the
following processing steps and processing solutions.
Basic processing steps:
~1) Color developing 38 C 3 min. 30 sec.
t2) Bleach-fixing 38 C 1 min. 30 sec.
30 ~3) Stabilizing processing 25 C-35 C 3 min.
(4) Drying 75 C-100 C about 2 min.
Processing solution compositions:
lColor developing tank solution~
~enzyl alcohol 15 ml
35 Ethylene glycol 15 ml
Potassium sulfite 2.0 g
Ir 1~ra~/c~ r~
1 307762
- 45 -
Potassium bromide 1.3 g
Sodium chloride 0.2 g
Potassium carbonate 24.0 g
3-Methyl-4-amino-N-ethyl-N-(B-
methanesulfoneamidethyl)aniline
sulfate 4.5 g
Fluorescent brightener (4,4'-
diaminostilbendisulfonic acid
derivative) (trade name: Kaicoll
PK-conc, produced by Shinnisso
Kako Co.)) 1.0 g
Hydroxylamine sulfate 3.0 g
l-Hydroxyethylidene-l,l-
diphosphonate 0.4 g
Hydroxyethyliminodiacetic acid 5.0 g
Magnesium chloride hexahydrate 0.7 g
1,2-Dihydroxybenzene-3,5-disulfonic
acid-disodium salt 0.2 g
~made up to one liter with addition of water, and
~0 adjusted to pH 10.20 with potassium hydroxide and
sulfuric acid).
[Color developing replenisher]
Benzyl alcohol 20 ml
Ethylene glycol 20 ml
Potassium sulfite 3.0 g
Potassium carbonate 30.0 g
Hydroxylamine sulfate 4.0 g
3-methyl-4-amino-N-ethyl-N-(B-
methanesulfoneamideethyl)aniline
sulfate 6.0 g
Fluorescent brightener ~4,4'-
diaminostilbendisulfonic acid
~ derivative) (trade ~ : Kaicoll
`` PK-conc, produced by Shinnisso
Xako Co.)) 2.5 g
l-Hydroxyethylidene-l,l-diphosphonic
1 307762
- 46 -
acid 0 5 g
Hydroxyethyliminodiacetic acid 5.0 g
Magnesium chloride hexahydrate 0.8 g
1,2-Dihydroxybenzene-3,5-disulfonic
acid disodium salt 0.3 g
~made up to one liter with addition of water, and
adjusted to pH 10.70 with potassium hydroxide).
[Bleach-fixing tank solution]
Ferric ammonium ethylenediamine-
tetraacetate dihydrate 60.0 g
Ethylenediaminetetraacetic acid 3.0 g
Ammonium thiosulfate
(70% solution) 100.0 ml
Ammonium sulfite ~40~ solution) 27.5 ml
1~ (made up to the total quantity of one liter with
addition of water and adjusted to pH 7.1 with
pottasium carbonate or glacial acetic acid).
[Bleach-fixing replenisher A]
Ferric ammonium ethylenediamine-
tetraacetate dihydrate 260.0 g
Potassium carbonate 42.0 g
~made up to the total quantity of one liter with
addition of water, the pH of this solution is
6.7+0.1).
tBleach-fixing replenisher B]
Ammonium thiosulfate
(70% solution) 500.0 ml
Ammonium sulfite (40% solution) 250.0 ml
Ethylenediaminetetraacetic acid l?.o g
Glacial acetic acid 85.0 ml
~made up to the total quantity of one liter with
addition of water, the pH of this solution is
5.3+0.1).
lStabilizing tan~ solution substituting for water washing
and its replenisher]
Ethylene glycol 1.0 g
1 307762
- 47 -
l-Hydroxyethylidene-l,l-diphosphonic
acid (60% aqueous solution) 1.0 g
Ammonia water (25% aqueous solution
of ammonium hydroxide) 2.0 g
(made up to one liter with water and adjusted to
pH 7.0 with sulfuric acid).
An automatic processing machine was filled with
the above color developing tank solution, the
bleach-fixing tank solution and the stabilizing tank
solution, and running test was performed while processing
the above Sakura Color SR paper sample and replenishing
the above color developing replenisher, the bleach-fixing
replenishers A, B and the stabilizing replenisher through
the constant volume cups every 3 minutes. The
replenished amounts were 190 ml as the replenished amount
into the color developing tank, each 50 ml of the
bleach-fixing replenishers A, B as the supplemented
amounts into the bleach-fixing tank and 250 ml of the
stabilizing replenisher substituting for water washing as
the supplemented amount into the stabilizing bath,
respectively per 1 m2 of the color paper. The
stabilizing tank of the automatic processing machine was
made a multi-tank countercurrent system, in which the
first to the third stabilizing tanks were provided in the
direction of the flow of the sample, and replenishment
was effected from the final tank, the overflowed solution
from the final tank was permitted to flow into the tank
in the preceding stage, and further the overflowed
solution from this tank was permitted to flow into the
tank preceding thereto.
Continuous processing was performed until the
total amount replenished of the stabil zing solution
substituting for water washing became 3-fold of the
stabilizing tank volume.
For the photographic processing waste solution (A~
which is the overflowed solution generated by the above
1 307762
- 48 -
processing, mixed at a ratio of [the overflowed solution
of the color developing solution] : ~the overflowed
solution of the bleach-fixing solution] : ~the overflowed
solution of the stabilizing solution substituting for
water washing] = 3 : 3 : 5, the following treatment was
practiced.
Example 1
Evaporation treatment of the photographic
processing waste solution (A) was carried out by use of
an evaporation kettle having two nichrome wire built-in
quartz tubes of 750 W placed therein, and the vapor Q
generated was cooled by means of a radiation plate device
62 shown in Fig. 3 to obtain distilled liquid R. Also,
the odor was found to be extremely little as compared
with the case when using no radiation plate device.
Examples 2 to 7
When the vapor Q obtained by the evaporation
treatment in Example 1 was subjected to cooling treatment
by use of the cooling means shown in Fig. 4 to Fig. 9,
the odor was found to be extremely little in any case
similarly as in Example 1 as compared with the case when
using no cooling means to give distilled liquid R without
any problem. In Fiq. 4 to Fiq. 6, the photoqraphic
processing waste solution had been preliminarily heated,
and the evaporation treatment in the evaporation kettle
was faster under the same conditions as compared with the
case without preliminary heating. Further, the cooling
means shown in Fig. 8 was found to be suitable for
temperature control of the color developinq tank.
Example 8
After evaporation of the 2 liter of the
photographic processinq waste solution (A) to dryness,
the lid 45 was taken off and the dried product was taken
out from the evaporation kettle. As the result, it had a
strong odor of hydroqen sulfide, with the dried product
beinq sticked to the bottom of the evaporation kettle and
also scattered on the wall, whereby all of them could be
~ 307762
- 49 -
removed with difficulty.
Example 9
An inner liner 44 comprising a carbon fiber fabric
was covered previously on the bottom of the evaporation
S kettle, and after evaporation concentration similarly as
in Example 1, 20 g of a high liquid absorbable resin
(Sumikagel N-100: trade name, produced by Sumitomo Kagaku
Co.) was added, and the solidified product was removed
together with the inner liner 44 comprising the above
fabric to be wholly removed with ease. When the inner
portion of the evaporation kettle was observed, it was
found to be clean without any trace of the so-called
sticking or scattering.
Example 10
Evaporation treatment was carried out with the
photographic processing waste solution (A) to be treated
fed first into the treating chamber 42 being 2 liter and
the heat capacity of the heater 1.5 KW, and the
photographic processing waste solution within the
treating means 40 was examined when the viscosity at the
evaporation temperature by a rotary viscometer was
increased by 10%. As the result, it was found to be
concentrated to about 3-fold.
Example 11
Evaporation treatment was carried out with the
photographic processing waste solution (A) to be treated
fed first into the treating chamber 42 being 2 liter and
the heat capacity of the heater 1.5 KW, and new feeding
of the` photographic processing waste solution (A) to be
3~ treated was repeated several times when the viscosity by
a rotary viscometer was increased by 10%, and then the
photographic processing waste solution in the treating
means 40 was examined to find that it was concentrated to
about 3-fold similarly as in Example 10.
Example 12
When the liquid surface level within the treating
1 307762
- 50 -
chamber 42 was detected to be leveled down to one tenth,
the thickened waste solution was taken out. Also, when
the liquid surface level within the treating chamber 42
was detected to be leveled down to one fifth, the
photographic processing waste solution (A) to be treated
was newly fed. Again, when the liquid level was leveled
down to one fifth, the thickened waste solution was taken
out.
Example 13
After desilverization treatment of the
photographic processing waste solution (A) by use of an
electrolytic silver recovering device (produced by San
Seiki Industry Ltd., BN-10 type) until the concentration
of silver complex salt is reduces to 0.2 g/lit., the
waste water was permitted to flow into the stock tank 30.
A mixture of such desilverized photographic processing
waste solution and overflowed solution of the color
developing solution (volume ratio = 3:8) was transferred
from the stock tank 30 to the treating means 40. In the
treating means 40, the waste solution was subjected to
evaporation treatment by use of an evaporation kettle
having two nichrome wire built-in ~uartz tubes of 750 W
placed therein. When the evaporation treatment was
continuously repeated, it was found that, in the case of
evaporation treatment of the photographic processing
waste solution which have not been desilverized, silver-
sulfate was sticked on the bottom of the evaporation
kettle, the evaporation efficiency was reduced and hence,
after one month, corrosion of the evaporation kettle was
caused. on the other hand, in the case of evaporation
treatment of the photographic processing waste solution
desilverized according to the present invention, such
defects were not found at all.
Example 14
Example 13 was repeated in the same manner except
for adding sodium sulfide into the photographic
- 51 - 1 3 07 7 62
processing waste water in place of employing the
electrolytic silver recovering device BN-10 in Example 13
to precipitate silver as silver sulfide. After
separation by decantation, the filtrate was permitted to
flow into the stock tank 30. Similar good result as in
Example 13 was obtained.
Example 15
Example 13 was repeated in the same manner except
for carrying out the silver recovering operation for the
concentrate which have been subjected to the evaporation
treatment. It was found that silver sulfide was sticked
to the bottom of the evaporation kettle and, after one
month, corrosion of the evaporation kettle was caused.
It was also found that, though the same amount of the
waste solution having the same silver concentration as in
Example 13 was employed, the obtained electrolytic
deposited silver was as low as about 20 % of that
obtained in Example 13 in which the silver recovery was
carried out bofore the evaporation treatment, and further
that the electrolytic deposited condition of the
electrolytic deposited silver was remarkably poor to
cause peel off.
Example 16
After evaporation treatment of the photographic
processing waste solution ~A) by use of an evaporation
kettle having two nichrome wire built-in quartz tubes of
750 W placed therein until the liquid surface level
become one tenth, the vapor generated was cooled and the
obtained distilled solution was used for preparation of a
bleach-fixing solution as a dissolving water. The
bleach-fixing solution was employed for processing a
ligh-sensitive photographic material. As a result, no
problem was caused on the photographic properties and no
bumping was seen.
Example 17
The distilled solution obtained by the evaporation
1 3~7762
- 52 -
treatment in Example 16 was treated through a column
being filled up with Cargon~granule-like activated
charcoal TYPE SGL ~produced by Toyo Cargon Co.) and the
obtained secondary treated solution was used for
preparation of a color developing solution. The color
developing solution was employed for processing a
ligh-sensitive photographic material. As a result, no
problem was caused on the photographic properties.
Example 18
After irradiation to the distilled solution
obtained in Example 16 by use of high pressure mercury
lump of 400 W for 4 hours, the secondary treated solution
obtained was used for preparation of a bleach-fixing
solution and subsecuently the bleach-fixing solution was
lS used for processing a light-sensitive photographic
material. As a result, no problem was caused on the
photographic properties.
Example 19
The distilled solution obtained by the evaporation
treatment in Example 16 was treated by use of an
electrolytic device and the obtained secondary treated
solution was used for preparation of a stabilizing
solution. The stabilizing solution was employed for
processing a light-sensitive photographic material. As a
result, no problem was caused on the photographic
properties.
Example 20
After the treatment of the distilled solution
obtained by the evaporation treatment in Example 16 by
permitting air to pass through the distilled solution by
use of an air pump for 1 hour, the obtained secondary
treated solution was used for preparation of a
stabili~ing solution substituting for water washing and
the stabilizing solution substituting for water washing
was employed for processing of the light-sensitive
photographic material. As a result, no problem was
* T~
1 307762
- 53 -
caused on the photographic properties.
Example 21
Example 1 was repeated in the same manner except
for employing a photographic processing waste solution
which was prepared by removing the overflowed solution of
the stabilizing solution substituting for water washing
from the photographic processing waste solution (A). On
the other hand, Example 1 was repeated in the same manner
for employing a photographic processing waste solution
which was prepared by containing the same quantity of
water in place of the overflowed solution of the
stabilizing solution substituting for water washing of
the photographic processing waste water (A). As the
resuls, a little sticking and scattering were found in
both of them.
As being seen from the comparison with Example 1,
in the case of employing the photographic processing
waste solution containing the overflowed solution of the
stabilizing solution substituting for water wahing, it is
understood that so-called sticking and scattering is very
little and good results can be obtained.
Example 22
Example 1 was repeated in the same manner except
for removing the gas adsorption means 50 in Figure 1 and
employing gas adsorbable means 51 being filled up with
granule-like activated charcoal (a mixture of activated
charcoals type AX and type BX, both are produced by
TSURUMICOAL Co. Ltd.). After continuous treatment for 10
hours, amounts of ammonia gas and hydrogen sulfide gas in
the discharging section 52 were measured by use of gas
detector tube (produced by GASTEC Co.). Subsequently,
the same experiment for comparison was carried out for 10
hours and the same measurements of gases were conducted
except for removing the cooling means 60.
As a result, in the case of employing the cooling
means 60 according to the present invention, the
1 307762
- 54 -
concentration of ammonia gas was 5 ppm and hydrogen
sulfide was 0 ppm. Both of these values are under the
value of allowable concentration advised by ACGIH
(American Conference of Governmental Industrial
Hygienists) (ammonia: 25 ppm, hydrogen sulfide: 10 ppm).
Odor was scarecely felt. On the contrary, in the case of
removing the cooling means 60, both of the concentrations
of ammonia gas and hydrogen sulfide were 0 ppm
immediately after the beggining of the eveporation
treatment. However, after 1 hour from the beggining of
the evaporation treatment, the concentration of ammonia
gas was 160 ppm and hydrogen sulfide was 240 ppm. The
air around the evaporation treatment device was heavy
with bad oder,